Spatial Patterns of Non-Structural Carbohydrates in Eucalyptus urophylla× E. grandis under Dry-Season Irrigation with Fertilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Plant Material
2.2. Index Measurements
2.2.1. Field Sampling and Initial Sample Processing
2.2.2. Chemical Analysis
2.3. Statistical Analysis
3. Results
3.1. Variation of Aboveground NSC Concentrations under Different Treatments
3.2. Variation of Underground NSC Concentrations under Different Treatment
3.3. Multivariate General Linear Model (MGLM) of the NSC Concentrations in Various Tissues
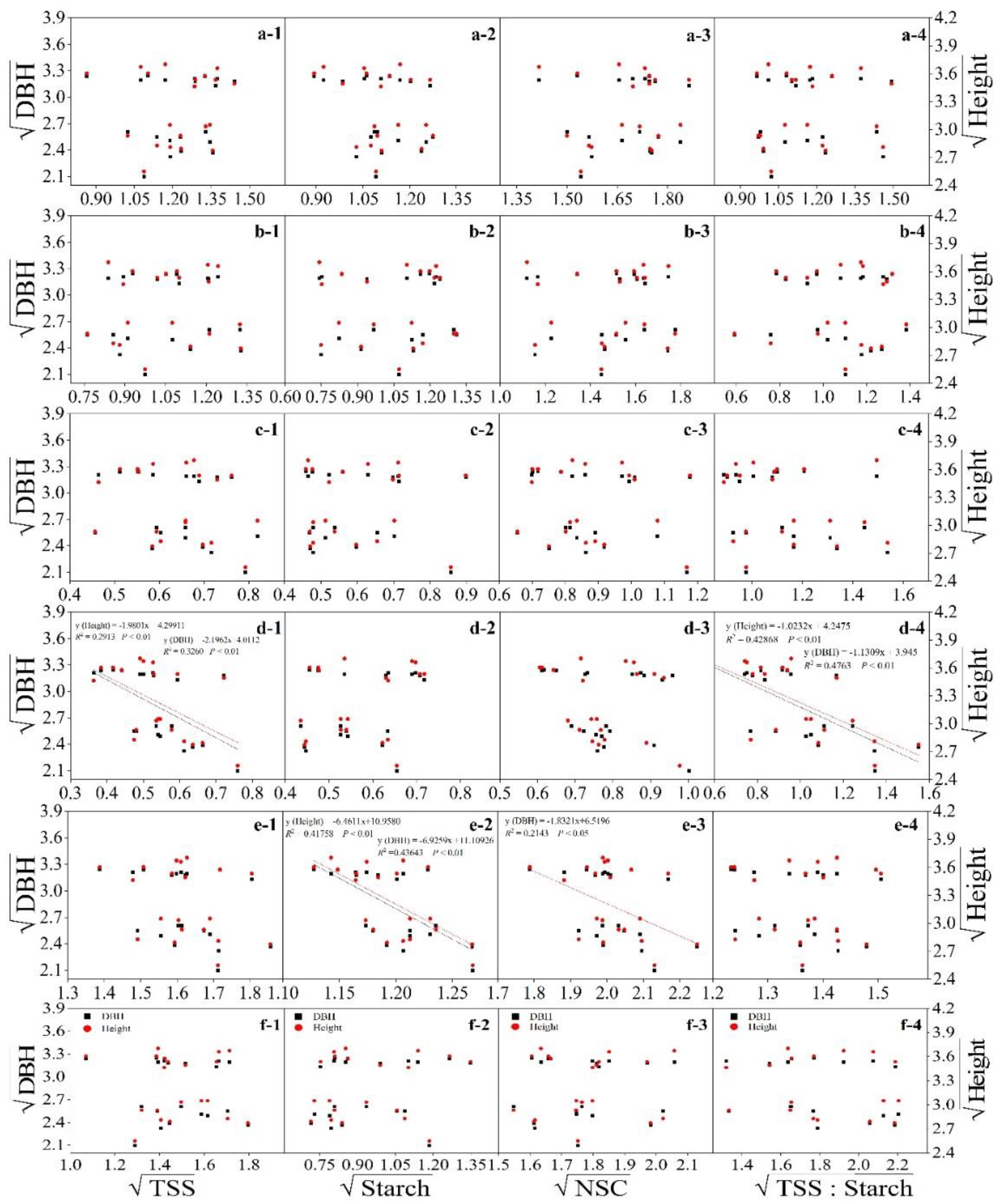
3.4. Relationship between Nonstructural Carbohydrates and Growth
3.5. Effects of Different Treatments on NSC
4. Discussion
4.1. Soluble Sugar as the Primarily Existing Form of NSC
4.2. NSC Concentrations of Tissues under Dry-Season Irrigation and Fertilization
4.3. TSS Concentrations Decreased with Height
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spinoni, J.; Barbosa, P.; De Jager, A.; McCormick, N.; Naumann, G.; Vogt, J.V.; Magni, D.; Masante, D.; Mazzeschi, M. A new global database of meteorological drought events from 1951 to 2016. J. Hydrol. 2019, 22, 100593. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, J.; Liu, X.; Zhou, G.; Yan, J. Decadal drought deaccelerated the increasing trend of annual net primary production in tropical or subtropical forests in southern China. Sci. Rep. 2016, 6, 28640. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, N.; Lee, M.; Reum Han, A.; Han, A.; Kim, H.S. Responses to drought stress in Prunus sargentii and Larix kaempferi seedlings using morphological and physiological parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Caurla, S.; Lobianco, A. Estimating climate service value in forestry: The case of climate information on drought for maritime pine in Southwestern France. Clim. Serv. 2020, 17, 100106. [Google Scholar] [CrossRef]
- Battie-Laclau, P.; Delgado-Rojas, J.S.; Christina, M.; Nouvellon, Y.; Bouillet, J.; Piccolo, M.D.C.; Moreira, M.Z.; Gonçalves, J.L.D.M.; Roupsard, O.; Laclau, J. Potassium fertilization increases water-use efficiency for stem biomass production without affecting intrinsic water-use efficiency in Eucalyptus grandis plantations. For. Ecol. Manag. 2016, 364, 77–89. [Google Scholar] [CrossRef]
- Christina, M.; le Maire, G.; Nouvellon, Y.; Vezy, R.; Bordon, B.; Battie-Laclau, P.; Gonçalves, J.L.M.; Delgado-Rojas, J.S.; Bouillet, J.P.; Laclau, J.P. Simulating the effects of different potassium and water supply regimes on soil water content and water table depth over a rotation of a tropical Eucalyptus grandis plantation. For. Ecol. Manag. 2018, 418, 4–14. [Google Scholar] [CrossRef]
- O’Connell, C.S.; Ruan, L.; Silver, W.L. Drought drives rapid shifts in tropical rainforest soil biogeochemistry and greenhouse gas emissions. Nat. Commun. 2018, 9, 1348. [Google Scholar] [CrossRef]
- Huang, S.; Ye, G.; Lin, J.; Chen, K.; Xu, X.; Ruan, H.; Tan, F.; Chen, H.Y.H. Autotrophic and heterotrophic soil respiration responds asymmetrically to drought in a subtropical forest in the Southeast China. Soil Biol. Biochem. 2018, 123, 242–249. [Google Scholar] [CrossRef]
- Bastida, F.; Torres, I.F.; Andres-Abellan, M.; Baldrian, P.; Lopez-Mondejar, R.; Vetrovsky, T.; Richnow, H.H.; Starke, R.; Ondono, S.; Garcia, C.; et al. Differential sensitivity of total and active soil microbial communities to drought and forest management. Glob. Chang. Biol. 2017, 23, 4185–4203. [Google Scholar] [CrossRef]
- Wang, Z.; Du, A.; Xu, Y.; Zhu, W.; Zhang, J. Factors Limiting the Growth of Eucalyptus and the Characteristics of Growth and Water Use under Water and Fertilizer Management in the Dry Season of Leizhou Peninsula, China. Agronomy 2019, 9, 590. [Google Scholar] [CrossRef]
- Wang, Z.; Zhong, R.; Lai, C.; Zeng, Z.; Lian, Y.; Bai, X. Climate change enhances the severity and variability of drought in the Pearl River Basin in South China in the 21st century. Agric. For. Meteorol. 2018, 249, 149–162. [Google Scholar] [CrossRef]
- Xie, Y.; Arnold, R.J.; Wu, Z.; Chen, S.; Du, A.; Luo, J. Advances in Eucalypt research in China. Front. Agric. Sci. Eng. 2017, 4, 380–390. [Google Scholar] [CrossRef]
- Pan, J.; Lin, N.; He, Q.; Su, Y.; Li, J. Factors influencing the productivity of three Eucalyptus plantation areas in China. Acta Ecol. Sinca 2018, 38, 6932–6940. [Google Scholar]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Muhr, J.; Messier, C.; Delagrange, S.; Trumbore, S.; Xu, X.; Hartmann, H. How fresh is maple syrup? Sugar maple trees mobilize carbon stored several years previously during early springtime sap-ascent. New Phytol. 2016, 209, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.S.; Czimczik, C.I.; Keenan, T.F.; Murakami, P.F.; Pederson, N.; Schaberg, P.G.; Xu, X.; Richardson, A.D. Age, allocation and availability of nonstructural carbon in mature red maple trees. New Phytol. 2013, 200, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Hoch, G.; Yakir, D.; Korner, C. Drought stress, growth and nonstructural carbohydrate dynamics of pine trees in a semi-arid forest. Tree Physiol. 2014, 34, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cao, Y.; Chen, Y.; Liu, G. Non-structural carbohydrate dynamics in Robinia pseudoacacia saplings under three levels of continuous drought stress. Trees 2015, 29, 1837–1849. [Google Scholar] [CrossRef]
- Kannenberg, S.A.; Phillips, R.P. Non-structural carbohydrate pools not linked to hydraulic strategies or carbon supply in tree saplings during severe drought and subsequent recovery. Tree Physiol. 2020, 40, 259–271. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, X.; Fu, Y.; Shao, J.; Zhou, L.; Li, S.; Zhou, G.; Hu, Z.; Hu, J.; Bai, S.H.; et al. Differential effects of drought on nonstructural carbohydrate storage in seedlings and mature trees of four species in a subtropical forest. For. Ecol. Manag. 2020, 469, 118159. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, C. Impacts of Tree Species and Tissue on Estimation of Nonstructural Carbohydrates Storage in Trunk. Sci. Silvae Sin. 2016, 52, 1–9. [Google Scholar]
- Furze, M.E.; Huggett, B.A.; Aubrecht, D.M.; Stolz, C.D.; Carbone, M.S.; Richardson, A.D. Whole-tree nonstructural carbohydrate storage and seasonal dynamics in five temperate species. New Phytol. 2019, 221, 1466–1477. [Google Scholar] [CrossRef] [PubMed]
- Schiestl-Aalto, P.; Ryhti, K.; Mäkelä, A.; Peltoniemi, M.; Bäck, J.; Kulmala, L. Analysis of the NSC Storage Dynamics in Tree Organs Reveals the Allocation to Belowground Symbionts in the Framework of Whole Tree Carbon Balance. Front. For. Glob. Chang. 2019, 2, 17. [Google Scholar] [CrossRef]
- Hartmann, H.; Bahn, M.; Carbone, M.; Richardson, A.D. Plant carbon allocation in a changing world—Challenges and progress: Introduction to a Virtual Issue on carbon allocation. New Phytol. 2020, 227, 981–988. [Google Scholar] [CrossRef]
- Piper, F.I. Drought induces opposite changes in the concentration of non-structural carbohydrates of two evergreen Nothofagus species of differential drought resistance. Ann. For. Sci. 2011, 68, 415–424. [Google Scholar] [CrossRef]
- Sala, A.; Hoch, G. Height-related growth declines in ponderosa pine are not due to carbon limitation. Plant Cell Environ. 2009, 32, 22–30. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees—From what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef] [PubMed]
- Kannenberg, S.A.; Novick, K.A.; Phillips, R.P. Coarse roots prevent declines in whole-tree non-structural carbohydrate pools during drought in an isohydric and an anisohydric species. Tree Physiol. 2018, 38, 582–590. [Google Scholar] [CrossRef]
- Chapin, F. The Ecology and Economics of Storage in Plants. Annu. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]
- Li, W.; Hartmann, H.; Adams, H.D.; Zhang, H.; Jin, C.; Zhao, C.; Guan, D.; Wang, A.; Yuan, F.; Wu, J. The sweet side of global change-dynamic responses of non-structural carbohydrates to drought, elevated CO2 and nitrogen fertilization in tree species. Tree Physiol. 2018, 38, 1706–1723. [Google Scholar] [CrossRef]
- Nikinmaa, E.; Hölttä, T.; Hari, P.; Kolari, P.; Mäkelä, A.; Sevanto, S.; Vesala, T. Assimilate transport in phloem sets conditions for leaf gas exchange. Plant Cell Environ. 2013, 36, 655–669. [Google Scholar] [CrossRef]
- Reinhardt, K.; Germino, M.J.; Kueppers, L.M.; Domec, J.; Mitton, J. Linking carbon and water relations to drought-induced mortality in Pinus flexilis seedlings. Tree Physiol. 2015, 35, 771–782. [Google Scholar] [CrossRef]
- Liu, H.; Shangguan, H.; Zhou, M.; Airebule, P.; Zhao, P.; He, W.; Xiang, C.; Wu, X. Differentiated responses of nonstructural carbohydrate allocation to climatic dryness and drought events in the Inner Asian arid timberline. Agric. For. Meteorol. 2019, 271, 355–361. [Google Scholar] [CrossRef]
- Oliver, T.H.; Heard, M.S.; Isaac, N.J.B.; Roy, D.B.; Procter, D.; Eigenbrod, F.; Freckleton, R.; Hector, A.; Orme, C.D.L.; Petchey, O.L.; et al. Biodiversity and Resilience of Ecosystem Functions. Trends Ecol. Evol. 2015, 30, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Link, R.M.; Schuldt, B. A whole-plant perspective of isohydry: Stem-level support for leaf-level plant water regulation. Tree Physiol. 2021, 41, 901–905. [Google Scholar] [CrossRef]
- Smith, M.G.; Miller, R.E.; Arndt, S.K.; Kasel, S.; Bennett, L.T. Whole-tree distribution and temporal variation of non-structural carbohydrates in broadleaf evergreen trees. Tree Physiol. 2018, 38, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, D.R.; Meinzer, F.C. Water stress, shoot growth and storage of non-structural carbohydrates along a tree height gradient in a tall conifer. Plant Cell Environ. 2011, 34, 1920–1930. [Google Scholar] [CrossRef]
- Hua, L.; Yu, F.; Qiu, Q.; He, Q.; Su, Y.; Liu, X.; Li, J. Relationships between diurnal and seasonal variation of photosynthetic characteristics of Eucalyptus plantation and environmental factors under dry-season irrigation with fertilization. Agric. Water Manag. 2021, 248, 106737. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Huang, G.; Liu, R.; Wu, H.; Zhao, C.; McDowell, N.G. Effects of nitrogen enrichment on tree carbon allocation: A global synthesis. Glob. Ecol. Biogeogr. 2019, 29, 573–589. [Google Scholar] [CrossRef]
- Zahoor, R.; Zhao, W.; Abid, M.; Dong, H.; Zhou, Z. Title: Potassium application regulates nitrogen metabolism and osmotic adjustment in cotton (Gossypium hirsutum L.) functional leaf under drought stress. J. Plant Physiol. 2017, 215, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.F.; Mateus, N.S.; Rosário, M.O.; Garcez, T.B.; Mazzafera, P.; Lavres, J. Enhancing potassium content in leaves and stems improves drought tolerance of Eucalyptus clones. Physiol. Plant. 2020, 170, 1–19. [Google Scholar]
- Goodsman, D.W.; Lieffers, V.J.; Landhäusser, S.M.; Erbilgin, N. Fertilization of lodgepole pine trees increased diameter growth but reduced root carbohydrate concentrations. For. Ecol. Manag. 2010, 260, 1914–1920. [Google Scholar] [CrossRef]
- Epron, D.; Laclau, J.P.; Almeida, J.C.R.; Goncalves, J.L.M.; Ponton, S.; Sette, C.R.; Delgado-Rojas, J.S.; Bouillet, J.P.; Nouvellon, Y. Do changes in carbon allocation account for the growth response to potassium and sodium applications in tropical Eucalyptus plantations? Tree Physiol. 2012, 32, 667–679. [Google Scholar] [CrossRef]
- Ryan, M.G.; Stape, J.L.; Binkley, D.; Fonseca, S.; Loos, R.A.; Takahashi, E.N.; Silva, C.R.; Silva, S.R.; Hakamada, R.E.; Ferreira, J.M.; et al. Factors controlling Eucalyptus productivity: How water availability and stand structure alter production and carbon allocation. For. Ecol. Manag. 2010, 259, 1695–1703. [Google Scholar] [CrossRef]
- Stape, J.L.; Binkley, D.; Ryan, M.G. Production and carbon allocation in a clonal Eucalyptus plantation with water and nutrient manipulations. For. Ecol. Manag. 2008, 255, 920–930. [Google Scholar] [CrossRef]
- Lu, H.; Xu, J.; Li, G.; Liu, W. Site Classification of Eucalyptus urophylla × Eucalyptus grandis Plantations in China. Forests 2020, 11, 871. [Google Scholar] [CrossRef]
- Bouillet, J.; Laclau, J.; Gonçalves, J.L.D.M.; Voigtlaender, M.; Gava, J.L.; Leite, F.P.; Hakamada, R.; Mareschal, L.; Mabiala, A.; Tardy, F.; et al. Eucalyptus and Acacia tree growth over entire rotation in single- and mixed-species plantations across five sites in Brazil and Congo. For. Ecol. Manag. 2013, 301, 89–101. [Google Scholar] [CrossRef]
- Viera, M.; Schumacher, M.V.; Bonacina, D.M.; de Oliveira Ramos, L.O.; Rodríguez-Soalleiro, R. Biomass and nutrient allocation to aboveground components in fertilized Eucalyptus saligna and E. urograndis plantations. New For. 2017, 48, 445–462. [Google Scholar] [CrossRef]
- Silva, P.H.M.D.; Poggiani, F.; Libardi, P.L.; Gonçalves, A.N. Fertilizer management of Eucalypt plantations on sandy soil in Brazil: Initial growth and nutrient cycling. For. Ecol. Manag. 2013, 301, 67–78. [Google Scholar] [CrossRef]
- Rowland, L.; Da Costa, A.C.L.; Galbraith, D.R.; Oliveira, R.S.; Binks, O.J.; Oliveira, A.A.R.; Pullen, A.M.; Doughty, C.E.; Metcalfe, D.B.; Vasconcelos, S.S.; et al. Death from drought in tropical forests is triggered by hydraulics not carbon starvation. Nature 2015, 528, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, P.; Ouyang, L.; Zhu, L.; Ni, G.; Schäfer, K.V.R. Whole-plant water hydraulic integrity to predict drought-induced Eucalyptus urophylla mortality under drought stress. For. Ecol. Manag. 2020, 468, 118179. [Google Scholar] [CrossRef]
- Christina, M.; Le Maire, G.; Battie Laclau, P.; Nouvellon, Y.; Bouillet, J.P.; Jourdan, C.; Moraes Gonçalves, J.L.; Laclau, J.P. Measured and modeled interactive effects of potassium deficiency and water deficit on gross primary productivity and light-use efficiency in Eucalyptus grandis plantations. Glob. Chang. Biol. 2015, 21, 2022–2039. [Google Scholar] [CrossRef]
- Battie-Laclau, P.; Laclau, J.P.; Domec, J.C.; Christina, M.; Bouillet, J.P.; de Cassia, P.M.; de Moraes, G.J.; Moreira, R.M.E.; Krusche, A.V.; Bouvet, J.M.; et al. Effects of potassium and sodium supply on drought-adaptive mechanisms in Eucalyptus grandis plantations. New Phytol. 2014, 203, 401–413. [Google Scholar] [CrossRef]
- Yu, F.; Truong, T.V.; He, Q.; Hua, L.; Su, Y.; Li, J. Dry Season Irrigation Promotes Leaf Growth in Eucalyptus urophylla × E. grandis under Fertilization. Forests 2019, 10, 67. [Google Scholar] [CrossRef]
- Weber, R.; Schwendener, A.; Schmid, S.; Lambert, S.; Wiley, E.; Landhausser, S.M.; Hartmann, H.; Hoch, G. Living on next to nothing: Tree seedlings can survive weeks with very low carbohydrate concentrations. New Phytol. 2018, 218, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Morris, D. Quantitative determination of carbohydrates with Dreywood’s anthrone reagent. Science 1948, 107, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.; Rose, C.L.; Omi, S.K.; Forry, K.R.; Durall, D.M.; Bigg, W.L. Starch determination by perchloric acid vs enzymes: Evaluating the accuracy and precision of six colorimetric methods. J. Agric. Food Chem. 1991, 39, 2–11. [Google Scholar] [CrossRef]
- Barry, K.M.; Quentin, A.; Eyles, A.; Pinkard, E.A. Consequences of resource limitation for recovery from repeated defoliation in Eucalyptus globulus Labilladiere. Tree Physiol. 2012, 32, 24–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mitchell, P.J.; O’Grady, A.P.; Tissue, D.T.; White, D.A.; Ottenschlaeger, M.L.; Pinkard, E.A. Drought response strategies define the relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality. New Phytol. 2013, 197, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Woodruff, D.R.; Meinzer, F.C. Carbon dynamics in trees: Feast or famine? Tree Physiol. 2012, 32, 764–775. [Google Scholar] [CrossRef]
- Maguire, A.J.; Kobe, R.K. Drought and shade deplete nonstructural carbohydrate reserves in seedlings of five temperate tree species. Ecol. Evol. 2015, 5, 5711–5721. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J.; Helmisaari, H.; Kaštovská, E.; Körner, C.; Lambers, H.; Meier, I.C.; Millard, P.; Ostonen, I. Surplus Carbon Drives Allocation and Plant—Soil Interactions. Trends Ecol. Evol. 2020, 35, 1110–1118. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Salami, S.A.; Babalar, M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9, 19250. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, N.; Lee, M.; Lee, H.; Adhikari, A.; Han, A.R.; Han, A.; Kim, H.S. Evaluation of morphological, physiological, and biochemical traits for assessing drought resistance in eleven tree species. Sci. Total Environ. 2021, 779, 146466. [Google Scholar] [CrossRef]
- Brodersen, C.R.; McElrone, A.J. Maintenance of xylem Network Transport Capacity: A Review of Embolism Repair in Vascular Plants. Front. Plant Sci. 2013, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Souden, S.; Ennajeh, M.; Ouledali, S.; Massoudi, N.; Cochard, H.; Khemira, H. Water relations, photosynthesis, xylem embolism and accumulation of carbohydrates and cyclitols in two Eucalyptus species (E. camaldulensis and E. torquata) subjected to dehydration–rehydration cycle. Trees 2020, 34, 1439–1452. [Google Scholar] [CrossRef]
- Warren, C.R.; Bleby, T.; Adams, M.A. Changes in gas exchange versus leaf solutes as a means to cope with summer drought in Eucalyptus marginata. Oecologia 2007, 154, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, X.; Zheng, M.; Mo, J. 13-year nitrogen addition increases nonstructural carbon pools in subtropical forest trees in southern China. For. Ecol. Manag. 2021, 481, 118748. [Google Scholar] [CrossRef]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef]
- Savage, J.A.; Clearwater, M.J.; Haines, D.F.; Klein, T.; Mencuccini, M.; Sevanto, S.; Turgeon, R.; Zhang, C. Allocation, stress tolerance and carbon transport in plants: How does phloem physiology affect plant ecology? Plant Cell Environ. 2016, 39, 709–725. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Hydraulic redistribution by plants and nutrient stoichiometry: Shifts under global change. Ecohydrology 2014, 7, 1–20. [Google Scholar] [CrossRef]
- Christina, M.; Nouvellon, Y.; Laclau, J.P.; Stape, J.L.; Bouillet, J.P.; Lambais, G.R.; Maire, G. Importance of deep water uptake in tropical Eucalypt forest. Funct. Ecol. 2016, 31, 509–519. [Google Scholar] [CrossRef]
- Klein, T.; Vitasse, Y.; Hoch, G. Coordination between growth, phenology and carbon storage in three coexisting deciduous tree species in a temperate forest. Tree Physiol. 2016, 36, 847–855. [Google Scholar] [CrossRef]
- Brunner, I.; Godbold, D.L. Tree roots in a changing world. J. For. Res. Jpn. 2007, 12, 78–82. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Goodger, J.Q.D. Chemical root to shoot signaling under drought. Trends Plant Sci. 2008, 13, 281–287. [Google Scholar] [CrossRef]
- Merchant, A.; Tausz, M.; Arndt, S.K.; Adams, M.A. Cyclitols and carbohydrates in leaves and roots of 13 Eucalyptus species suggest contrasting physiological responses to water deficit. Plant Cell Environ. 2006, 29, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Nóia Júnior, R.D.S.; Amaral, G.C.; Pezzopane, J.E.M.; Fonseca, M.D.S.; Câmara Da Silva, A.P.; Xavier, T.M.T. Ecophysiological acclimatization to cyclic water stress in Eucalyptus. J. For. Res. 2020, 31, 797–806. [Google Scholar] [CrossRef]
- Ferraz, S.F.D.B.; Rodrigues, C.B.; Garcia, L.G.; Alvares, C.A.; Lima, W.D.P. Effects of Eucalyptus plantations on streamflow in Brazil: Moving beyond the water use debate. For. Ecol. Manag. 2019, 453, 117571. [Google Scholar] [CrossRef]
- Kannenberg, S.A.; Phillips, R.P. Plant responses to stress impacts: The C we do not see. Tree Physiol. 2017, 37, 151–153. [Google Scholar] [CrossRef]
- Binkley, D.; Campoe, O.C.; Alvares, C.A.; Carneiro, R.L.; Stape, J.L. Variation in whole-rotation yield among Eucalyptus genotypes in response to water and heat stresses: The TECHS project. For. Ecol. Manag. 2020, 462, 117953. [Google Scholar] [CrossRef]
- Schonbeck, L.; Gessler, A.; Hoch, G.; McDowell, N.G.; Rigling, A.; Schaub, M.; Li, M.H. Homeostatic levels of nonstructural carbohydrates after 13 yr of drought and irrigation in Pinus sylvestris. New Phytol. 2018, 219, 1314–1324. [Google Scholar] [CrossRef] [PubMed]
- Kebrom, T.H. A growing stem inhibits bud outgrowth—The overlooked theory of apical dominance. Front. Plant Sci. 2017, 8, 1874. [Google Scholar] [CrossRef] [PubMed]
- Han, Q. Height-related decreases in mesophyll conductance, leaf photosynthesis and compensating adjustments associated with leaf nitrogen concentrations in Pinus densiflora. Tree Physiol. 2011, 31, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Hoch, G.; Siegwolf, R.T.W.; Keel, S.G.; Körner, C.; Han, Q. Fruit production in three masting tree species does not rely on stored carbon reserves. Oecologia 2013, 171, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Münch, E. Die Stoffbewegungen in der Pflanze. Protoplasma 1932, 15, 488–489. [Google Scholar]
- Givnish, T.J.; Wong, S.C.; Stuart-Williams, H.; Holloway-Phillips, M.; Farquhar, G.D. Determinants of maximum tree height in Eucalyptus species along a rainfall gradient in Victoria, Australia. Ecology 2014, 95, 2991–3007. [Google Scholar] [CrossRef]
- Palacio, S.; Camarero, J.J.; Maestro, M.; Alla, A.Q.; Lahoz, E.; Montserrat-Martí, G. Are storage and tree growth related? Seasonal nutrient and carbohydrate dynamics in evergreen and deciduous Mediterranean oaks. Trees 2018, 32, 777–790. [Google Scholar] [CrossRef]
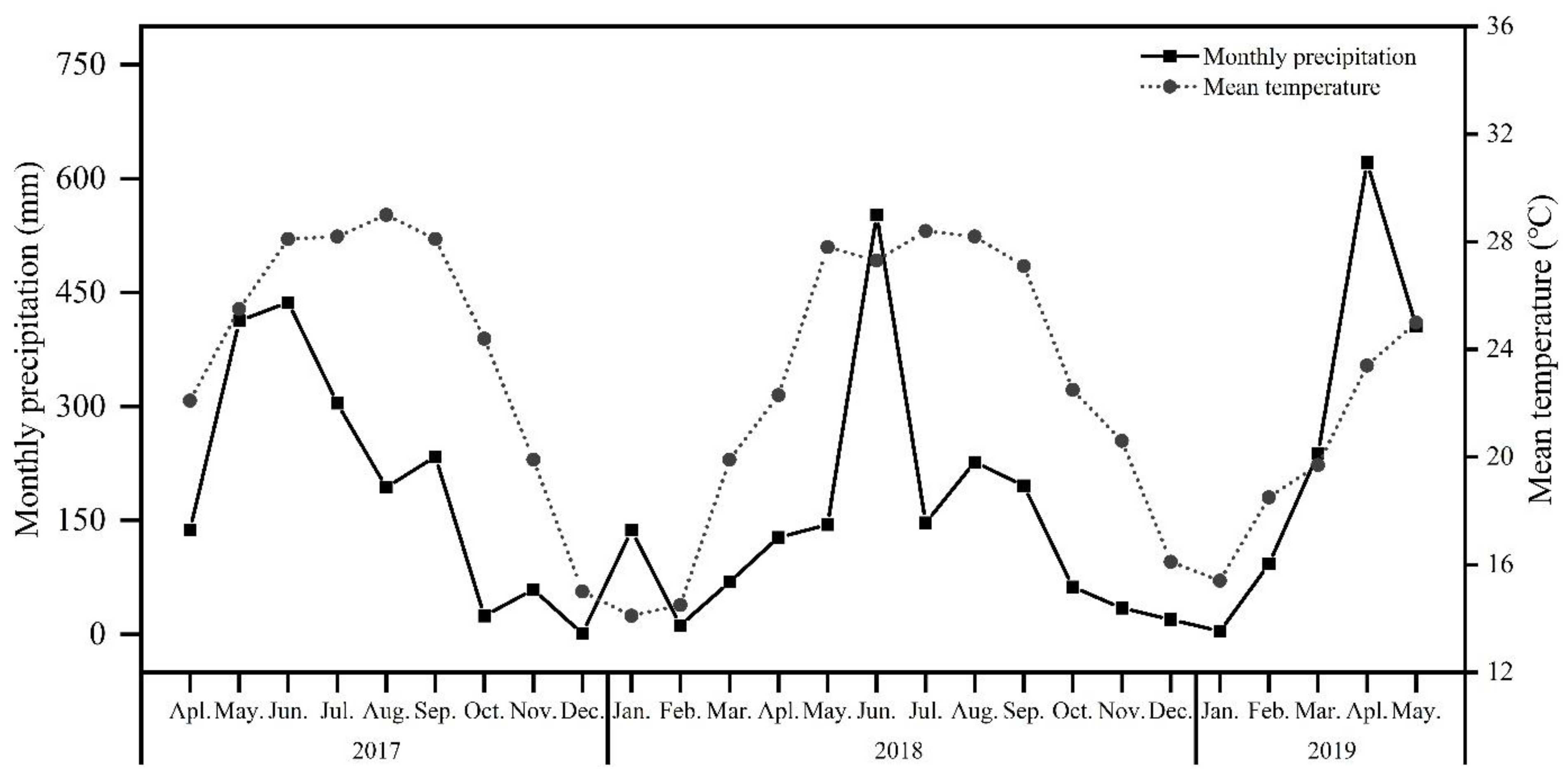
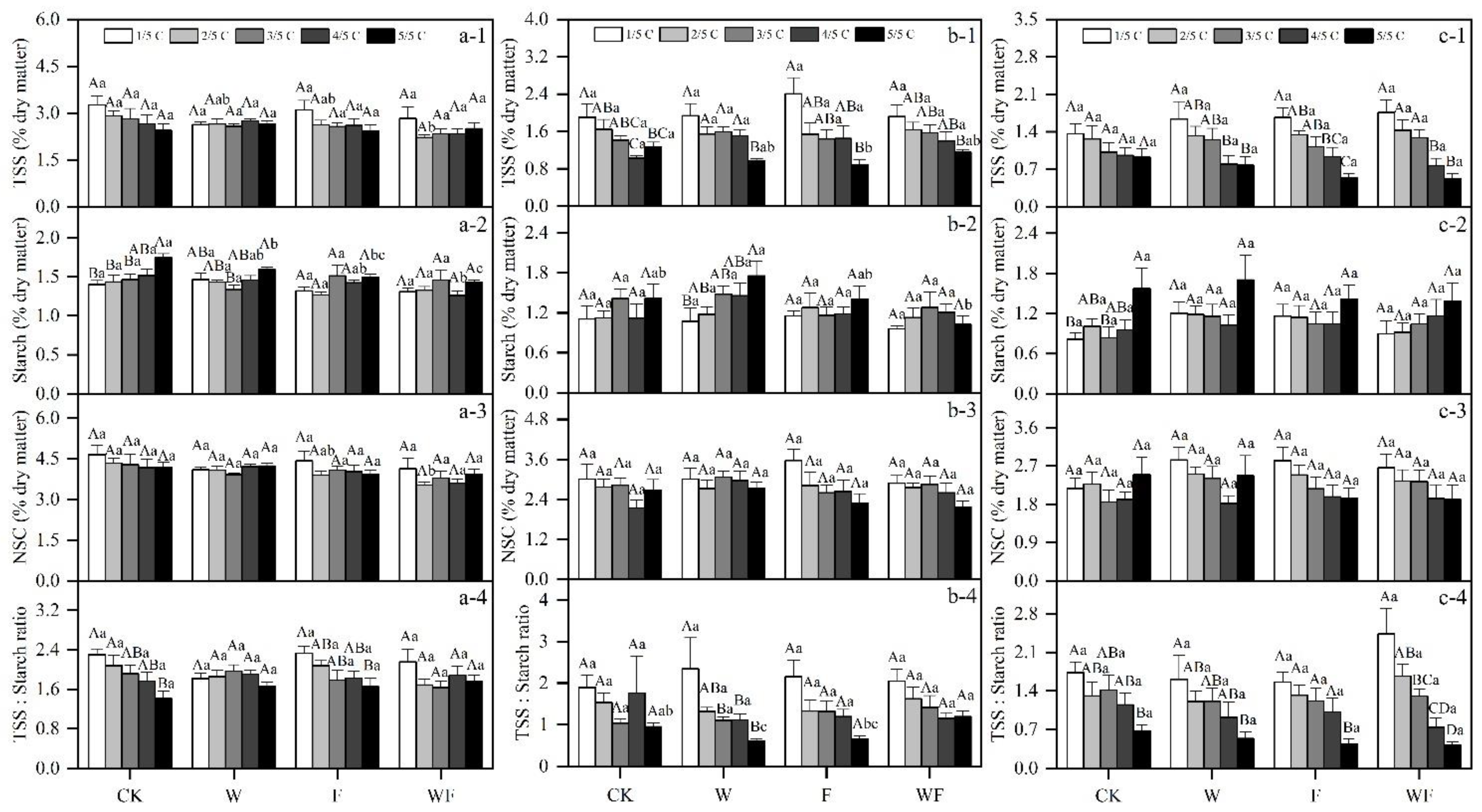
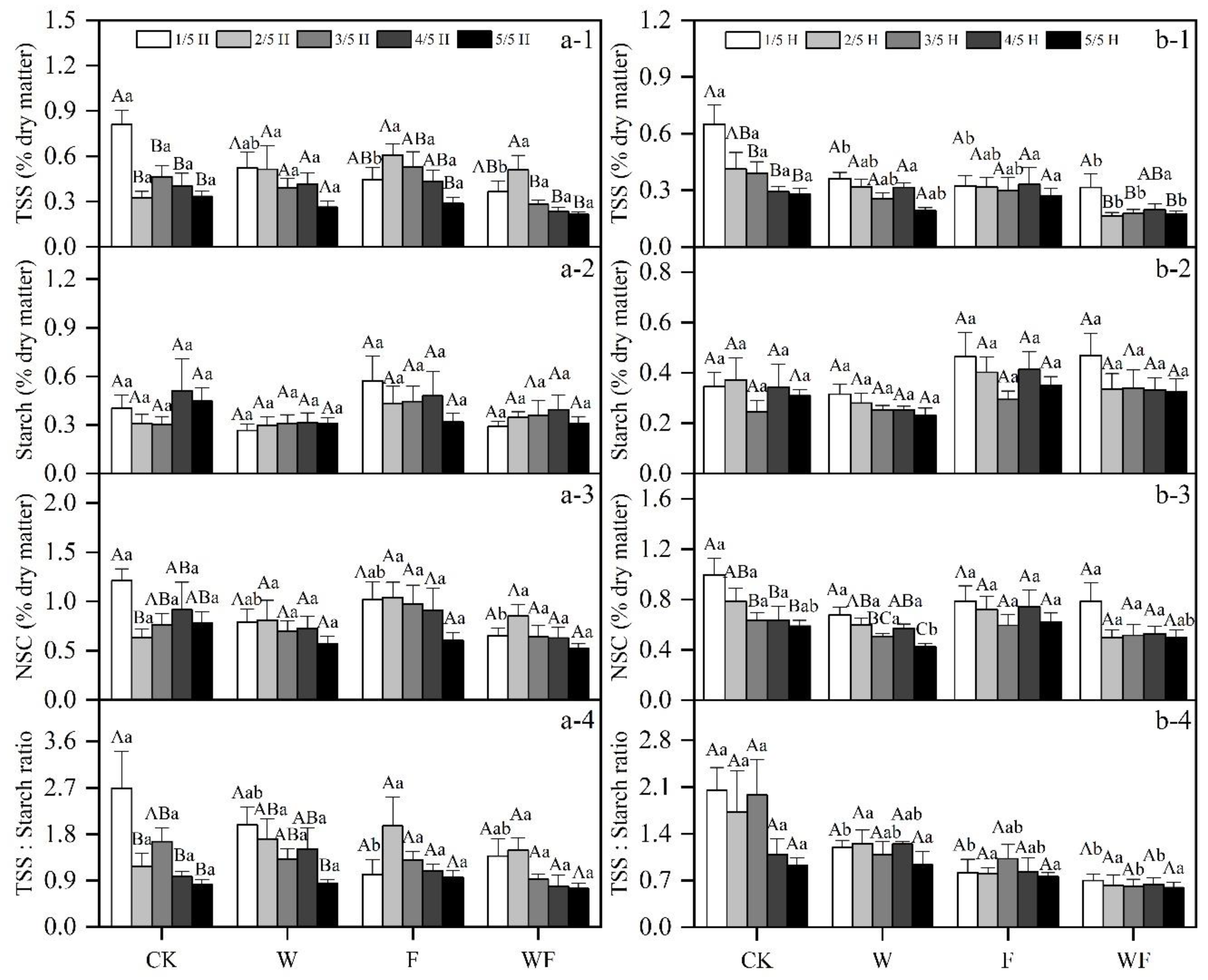
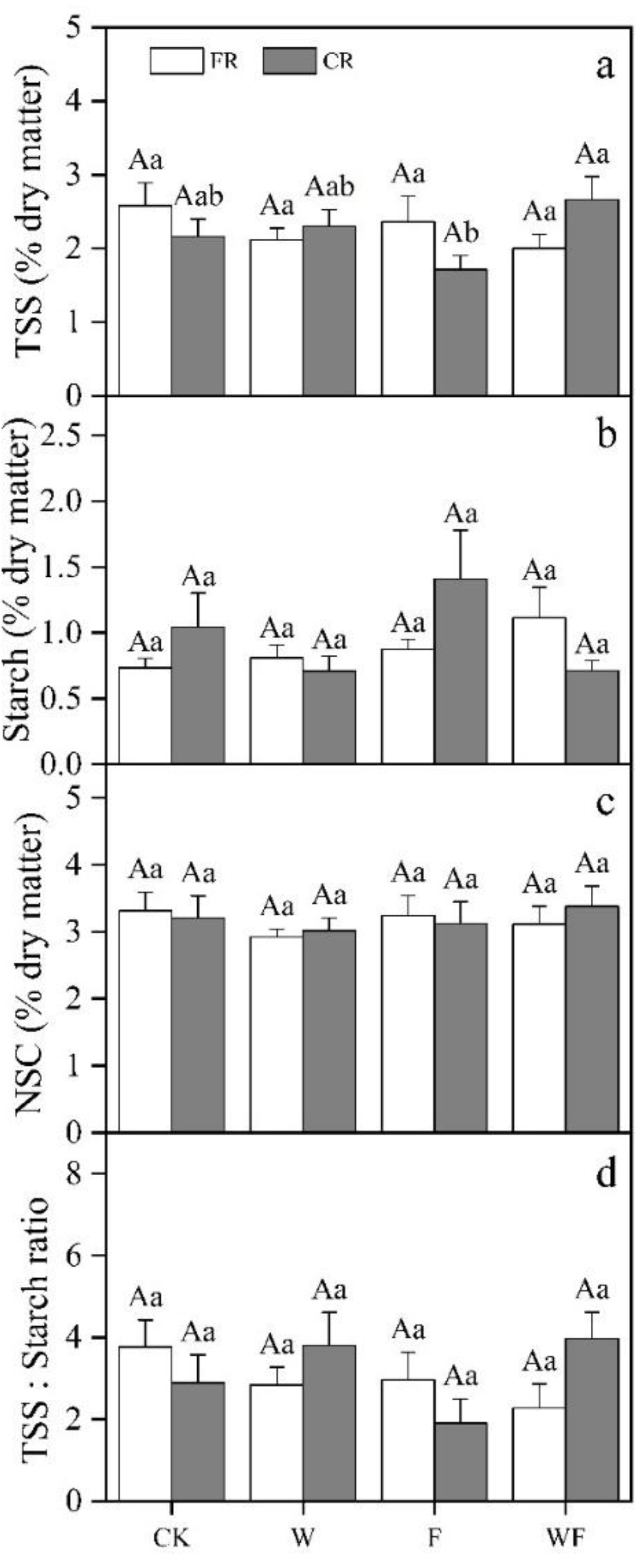

| Season | Treatment | May 2017–May 2018 | June 2018–May 2019 |
|---|---|---|---|
| Dry season | CK | 67.48 ± 0.73 | 77.11 ± 1.84 |
| W | 90.56 ± 0.42 | 89.06 ± 1.29 | |
| F | 57.40 ± 0.51 | 68.06 ± 1.68 | |
| WF | 83.44 ± 0.43 | 84.06 ± 1.53 | |
| Rainy season | CK | 85.82 ± 0.47 | 80.80 ± 1.27 |
| W | 85.64 ± 0.47 | 85.74 ± 1.49 | |
| F | 81.95 ± 0.55 | 75.83 ± 1.60 | |
| WF | 83.62 ± 0.51 | 83.09 ± 2.03 |
| Treatment | DBH (cm) | Tree Height (m) | Under-Crown Height (m) |
|---|---|---|---|
| CK | 5.52 ± 0.30 | 7.58 ± 0.25 | 3.66 ± 0.28 |
| W | 6.52 ± 0.11 | 9.00 ± 0.15 | 6.28 ± 1.07 |
| F | 10.14 ± 0.10 | 12.78 ± 0.24 | 4.92 ± 0.24 |
| WF | 10.37 ± 0.06 | 12.94 ± 0.24 | 4.96 ± 0.81 |
| Tissue | Variables | Fixed Factors | Covariates | R2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Height | Dry-Season Irrigation (W) | Fertilization (F) | Height × W | Height × F | W × F | Height × W × F | Block | |||
| Leaf | TSS | 0.050 * | 0.060 | 0.057 | 0.319 | 0.772 | 0.600 | 0.859 | 0.002 ** | 0.310 |
| Starch | 0.002 ** | 0.139 | 0.002 ** | 0.467 | 0.104 | 0.942 | 0.817 | 0.481 | 0.356 | |
| NSC | 0.239 | 0.025 * | 0.007 ** | 0.706 | 0.757 | 0.686 | 0.871 | 0.002 ** | 0.299 | |
| Ratio | 0.001 ** | 0.351 | 0.924 | 0.093 | 0.373 | 0.595 | 0.833 | 0.013 * | 0.352 | |
| Branchlet | TSS | 0.000 ** | 0.594 | 0.719 | 0.663 | 0.812 | 0.907 | 0.228 | 0.090 | 0.421 |
| Starch | 0.118 | 0.802 | 0.170 | 0.757 | 0.523 | 0.100 | 0.708 | 0.022 * | 0.241 | |
| NSC | 0.064 | 0.625 | 0.571 | 0.594 | 0.551 | 0.271 | 0.789 | 0.941 | 0.191 | |
| Ratio | 0.000 ** | 0.857 | 0.521 | 0.960 | 0.905 | 0.206 | 0.434 | 0.002 ** | 0.420 | |
| Old branch | TSS | 0.000 ** | 0.807 | 0.937 | 0.465 | 0.135 | 0.966 | 0.982 | 0.000 ** | 0.585 |
| Starch | 0.016 * | 0.486 | 0.829 | 0.967 | 0.909 | 0.111 | 0.790 | 0.007 ** | 0.256 | |
| NSC | 0.041 * | 0.480 | 0.883 | 0.833 | 0.582 | 0.295 | 0.913 | 0.508 | 0.185 | |
| Ratio | 0.000 ** | 0.759 | 0.875 | 0.511 | 0.297 | 0.069 | 0.711 | 0.000 ** | 0.656 | |
| Stem xylem | TSS | 0.000 ** | 0.008 ** | 0.179 | 0.458 | 0.028 * | 0.179 | 0.344 | 0.095 | 0.522 |
| Starch | 0.909 | 0.036 * | 0.273 | 0.751 | 0.729 | 0.933 | 0.844 | 0.181 | 0.150 | |
| NSC | 0.112 | 0.008 ** | 0.942 | 0.584 | 0.361 | 0.472 | 0.802 | 0.853 | 0.238 | |
| Ratio | 0.000 ** | 0.530 | 0.024 * | 0.823 | 0.030 * | 0.297 | 0.218 | 0.001 ** | 0.432 | |
| Stem cambium | TSS | 0.000 ** | 0.000 ** | 0.000 ** | 0.827 | 0.328 | 0.825 | 0.154 | 0.001 ** | 0.517 |
| Starch | 0.132 | 0.173 | 0.006 ** | 0.723 | 0.954 | 0.692 | 0.997 | 0.004 ** | 0.569 | |
| NSC | 0.002 ** | 0.001 ** | 0.679 | 0.975 | 0.827 | 0.704 | 0.648 | 0.985 | 0.304 | |
| Ratio | 0.122 | 0.004 ** | 0.000 ** | 0.300 | 0.381 | 0.884 | 0.478 | 0.000 ** | 0.580 | |
| Tissue | Variables | Fixed Factors | Covariates | R2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Classification (C) | Dry-Season Irrigation (W) | Fertilization (F) | C × W | C × F | W × F | C × W × F | Block | |||
| Root | TSS | 0.776 | 0.661 | 0.531 | 0.026 * | 0.798 | 0.257 | 0.382 | 0.441 | 0.224 |
| Starch | 0.770 | 0.276 | 0.181 | 0.041 * | 0.855 | 0.754 | 0.466 | 0.081 | 0.273 | |
| NSC | 0.900 | 0.649 | 0.682 | 0.510 | 0.836 | 0.428 | 0.845 | 0.614 | 0.056 | |
| Ratio | 0.880 | 0.423 | 0.230 | 0.023 * | 0.771 | 0.465 | 0.520 | 0.162 | 0.263 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, J.-J.; Liu, W.-Q.; Huang, F.; Hua, L.; Yu, F.; He, Q.; Su, Y.; Li, J.-Y.; Qiu, Q. Spatial Patterns of Non-Structural Carbohydrates in Eucalyptus urophylla× E. grandis under Dry-Season Irrigation with Fertilization. Forests 2021, 12, 1049. https://doi.org/10.3390/f12081049
Kong J-J, Liu W-Q, Huang F, Hua L, Yu F, He Q, Su Y, Li J-Y, Qiu Q. Spatial Patterns of Non-Structural Carbohydrates in Eucalyptus urophylla× E. grandis under Dry-Season Irrigation with Fertilization. Forests. 2021; 12(8):1049. https://doi.org/10.3390/f12081049
Chicago/Turabian StyleKong, Jie-Jun, Wen-Quan Liu, Feng Huang, Lei Hua, Fei Yu, Qian He, Yan Su, Ji-Yue Li, and Quan Qiu. 2021. "Spatial Patterns of Non-Structural Carbohydrates in Eucalyptus urophylla× E. grandis under Dry-Season Irrigation with Fertilization" Forests 12, no. 8: 1049. https://doi.org/10.3390/f12081049
APA StyleKong, J.-J., Liu, W.-Q., Huang, F., Hua, L., Yu, F., He, Q., Su, Y., Li, J.-Y., & Qiu, Q. (2021). Spatial Patterns of Non-Structural Carbohydrates in Eucalyptus urophylla× E. grandis under Dry-Season Irrigation with Fertilization. Forests, 12(8), 1049. https://doi.org/10.3390/f12081049






