3.1. Basal Area
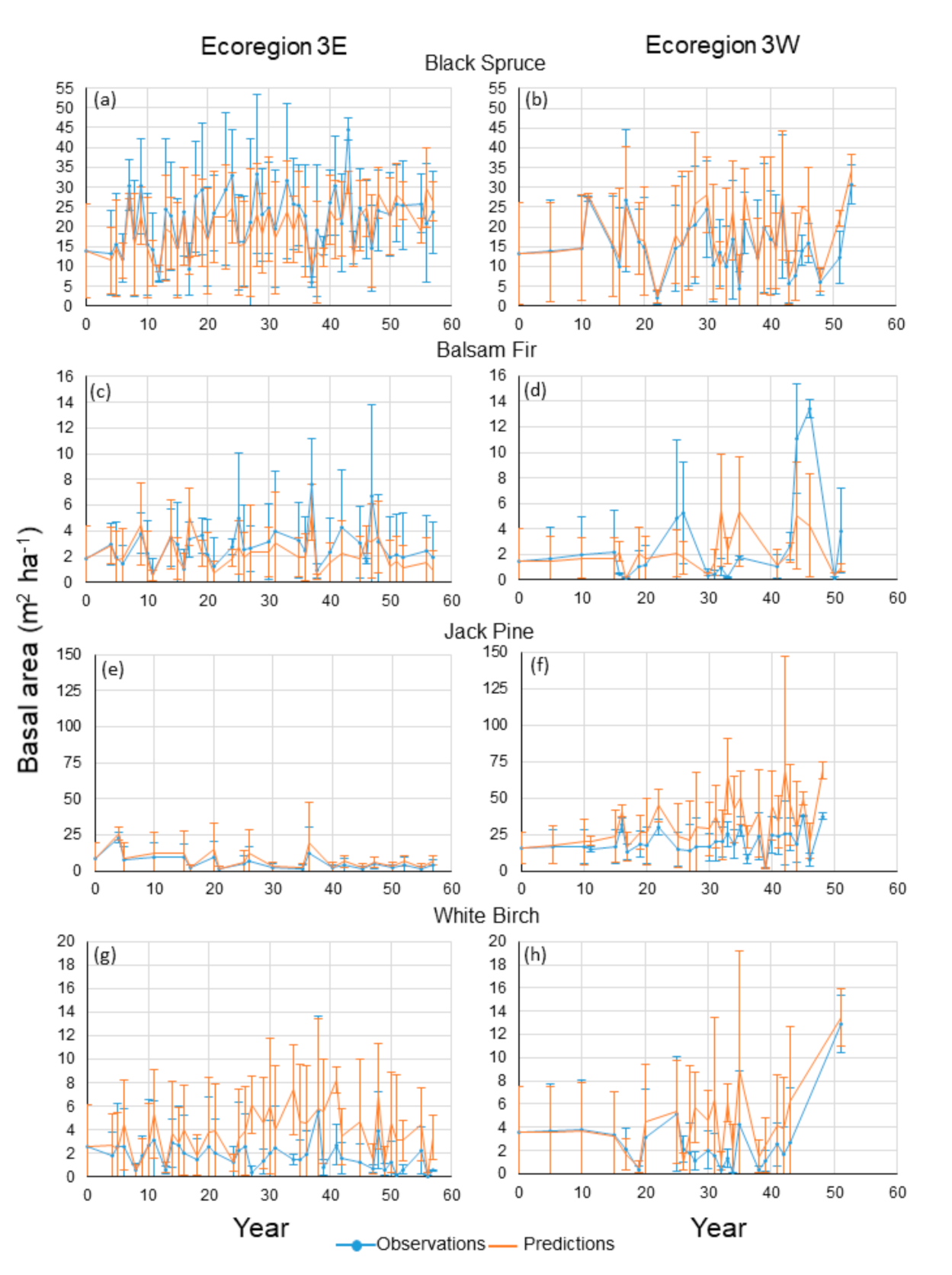
Agreement between mean predicted and observed basal areas was high for black spruce in the two ecoregions for nearly every year and the standard deviations between observations and predictions overlapped considerably (
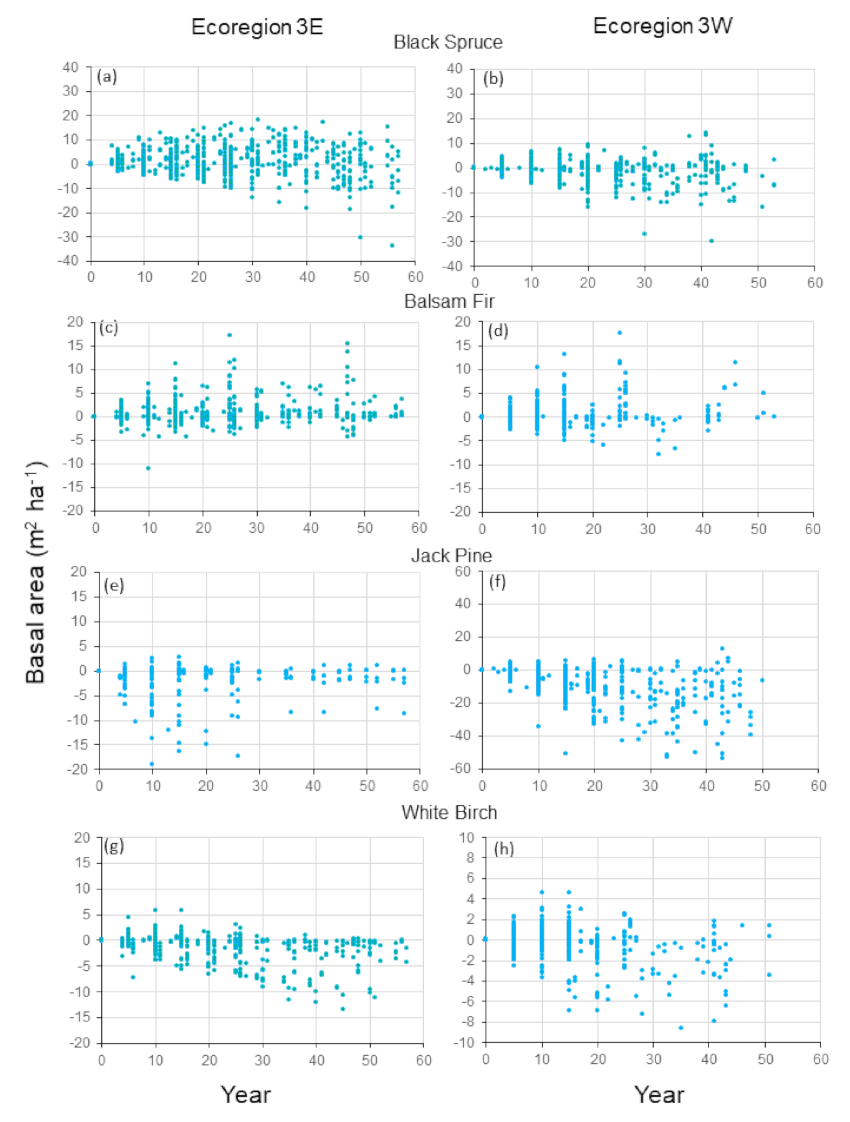
Figure 2a,b). For Ecoregion 3E, percentages of differences between observations and predictions smaller than 2 and 5 m
2 ha
−1 in absolute values were 54 and 78%, respectively (
Figure 3a). The maximum amplitudes of the majority of the differences were ±10 m
2 ha
−1 over years and the number of differences smaller or higher than −10 or 10 m
2 ha
−1 were marginal, representing only 6% of the differences. The same statistics for differences smaller than 2 and 5 m
2 ha
−1 in absolute values for Ecoregion 3W were 73 and 90%, with a pronounced proportion of negative differences (
Figure 3b). Only 2.9% of the differences between observations predictions were smaller or higher than 10 m
2 ha
−1 over years and were observed in the past 30 years. Fluctuations were important for both ecoregions, but most of them occurred in the same years for predictions and observations (
Figure 3a,b). Mean basal area increased slightly over time in Ecoregion 3E. For Ecoregion 3W, there was a relatively stable pattern of change over time, despite fluctuations.
The pattern of change over years in mean basal area for balsam fir was relatively stable for both ecoregions, but fluctuations were more pronounced than those for black spruce and did not always occur in the same years, particularly in Ecoregion 3W (
Figure 2c,d). For Ecoregion 3W, agreement between predictions and observations was reasonably high from years 0 to 15, and for years 19, 20, 30, 41, 41 and 50, with large overlaps between the standard deviations of observations and predictions. At the plot scale and by year, 82 and 94% of the differences between predictions and observations in Ecoregion 3W were less than 2 and 5 m
2 ha
−1, respectively, and were within the same amplitude of differences over years (
Figure 3d). Only 6% of the differences between observations and predictions were higher than 5 m
2 ha
−1. A close examination of the sample plot data in observation years 25 and 26 indicated that the large differences between observations and predictions were caused by extreme values in nine sample plots. The pronounced increases in basal area that was observed from years 25 to 26 and from years 44 to 46, which increased the differences between observations and predictions, were probably caused by measurement or record errors in the historical data. Differences between mean predictions and observations for Ecoregion 3E were generally less pronounced than in Ecoregion 3W, but comparable percentages of differences among sample plots and years were observed: 83 and 95% were less than 2 and 5 m
2 ha
−1 in absolute values, respectively (
Figure 3c). The range of variation was the same over years, except for some large differences at years 10, 25, 26 and 47, which represented 2.5% of the total number of differences. Substantial overlaps in the standard deviations of observations and predictions and the occurrences of fluctuations were evident at the same time for most years.
Agreement between mean observations and predictions was high for jack pine in Ecoregion 3E, which was characterized by a slight pattern of decrease over time (
Figure 2e). Differences between predictions and observations smaller than 2 and 5 m
2 ha
−1 represented 77 and 87% of the differences at the plot scale and by year. The amplitude of variation was relatively homogeneous over years, except for some large negative differences from year 10 to 60 (
Figure 3e), representing 13% of the total number of differences. For Ecoregion 3W, mean observed and predicted basal areas for jack pine more than doubled in 48 years, despite fluctuations (
Figure 2f). Differences between mean predictions and observations were generally higher than those in Ecoregion 3E (
Figure 3f), but their standard deviations overlapped considerably for most years: 50 and 63% of the differences between observations and predictions were less than 2 and 5 m
2 ha
−1 in absolute values, respectively, over years.
Mean predicted and observed basal areas for white birch in Ecoregion 3E fluctuated considerably and generally did not occur the same years (
Figure 2g). Agreement was high between mean observations and predictions for the first 25 years. Then, differences generally increased until year 50, but were all not pronounced: 80 and 91% of the differences between observations and predictions were smaller than 2 and 5 m
2 ha
−1 in absolute values, respectively (
Figure 3g). The greatest differences between observations and predictions were observed from years 27 to 51 (8%). Mean observed and predicted basal areas in Ecoregion 3W were characterized by a pattern of decrease in the first 40 years, followed by a sharp increase (
Figure 2h). Agreement was high between predictions and observations for the first 15 years. Then, relatively large negative differences were observed between years 15 and 43, with no strong overlap between the standard deviations of predictions and observations. When considered at the plot scale and by year, these relatively large differences can be attributed to sharp decreases in observations for some years only (
Figure 3h), considerably decreasing the mean values. Despite these discrepancies, 86 and 96% of the differences between predicted and observed basal areas were less than 2 and 5 m
2 ha
−1 over years, respectively.
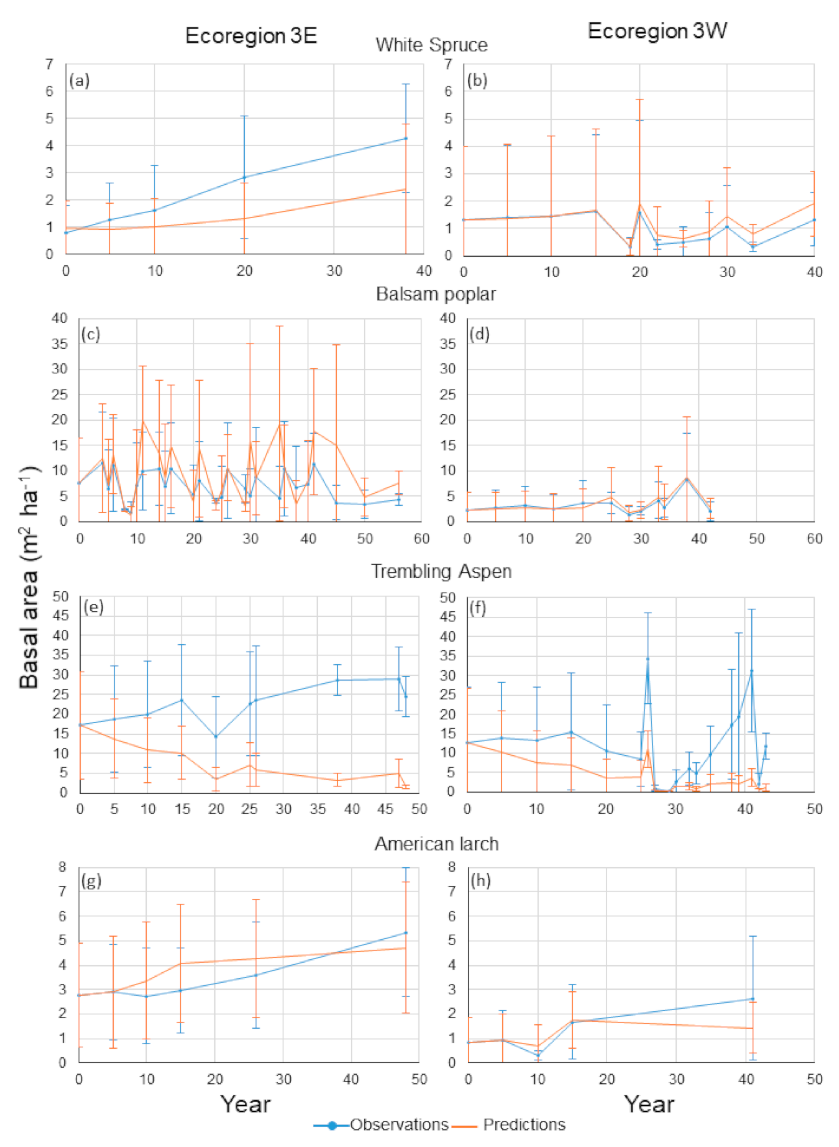
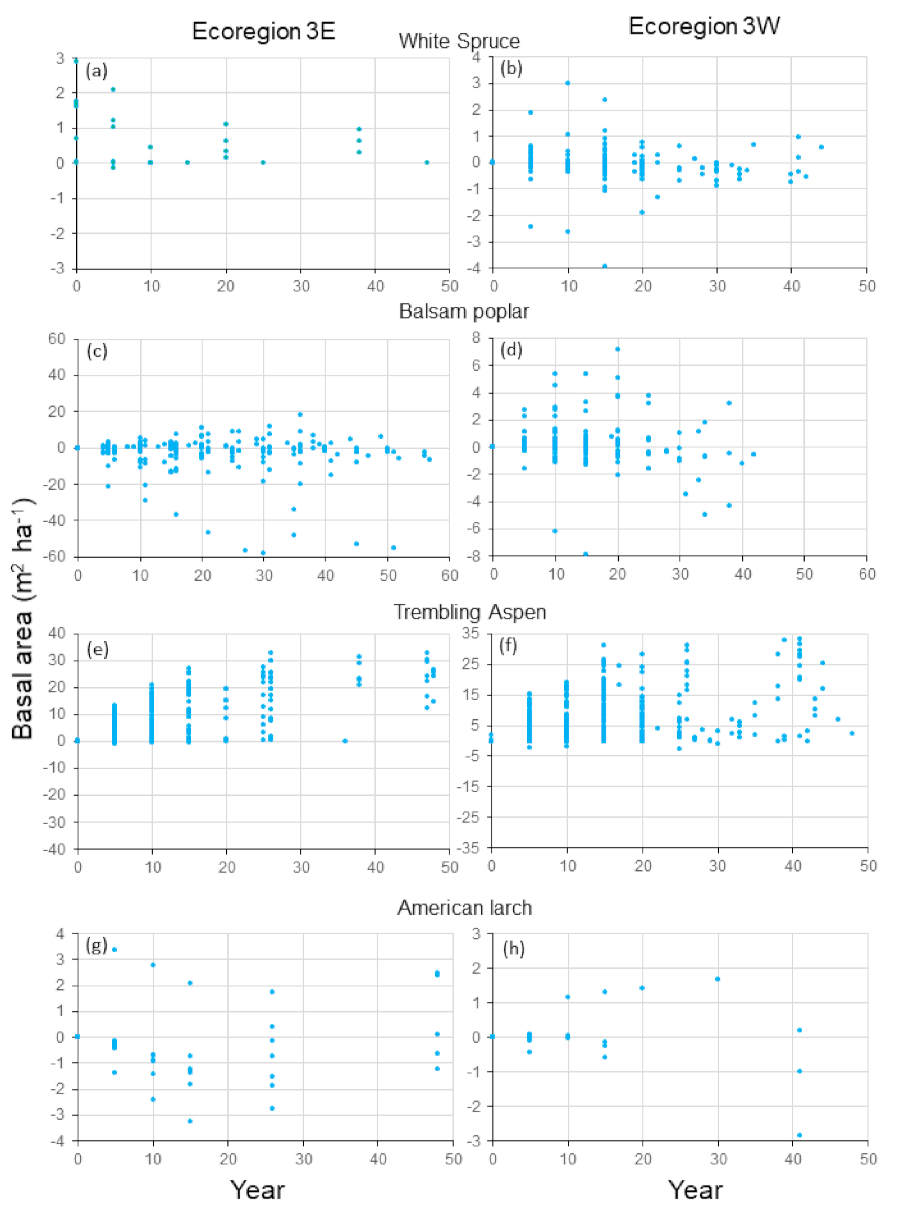
Compared to the other species, white spruce occupied small proportions of the stands, as indicated by the relatively small basal area values (
Figure 4a,b). Mean predictions and observations were similar until year 10 and their standard deviations overlapped considerably for Ecoregion 3E (
Figure 4a). However, compared to other species, there was a higher agreement between observations and predictions: 72% of the differences between predictions and observationsover years were smaller than 1 m
2 ha
−1 and only 2 differences were greater than 2 m
2 ha
−1 (
Figure 5a). Even though differences between mean predictions and observations increased over years (
Figure 4a), they remained relatively small (
Figure 5a). Agreement was high between mean predicted and observed basal areas in Ecoregion 3W for all years (
Figure 4b). The standard deviations for both mean predictions and observations overlapped considerably and fluctuations occurred in the same years. Deviations between observations and predictions were similar to those in Ecoregion 3E: 95% of the differences between predictions and observations were smaller than 1 m
2 ha
−1 in absolute values (
Figure 5b).
Mean predictions and observations for balsam poplar in Ecoregion 3E fluctuated, with no obvious pattern of increase or decrease over time (
Figure 4c). Despite apparent large discrepancies, the percentages of differences smaller than 2 and 5 m
2 ha
−1 between predictions and observations were 62 and 81%, respectively, over years (
Figure 5c). Very large differences between mean predictions and observations at years 11, 21, 30, 35 and 45 also resulted from sudden decreases in observed basal area relative to previous years in some sample plots. However, the number of large differences between observations and predictions represented only 12% of these values. Mean predictions and observations for balsam poplar in Ecoregion 3W were very close and their standard deviations overlapped considerably (
Figure 4d) and 76% of the differences between predictions and observations over years were less than 1 m
2 ha
−1 (
Figure 5d).
Results for trembling aspen were not as consistent as those for the other species. Large differences between mean predictions and observations were observed for nearly every year in Ecoregion 3E and standard deviations did not much overlap (
Figure 4e). Large positive differences between predictions and observations were observed nearly every year (
Figure 5e). For Ecoregion 3W, mean predicted basal area decreased over years and mean observed basal area fluctuated considerably (
Figure 4f). However, agreement was relatively high for years 29, 30 and 42. In years 5, 10, 15, 25 and 32, the standard deviations for both predictions and observations overlapped. Despite large differences between mean predictions and observations for several years, 52 and 66% of the differences between observations and predictions were less than 2 and 5 m
2 ha
−1, respectively (
Figure 5f). The large differences for years 26, 35 to 41 and 43 were caused by high mortality rates predicted by ZELIG-CFS for some sample plots.
American larch occupied a small proportion of the forests compared to the other species (
Figure 4g,h). For the two ecoregions, agreement was high between mean predictions and observations and their standard deviations largely overlapped. Over years, 57 and 81% of the differences between observations and predictions were less than 1 m
2 ha
−1 for ecoregions 3E and #w, respectively (
Figure 5g,h).
Bias values indicated that, on average, ZELIG-CFS slightly underestimated basal area for black spruce in ecoregions 3E and 3W, but slightly overestimated basal area for jack pine in Ecoregion 3E (
Table 7). In Ecoregion 3E, high positive bias values were observed for balsam fir, white birch, balsam poplar and American larch, but relatively high negative bias for white spruce and trembling aspen. Balsam fir and balsam poplar had high average bias values in Ecoregion 3E, but lower average bias values in Ecoregion 3W. The reverse was observed for jack pine between Ecoregions. In absolute values, lower bias values were observed for white birch, trembling aspen and American larch in Ecoregion 3W than in 3E. While a negative bias was observed for white spruce in Ecoregion 3E, a positive bias was observed in Ecoregion 3W. In many cases, high bias values were caused by extremes that were not necessarily associated with many substantial differences between predictions and observations. For instance, 25% of the bias values at the combined plot scale and by year for balsam fir in Ecoregion 3E were less than 10% and most of the high bias values occurred in simulation results past 20 years. Additionally, high bias values were observed in many sample plots that did not contain many trees of particular species. For instance, predicted basal area for balsam fir in a sample plot was 0.011 m
2 ha
−1, but observed basal area was 0.056 m
2 ha
−1, which resulted in a bias of 80% in absolute value. The predicted basal area represented 15 small trees with average dbh of 2.5 cm over 184 trees in a 809 m
2 sample plot. For the other five species in the sample plot, average dbh was 9 cm, with 42 trees larger than 15 cm in dbh. This pattern was observed in several sample plots for species with high average bias values (data now shown).
Model efficiency was fairly high for black spruce and white spruce in both ecoregions and for white birch and balsam poplar in Ecoregion 3W (
Table 7). It was greater than 0.50 for American larch in both ecoregions, and trembling aspen in Ecoregion 3W, between 0.40 and 0.50 for balsam fir in both ecoregions and jack pine in Ecoregion 3E, and very low for trembling aspen in Ecoregion 3E. Only two negative values were observed: balsam poplar in Ecoregion 3E and jack pine in Ecoregion 3W. Low or negative values of model efficiency observed for some species can be associated with the occurrence of substantial differences between observations and predictions, but they did not affect the general patterns of similarity of changes in basal area over time for most species (
Figure 2 and
Figure 4).
3.2. Stand Density
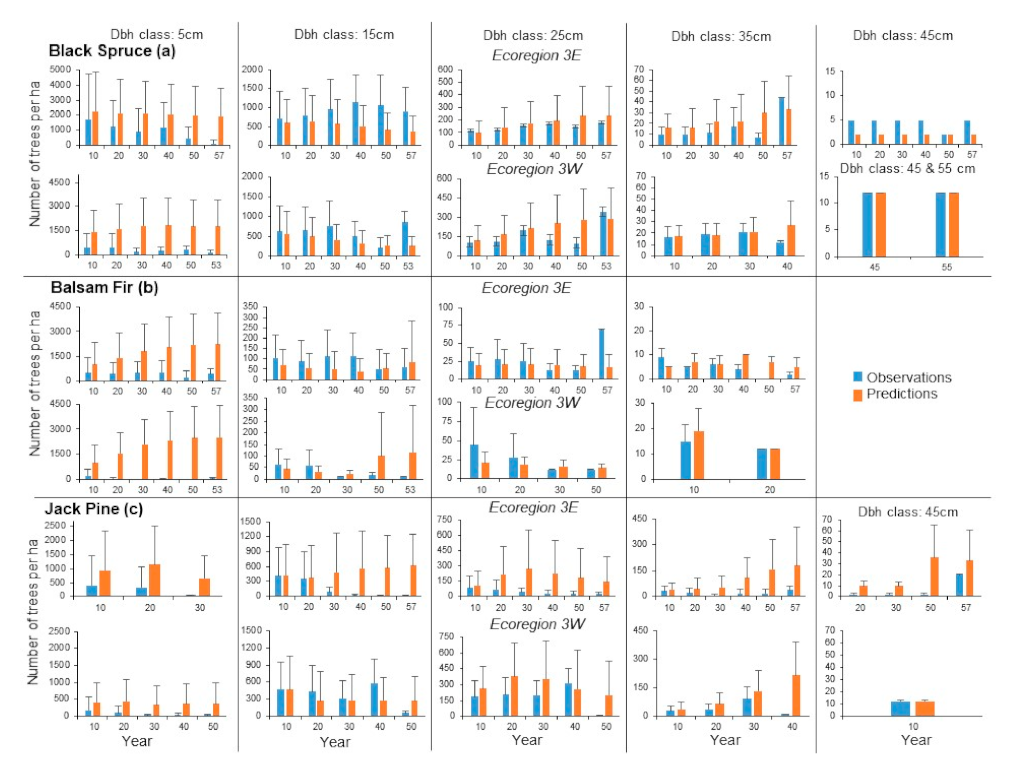
Agreement for dbh class distributions for black spruce in Ecoregion 3E was relatively high between mean observed and predicted stand densities for several years and dbh classes (
Figure 6a). Differences between mean observations and predictions were generally more pronounced in older years and large dbh classes. However, for most dbh classes and years, the standard deviations between observations and predictions largely overlapped. For the 45 cm dbh class, the absence of standard deviations was the result of lack of variation in the observed and predicted numbers of trees. These results are consistent with the similarity coefficients computed at the plot scale, which indicated a gradual decrease over year, but with relatively high coefficients in the first 40 years (
Figure 7). Differences between mean observed and predicted stand densities in Ecoregion 3W were larger than those in Ecoregion 3E for the 5 cm dbh class, but agreement was relatively high for the 15, 25 and 35 cm dbh classes for most years (
Figure 6a). For the 45 and 55 cm dbh classes, observed and predicted mean stand densities at years 45 and 55 were the same, which explains the absence of standard deviations. The similarity coefficients were generally smaller than those in Ecoregion 3E, particularly in the first few years, and were also characterized by a pattern of decrease over time (
Figure 7). When analyzed by combining plots, years and dbh classes, results indicated that 47.3 and 61.2% of the differences between observed and predicted stand densities were less than 100 trees ha
−1 for ecoregions 3E and 3W, respectively (
Table 8 and
Table 9). Differences greater than 100 trees ha
−1 may appear considerable, but, in reality, they are less important when adjusted to sample plot area That is, a difference of 100 trees ha
−1 means a difference of 4 trees in a 400 m
2 sample plot. Differences greater than 100 trees ha
−1 were observed for sample plots combined with years and dbh classes with high densities.
For balsam fir in Ecoregion 3E, relatively good agreements were observed between mean observed and predicted densities for some years in the 15, 25 and 35 cm dbh classes (
Figure 6b). Low agreement was evident for all years for the 5 cm dbh class. For year 50 for the 35 cm dbh class, ZELIG-CFS predicted the presence of 7 trees ha
−1, but none were present in the observations. However, 7 years later (year 57), trees were present in this dbh class, suggesting an error in the observation data set. No differences were observed between 22% of the observed and predicted stand densities and 37% were greater than 200 trees ha
−1 (
Table 8). Percent differences decreased gradually from the lowest (1–20 trees ha
−1) to the highest (181–200 trees per plot) density classes. Except for 11 years, the similarity coefficients were higher than 0.50 (
Figure 7). Differences between observed and predicted mean stand densities were more pronounced in Ecoregion 3W than 3E, particularly for the 5 and 15 cm dbh classes (
Figure 6b), which resulted in lower similarity coefficients (
Figure 7). ZELIG-CFS predicted high densities for both dbh classes, but observations indicated that few trees were present. It is possible that errors in the observation data set may be the cause of the disparity. Despite the large differences between observations and predictions, agreement was high in many instances. The absence of differences between observations and predictions were evident for 33.8% of the combinations of sample plots, years and dbh classes, 33.7% of the differences varied between 1 and 200 trees ha
−1 and 32.5% of the differences were greater than 200 trees ha
−1 (
Table 8).
Larges differences between mean observed and predicted stand densities were observed for jack pine in Ecoregion 3E for most years in each dbh class (
Figure 6c). However, the percentages of differences between observed and predicted stand densities suggest that the large differences were caused by only a relatively small number of combinations of sample plots, years and dbh classes in the results (
Table 8). As much as 32.7% of the differences between observed and predicted stand densities were less than 1 tree ha
−1, 56.8% of them were between 1 and 200 trees ha
−1 and only 10.6% of them were greater than 200 trees ha
−1 (
Table 8). The relatively high percentage of the absence of differences between observations and predictions may explain the occurrence of relatively high similarity coefficients (
Figure 7). Except for the 5 cm dbh class, differences between observed and predicted stand densities were smaller for Ecoregion 3W than for 3E (
Figure 6c). Substantial overlaps between the standard deviations of observations and predictions suggested that agreement was high in several instances. As much as 27.5% of the differences between observations and predictions were negligible, 47.2% were between 1 and 200 stems ha
−1, and 25.3% were greater than 200 stems ha
−1 (
Table 9). Similarity coefficients were greater than 0.50 for 13 years over a total of 40 years (
Figure 7).
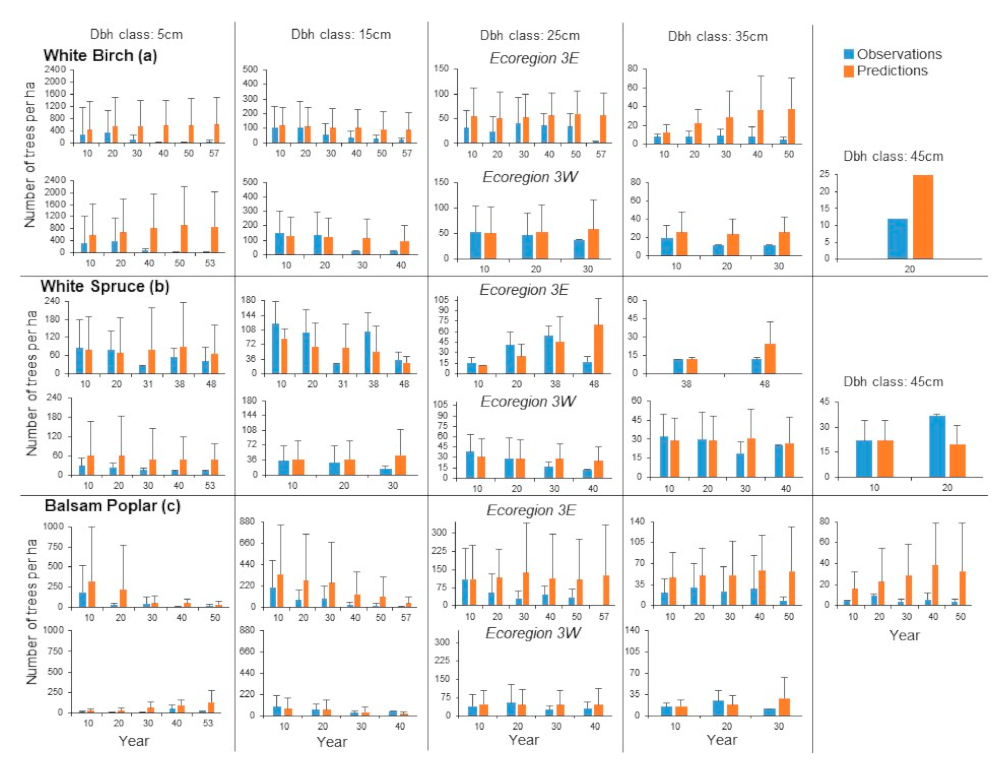
The patterns of mean dbh class distribution for white birch in Ecoregion 3E indicated high agreement between observations and predictions for some years and dbh classes (
Figure 8a). Substantial differences were observed for the older years in the 5, 15 and 35 cm dbh classes and only for year 57 in the 25 cm dbh class. Large overlaps in the standard deviations between observations and predictions indicated agreement was high in several cases: differences between observations and predictions did not differ for 29.3% of the combinations of sample plots, years and dbh classes and only 16.2% of them were greater than 200 trees ha
−1 (
Table 8). Similarity coefficients were relatively low for most years (
Figure 7). Results for Ecoregion 3W differed considerably from those for Ecoregion 3E (
Figure 8a). Most of the large differences between mean observed and predicted stand densities were observed in the older years for most dbh classes, except for the 25 cm dbh class. As suggested by the large overlaps between the standard deviations of observations and predictions, agreement was high for many combinations of sample plots, years and dbh classes: no difference between observed and predicted stand densities were observed in 37.8% of the cases and only 12.9% had differences greater than 200 trees ha
−1 (
Table 9). Most of the similarity coefficients were smaller than 0.50 (
Figure 7).
For white spruce in Ecoregion 3E, agreement between observed and predicted mean stand densities were high for most dbh classes and years (
Figure 8b). There were no differences between observed and predicted stand densities for 36.7% of the combinations of sample plots, years and dbh class distributions and only 1.7% of them were greater than 200 trees ha
−1 (
Table 8). The similarity coefficients were all greater than 0.60 (
Figure 7). Except for the 5 cm dbh class, agreement between observed and predicted stand densities was high for Ecoregion 3W (
Figure 8b). In general, differences between observed and predicted stand densities were smaller than those in Ecoregion 3E (
Figure 8b): 58.6% of the differences between observed and predicted stand densities were negligible and only 0.2% of them were higher than 200 trees ha
−1. The similarity coefficients were all greater than 0.60.
Differences between observed and predicted mean stand densities were large for balsam poplar in Ecoregion 3E for most of the dbh classes and years (
Figure 8c). Relatively high agreement was observed only for year 10 in the 5, 15 and 25 cm dbh classes. However, despite the pronounced differences in the mean densities, the results at the sample plot scale and by dbh class and year indicated that 31.9% of observed stand densities coincided with the predicted densities and 14.5% of them were higher than 200 trees ha
−1 (
Table 8). The similarity coefficients were low beyond year 20 (
Figure 7). The comparison of mean stand densities for Ecoregion 3W indicated better agreement than that for Ecoregion 3E between observations and predictions (
Figure 8c). This can be seen in the percent differences between observed and predicted stand densities (
Table 9). Only 0.3 % of the differences between observed and predicted stand densities were higher than 200 trees ha
−1 and as much as 56.6% of them were negligible. The similarity coefficients were all greater than 0.60, except for years 31 and 33 (
Figure 7).
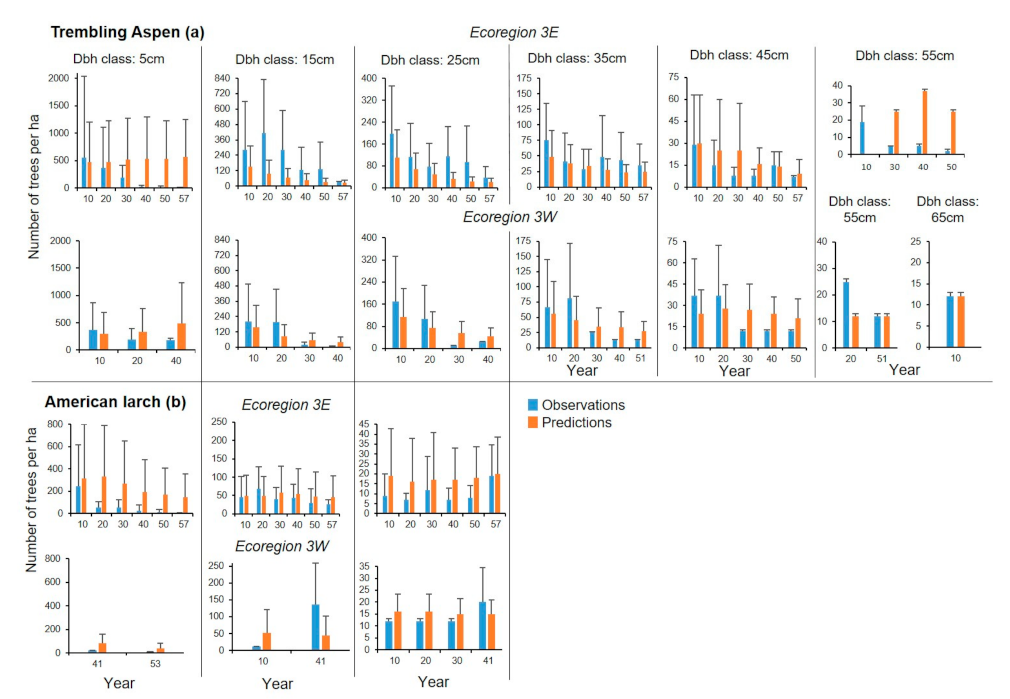
In Ecoregion 3E, observed and predicted mean stand densities for trembling aspen differed considerably in the 5, 15, 25 and 55 cm dbh classes for nearly all years (
Figure 9a). Relatively high agreements between observations and predictions were observed for years 10 and 20 in the 5 cm class and for most years in the 35 and the 45 cm dbh classes. Similar to the other species, there were large overlaps between the standard deviations for most combinations of sample plots, years and dbh classes that can be associated with several high agreements between observations and predictions. As much of 28.2% of the differences between observed and predicted stand densities did not differ and 54.9% of the percent differences were between 1 and 200 trees ha
−1 (
Table 8). Most of the similarity coefficients were smaller than 0.50 (
Figure 7). Higher agreements between observed and predicted mean stand densities were observed for Ecoregion 3W than that for 3E for most years and dbh classes, except for year 30 in the 15, 25 and 45 cm dbh classes, year 40 in the 5, 15, 35 and 45 cm dbh classes (
Figure 9a). Despite the large departures, agreement was high between observed and predicted stand densities in many cases (
Table 9). There were no differences between observed and predicted stand densities for 36.9% of the combinations of sample plots, years and dbh classes and only 12.4 of them were greater than 200 trees ha
−1. Similarity coefficients were higher than 0.40 until year 35, except for years 29 and 33, then gradually declined, except for year 46 (
Figure 7).
For American larch, agreement was high between observed and predicted mean stand densities in Ecoregion 3E, except for years 20 to 57 in the 5 cm dbh class and years 10, 20, 40 and 50 in the 25 cm dbh class (
Figure 9b). As much as 29.8% of the differences between observed and predicted stand densities were negligible and only 7.9% of them were greater than 200 trees ha
−1 (
Table 8). Most similarity coefficients were greater than 0.40 for most years (
Figure 5d). American larch was less present in Ecoregion 3W (
Figure 9b). Agreement was high between mean observations and predictions only in the 25 cm dbh class. As much of 66.7% of the differences between observations and predictions were negligible and 11.1% of them were higher than 21 trees ha
−1 (
Table 9). These results can be associated with relatively high similarity coefficients (
Figure 7).