Nutrient Balance as a Tool for Maintaining Yield and Mitigating Environmental Impacts of Acacia Plantation in Drained Tropical Peatland—Description of Plantation Simulator
Abstract
1. Introduction
- how the nutrient balance of an Acacia stand will change under a higher WT regime;
- what kind of nutrient management should be applied to maintain the yield under higher WT management; and
- how much the rate of peat subsidence and CO2 efflux will be decreased under this management regime?
2. Materials and Methods
2.1. Acacia Plantation in Drained Tropical Peatlands
2.2. Study Outline
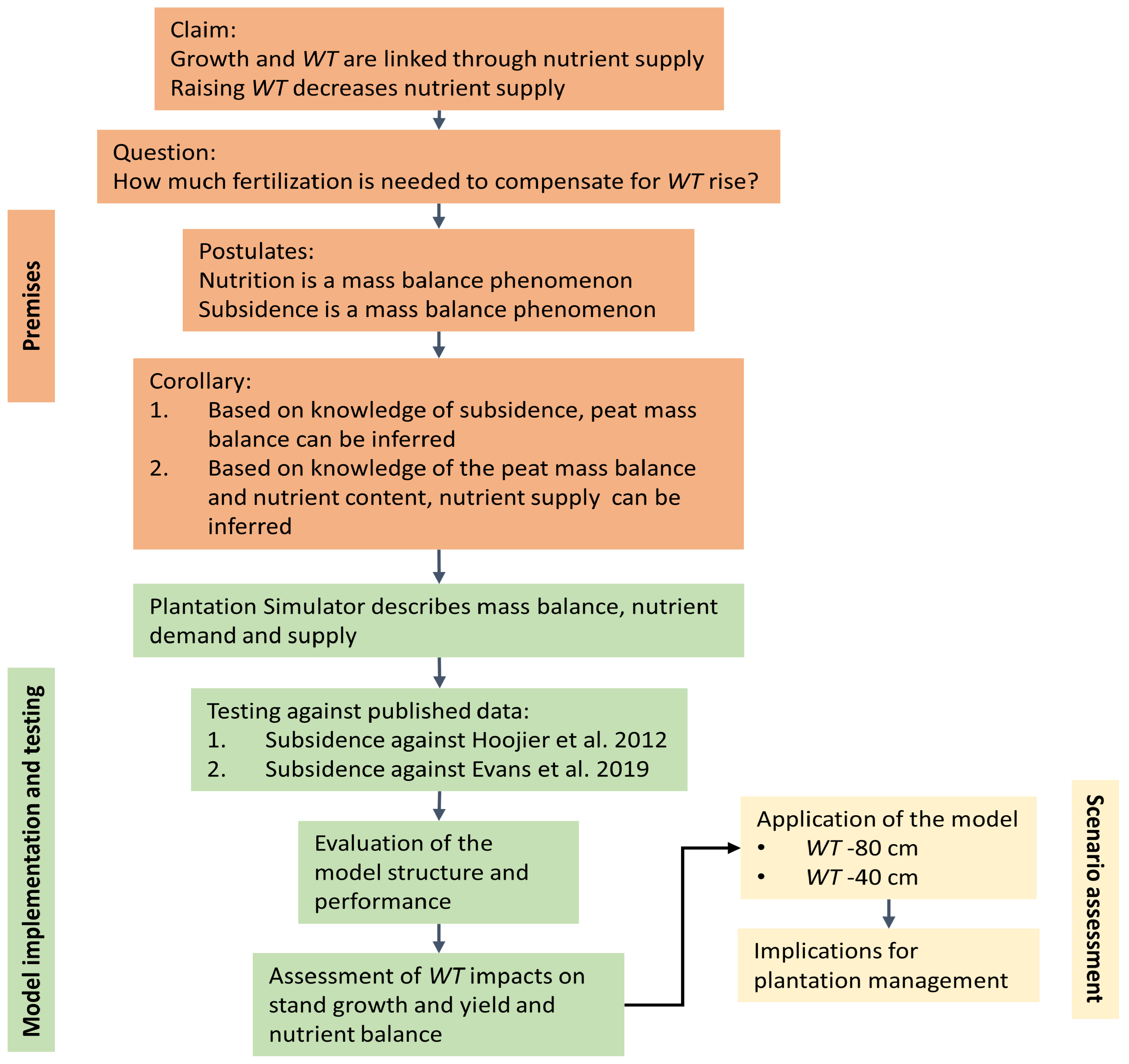
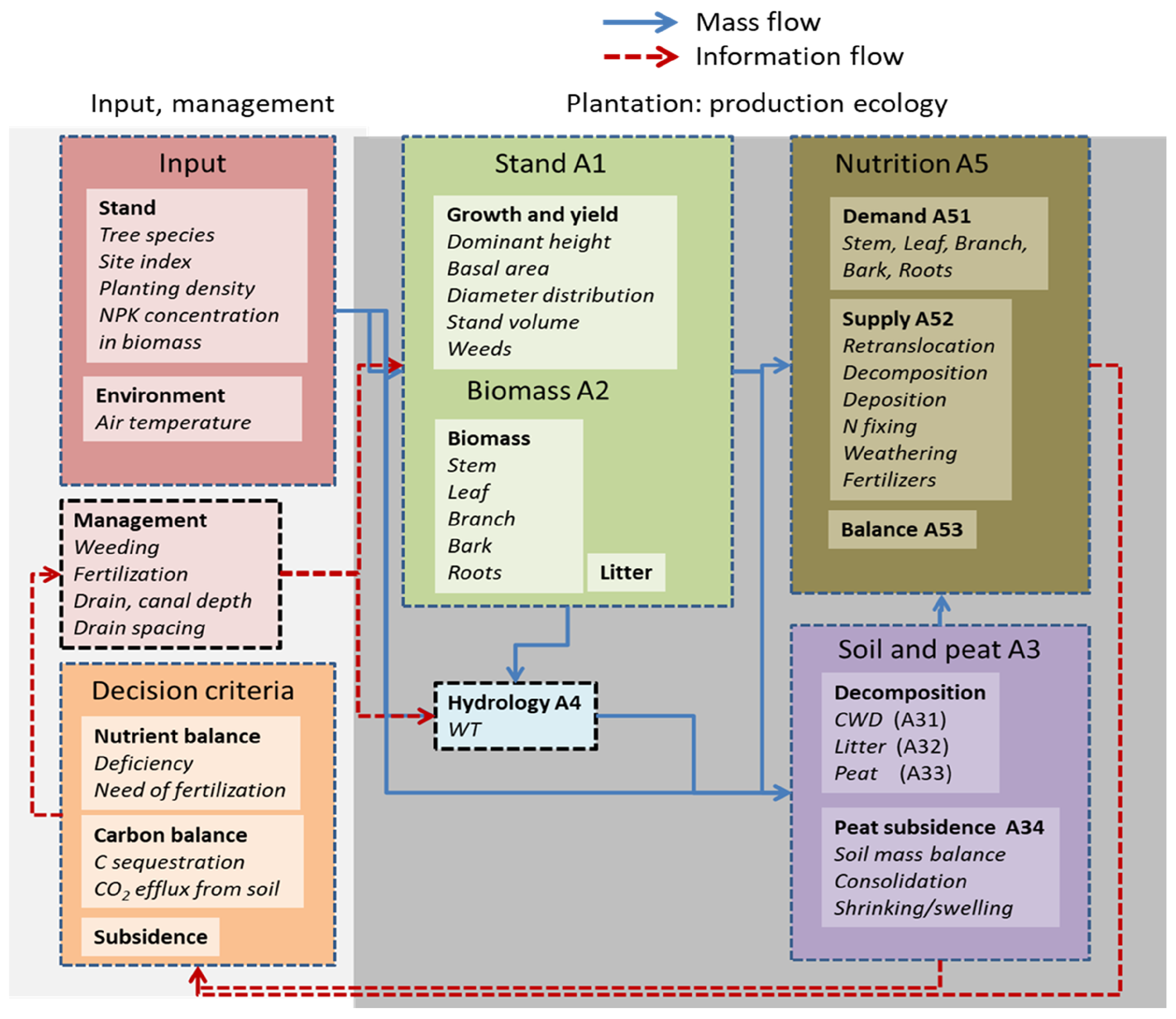
2.3. Overall Model Description
2.4. Deduction with Plantation Simulator
2.5. Model Testing
2.6. Scenario Assessment
3. Results
3.1. Testing the Mass Balance Using Published Subsidence Data
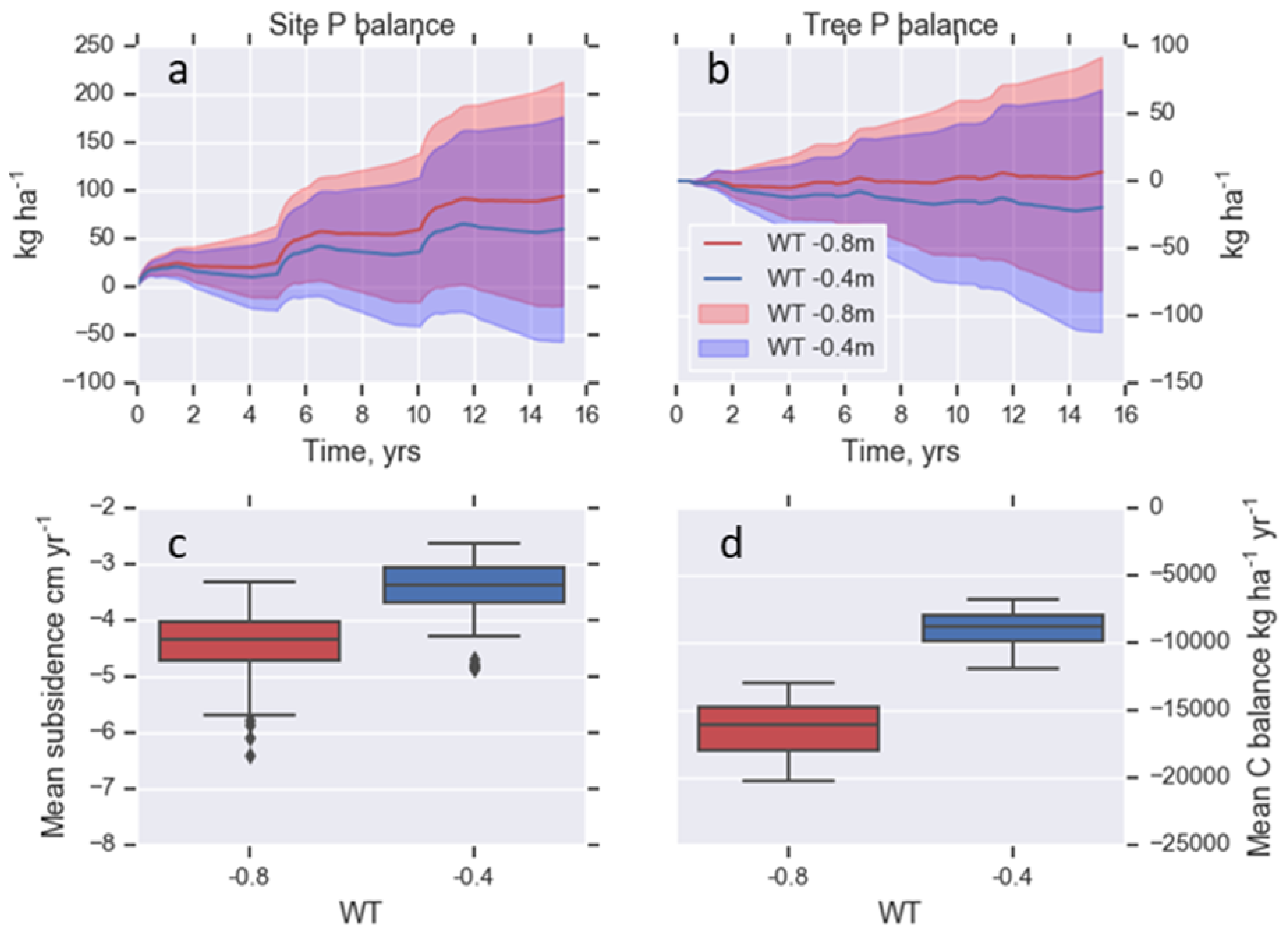
3.2. Nutrient and C Balances
3.3. Scenario Assessment: Raising the Water Table
4. Discussion
4.1. Need for a Simulation Model
4.2. Model Performance
4.3. Scenario Assessment
4.4. Uncertainty
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Plantation Simulator Description
Appendix A.1. Stand
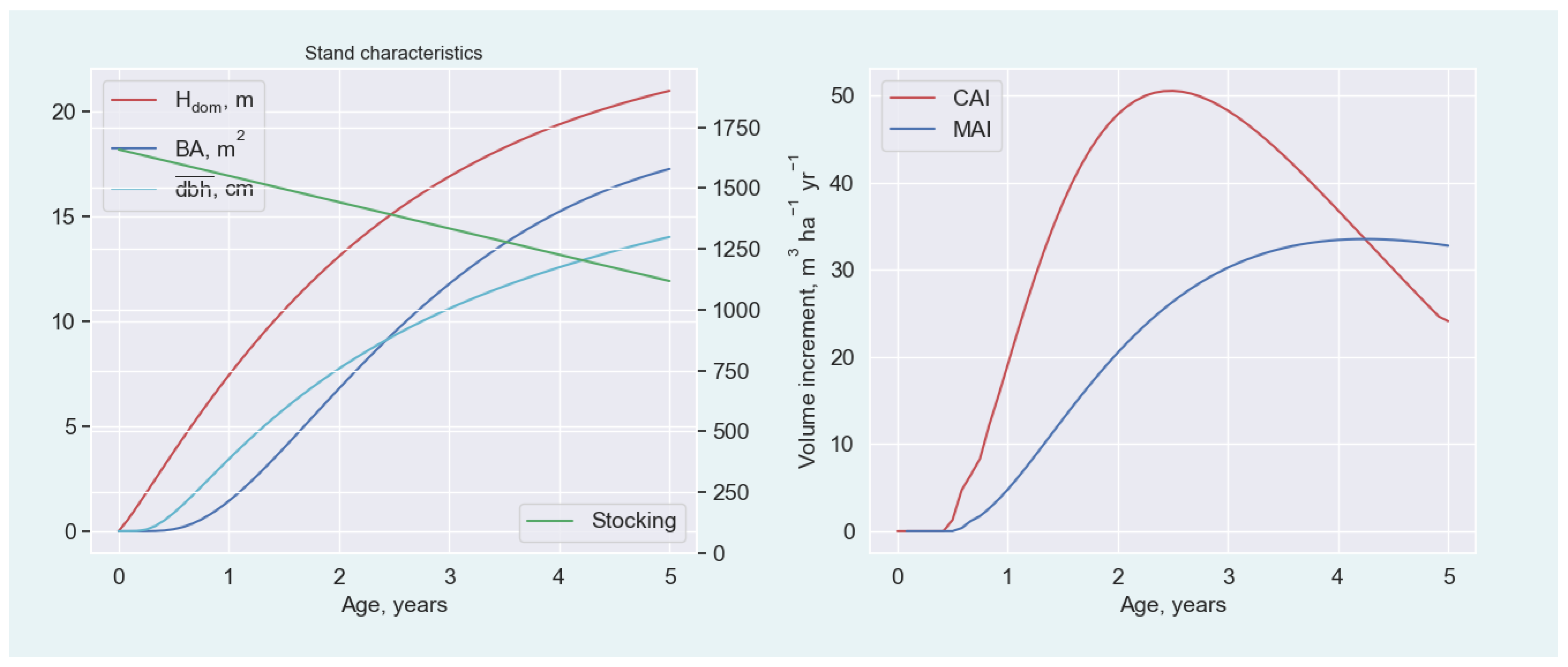
Appendix A.1.1. Stand Growth and Yield
Appendix A.1.2. Parameterization of H dom Model
| Symbol | Description | Unit | Value |
|---|---|---|---|
| Initial stocking | stems ha−1 | 1666 | |
| Site index | m at | 17…25 | |
| Mortality rate | stems ha−1 yr−1 | 12…240 | |
| Stocking | stems ha −1 | 500…1666 | |
| A | Stand age | yrs | 0…5 |
| Dominant height | m | 0…25 | |
| G | Basal area | m2 ha−1 | 0…25 |
| Weighted mean diameter | cm | 0…25 | |
| Diameter distribution | in diameter d | - | |
| Index age | yrs | 5 | |
| d | Tree diameter | cm | 0…40 |
| h | Tree height | m | 0…30 |
| parameter | - | 0.5 | |
| parameter | - | 1.1 | |
| G parameter | - | −4.14724 | |
| G parameter | - | −2.07358 | |
| G parameter | - | 0.99584 | |
| G parameter | - | 0.62386 | |
| Diameter disrtibution parameter | - | Equation (A5) | |
| Diameter disrtibution parameter | - | Equation (A6) | |
| Diameter disrtibution parameter | - | 0.00378 | |
| Diameter disrtibution parameter | - | 1.06688 | |
| Diameter disrtibution parameter | - | 0.09209 | |
| Diameter disrtibution parameter | - | 1.49093 | |
| Diameter disrtibution parameter | - | −0.03053 | |
| Tree height parameter | - | −0.5929 | |
| Tree height parameter | - | −0.31894 | |
| Tree height parameter | - | 1.48904 | |
| Tree height parameter | - | 6.77843 | |
| Tree height parameter | - | −0.13929 | |
| Tree height parameter | - | 0.2971 | |
| Tree height parameter | - | −0.72857 | |
| Tree height parameter | - | Equation (A8) | |
| Tree height parameter | - | Equation (A9) | |
| Tree volume parameter | - | −9.83466 | |
| Tree volume parameter | - | 1.70518 | |
| Tree volume parameter | - | 1.14496 |
Appendix A.2. Biomass and Litter
| Symbol | Description | Unit | Value |
|---|---|---|---|
| Biomass parameters | |||
| Wood density | kg m−3 | 500 | |
| Maximum green mass | kg ha−1 | 8000 | |
| Bark parameter | - | −3.421 | |
| Bark parameter | - | 1.848 | |
| Branch parameter | - | −0.576 | |
| Branch parameter | - | 1.282 | |
| Foliage parameter | - | −0.143 | |
| Foliage parameter | - | 0.659 | |
| Weed parameter | kg ha−1 | 6000.0 | |
| Weed parameter | - | 1.0 | |
| Weed age | years | ||
| Litter parameters | |||
| Mass in component i 1 | kg ha−1 | ||
| Bark longevity | years | 5 | |
| Branch longevity | years | 2 | |
| Foliage longevity | years | 0.5 | |
| Coarseroot longevity | years | 5 | |
| fineroot longevity | years | 0.25 | |
| Litter mass from living biomass | kg ha−1 | ||
| Litter mass from dead biomass | kg ha−1 | ||
| Coarse woody debris | kg ha−1 | ||
| Nutrient parameters | Nutrient J 2 | ||
| J concentration in bark | mass-% | 1.30, 0.10, 0.33 | |
| J concentration in branch | mass-% | 0.30, 0.10, 0.15 | |
| J concentration in foliage | mass-% | 2.20, 0.10, 0.40 | |
| J concentration in coarse roots | mass-% | 0.30, 0.02, 0.05 | |
| J concentration in fine roots | mass-% | 3.00, 0.02, 0.05 | |
| J concentration in stem | mass-% | 0.30, 0.03, 0.03 | |
| J concentration in weeds | mass-% | 1.30, 0.09, 0.45 | |
| J retranslocation Acacia | kg kg−1 | 0.20, 0.33, 0.64 | |
| J retranslocation weeds | kg kg−1 | 0.50, 0.5, 0.5 | |
| Litter nutrients from living biomass | kg ha−1 time step−1 | Equation (A17) | |
| Litter nutrients from dead biomass | kg ha−1 time step−1 | Equation (A18) |
Appendix A.3. Soil and Peat
Appendix A.3.1. Coarse Woody Debris (CWD) Decomposition Module
| Symbol | Description | Unit | Value |
|---|---|---|---|
| Soil temperature in open area | °C | 29 | |
| Soil temperature under canopy | °C | 32 | |
| Water table | m | ||
| Coarse woody debris | |||
| Coarse woody debris | kg ha−1 | timestep−1 | |
| Mean air temperature | °C | ||
| Decay rate of CWD | year−1 | ||
| Remaining CWD in the cohort | kg ha−1 | ||
| Decayed CWD in the cohort | kg ha−1 time step−1 | ||
| Carbon content in CWD | kg kg−1 | 0.5 | |
| C emission from CWD | kg ha−1 | ||
| N in CWD | kg ha−1 | ||
| P in CWD | kg ha−1 | ||
| K in CWD | kg ha−1 | ||
| N release from CWD | kg ha−1 time step−1 | ||
| P release from CWD | kg ha−1 time step−1 | ||
| K release from CWD | kg ha−1 time step−1 | ||
| Romul: Litter decomposition | |||
| W | Soil moisture relative to field capacity | - | 1.28 |
| Soil pH | pH-unit | 3.5 | |
| Soi temperature | °C | 29…32 | |
| Soil organic matter pools | (J = mass, N, P, K) | ||
| Fresh litter, above ground | kg m−2 | ||
| Fresh litter, below ground | kg m−2 | ||
| Partly decomposed OM, above ground | kg m−2 | ||
| Partly decomposed OM, below ground | kg m−2 | ||
| Humus | kg m−2 | ||
| Release of CO2 and nutrients | kg ha−1 time step−1 | ||
| Temperature sensitivity | - | 2 | |
| Reference temperature | °C | 28 | |
| Time step in peat decomposition | years | 30/365 | |
| Total CO2 from soil | kg m−2 | ||
| Release of nutrient J from peat | kg m−2 | ||
| Conversion factor from CO2 to C | 0.2727 | ||
| Conversion farcor from C to dry biomass | 2 |
Appendix A.3.2. Stand Litter Decomposition, Romul Module

Appendix A.3.3. Peat Decomposition Module
| Symbol | Description | Unit | Value |
|---|---|---|---|
| Net demand | kg ha−1 timestep−1 | ||
| Gross demand | kg ha−1 timestep−1 | ||
| Share of microbial fixing from N demand | kg kg−1 | 0.4 | |
| Atmospheric deposition of N, P, K | kg ha−1 yr−1 | 15.0, 0.1, 6.2 | |
| Concentration of of N, P, K in peat | % mass | 1.6, 0.015, 0.03 | |
| Microbial fixation of N | kg ha−1 timestep−1 | ||
| Restranslocation of nutrients | kg ha−1 timestep−1 | ||
| Release of J from fertilizers | kg ha−1 timestep−1 | ||
| Fertilizar nutrient content | % of mass in N, P2O5, K2O | ||
| Fertilizar dose | g tree−1 | ||
| Decay rate of fertilizer | time step−1 | ||
| Total nutrient supply | kg ha−1 timestep−1 | ||
| Tree-wise nutrient supply | kg ha−1 timestep−1 | ||
| Total nutrient balance | kg ha−1 timestep−1 | ||
| Tree coverage | m2 m−2 |
Appendix A.3.4. Subsidence Module
Appendix A.3.5. Carbon Balance
Appendix A.4. Hydrology
Appendix A.5. Nutrition
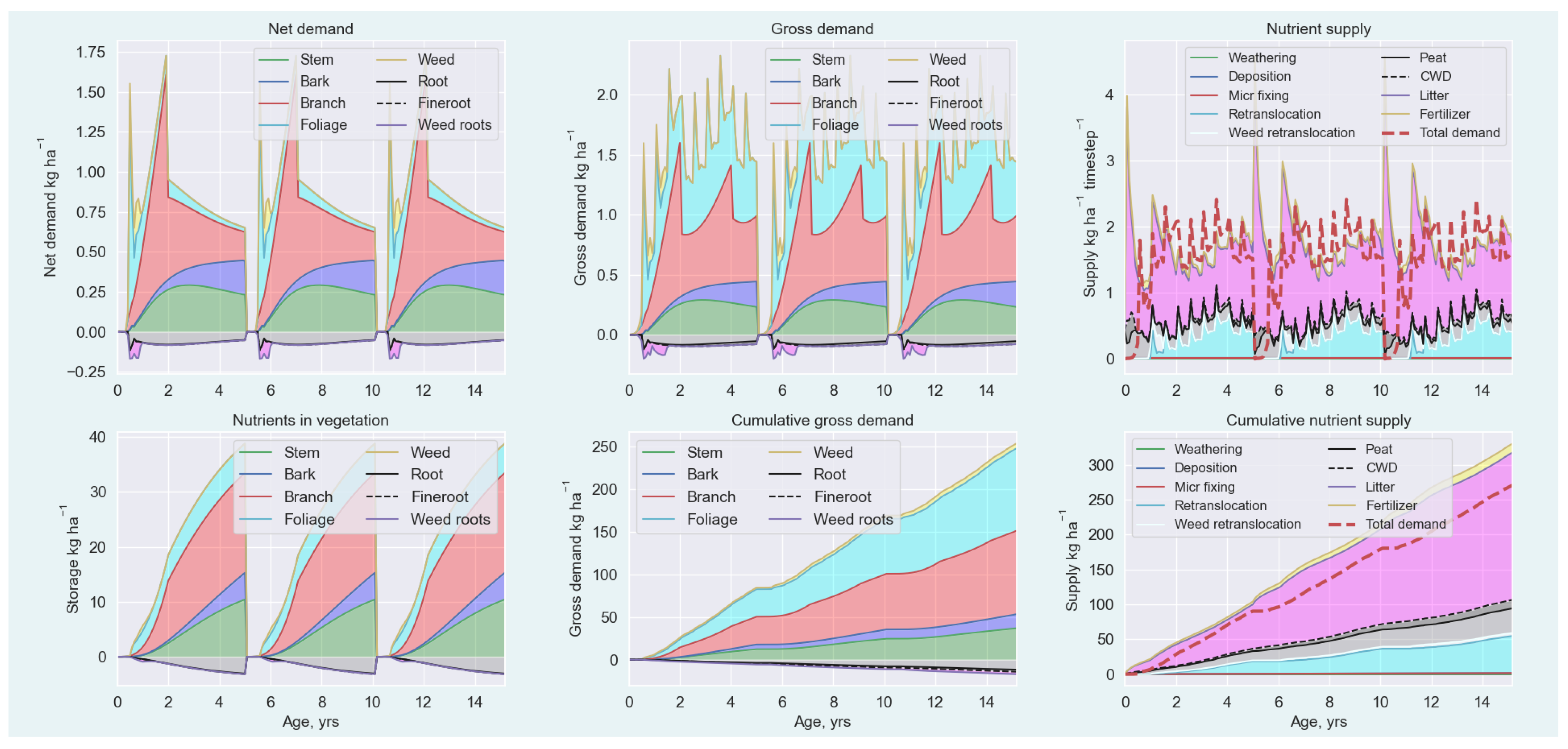
Appendix A.5.1. Nutrient Demand
Appendix A.5.2. Nutrient Supply
Appendix A.5.3. Nutrient Balance

References
- Nichols, J.E.; Peteet, D.M. Rapid expansion of northern peatlands and doubled estimate of carbon storage. Nat. Geosci. 2019, 12, 917–921. [Google Scholar] [CrossRef]
- Dargie, G.; Lewis, S.; Lawson, I.; Mitchard, E.; Page, S.; Bocko, Y.; Ifo, S. Age, extent and carbon storage of the central Congo Basin peatland complex. Nature 2017, 542, 86–90. [Google Scholar] [CrossRef]
- Reddy, K.; Delaune, R. Biogeochemistry of Wetlands: Science and Applications; CRC Press: Boca Raton, FL, USA, 2008; pp. 1–781. [Google Scholar] [CrossRef]
- Minkkinen, K.; Laine, J. Long-term effect of forest drainage on the peat carbon stores of pine mires in Finland. Can. J. For. Res. 1998, 28, 1267–1275. [Google Scholar] [CrossRef]
- Hooijer, A.; Page, S.; Jauhiainen, J.; Lee, W.A.; Lu, X.X.; Idris, A.; Anshari, G. Subsidence and carbon loss in drained tropical peatlands. Biogeosciences 2012, 9, 1053–1071. [Google Scholar] [CrossRef]
- Ojanen, P.; Minkkinen, K.; Alm, J.; Penttilä, T. Soil–atmosphere CO2, CH4 and N2O fluxes in boreal forestry-drained peatlands. For. Ecol. Manag. 2010, 260, 411–421. [Google Scholar] [CrossRef]
- Jauhiainen, J.; Hooijer, A.; Page, S.E. Carbon dioxide emissions from an Acacia plantation on peatland in Sumatra, Indonesia. Biogeosciences 2012, 9, 617–630. [Google Scholar] [CrossRef]
- Miettinen, J.; Shi, C.; Liew, S.C. Fire Distribution in Peninsular Malaysia, Sumatra and Borneo in 2015 with Special Emphasis on Peatland Fires. Environ. Manag. 2017, 60. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.D.; Williamson, J.M.; Kacaribu, F.; Irawan, D.; Suardiwerianto, Y.; Hidayat, M.F.; Laurén, A.; Page, S.E. Rates and spatial variability of peat subsidence in Acacia plantation and forest landscapes in Sumatra, Indonesia. Geoderma 2019, 338, 410–421. [Google Scholar] [CrossRef]
- Nieminen, M.; Sallantaus, T.; Ukonmaanaho, L.; Nieminen, T.M.; Sarkkola, S. Nitrogen and phosphorus concentrations in discharge from drained peatland forests are increasing. Sci. Total. Environ. 2017, 609, 974–981. [Google Scholar] [CrossRef]
- Konecny, K.; Ballhorn, U.; Navratil, P.; Jubanski, J.; Page, S.E.; Tansey, K.; Hooijer, A.; Vernimmen, R.; Siegert, F. Variable carbon losses from recurrent fires in drained tropical peatlands. Glob. Chang. Biol. 2016, 22, 1469–1480. [Google Scholar] [CrossRef]
- Page, S.; Rieley, J.; Banks, C. Global and regional importance of the tropical peatland carbon pool. Glob. Chang. Biol. 2011, 17, 798–818. [Google Scholar] [CrossRef]
- Miettinen, J.; Shi, C.; Liew, S.C. Land cover distribution in the peatlands of Peninsular Malaysia, Sumatra and Borneo in 2015 with changes since 1990. Glob. Ecol. Conserv. 2016, 6, 67–78. [Google Scholar] [CrossRef]
- Wijedasa, L.; Jauhiainen, J.; Könönen, M.; Lampela, M.; Vasander, H.; Leblanc, M.C.; Evers, S.; Smith, T.; Yule, C.; Varkkey, H.; et al. Denial of long-term issues with agriculture on tropical peatlands will have devastating consequences. Glob. Chang. Biol. 2017, 23. [Google Scholar] [CrossRef] [PubMed]
- Finér, L. Biomass and nutrient dynamics of Scots pine on a drained ombrotrophic bog. Finn. For. Res. Inst. Res. Pap. 1992, 420, 1–43. [Google Scholar]
- Lauhanen, R.; Kaunisto, S. Effect of drainage maintenance on the nutrient status on drained Scots pine mires. Suo 1999, 50, 119–132. [Google Scholar]
- Silvola, J. Effect of drainage and fertilization on carbon output and nutrient mineralization of peat. Suo 1988, 1–2, 27–37. [Google Scholar]
- Wang, M.; Talbot, J.; Moore, T.R. Drainage and fertilization effects on nutrient availability in an ombrotrophic peatland. Sci. Total. Environ. 2018, 621, 1255–1263. [Google Scholar] [CrossRef]
- Kaunisto, S.; Paavilainen, E. Nutrient stores in old drainage areas and growth of stands. Comminicationes Inst. For. Fenn. 1988, 145, 1–39. [Google Scholar]
- Laiho, R.; Laine, J. Nitrogen and phosphorus stores in Peatlands drained for forestry in Finland. Scand. J. For. Res. 1994, 9, 251–260. [Google Scholar] [CrossRef]
- Sarkkola, S.; Ukonmaanaho, L.; Nieminen, T.; Laiho, R.; Laurén, A.; Finér, L.; Nieminen, M. Should harvest residues be left on site in peatland forests to decrease the risk of potassium depletion? For. Ecol. Manag. 2016, 374, 136–145. [Google Scholar] [CrossRef]
- Marwanto, S.; Watanabe, T.; Iskandar, W.; Sabiham, S.; Funakawa, S. Effects of seasonal rainfall and water table movement on the soil solution composition of tropical peatland. Soil Sci. Plant Nutr. 2018, 64, 386–395. [Google Scholar] [CrossRef]
- Arnold, R.; Cuevas, E. Genetic variation in early growth, stem straightness and survival in Acacia crassicarpa, A. mangium and Eucalyptus urophylla in Bukbdnon province, Philippines. J. Trop. For. Sci. 2003, 15, 332–351. [Google Scholar]
- Mendham, D.S.; White, D.A. A review of nutrient, water and organic matter dynamics of tropical acacias on mineral soils for improved management in Southeast Asia. Aust. For. 2019, 82, 45–56. [Google Scholar] [CrossRef]
- Fabrika, M.; Pretzsch, H. Forest Ecosystem Analysis and Modelling; Technical University in Zvolen, Faculty of Forestry, Department of Forest Management and Geodesy: Zvolen, Slovakia, 2013; pp. 1–620. [Google Scholar]
- Forss, E.; Gadow, K.; Saborowski, J. Growth models for unthinned Acacia mangium plantations in South Kalimantan, Indonesia. J. Trop. For. Res. 1996, 8, 449–462. [Google Scholar]
- Forss, E.; Maltamo, M.; Saramäki, J. Static stand and tree characteristics model for Acacia mangium plantations. J. Trop. For. Sci. 1998, 10, 318–336. [Google Scholar]
- Chen, Y.; Liu, Z.; Rao, X.; Wang, X.; Liang, C.; Lin, Y.; Zhou, L.; Cai, X.a.; Fu, S. Carbon Storage and Allocation Pattern in Plant Biomass among Different Forest Plantation Stands in Guangdong, China. Forests 2015, 6, 794–808. [Google Scholar] [CrossRef]
- Krisnawati, H.; Kallio, M.; Kanninen, M. Acacia Mangium Willd.: Ecology, Silviculture and Productivity; Center for International Forestry Research: Bogor, Indonesia, 2011. [Google Scholar] [CrossRef]
- Alvarado, A. Plant Nutrition in Tropical Forestry. In Tropical Forestry Handbook; Pancel, L., Köhl, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1113–1202. [Google Scholar]
- Laclau, J.P.; Ranger, J.; Gonçalves, J.; Maquere, V.; Krusche, A.; M’Bou, A.; Nouvellon, Y.; Saint-Andre, L.; Jean-Pierre, B.; Piccolo, M.; et al. Biogeochemical cycles of nutrients in tropical Eucalyptus plantations: Main features shown by intensive monitoring in Congo and Brazil. For. Ecol. Manag. 2010, 259, 1771–1785. [Google Scholar] [CrossRef]
- Mendham, D.S.; Hardiyanto, E.B.; Wicaksono, A.; Nurudin, M. Nutrient management of contrasting Acacia mangium genotypes and weed management strategies in South Sumatra, Indonesia. Aust. For. 2017, 80, 127–134. [Google Scholar] [CrossRef]
- Othman, H.; Mohammed, A.; Mohamad Darus, F.; Harun, M.; Zambri, M. Best management practices for oil palm cultivati on peat: Ground water-table maintenance in relation to peat subsidence and estimation of CO2 emissions at Sessang, Sarawak. J. Oil Palm Res. 2011, 23, 1078–1086. [Google Scholar]
- Couwenberg, J.; Hooijer, A. Towards robust subsidence-based soil carbon emission factors for peat soils in South-East Asia, with special reference to oil palm plantations. Mires Peat 2013, 12, 1–13. [Google Scholar]
- Ardiansyah, M.; Rusdi, M.; Rosalina, U.; Boer, R. Biomass Estimation and Carbon Stock Calculation in the Industrial Forest Plantation of PT WKS Jambi Using Landsat Data. 2005. Available online: https://www.researchgate.net/profile/Muhammad-Rusdi/publication/279851174_Biomass_estimation_and_carbon_stock_calculation_in_the_industrial_forest_plantation_of_PT_WKS_Jambi_using_Landsat_data/links/559bf03408ae7f3eb4cee337/Biomass-estimation-and-carbon-stock-calculation-in-the-industrial-forest-plantation-of-PT-WKS-Jambi-using-Landsat-data.pdf (accessed on 1 March 2021).
- Nambiar, S. Management of Soil, Nutrients and Water in Tropical Plantation Forests; ACIAR; CSIRO Australia; CIFOR Indonesia: Canberra, Australia; Bogor, Indonesia, 1997. [Google Scholar]
- Van Bich, N.; Eyles, A.; Mendham, D.; Dong, T.L.; Ratkowsky, D.; Evans, K.J.; Hai, V.D.; Thanh, H.V.; Thinh, N.V.; Mohammed, C. Contribution of Harvest Residues to Nutrient Cycling in a Tropical Acacia mangium Willd. Plantation. Forests 2018, 9, 577. [Google Scholar] [CrossRef]
- Weiskittel, A.; Hann, D.W.; Kershaw, J.A., Jr.; Vanclay, J.K. Forest Growth and Yield Modeling; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 1–415. [Google Scholar]
- Berg, B.; McClaugherty, C. Plant Litter. Decomposition, Humus Formation, Carbon Sequestration; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Hararuk, O.; Smith, M.J.; Luo, Y. Microbial models with data-driven parameters predict stronger soil carbon responses to climate change. Glob. Chang. Biol. 2015, 21, 2439–2453. [Google Scholar] [CrossRef] [PubMed]
- Mackensen, J.; Bauhus, J.; Webber, E. Decomposition rates of coarse woody debris—A review with particular emphasis on Australian tree species. Aust. J. Bot. 2003, 51, 27–37. [Google Scholar] [CrossRef]
- Chertov, O.; Komarov, A.; Nadporozhskaya, M.; Bykhovets, S.; Zudin, S. ROMUL—A model of forest soil organic matter dynamics as a substantial tool for forest ecosystem modeling. Ecol. Model. 2001, 138, 289–308. [Google Scholar] [CrossRef]
- Basuki, S.; Munoz, C.P. Emission of CO2 and CH4 from plantation forest of Acacia crassicarpa on peatlands in Indonesia. In Proceedings of the 14th International Peat Congress, Stockholm, Sweden, 3–8 June 2012; pp. 1–7. [Google Scholar]
- Warren, M.W.; Kauffman, J.B.; Murdiyarso, D.; Anshari, G.; Hergoualc’h, K.; Kurnianto, S.; Purbopuspito, J.; Gusmayanti, E.; Afifudin, M.; Rahajoe, J.; et al. A cost-efficient method to assess carbon stocks in tropical peat soil. Biogeosciences 2012, 9, 4477–4485. [Google Scholar] [CrossRef]
- Bi, H.; Turner, J.; Lambert, M. Additive biomass equations for native eucalypt forest trees of temperate Australia. Trees 2004, 18, 467–479. [Google Scholar] [CrossRef]
- Palviainen, M.; Finér, L. Decomposition and nutrient release from Norway spruce coarse roots and stumps—A 40-year chronosequence study. For. Ecol. Manag. 2015, 358, 1–11. [Google Scholar] [CrossRef]
- Anonymous. Description and Manual of Romul. Available online: http://xxx.lanl.gov/abs/http://ecomodelling.ru/images/dles/en_romul_description_manual.pdf (accessed on 11 November 2020).
- Camporese, M.; Ferraris, S.; Putti, M.; Salandin, P.; Teatini, P. Hydrological modeling in swelling/shrinking peat soils. Water Resour. Res. 2006, 42. [Google Scholar] [CrossRef]

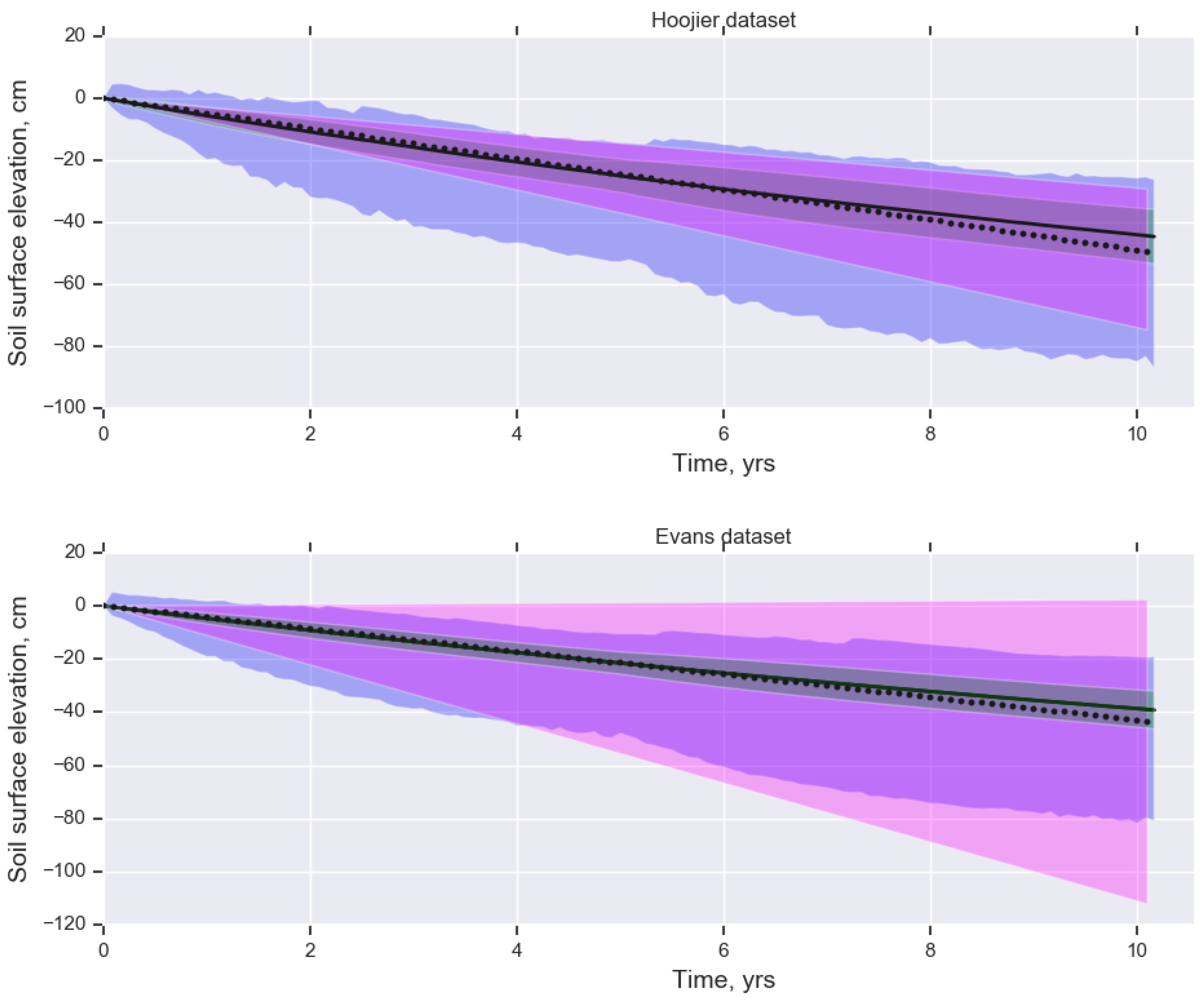


| Hoojier et al. [5] | Evans et al. [9] | |
|---|---|---|
| Number of sites | 21 | 210 |
| Number of runs per site | 50 | 50 |
| Rotation length, yrs | 5 | 5 |
| Number of rotations | 3 | 3 |
| Site index | 20 (3.5) | 20 (3.5) |
| Mortality rate, stems month−1 | range 1…20 | range 1…20 |
| Initial bulk density, kg m3 | 89 (18) | 110 (22) |
| WT | as reported | as reported |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurén, A.; Palviainen, M.; Page, S.; Evans, C.; Urzainki, I.; Hökkä, H. Nutrient Balance as a Tool for Maintaining Yield and Mitigating Environmental Impacts of Acacia Plantation in Drained Tropical Peatland—Description of Plantation Simulator. Forests 2021, 12, 312. https://doi.org/10.3390/f12030312
Laurén A, Palviainen M, Page S, Evans C, Urzainki I, Hökkä H. Nutrient Balance as a Tool for Maintaining Yield and Mitigating Environmental Impacts of Acacia Plantation in Drained Tropical Peatland—Description of Plantation Simulator. Forests. 2021; 12(3):312. https://doi.org/10.3390/f12030312
Chicago/Turabian StyleLaurén, Ari, Marjo Palviainen, Susan Page, Chris Evans, Iñaki Urzainki, and Hannu Hökkä. 2021. "Nutrient Balance as a Tool for Maintaining Yield and Mitigating Environmental Impacts of Acacia Plantation in Drained Tropical Peatland—Description of Plantation Simulator" Forests 12, no. 3: 312. https://doi.org/10.3390/f12030312
APA StyleLaurén, A., Palviainen, M., Page, S., Evans, C., Urzainki, I., & Hökkä, H. (2021). Nutrient Balance as a Tool for Maintaining Yield and Mitigating Environmental Impacts of Acacia Plantation in Drained Tropical Peatland—Description of Plantation Simulator. Forests, 12(3), 312. https://doi.org/10.3390/f12030312





