Allocation of Wood Density in European Oak (Quercus robur L.) Trees Grown under a Canopy of Scots Pine
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Tree Selection and Wood Sampling Procedure
2.2. Measurement of Wood Density
2.3. Statistical Analysis
3. Results and Discussion
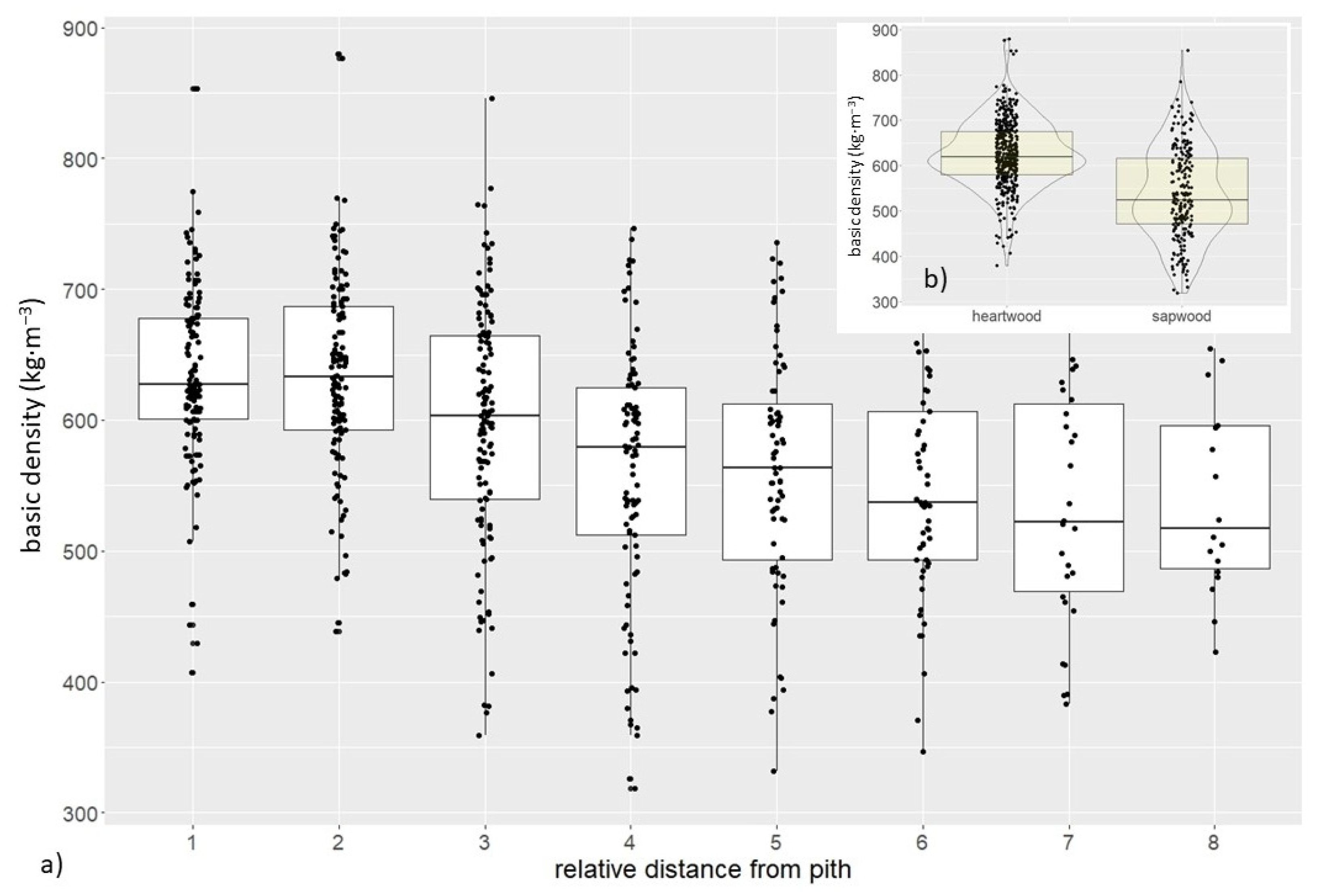
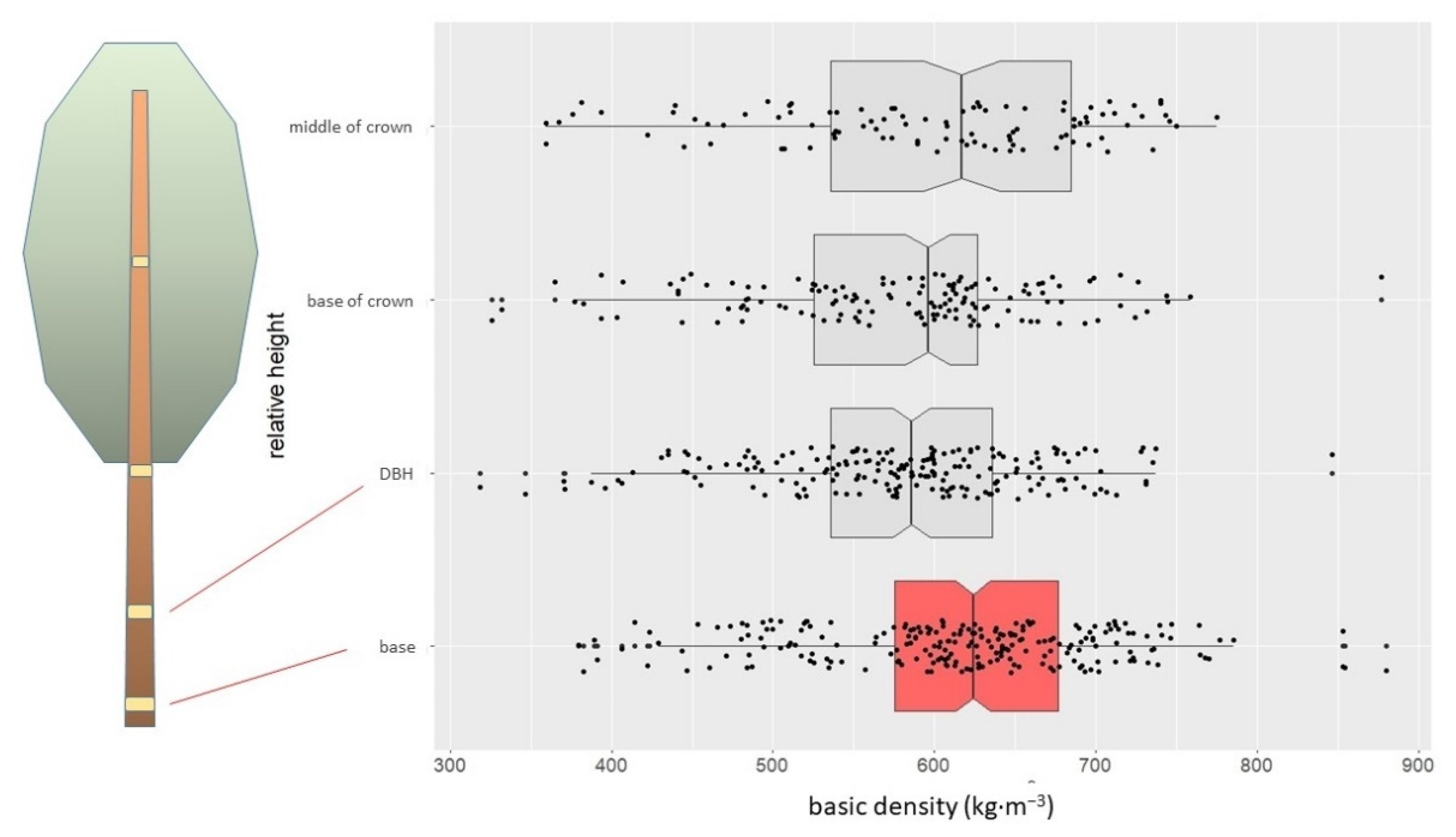
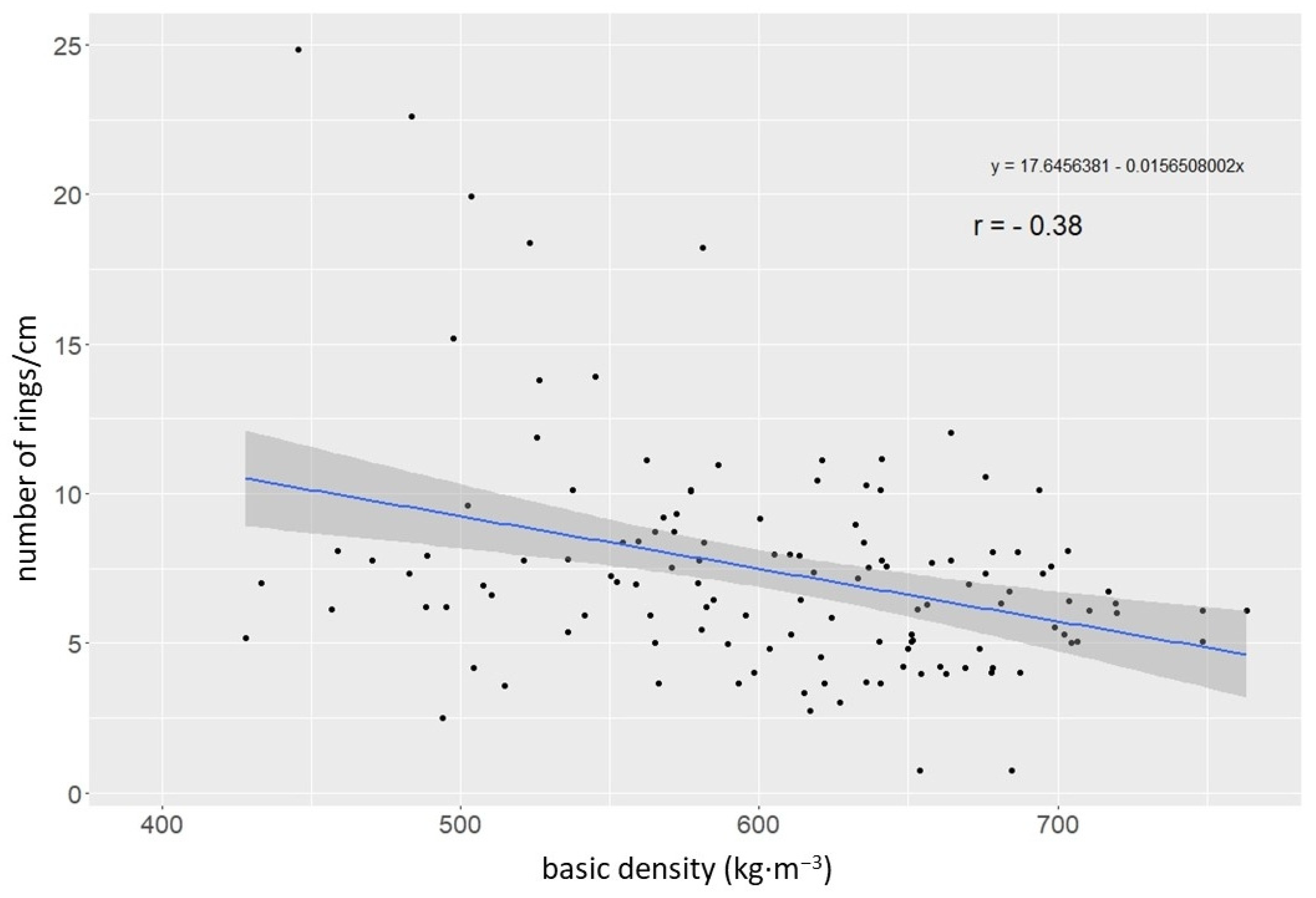
3.1. Wood Density of the Trunk
3.2. Wood Density of the Branches
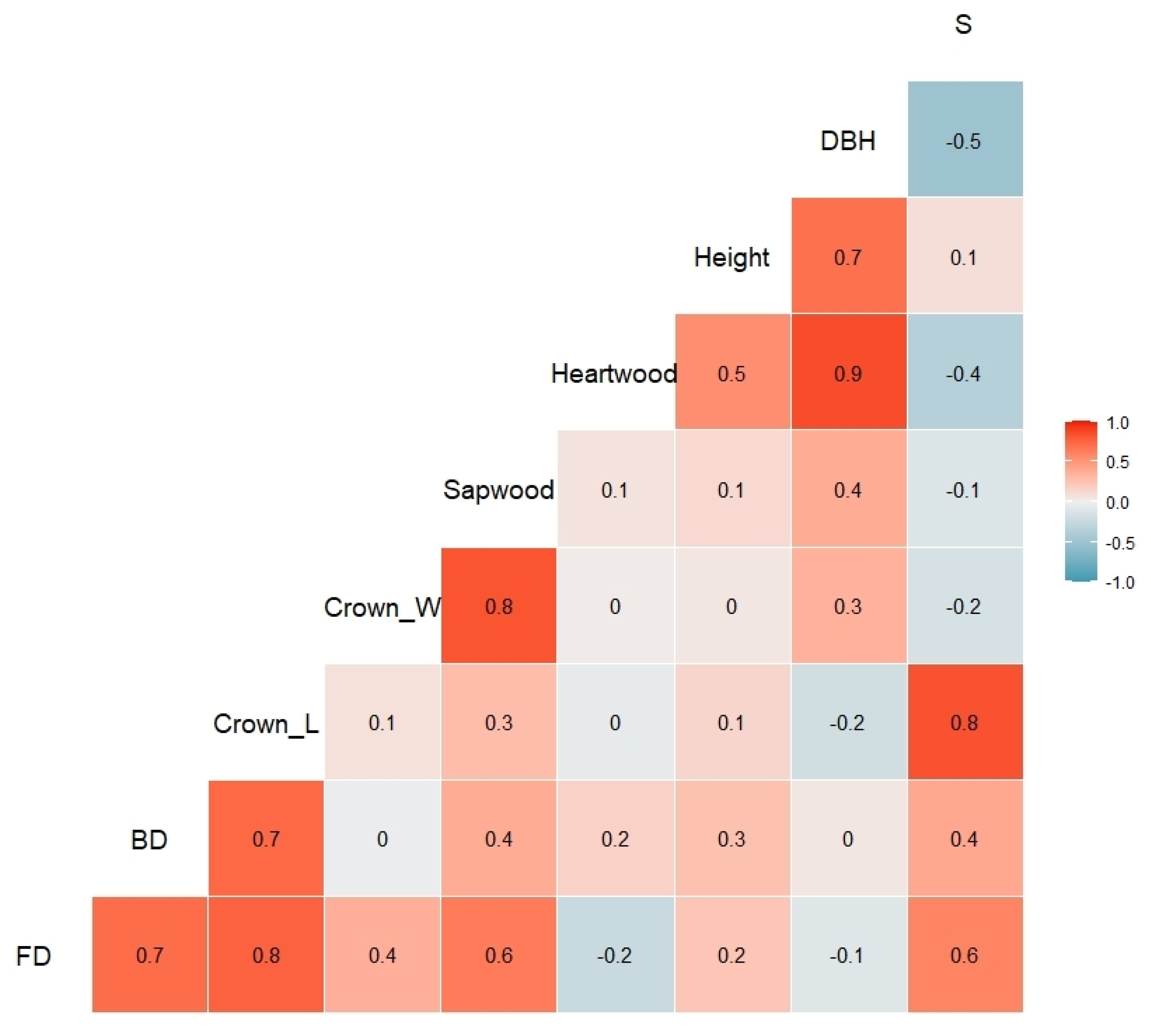
3.3. Biometric Tree Relationships
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hacke, U.G.; Sperry, J.S.; Pockman, W.T.; Davis, S.D.; McCulloh, K.A. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 2001, 126, 457–461. [Google Scholar] [CrossRef]
- Hoffmann, W.A.; Marchin, R.M.; Abit, P.; Lau, O.L. Hydraulic failure and tree dieback are associated with high wood density in a temperate forest under extreme drought. Glob. Chang. Biol. 2011, 17, 2731–2742. [Google Scholar] [CrossRef]
- Krajnc, L.; Hafner, P.; Gričar, J. The effect of bedrock and species mixture on wood density and radial wood increment in pubescent oak and black pine. For. Ecol. Manag. 2021, 481, 118753. [Google Scholar] [CrossRef]
- Fries, A. Genetic parameters, genetic gain and correlated responses in growth, fibre dimensions and wood density in a Scots pine breeding population. Ann. For. Sci. 2012, 69, 783–794. [Google Scholar] [CrossRef]
- Nazari, N.; Bahmani, M.; Kahyani, S.; Humar, M.; Koch, G. Geographic Variations of the Wood Density and Fiber Dimensions of the Persian Oak Wood. Forests 2020, 11, 1003. [Google Scholar] [CrossRef]
- Jakubowski, M.; Tomczak, A.; Jelonek, T.; Grzywiński, W. Variations of wood properties of birch (betula pendula roth) from a 23-year old seed orchard. Wood Res. 2020, 65, 75–86. [Google Scholar] [CrossRef]
- Lachowicz, H.; Bieniasz, A.; Wojtan, R. Variability in the basic density of silver birch wood in Poland. Silva Fenn 2019, 53, 13. [Google Scholar] [CrossRef]
- Fearnside, P.M. Wood density for estimating forest biomass in Brazilian Amazonia. For. Ecol. Manag. 1997, 90, 59–87. [Google Scholar] [CrossRef]
- Baker, T.R.; Phillips, O.L.; Malhi, Y.; Almeida, S.; Arroyo, L.; Di Fiore, A.; Erwin, T.; Killeen, T.J.; Laurance, S.G.; Laurance, W.F.; et al. Variation in wood density determines spatial patterns in Amazonian forest biomass. Glob. Chang. Biol. 2004, 10, 545–562. [Google Scholar] [CrossRef]
- Nogueira, E.M.; Fearnside, P.M.; Nelson, B.W. Normalization of wood density in biomass estimates of Amazon forests. For. Ecol. Manag. 2008, 256, 990–996. [Google Scholar] [CrossRef]
- Liang, X.; Ye, Q.; Liu, H.; Brodribb, T.J. Wood density predicts mortality threshold for diverse trees. New Phytol. 2020, 229, 3053–3057. [Google Scholar]
- Pásztory, Z.; Börcsök, Z.; Ronyecz, I.; Mohácsi, K.; Molnár, S.; Kis, S. Oven dry density of sessile oak, turkey oak and hornbeam in different region of Mecsek Mountain. Wood Res. 2014, 59, 683–694. [Google Scholar]
- Inagaki, T.; Hartley, I.D.; Tsuchikawa, S.; Reid, M. Prediction of oven-dry density of wood by time-domain terahertz spectroscopy. Holzforschung 2014, 68, 61–68. [Google Scholar] [CrossRef]
- Zeidler, A.; Borůvka, V. Wood density of northern red oak and pedunculate oak grown in former brown coal mine in the Czech Republic. BioResources 2016, 11, 9373–9385. [Google Scholar] [CrossRef]
- Lundström, T.; Heiz, U.; Stoffel, M.; Stöckli, V. Fresh-wood bending: Linking the mechanical and growth properties of a Norway spruce stem. Tree Physiol. 2007, 27, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Gryc, V.; Horáček, P. Variability in density of spruce (Picea abies [L.] Karst.) wood with the presence of reaction wood. J. For. Sci. 2007, 53, 129–137. [Google Scholar] [CrossRef]
- Gryc, V.; Vavrčik, H.; Horn, K. Density of juvenile and mature wood of selected coniferous species. J. For. Sci. 2011, 57, 123–130. [Google Scholar] [CrossRef]
- Niklas, K.J.; Spatz, H.C. Worldwide correlations of mechanical properties and green wood density. Am. J. Bot. 2010, 97, 1587–1594. [Google Scholar] [CrossRef]
- Goy, B.; Martin, P.; Leban, J.M. The measurement of wood density by microwave sensor. Holz als Roh-und Werkstoff. 1992, 50, 163–166. [Google Scholar] [CrossRef]
- Rinn, F.; Schweingruber, F.H.; Schär, E. Resistograph and x-ray density charts of wood. Comparative evaluation of drill-resistance profiles and x-ray density charts of different wood species. Holzforschung 1996, 50, 303–311. [Google Scholar] [CrossRef]
- Macedo, A.; Vaz, C.M.P.; Pereira, J.C.D.; Naime, J.M.; Cruvinel, P.E.; Crestana, S. Wood density determination by X-and gamma-ray tomography. Holzforschung 2002, 56, 535–540. [Google Scholar] [CrossRef]
- Gwaze, D.; Stevenson, A. Genetic variation of wood density and its relationship with drill resistance in shortleaf pine. South. J. Appl. For. 2008, 32, 130–133. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Karlsson, B.; Lundqvist, S.O.; Gil, M.R.G.; Olsson, L.; Wu, H.X. Estimating solid wood properties using Pilodyn and acoustic velocity on standing trees of Norway spruce. Ann. For. Sci. 2015, 72, 499–508. [Google Scholar] [CrossRef]
- Gao, S.; Wang, X.; Brashaw, B.K.; Ross, R.J.; Wang, L. Rapid assessment of wood density of standing tre with nondestructive methods—A review. In Proceedings of the 2012 International Conference on Biobase Material Science and Engineering, Changsha, China, 21–23 October 2012; pp. 262–267. [Google Scholar] [CrossRef]
- Gao, S.; Wang, X.; Wiemann, M.C.; Brashaw, B.K.; Ross, R.J.; Wang, L. A critical analysis of methods for rapid and nondestructive determination of wood density in standing trees. Ann. For. Sci. 2017, 74, 27. [Google Scholar] [CrossRef]
- Mannes, D.; Lehmann, E.; Cherubini, P.; Niemz, P. Neutron imaging versus standard X-ray densitometry as method to measure tree-ring wood density. Trees 2007, 21, 605–612. [Google Scholar] [CrossRef]
- Jacquin, P.; Longuetaud, F.; Leban, J.M.; Mothe, F. X-ray microdensitometry of wood: A review of existing principles and devices. Dendrochronologia 2017, 42, 42–50. [Google Scholar] [CrossRef]
- Schönfelder, O.; Zeidler, A.; Borůvka, V.; Bílek, L.; Vítámvás, J. Effect of Shelterwood and Clear-Cutting Regeneration Method on Wood Density of Scots Pine. Forests 2020, 11, 868. [Google Scholar] [CrossRef]
- Larjavaara, M.; Muller-Landau, H.C. Still rethinking the value of high wood density. Am. J. Bot. 2012, 99, 165–168. [Google Scholar] [CrossRef]
- Williamson, G.B.; Wiemann, M.C. Measuring wood specific gravity… correctly. Am. J. Bot. 2010, 97, 519–524. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Owoundi, R.E.; Nepveu, G.; Mothe, F.; Dhote, J.F. Modelling wood density in European Oak (Quercus petraea and Quercus robur) and simulating the silvicultural influence. Can. J. For. Res. 1993, 23, 2587–2593. [Google Scholar] [CrossRef]
- Degron, R.; Nepveu, G. Methodology of microdensitometric measurement for oak wood (Quercus robur L. and Quercus petraea Liebl): Proposal for a representative sampling of oak wood density within ring. Holzforschung 1996, 50, 491–496. [Google Scholar] [CrossRef]
- Bergès, L.; Dupouey, J.L.; Franc, A. Long-term changes in wood density and radial growth of Quercus petraea Liebl. in northern France since the middle of the nineteenth century. Trees 2000, 14, 398–408. [Google Scholar] [CrossRef]
- Fromm, J.H.; Sautter, I.; Matthies, D.; Kremer, J.; Schumacher, P.; Ganter, C. Xylem water content and wood density in spruce and oak trees detected by high-resolution computed tomography. Plant. Physiol. 2001, 127, 416–425. [Google Scholar] [CrossRef]
- Knapic, S.; Louzada, J.L.; Leal, S.; Pereira, H. Radial variation of wood density components and ring width in cork oak trees. Ann. For. Sci. 2007, 64, 211–218. [Google Scholar] [CrossRef]
- Genet, A.; Auty, D.; Achim, A.; Bernier, M.; Pothier, D.; Cogliastro, A. Consequences of faster growth for wood density in northern red oak (Quercus rubra Liebl.). Forestry 2013, 86, 99–110. [Google Scholar] [CrossRef]
- Sousa, V.B.; Louzada, J.L.; Pereira, H. Variation of ring width and wood density in two unmanaged stands of the Mediterranean Oak Quercus faginea. Forests 2018, 9, 44. [Google Scholar] [CrossRef]
- Humar, M.; Fabčič, B.; Zupančič, M.; Pohleven, F.; Oven, P. Influence of xylem growth ring width and wood density on durability of oak heartwood. Int. Biodeterior. Biodegrad. 2008, 62, 368–371. [Google Scholar] [CrossRef]
- Ackermann, F. Influence du type de station forestière sur les composantes intracernes de la densité du bois du chêne pédonculé (Quercus robur L) dans les chênaies de l’Adour et des coteaux basco-béarnais. In Annales Des Sciences Forestières; EDP Sciences: Les Ulis, France, 1995; Volume 52, pp. 635–652. [Google Scholar]
- Degron, R.; Nepveu, G. Prévision de la variabilité intra-et interarbre de la densité du bois de chêne rouvre (Quercus petraea Liebl) par modélisation des largeurs et des densités des bois initial et final en fonction de l’âge cambial, de la largeur de cerne et du niveau dans l’arbre. In Annales Des Sciences Forestières; EDP Sciences: Les Ulis, France, 1996; Volume 53, pp. 1019–1030. [Google Scholar]
- Kollmann, F.F.P.; Côté, W.A. Principles of Wood Science and Technology I Solid Wood; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1968; p. 592. [Google Scholar]
- Kiaei, M. Wood density of naturally growing oak trees in north Iran. Res. Crop. 2012, 13, 776–777. [Google Scholar]
- Tomczak, A.; Jelonek, T.; Jakubowski, M. Changes in the structure and properties of wood as an effect of the impact of wind on trees. Sylwan 2012, 156, 776–783. [Google Scholar]
- Bahmani, M.; Saeedi, S.; Humar, M.; Kool, F.; Zabol, I. Effect of tree diameter classes on the properties of Persian oak (Quercus brantii Lindl.) wood. Wood Res. Slovak 2018, 63, 755–762. [Google Scholar]
- Guilley, E.; Hervé, J.C.; Nepveu, G. The influence of site quality, silviculture and region on wood density mixed model in Quercus petraea Liebl. For. Ecol. Manag. 2004, 189, 111–121. [Google Scholar] [CrossRef]
- Bergès, L.; Nepveu, G.; Franc, A. Effects of ecological factors on radial growth and wood density components of sessile oak (Quercus petraea Liebl.) in Northern France. For. Ecol. Manag. 2008, 255, 567–579. [Google Scholar] [CrossRef]
- Sousa, V.B.; Cardoso, S.; Pereira, H. Ring width variation and heartwood development in Quercus faginea. Wood Fiber Sci. 2013, 45, 405–414. [Google Scholar]
- Mosedale, J.R.; Charrier, B.; Janin, G. Genetic control of wood colour, density and heartwood ellagitannin concentration in European oak (Quercus petraea and Q. robur). For. Int. J. For. Res. 1996, 69, 111–124. [Google Scholar] [CrossRef]
- Kelly, D.L. The regeneration of Quercus petraea (sessile oak) in southwest Ireland: A 25-year experimental study. For. Ecol. Manag. 2002, 166, 207–226. [Google Scholar] [CrossRef]
- Olesen, P.O. Water displacement method; a fast and accurate method of determining the green volume of wood samples. For. Tree Improv. 1971, 3, 3–23. [Google Scholar]
- Lei, H.; Milota, M.R.; Gartner, B.L. Between-and within-tree variation in the anatomy and specific gravity of wood in Oregon white oak (Quercus garryana Dougl.). IAWA J. 1996, 17, 445–461. [Google Scholar] [CrossRef]
- Zheng, J.; Martínez-Cabrera, H.I. Wood anatomical correlates with theoretical conductivity and wood density across China: Evolutionary evidence of the functional differentiation of axial and radial parenchyma. Ann. Bot. 2013, 112, 927–935. [Google Scholar] [CrossRef] [PubMed]
- King, D.A.; Davies, S.J.; Tan, S.; Noor, N.S.M. The role of wood density and stem support costs in the growth and mortality of tropical trees. J. Ecol. 2006, 94, 670–680. [Google Scholar] [CrossRef]
- Yang, X.Z.; Zhang, W.H.; He, Q.Y. Effects of intraspecific competition on growth, architecture and biomass allocation of Quercus liaotungensis. J. Plant. Interact. 2019, 14, 284–294. [Google Scholar] [CrossRef]
- Alfaro-Sánchez, R.; Valdés-Correcher, E.; Espelta, J.M.; Hampe, A.; Bert, D. How do social status and tree architecture influence radial growth, wood density and drought response in spontaneously established oak forests? Ann. For. Sci. 2020, 77, 1–17. [Google Scholar] [CrossRef]
- Kaźmierczak, K.; Pazdrowski, W.; Mańka, K.; Szymański, M.; Nawrot, M. Forming slendernes of pedunculate oak stems (Quercus robur L.) in dependence of age of trees. Sylwan 2008, 7, 39–45. [Google Scholar]
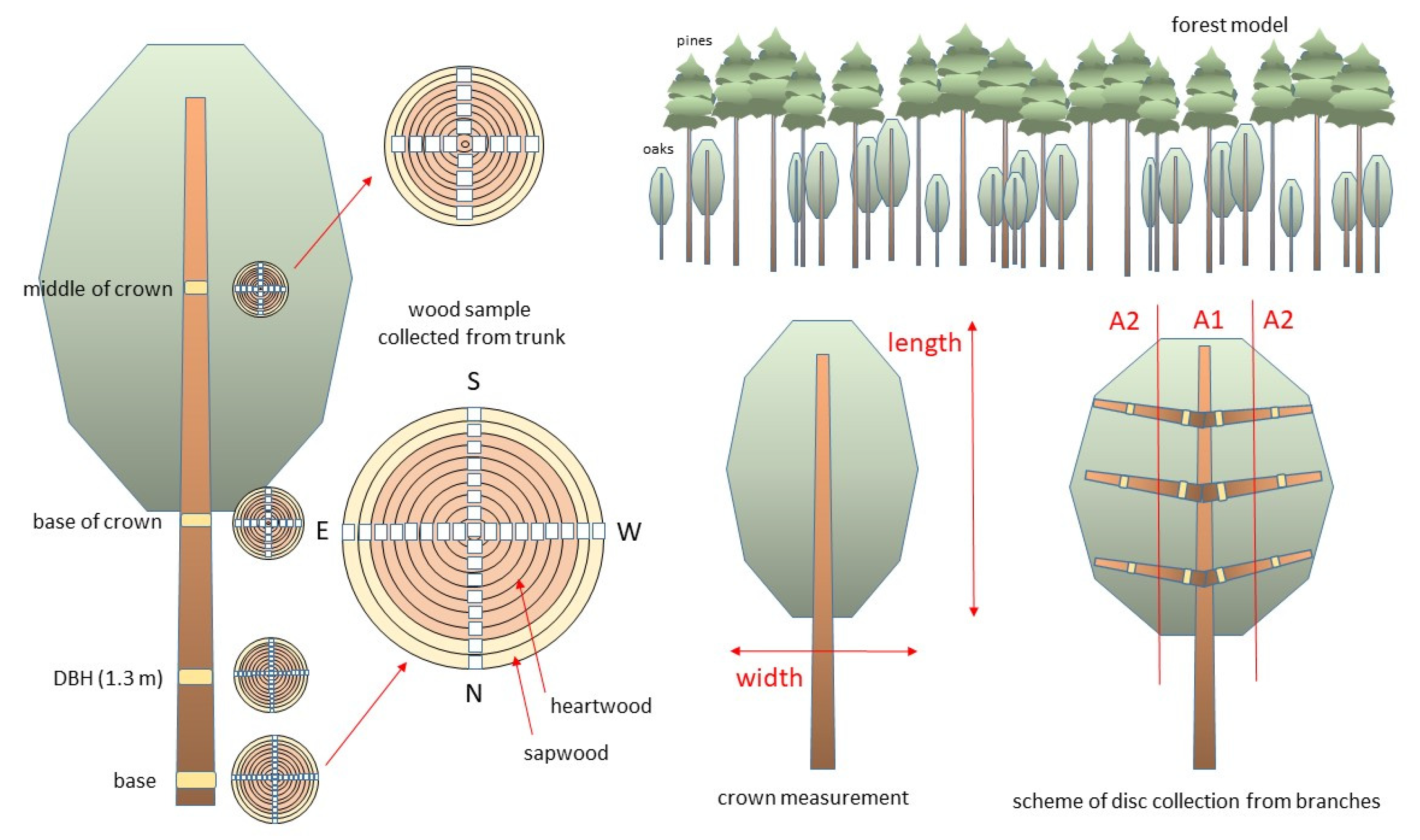
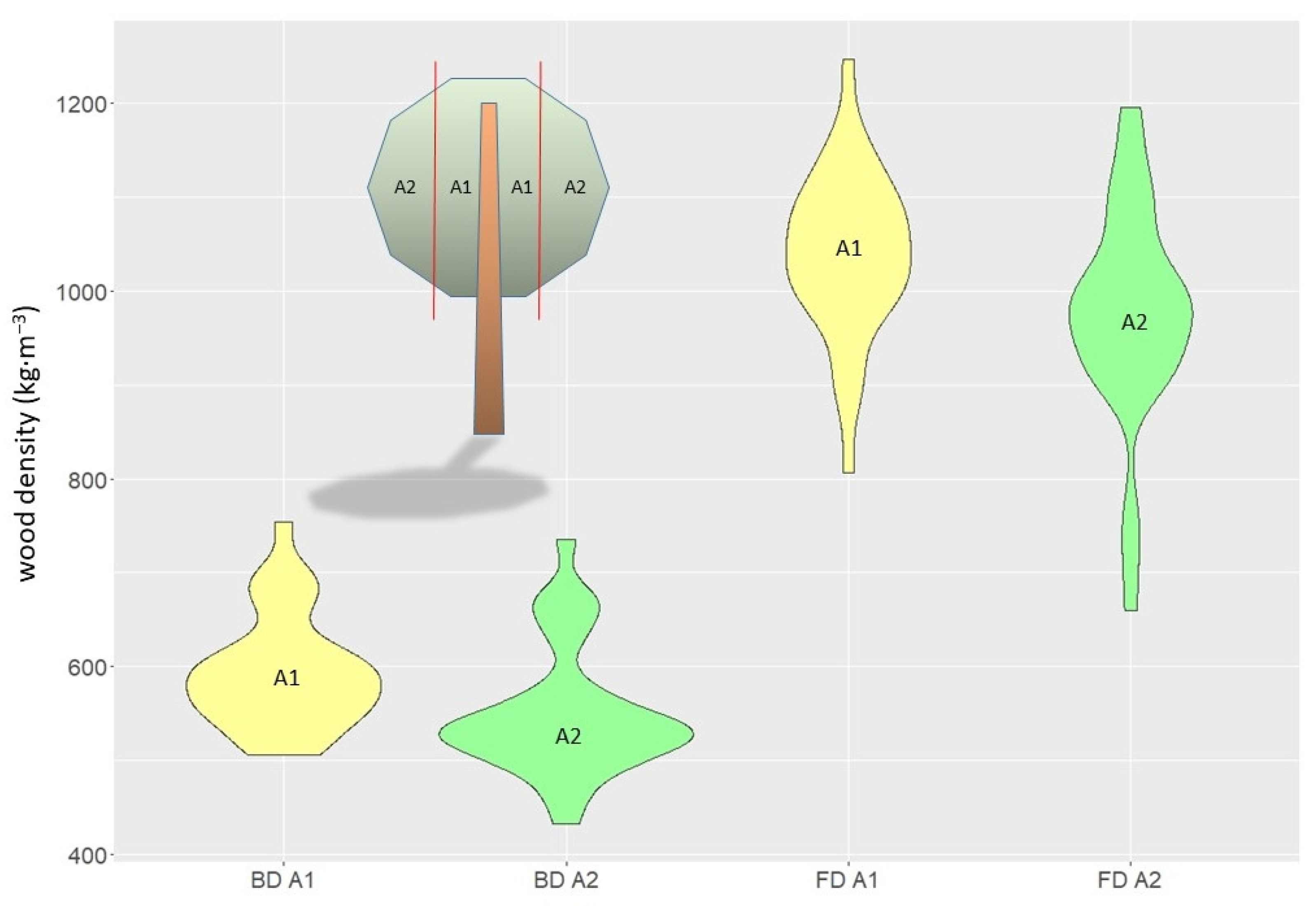

| Tree Features | Mean ± SD |
|---|---|
| DBH (cm) | 17.8 ± 3.9 |
| Tree height (m) | 13.6 ± 2.7 |
| Heartwood area at DBH (cm2) | 163 ± 86 |
| Sapwood area at DBH (cm2) | 48 ± 26 |
| Crown width (m) | 5.8 ± 2.1 |
| Crown length (m) | 9.6 ± 2.9 |
| Number of rings in basal disc | 65 ± 12 |
| Location | Density Category | Mean | Q25 | Median | Q75 | SD | CV (%) | Max | Min |
|---|---|---|---|---|---|---|---|---|---|
| trunk (samples) | basic (kg·m−3) | 595 | 537 | 605 | 655 | 93 | 15.6 | 880 | 318 |
| trunk (discs) | fresh (kg·m−3) | 1031 | 983 | 1032 | 1091 | 75 | 7.2 | 1183 | 885 |
| branches (discs) | basic (kg·m−3) | 573 | 524 | 558 | 610 | 68 | 11.9 | 754 | 433 |
| branches (discs) | fresh (kg·m−3) | 1007 | 959 | 1011 | 1081 | 109 | 10.8 | 1247 | 660 |
| Age (y) | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | 110 | 120 | 130 | 140 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 1.09 | 1.07 | 1.02 | 0.97 | 0.93 | 0.89 | 0.85 | 0.83 | 0.76 | 0.72 | 0.75 | 0.76 |
| Min. | 0.50 | 0.56 | 0.59 | 0.54 | 0.53 | 0.57 | 0.6 | 0.60 | 0.61 | 0.59 | 0.58 | 0.61 |
| Max. | 1.60 | 1.59 | 1.49 | 1.25 | 1.23 | 1.18 | 1.09 | 1.07 | 1.00 | 0.96 | 0.92 | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubowski, M.; Dobroczyński, M. Allocation of Wood Density in European Oak (Quercus robur L.) Trees Grown under a Canopy of Scots Pine. Forests 2021, 12, 712. https://doi.org/10.3390/f12060712
Jakubowski M, Dobroczyński M. Allocation of Wood Density in European Oak (Quercus robur L.) Trees Grown under a Canopy of Scots Pine. Forests. 2021; 12(6):712. https://doi.org/10.3390/f12060712
Chicago/Turabian StyleJakubowski, Marcin, and Marek Dobroczyński. 2021. "Allocation of Wood Density in European Oak (Quercus robur L.) Trees Grown under a Canopy of Scots Pine" Forests 12, no. 6: 712. https://doi.org/10.3390/f12060712
APA StyleJakubowski, M., & Dobroczyński, M. (2021). Allocation of Wood Density in European Oak (Quercus robur L.) Trees Grown under a Canopy of Scots Pine. Forests, 12(6), 712. https://doi.org/10.3390/f12060712







