Abstract
Arbutus unedo L. is a small Ericaceae tree with a circum-Mediterranean distribution. It has a huge ecological impact on southern Europe forests and a great economic importance as a source of phytochemicals with bioactive properties and for fruit production. On the foreseen climate change context, breeding toward drought tolerance is necessary in order to ameliorate plant performance. Therefore, the aim of this work was to study the reproduction mechanisms of the strawberry tree, obtain new genetic combinations by hybridization, and select genotypes more tolerant to drought stress. A morphological analysis of flowers and pollen was carried out, and controlled pollinations were performed both in vitro and ex vitro. The very first approach on strawberry tree breeding by means of hybridization is also presented. Several physiological parameters were evaluated on 26 genotypes submitted to a water-deficit regime. Plant behavior under drought greatly varied among genotypes, which showed high phenotype plasticity. Three genotypes that were able to cope with water restriction without compromising net CO2 assimilation were identified as highly tolerant to drought stress. The results obtained elucidate the reproduction mechanisms of the strawberry tree and open the way for a long-term breeding program based on the selection of drought-tolerant plants.
1. Introduction
Strawberry tree (Arbutus unedo L.) is an Ericaceae widely distributed mainly around the Mediterranean basin, but also in the Atlantic coasts of Portugal, France, and Southern Ireland [1]. Members of this species grow spontaneously on poor rocky and well-drained acidic soils and can stand a wide range of temperatures, easily thriving in marginal soils where other species of trees or bushes can hardly survive. Moreover, its ability to rapidly regenerate after forests fires prevents the spread of common invasive species in Mediterranean ecosystems such as Acacia spp. or Ailanthus altissima, avoids soil degradation, and helps retain water, which are aspects that demonstrate the relevant ecological role of this species. Strawberry tree has several uses, mainly on pharmaceutical, cosmetics, and food industries, due to the variability and amount of phytochemicals present in their tissues and organs [2]. However, the edible berries used to produce an alcoholic distillate that reaches high prices in the market still remain the principal income source for farmers and other stakeholders [3].
Strawberry tree orchards are usually established using seed-derived plantlets on marginal dry areas where water is usually a scarce resource. Considering the expected increase on the frequency and severity of drought events in southern Europe in the near future [4], it is urgent to obtain new genotypes more tolerant to drought stress, in order to ameliorate their performance and increase productivity. Several studies have been carried out to study the effects of drought stress on water relations, growth rate, and photosynthesis in A. unedo under field conditions [5]. Although crucial, data focusing on drought performance at early stages of plant development are missing. Thus, early selection decisions are currently only based on productivity/fruit quality traits. Considering the increasing demand of high-quality plant stocks of A. unedo, in vitro propagation protocols were developed to clone selected genotypes [6,7,8], and studies to evaluate how these in vitro propagation systems change drought tolerance of regenerated plants have been carried out [9].
Some studies have been conducted on strawberry tree pollen, and a morphological description has been provided [10,11]. However, as far as is known, no work has been done in order to improve strawberry tree throughout conventional breeding, although some extensive experiments have been carried out on other Ericaceae, such as Rhododendron [12,13] and Vaccinium [14,15] species. Although conventional breeding is a lengthy process, particularly in tree species with long life-cycles, improved varieties of several tree species such as Populus spp., Platanus spp., and Malus x domestica have been produced through classical breeding [16,17]. The first step to initiate the development of new cultivars based on conventional breeding is a deep knowledge of the mechanisms of sexual plant reproduction, in particular the compatibility between the male and female reproductive structures, as well as the time of their maturation and phenology [18].
In order to set up the basis for a long-term breeding program on strawberry tree, the aim of this work was to study the reproduction system of A. unedo, from pollen morphology to pollen–stigma interactions, and analyze the tolerance of the F1 plants toward drought. For this purpose, a morphological analysis of flowers and pollen was carried out, and controlled pollinations were made in vitro and in situ to obtain hybrid plants. Moreover, the plants obtained by the artificial crossings were tentatively selected based on its drought tolerance. For this purpose, they were submitted to a water deficit regime in order to identify individuals able to maintain higher photosynthetic levels under water deficit conditions, which might be used on future micropropagation and/or breeding programs.
2. Materials and Methods
2.1. Plant Material
Flowers from three different populations were used in this study: CH (N 41°42′31.868″ W 7°26′32.506″, altitude 579 m), from Chaves (North Portugal) and populations C1 (N 40°12′17.472″ W 8°23′40.929″, altitude 103 m) and C2 (N 40°11′33.604″ W 8°23′37.163″, altitude 123 m) from Coimbra (Central Portugal). Plants were selected based on their fruit quality and production. A tree from population CH was used as a pollen donor for morpho-histological analysis (Section 2.2), germination studies (Section 2.3), and in vitro and in situ pollination assays (Section 2.4). For pollen release and gathering, anthers were removed from the flowers and placed on a Petri dish coated with aluminum foil for 1–2 days at room temperature. In vitro and in vivo pollinations (Section 2.4) were carried out using emasculated flowers from ten trees from C1 and C2 populations (5 from each population) and the collected pollen as described before (Figure 1).
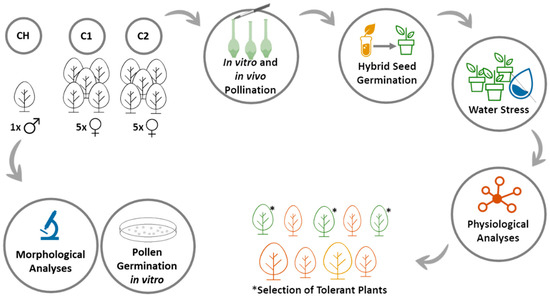
Figure 1.
Methodology scheme applied in this work to study the phenology and reproductive anatomy of strawberry tree as well as selection of drought tolerant plants.
2.2. Reproductive Phenology and Anatomy
Trees from populations C1 and C2 were monthly monitored throughout the year in order to characterize A. unedo reproductive phenology. Flowers and fruits were gathered and characterized, including anther position and fruit maturation stages. To analyze its morphology, pollen from CH was treated by the standard method of acetolysis [19]. Briefly, after being washed in water and acetic glacial acid (100%, v/v), pollen grains were treated with the classic acetolysis mixture (9:1, acetic anhydride:sulphuric acid) and heated in a water bath at 70 °C for 5 min. After being treated with acetone (100%, v/v), acetolyzed pollen material was mounted in glycerin jelly (Sigma-Aldrich, St. Louis, MO, USA). Measurements (D: diameter of the tetrad; d: single grain diameter; and the ratio D/d) were taken under light microscopy (Nikon EclipseCi, Nikon Instruments Europe BV, Amsterdam, Netherlands) with an ocular micrometer, from 30 randomly chosen pollen tetrads from 3 different slides (10 per slide). Terminology based on that of Punt et al. [20] was used for pollen morphology characterization. For scanning electron microscopy, pollen was placed on stubs and coated with gold on a JEOL JFC 1100 apparatus (JEOL, Musashino, Japan). Pollen observations were performed on a JEOL JSM 5400 microscope (JEOL, Murashino, Japan). For another anatomy studies, whole anthers were fixed for 3 h at room temperature in glutaraldehyde (1.5%, v/v, Sigma), prepared with phosphate buffer (0.1 M), and postfixed in osmium tetroxide (1%, w/v, Sigma) prepared with the same buffer. Samples were further dehydrated with ethanol and embedded with Spurr resin [21]. After the polymerization, ultrathin sections (1.5 μm) were obtained on an LKB Ultratome III and the cross-sections were stained with toluidine blue (1%, w/v) [22] and observed on a light microscope (Nikon EclipseCi, Nikon Instruments Europe BV), and photographs were collected with a Nikon DS-Fi3 camera (Nikon Instruments Europe BV)and processed with the software NIS-Elements D (version 4.60, Nikon Instruments Europe BV).
2.3. Pollen Germination
Previously to the pollination assays, the viability of the collected pollen was checked, and the effect of sucrose concentration was evaluated. Mature pollen was cultured on Petri dishes containing a basal germination medium [23] composed of H3BO3 (5 mg L−1), CaCl2 (15 mg L−1), KNO3 (10 mg L−1), agar (8%, w/v, Duchefa Biochemie BV, Haarlem, The Netherlands), and different concentrations of sucrose (0, 3, 6, 9, 12, 15, and 18%, w/v, Duchefa), for 6 and 24 h, at room temperature. Then, pollen grains were stained with aceto-carmine and observed under a light microscope. As A. unedo pollen grains are dispersed as tetrad units [11], germination rates were determined by scoring 100 pollen tetrads from 5 replicates (a total of 500 tetrads and 2000 pollen grains). A pollen tetrad was considered germinated when the pollen tube length of at least a pollen grain surpassed the diameter of a pollen grain. In a second experiment, different carbon sources as well as the effect of some plant growth regulators on pollen germination were also tested. For this purpose, pollen was cultivated for 6 h on the medium described before, with sucrose, glucose, and fructose at three different concentrations (3%, 9%, and 15%). The effect of NAA (1-naphthaleneacetic acid, Sigma), IBA (indole-3-butyric acid, Sigma), and GA3 (gibberellic acid, Sigma) was also tested in three concentrations (10, 100, and 500 mg L−1) on the same basal germination medium containing 15% sucrose.
2.4. In Vitro and In Situ Pollination Assays
For in vitro pollination, flowers immediately before anthesis from C1 and C2 populations were used. After emasculation, a total of 120 pistils from each population was placed on baby food jars (5 pistils per container) with a jellified medium for support (with water and 8 g L−1 agar). Then, pollen from population CH collected as described in Section 2.2. was carefully placed at the stigma using a spatula. Open and closed non-pollinated flowers were used as controls and all treatments were done in triplicate. From each population, a total of 75 pistils were crosspollinated (15 pistils/tree from 5 different trees), 15 autopollinated (from a single tree), and 30 used as negative and positive controls (15 each from a single tree). Following artificial pollination, the pistils were kept in the dark at 25 °C, for 24 h, and the efficiency of the pollination was evaluated. For this purpose, pistils were fixed in FAA (formalin:acetic acid:ethanol, 5:5:90, v/v/v) at room temperature for 24 h, washed in water, softened on a NaOH solution (8 N), and mounted with aniline blue (0.1%, w/v, Sigma) as described by Martin [24]. The observations were carried out in a fluorescence microscope (ex: 370 nm, Leica DM4000 B, Leica Microsystems GmbH, Wetzlar, Germany), and pollination was considered efficient when pollen germination was observed on the stigma, and pollen tubes were grown along the style and reached the ovaries.
For in situ pollination assays, pollen with viability over 80% from a single tree (population CH) was used to hand-pollinate flowers from the trees used for in vitro pollination. After the emasculation with forceps, the pollen was carefully placed on the stigma, and the pollinated flowers were covered with polypropylene pollination bags for 7 days in order to avoid pollen contamination. Then, 10 flowers from three different inflorescences (a total of 30 per tree) were pollinated on each of the 10 trees. All the immature and old flowers from the pollinated inflorescences were removed. During the assays, the minimum absolute temperature was 7.1 °C, and the maximum absolute temperature was 26.9 °C, while the total precipitation recorded was 145.5 mm, according to the data provided by the meteorological station of Coimbra/Cernache (www.ipma.pt).
2.5. Seed Germination and Plant Development
Mature fruits resulting from hand-pollination were gathered and washed with tap water. Isolated seeds were washed with distilled water for 10 min. Following a 30 s surface sterilization with ethanol (70%, v/v, Merck), the seeds were sterilized in a calcium hypochlorite solution (5%, w/v, Sigma) and 2–3 drops of Tween 20 for 10 min, washed 3 times with distilled sterilized water, and sowed on sterilized Petri dishes (9 cm) with cotton wool imbibed with sterile distilled water and covered with filter paper. The seeds were kept at 4 °C for 30 days and then transferred to a culture chamber (25 °C) for another 60 days. After this period, the germination rate was recorded, and Relative Germinability (RG) was calculated: RG = (number of seeds produced × 100)/number of viable seeds germinated [25]. Then, seedlings were transferred to acclimatization containers with sterilized perlite and kept in a growth chamber at 25 °C and 70% relative humidity, under a 16 h daily illumination regime of 15–20 μmol m−2 s−1 photosynthetically active radiation (PAR, cool-white fluorescent lamps). After 15–30 days, the plants were transferred to individual containers (5 dm3) with a substrate composed of peat and perlite (3:1, v/v, Siro, Mira, Portugal) for further growth on a greenhouse.
2.6. Drought Stress Assays
Then, three-year-old plants resulting from cross-pollination (a tree from each population) were submitted to water stress. A total of 26 plants (#1–13 from population C1 and #14–26 from population C2) were watered to full field capacity, and plant performance was evaluated after 24 h (t0). After that period, watering was interrupted, and plants were submitted to 3 weeks (t3) of water deficit. Leaf gas exchange was evaluated on t0 and t3, while due to the destructive nature of leaf water potential (Ψw) and leaf relative water content (RWC) measurements, sampling was performed at the end of the experiment (t3). The experiment was conducted during July, and the temperature ranged from 13 °C (15.8 ± 1.4) to 32 °C (24.1 ± 2.7). The average temperature at each sampling point was 20.5 ± 4.5 °C (t0) and 17.5 ± 6.4 °C (t3).
In situ leaf gas exchange measurements (net CO2 assimilation rate: A, transpiration rate: E, stomatal conductance: gs and intercellular CO2 concentration: ci) were measured on a young and fully expanded leaf (normally the fifth leaf from the top) using a portable infrared gas analyzer coupled to a broad leaf chamber (LCpro+, ADC, Hoddesdon, UK), operating in open mode and under the following conditions: photosynthetic photon flux density—650 µmol m−2 s−1 (based on a light curve: 0–1750 µmol m−2 s−1); air flux—200 mol s−1; block temperature—25 °C; and atmospheric CO2 and H2O concentration. Data were recorded when the measured parameters were stable (2–6 min). Water potential was measured with a Scholander-type pressure chamber (PMS Instrument Co., OR, USA). Relative water content (RWC) was calculated as: RWC (%) = (FW − DW)/(TW − DW) × 100, where FW is the fresh weight of the leaf, TW is the turgid weight (after 24 h on distilled water at 4 °C), and DW is the dry weight (after drying at 70 °C for 48 h).
2.7. Statistical Analysis
Pollen germination and physiological data were analyzed using ANOVA (GraphPad Prism for Windows v. 6.01) followed by Tukey’s multiple comparison test (p < 0.05). Data expressed as percentages were first submitted to arcsine transformation. A heatmap with a dendrogram and principal component analysis (PCA) were carried out using R software (version 4.0.3, R Foundation for Statistical Computing, Vienna, Austria) [26] to evaluate the interaction and significance of all the physiological parameters measured on the analyzed trees. A heatmap with physiological data from all the samples was constructed using the Heatmap function and the package ComplexHeatmap [27]. The dendrogram within the heatmap was calculated with Euclidean distance as a dissimilarity measure. Finally, data were classified with a PCA, using the prcomp function and the package ggbiplot [28].
3. Results
3.1. Reproductive Phenology and Anatomy
The reproductive cycle of strawberry tree is long, and it lasts for almost two years (Figure 2A). During this period, three distinct stages can be identified: flower buds, flowers at anthesis, and fruit development. During June, the inflorescences (panicles) start to appear from terminal meristems of young stems (Figure 2B). Flower development proceeds through summer months, and flower anthesis usually begins in October (Figure 2C). The flowering period can be long, from early October to late January depending on the trees and location. The flower is complete, bell-shaped, sympetalous, and white to slightly pink (Figure 2C). Each pistil is formed by a pentalocular ovary, a style, and a stigma that becomes receptive to pollen just before flower anthesis. Each stamen possesses a hairy filament and an anther with two pores located at the top. During flower development and just before flower anthesis, anthers suffer an inversion process from an extrorse to an introrse position and develop two appendages on the apical end (Figure 2D). After pollination, the slow fruit development process begins. Each infructescence will usually bear between 1 and 20 fruits (Figure 2E) that will develop along the year until fully ripened (Figure 2F). Consequently, fruit ripening occurs simultaneously with the next flowering period, during autumn (Figure 2A). Fruits at different developmental and ripening stages can be found at the same time on a tree. When fully ripped, fruits present a variable size and shape and a bright red color (Figure 2G).
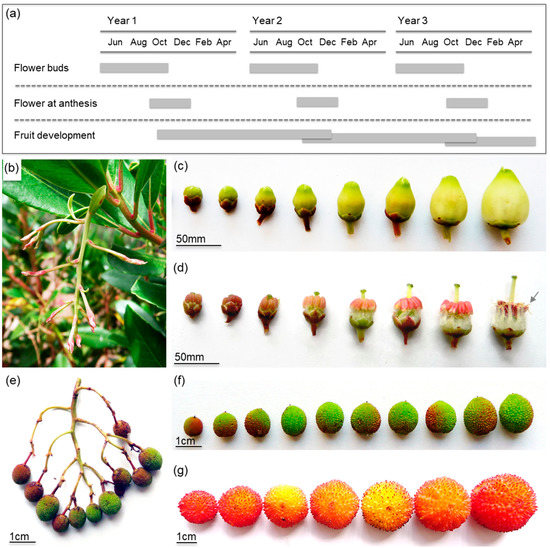
Figure 2.
Different aspects of strawberry tree phenology and reproductive anatomy: (a) phenological cycle of strawberry tree; (b) terminal hanging panicle at an early development stage during June; (c) general morphology of the bell-shaped flowers from early stages of development to flower anthesis; (d) developmental process of the stamens (arrow indicates anther appendage); (e) infructescence at an early development stage; (f) fruit developmental stages before ripening; (g) mature futures with different sizes and shapes. Feb—February, Apr—April, Jun—June, Aug—August, Oct—October, Dec—December.
Anthers of A. unedo have four microsporangia or pollen sacs arranged in pairs (Figure 3A). Pollen were dispersed in groups of four, and each anther contains on average 500 pollen units. At the earlier developmental stages, some pollen grains were found to be aborted on the pollen tetrad (Figure 3B). Pollen tetrads became mature and are released just after flower anthesis. Single pollen grains are 3-zonocolporate and have a circular or slightly elliptic outline on optical slice (Figure 3C,D). The ectoapertures are long colpus with a granulate membrane, and the endoapertures are pores with a regular outline (Figure 3E,F). The exine (≈ 1.5 μm) has a psilate surface and is tectate and slightly columellate. The size of the pollen tetrads (D) ranged from 42 to 67 μm (53.8 μm ± 3.6), whereas the size of single pollen grains (d) varied from 22 to 36 μm (29.5 μm ± 0.8). The relation D/d was between 1.6 and 2.2 (1.8 ± 0.1).
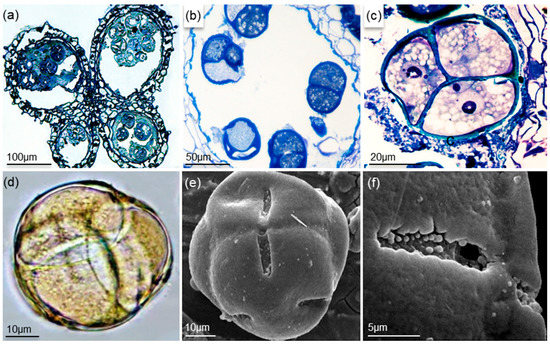
Figure 3.
Anther and pollen morphology of strawberry tree: (a) anther cross-section stained with toluidine blue; (b) pollen sac with aborted pollen grains; (c) pollen tetrad section stained with toluidine blue showing a bi-nucleated pollen grain; (d) non-acetolized pollen tetrad; (e) pollen tetrad on SEM; (f) aperture and ornamentation detail of the pollen tetrad on SEM.
3.2. Pollen Germination
Pollen germination was higher on media with higher sucrose concentrations for both periods analyzed (6 and 24 h). After 24 h, the best germination rates were obtained on 15%, 18%, and 21% sucrose, without statistical differences. However, a decrease on germination was observed on the medium with 18% sucrose after 6 h (Figure 4A,B). The highest germination rates were obtained on a medium with 21% sucrose, after 24 h (83.29% ± 10.85), and 15% sucrose after 6 h (80.52% ± 12.55; Figure 4A,B). In most of the pollen tetrads scored as germinated, only one of the pollen grains developed a pollen tube (70.98% ± 1.81), whereas the germination of more than two pollen grains was only observed occasionally (Figure 5A). When different carbon sources were tested, sucrose gave the best results on the three concentrations tested, with statistical differences when compared to glucose and fructose. The maximum germination rate was obtained with 15% sucrose (70.33% ± 1.89). Although the glucose was not as efficient as sucrose, a germination rate of 57.00% ± 4.55 was obtained with the maximum concentration tested (Figure 4C). No pollen germination was observed when fructose was used as a carbon source. Likewise, NAA and IBA had an inhibitory effect on pollen germination, even on the lowest concentration tested (10 mg L−1). When these two auxins were applied at higher concentrations, pollen germination was completely inhibited with statistical difference between concentrations for both NAA and IBA (Figure 4D). On the other hand, GA3 highly promoted pollen germination, and germination rates of 93.33 ± 3.09 and 94.67 ± 1.25 were obtained with 100 mg L−1 and 500 mg L−1 of GA3, respectively (Figure 4D). These values are higher than those obtained when a concentration of 10 mg L−1 was applied, with statistic significant differences. When the concentration of CaCl2 was highly incremented on the germination medium (10× increase), similar results were obtained.
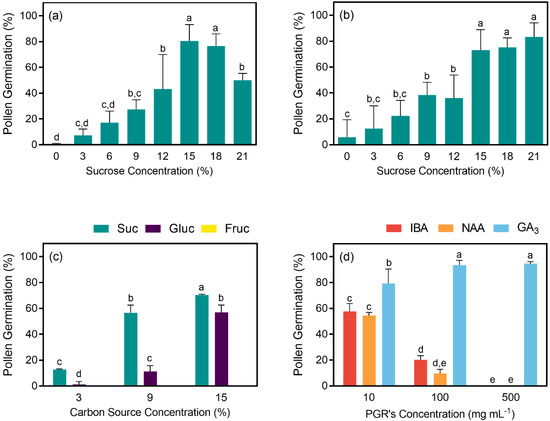
Figure 4.
(a) Effect of different sucrose concentrations (0, 3%, 6%, 9%, 12%, 15%, and 18%) on pollen germination rates after 6 h and (b) 24 h; (c) effect of different carbon sources (sucrose, glucose, and fructose) at three different concentrations (3%, 9%, and 15%) on pollen germination after 24 h; (d) effect of plant growth regulators (indole-3-butyric acid (IBA), 1-naphthaleneacetic acid (NAA), and gibberellic acid (GA3)) on germination rates at different concentrations (10, 100, and 500 mg L−1) after 24 h. Values (%) are means ± standard deviations, n = 5, different letters indicate significant differences between treatments at p ≤ 0.05.
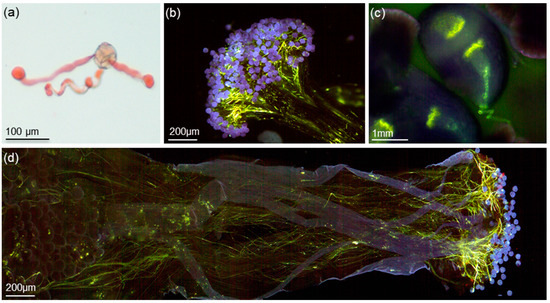
Figure 5.
Strawberry tree pollen germination: (a) germinated pollen tetrad stained with aceto-carmine; (b) germination of pollen grains on stigma; (c) pollen tube entering the ovule micropyle; (d) pollen germination on the stigma, and pollen tube growth along the pistil.
3.3. In Vitro and In Situ Pollination Assays
From the 12 combinations of crosses carried out in vitro, including two auto-pollinations, the average success rate obtained was 78.9% ± 22.7. Pollen germination was observed on stigma 1–2 h after the pollination (Figure 5B). The pollen tubes grow along the stile, reaching the ovary in 24 h (Figure 5D), and the tips of pollen tubes enter the micropyle (Figure 5C). The effectiveness of the cross-pollinations was 82.51% ± 19.81, while the effectiveness of the self-pollinations was 71.65 ± 29.50. We observed the accumulation of callose along the pollen tubes as well as on the tips. In some cases, pollen showed no signs of germination, both on self- and cross-pollinations. The growing pattern of pollen tubes seemed to be very similar on all the crosses made. In most of the flowers from the positive control (open flowers), pollen germination and pollen tube growth were observed, while all the flowers from the negative control (closed flowers) showed no signs of pollen in the stigma. Most of the pollinated flowers in situ were lost along the fruit developmental process. From the total of 300 pollinated flowers, after one year under development, only three fruits reached the mature stage, which represents a very low success rate of only 1%.
3.4. Seed Germination and Plant Development
From the three fruits retrieved from the field, a relative germination rate of 85.0% was obtained for group C1 and 86.7% was obtained for C2. After in vitro germination, seedling development proceeded rapidly, and after the acclimatization period, the root system was well developed (Figure 6A). The hybrid plants were morphological diverse (Figure 6B) in terms of height and leaf morphology. A total of 35 plants were obtained, 17 from group C1 and 13 from C2. After 3 years under development, 13 plants from each group were submitted to drought stress.
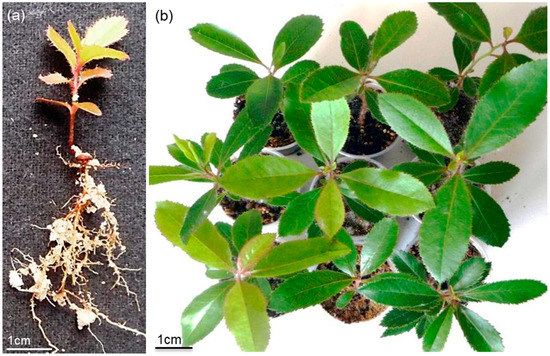
Figure 6.
Strawberry tree hybrid plants development: (a) two-month-old hybrid seedlings; (b) six-month-old hybrid plants with distinct morphological characteristics.
3.5. Plant Water Status and Gas Exchange
Before the imposed water stress deficit regime (t0), a considerable variance was found among genotypes on all the physiological parameters measured. While some of the tested genotypes presented higher net CO2 assimilation rates (e.g., 3, 5, 13, 21, and 25), others had considerably lower values (e.g., 2 and 17) (Table 1, Figure 7A). After 3 weeks under water deficit, stomatal conductance, net CO2 assimilation, and transpiration rates decreased (Table 1, Figure 7B). On the other hand, intercellular CO2 concentration increased on most of the plants throughout the imposed water stress. Although a great reduction on stomatal conductance, transpiration and net CO2 assimilation rates was observed on genotype 13 after drought stress, these parameters are still considerably higher than on most of the evaluated genotypes. A similar behavior was observed on genotypes 12 and 17, but with a less marked decreased on net CO2 assimilation rate. Meanwhile, these parameters remained unchanged or slightly increased on genotypes 14, 15, and 18. In general, relative water content and water potential were higher on plants with a higher net CO2 assimilation rate.

Table 1.
Gas exchange related parameters water status of 26 hybrid plants on t0 (control) and t3 (3 weeks under water deficit). Ci—intercellular CO2 concentration, E—transpiration rate, gs—stomatal conductance, A—net CO2 assimilation rate, RWC—relative water content, WP—water potential. + plants with an intermediate performance. ++ plants identified with the best performance. * equipment detection limit.
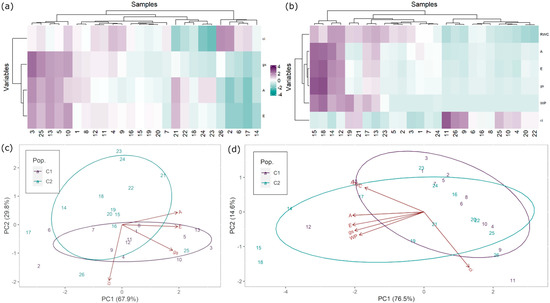
Figure 7.
Physiological parameters measured on 26 hybrid plants from populations C1 and C2 on t0 and t3 (3 weeks under water deficit): (a,b) heatmap with dendrogram on t0 and t3; (c,d) principal component analysis (PCA) on t0 and t3. ci—intercellular CO2 concentration, E—transpiration rate, gs—stomatal conductance, A—net CO2 assimilation rate, RWC—relative water content, WP—water potential.
This result is confirmed by the dendrogram within the heatmap (Figure 7A,B) as well as the PCA biplot (Figure 7C,D), which revealed a high positive correlation between E, gs, and A with relative water content and water potential (Figure 7B). Moreover, a negative correlation was found between these parameters and ci. On most of the plants, water potential was below the detection limit (−50 MPa). The PCA analysis has also revealed a very diverse behavior of plants, regardless of their provenience either on t0 or t3 (Figure 7C,D). Thus, plants with the best and worst performance under water stress are from both proveniences. On t0, principal component 1 (PC1) contributes 67.8% to the total variance and A, gs, and E are the parameters with a higher weight on this component, whereas PC2 contributes 29.8% to the total variance, and ci is the variable that most contributes to this variance. The genotypes identified due to a better performance (3, 5, 13, 21, and 25) are grouped (Figure 7C). On t3, principal component 1 (PC1) contributes 76.5% to the total variance. A, gs, E, relative water content, and water potential are the parameters with a higher weight on this component. PC2 contributes 14.6% to the total variance. As mentioned before, some of the tested plants showed a better overall performance in terms of net CO2 assimilation under drought. Thus, genotypes 12, 13, 14, 15, 17, and 18 are grouped together by the influence of some gas exchange parameters (gs, E, and A), relative water content, and water potential. Genotype 19 is also on this cluster, as it was able to maintain relatively high values of relative water content and water content, in spite of its low performance in terms of net CO2 assimilation. Plants with a worst overall performance are grouped together by the influence of ci (Figure 7D).
When comparing the two groups (C1 and C2), although no statistical differences were found between groups (Figure 8A), transpiration rates greatly decreased after 3 weeks on both groups (Figure 8B), as well as stomatal conductance (Figure 8C) and net CO2 assimilation rate (Figure 8D). No statistical difference was found for relative water content as well, with values of 62.4 ± 13.0% on C1 and 65.0 ± 14.7% on group C2.
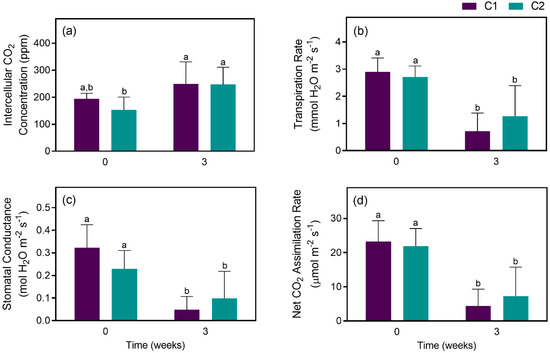
Figure 8.
Gas exchange-related parameters of hybrid plants from populations C1 and C2 on t0 (plants watered to full field capacity) and t3 (3 weeks under water deficit): (a) intercellular CO2 concentration; (b) transpiration rate; (c) stomatal conductance; (d) net CO2 assimilation rate (D). Values are means ± SDs, n = 13, different letters indicate significant differences between treatments at p ≤ 0.05.
4. Discussion
The long phenological cycle observed on the trees analyzed on this work is similar to the one that has been reported by Villa [10]. The inversion process of the anthers observed, as well as the development of the two appendages, has been described as a characteristic feature of the Ericaceae family [29]. The pollen morphology observed is similar to data reported by Villa [10], but it slightly differs from that described by Mateus [11]. According to this author, the endoapertures of strawberry tree pollen are endocolpus. In contrast, in this study, the endoapertures observed were endopores. The size of the pollen tetrads is slightly different as well: the diameter of the tetrads (D) determined on this work range from 42 to 67 μm, compared to 49–66 μm obtained by Mateus [11], while the size of single pollen grains (d) range from 22 to 36 μm compared to 33–41 μm.
The pollen germination rates obtained on the germination medium with 12% sucrose (89.40%) are similar to the ones obtained on other species, such as Prunus domestica L. [30] and Pistacia spp. L. [31], and they are much higher than the germination rates obtained on Annona cherimola Mill. [32] and Olea europaea L. [33]. Therefore, the germination medium used is adequate for strawberry trees and should be used for pollen viability tests. Although it has been reported by Cane [34] that 90% of the tetrads generated 3–4 pollen tubes on another member of the Ericaceae (Vaccinium macrocarpum Aiton), we found that most of the strawberry tree pollen tetrads analyzed had only one pollen grain germinated. This can be due to the fact that some of the grains on the pollen tetrads were found aborted, even in the initial developmental stages. The inhibition of pollen germination caused by fructose has been reported on the literature by Okusaka and Hiratsuka [35]. According to these authors, fructose completely inhibited pear pollen germination but without pollen viability loss. Thus, fructose is not an adequate sugar for strawberry tree pollen germination, and other carbon sources should be used instead, preferably sucrose. Plant growth regulators (PGRs) can be an extremely useful tool for plant breeders, either as gametocides or on the contrary by promoting pollen germination and eventually increasing fruit seed-set. Different PGRs have been tested on diverse crop species including rye [36], barley [37], onion, tomato, eggplant, pepper, watermelon [38], and wheat [39], on most cases in concentrations similar to the ones tested on this study (10–500 mgL−1). Our results showed an inhibitory effect of IBA and NAA on strawberry tree pollen germination in similar concentrations to those reported in the literature: the application of NAA (50 mg L−1) on eggplant and IBA or NAA (10–100 mg L−1) on onion proved to have an efficient gametocide effect [38]. On the other hand, the applications of at least 100 mg L−1 of GA3 greatly promoted pollen germination, which is an effect that has also been observed on strawberry with a similar concentration (50 mg L−1) [40] and blueberry where the application of GA3 on flowers (on concentrations approximately between 30 and 500 mg L−1) lead to an increased fruit set [41]. For this reason, the effect of GA3 on strawberry tree pollen germination and fruit set should be further tested due to its potential as a breeding aiding tool.
Although a previous study had suggested that pollen tubes growth speed is slower on self-pollination due to higher rates of attrition [42], this was not the case in strawberry trees, and similar pollen tube growth patterns on self- and cross-pollinations was observed. Moreover, no difference was found between the effectiveness of self- and cross-pollinations. The observed accumulation of callose on pollen tubes of a strawberry tree has also been observed in Chaenomeles japonica (Thunb.) Lindl. ex Spach [43]. Only a small portion of the hand-pollinated flowers on the field were able to complete the long development process and bear fruits. Due to the long development process that takes a year to be completed, strawberry tree pollinated flowers and fruits under development are subjected to a wide range of environmental conditions and interferences, and only a small portion of fruits is able to complete its development. This might help explain the low success rate of hand-pollinations along with the high manipulation required to carry out the pollination procedure. In fact, it has been reported that fruit production on Vaccinium spp. is lower when plants were cross- or self-pollinated by hand than when natural pollination occurs [15]. Thus, the improvement of pollinations conditions is something to be pursued in the near future in order to increase success rates of hand-pollinations. The increase of the amount of pollen placed on the stigma and/or the use of PGRs (e.g., GA3) should be considered, as well as the implementation of open pollinated seed orchards. The size and seediness of fruits may also be affected when hand-pollination is carried out, as referred by Usui et al. (2005). In this work, the average of viable seeds obtained on hand-pollinated fruits was 50%, which is much lower than the 77% obtained on open pollinated trees. Nonetheless, due to the low amount of hybrid fruits obtained, these results are not significant, and further analyses should be carried out in the future. The high germination rates obtained are similar to other works [44,45,46] indicating that the germination ability of the hybrid seeds is not compromised. However, such rates were obtained after cold stratification, which is a procedure that should be followed in order to break seed dormancy.
When the hybrid plants were submitted to drought stress (t3), a decrease on transpiration rates, stomatal conductance, as well as net CO2 assimilation rate was observed, which has a consequence on the efficient conservative water use strategy adopted by strawberry trees [47]. As mentioned before, the strawberry tree is an isohydric species with a tight stomatal control. When under water-deficit conditions, plants will adopt a conservative water-use strategy by closing stomata, thus keeping a low gas exchange rate [48,49]. The increase of intercellular CO2 concentration throughout the imposed water stress is caused by a reduction of the photosynthetic machinery due to stomatal limitations and probably oxidative stress [5]. The high positive correlation found between water availability (relative water content and water potential) and gas exchange parameters evaluated is not surprising, as water is one of the most limiting factors on the entire photosynthetic process. Thus, plants with a higher ability to maintain their water status have as expected a better overall performance.
Overall, the measured values of physiological parameters are in accordance with those obtained on a previous study [9]. However, net CO2 assimilation rates measured were higher than those obtained on previous reports [9,49]. This difference can be related to the older age of the plants evaluated on this study, which hypothetically might have a higher photosynthetic ability. Considerably lower values of water potential were also measured on this work, which is probably related with the period under water stress as well as the age of the plants that might have more lignified tissues and a higher resistance to cavitation and low water potential. Still, this hypothesis should be further tested and confirmed on future analysis.
Although most of the tested plants showed a poor performance under drought stress, we successfully identified two groups of plants that followed a different strategy to cope with water deficit and were able to maintain a high stomatal conductance and consequently higher net CO2 assimilation rates. Genotypes 14, 15, and 18 were able to maintain their basal levels of photosynthesis, which was accomplished by maintaining stomata open, as these plants were able to maintain relatively high levels of water (relative water content and water potential). Genotypes 12, 13, and 17 were shown to have an intermediate performance under drought stress. Although these plants were able to maintain the photosynthetic mechanisms active after 3 weeks under water-deficit conditions, they were already probably close to their resistance limits, and a significant drop on net CO2 assimilation rates was expected after a few more days under stress. Finally, some genotypes (e.g., 19) were able to maintain relatively high levels of water (relative water content and water potential), but they were unable to maintain satisfactory levels of net CO2 assimilation, which might be due to the biochemical limitation of photosynthesis rather than stomatal constraints. From the genotypes identified on t0 that have higher net CO2 assimilation rates, only genotype 13 was able to maintain a similar performance under stress. On the other hand, genotypes with lower net CO2 assimilation rates on t0 (e.g., 17) were able to cope with drought stress and maintain the levels measured at t0. The genotypes identified on t0 for its high net CO2 assimilation rates (3, 5, 13, 21, and 25) might have high potential, and their productivity should be evaluated. Nonetheless, on the water restriction scenario we hypothesize on this work, with the exception of genotype 13, they generally fail to cope with water stress.
In addition to revealing the importance of genotype on strawberry tree physiological performance and response to drought, these results show that strawberry tree plants have a high phenotypic plasticity and are able to adjust differential strategies to cope with stress. In order to facilitate and considerably reduce the necessary required time for selection, the identification of other adequate selection parameters should be pursued. In particular, metabolites such as phenols, proline, chlorophyll, anthocyanins, and several hormones (e.g., abscisic acid, jasmonic acid, and salicylic acid) that are known to be essential on plant response mechanisms to drought stress, might be used as markers to identify plants with a better appetence to undergo extreme drought events.
Although a great variance was observed between individuals from the same population, no differences were observed between populations on all the tested parameters. These results suggest that intra-population variation should be take into account and prioritized over inter-population on future selection endeavors, and a large number of individuals from within a population must be sampled. In contrast, results obtained by Vasques et al. [49] showed that seedlings provenience might influence the tolerance of plants under water stress, thus suggesting local adaptations of plant populations, which reinforces the importance of inter-population variance on plant behavior. Due to its implication on plant selection, this hypothesis should be further investigated. Overall, the obtained results will have important repercussions on strawberry tree phenotyping and early plant selection as well as breeding toward the obtention of drought stress resistant genotypes.
5. Conclusions
As a basis for any breeding program that includes plant hybridization, a deep knowledge of the plant reproduction system is necessary. This work provides the first insights of a strawberry tree reproduction system, which will be crucial on future breeding attempts. As a tool for plant selection, the physiological parameters used in this study proved to be adequate. However, the analysis of biochemical parameters could not only elucidate the tolerance mechanism of A. unedo but also identify key metabolites (e.g., phenols, hormones, and pigments) that could be used as markers for early plant selection. Three genotypes (14, 15, and 18) showed a particular appetence to cope with water stress and may be the basis for a future breeding program. However, due to the influence of genotype on plant response to water stress and the observed phenotypic plasticity, the analysis of a large number of individuals should be carried out in order to develop a long-term breeding program. The selection and breeding of strawberry tree genotypes more tolerant to drought stress is essential in order to maintain species sustainability and our promising results are a step forward in order to ameliorate strawberry tree adaptation while preserving productivity on drought prone areas.
Author Contributions
Conceptualization, J.M. and J.C.; formal analysis, J.M. and P.M.; data analysis, J.M.; writing—original draft preparation, J.M.; writing—review and editing, G.P. and J.C.; supervision, G.P. and J.C.; funding acquisition, G.P. and J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Foundation for Science and Technology (Portugal), who supported J. Martins PhD fellowship (SFRH/BD/122478/2016), ReNATURE project (CENTRO-01-0145-FEDER-000007) and F4F-Forest for the future (CENTRO-08-5864-FSE-000031, Programa Operacional Regional do Centro, Fundo Social Europeu). Thanks to CESAM (UIDB/50017/2020+UIDP/50017/2020), to FCT/MEC through national funds, and the co-funding by the FEDER, within the PT2020 Partnership Agreement and Compete 2020. This work was carried out at the R&D Unit Center for Functional Ecology—Science for People and the Planet (CFE), with reference UIDB/04004/2020, financed by FCT/MCTES through national funds (PIDDAC).
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Torres, J.A.; Valle, F.; Pinto, C.; García-Fuentes, A.; Salazar, C.; Cano, E. Arbutus unedo L. communities in southern Iberian Peninsula mountains. Plant Ecol. 2002, 160, 207–223. [Google Scholar] [CrossRef]
- Morgado, S.; Morgado, M.; Plácido, A.I.; Roque, F.; Duarte, A.P. Arbutus unedo L.: From traditional medicine to potential uses in modern pharmacotherapy. J. Ethnopharmacol. 2018, 225, 90–102. [Google Scholar] [CrossRef]
- Alarcão-e-Silva, M.L.C.M.M.; Leitão, A.E.B.; Azinheira, H.G.; Leitão, M.C.A. The Arbutus Berry: Studies on its color and chemical characteristics at two mature stages. J. Food Compos. Anal. 2001, 14, 27–35. [Google Scholar] [CrossRef]
- Bussotti, F.; Ferrini, F.; Pollastrini, M.; Fini, A. The challenge of Mediterranean sclerophyllous vegetation under climate change: From acclimation to adaptation. Environ. Exp. Bot. 2014, 103, 80–98. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Peñuelas, J. Drought-induced oxidative stress in strawberry tree (Arbutus unedo L.) growing in Mediterranean field conditions. Plant Sci. 2004, 166, 1105–1110. [Google Scholar] [CrossRef]
- Martins, J.F.; Correia, S.I.; Canhoto, J.M. Somatic embryogenesis induction and plant regeneration in strawberry tree (Arbutus unedo L.). In Methods in Molecular Biology; Germana, M., Lambardi, M., Eds.; Humana Press: New York, NY, USA, 2016; Volume 1359, pp. 329–339. [Google Scholar]
- Martins, J.F.; Santos, T.; Correia, S.I.; Canhoto, J.M. Somatic embryogenesis in Arbutus unedo L. and other Ericaceae. In Vegetative Propagation of Forest Trees; Park, Y.S., Bonga, J., Moon, H.K., Eds.; National Institute of Forest Science: Seoul, Korea, 2016; pp. 565–590. [Google Scholar]
- Gomes, F.; Canhoto, J.M. Micropropagation of strawberry tree (Arbutus unedo L.) from adult plants. Vitr. Cell. Dev. Biol. Plant 2009, 45, 72–82. [Google Scholar] [CrossRef]
- Martins, J.F.; Correia, S.; Correia, B.; Pinto, G.; Canhoto, J.M. Shoot proliferation and organogenesis on Arbutus unedo: Physiological analysis under water stress. Biol. Plant. 2019, 63, 278–286. [Google Scholar] [CrossRef]
- Villa, R.S. Ricerche sulla biologia di Arbutus unedo L. (Ericaceae): Ciclo di sviluppo. Boll. Soc. Sarda Sci. Nat. 1982, 21, 309–317. [Google Scholar]
- Mateus, J. Pollen morphology of Portuguese Ericales. Rev. Biol. 1989, 14, 135–208. [Google Scholar]
- Doorenbos, J. Shortening the breeding cycle of rhododendron. Euphytica 1955, 4, 141–146. [Google Scholar] [CrossRef]
- Escaravage, N.; Pornon, A.; Doche, B.; Till-Bottraud, I. Breeding system in an alpine species: Rhododendron ferrugineum L. (Ericaceae) in the French northern Alps. Can. J. Bot. 1997, 75, 736–743. [Google Scholar] [CrossRef]
- Lyrene, P.M. Value of various taxa in breeding tetraploid blueberries in Florida. Euphytica 1997, 94, 15–22. [Google Scholar] [CrossRef]
- Usui, M.; Kevan, P.G.; Obbard, M. Pollination and breeding system of lowbush blueberries, Vaccinium angustifolium Ait. and V. myrtilloides Michx. (Ericacaeae), in the boreal forest. Can. Field Nat. 2005, 119, 48–57. [Google Scholar] [CrossRef]
- Igarashi, M.; Hatsuyama, Y.; Harada, T.; Fukasawa-Akada, T. Biotechnology and apple breeding in Japan. Breed. Sci. 2016, 66, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Aravanopoulos, F.A. Breeding of fast growing forest tree species for biomass production in Greece. Biomass Bioenergy 2010, 34, 1531–1537. [Google Scholar] [CrossRef]
- Fryxell, P. Mode of reproduction of higher plants. Bot. Rev. 1957, 23, 135–233. [Google Scholar] [CrossRef]
- Erdtman, G. The acetolysis method in a revised description. Sven. Bot. Tidskr. Lund 1960, 54, 561–564. [Google Scholar]
- Punt, W.; Hoen, P.P.; Blackmore, S.; Nilsson†, S.; Le Thomas, A. Glossary of pollen and spore terminology. Rev. Palaeobot. Palynol. 2007, 143, 1–81. [Google Scholar] [CrossRef]
- Spurr, A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 1969, 26, 31–43. [Google Scholar] [CrossRef]
- Roland, J.C. General preparation and staining of thin sections. In Electron Microscopy and Cytochemistry of Plant Cells; Hall, J.L., Ed.; Elsevier/North-Holland Biomedical Press: Amsterdam, The Netherlands, 1978; pp. 1–62. [Google Scholar]
- Jahier, J.; Chevre, A.M.; Eber, F.; Delourme, R.; Tanguy, A.M. Techniques de Cytogenetique Vegetale; INRA: Paris, France, 1992; ISBN 9782738003966. [Google Scholar]
- Martin, F.W. Staining and observing pollen tubes in the style by means of fluorescence. Stain Technol. 1959, 34, 125–128. [Google Scholar] [CrossRef]
- Yang, J.; Lovett-Doust, J.; Lovett-Doust, L. Seed germination patterns in green dragon (Arisaema dracontium, Araceae). Am. J. Bot. 1999, 86, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/2017 (accessed on 10 October 2020).
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed]
- Vu, V.Q. Ggbiplot: A Ggplot2 Based Biplot. R Package Version 0.55. Available online: http://github.com/vqv/ggbiplot (accessed on 10 October 2020).
- Hermann, P.M.; Palser, B.F. Stamen development in the Ericaceae. I. Anther wall, microsporogenesis, inversion, and appendages. Am. J. Bot. 2000, 87, 934–957. [Google Scholar] [CrossRef] [PubMed]
- Ćalić, D.; Devrnja, N.; Kostić, I.; Kostić, M. Pollen morphology, viability, and germination of Prunus domestica cv. PoŽegača. Sci. Hortic. 2013, 155, 118–122. [Google Scholar] [CrossRef]
- Acar, I.; Gopal, V. The effects of temperature on in vitro pollen germination and pollen tube growth of Pistacia spp. Sci. Hortic. 2010, 125, 569–572. [Google Scholar] [CrossRef]
- Rosell, P.; Herrero, M.; Galán Saúco, V. Pollen germination of cherimoya (Annona cherimola Mill.). Sci. Hortic. 1999, 81, 251–265. [Google Scholar] [CrossRef]
- Vuletin Selak, G.; Perica, S.; Goreta Ban, S.; Poljak, M. The effect of temperature and genotype on pollen performance in olive (Olea europaea L.). Sci. Hortic. 2013, 156, 38–46. [Google Scholar] [CrossRef]
- Cane, J.H. Pollen viability and pollen tube attrition in cranberry (Vaccinium macrocarpon Aiton). Acta Hortic. 2009, 810, 563–566. [Google Scholar] [CrossRef]
- Okusaka, K.; Hiratsuka, S. Fructose inhibits pear pollen germination on agar medium without loss of viability. Sci. Hortic. 2009, 122, 51–55. [Google Scholar] [CrossRef]
- Nátrová, Z.; Hlaváč, M. The effect of gametocides on microsporogenesis of winter rye. Biol. Plant. 1975, 17, 256–262. [Google Scholar] [CrossRef]
- Verma, M.M.; Kumar, J. Ethrel-a male gametocide that can replace the male sterility genes in barley. Euphytica 1978, 27, 865–868. [Google Scholar] [CrossRef]
- Saimbhi, M.S.; Brar, J.S. A review of the practical use of gametocides on vegetable crops. Sci. Hortic. 1978, 8, 11–17. [Google Scholar] [CrossRef]
- Chakraborty, K.; Devakumar, C. Ethyloxanilates as specific male gametocides for wheat (Triticum aestivum L.). Plant Breed. 2006, 125, 441–447. [Google Scholar] [CrossRef]
- Voyiatzis, D.G.; Paraskevopoulou-Paroussi, G. Factors affecting the quality and in vitro germination capacity of strawberry pollen. J. Hortic. Sci. Biotechnol. 2002, 77, 200–203. [Google Scholar] [CrossRef]
- Cano-Medrano, R.; Darnell, R.L. Effect of GA3 and pollination on fruit set and development in rabbiteye blueberry. Hortic. Sci. 1998, 33, 632–635. [Google Scholar] [CrossRef]
- Horsley, T.N.; Johnson, S.D. Is Eucalyptus cryptically self-incompatible? Ann. Bot. 2007, 100, 1373–1378. [Google Scholar] [CrossRef]
- Kaufmane, E.; Rumpunen, K. Pollination, pollen tube growth and fertilization in Chaenomeles japonica (Japanese quince). Sci. Hortic. 2002, 94, 257–271. [Google Scholar] [CrossRef]
- Demirsoy, L.; Demirsoy, H.; Celikel, G.; Macit, I.; Ersoy, B. Seed treatment with GA3 or stratification enhances emergence of some strawberry tree genotypes—Short communication. Hortic. Sci. 2010, 37, 34–37. [Google Scholar] [CrossRef]
- Ertekin, M.; Kirdar, E. Breaking seed dormancy of strawberry tree (Arbutus unedo). Int. J. Agric. Biol. 2010, 12, 57–60. [Google Scholar]
- Tilki, F. Improvement in seed germination of Arbutus unedo L. Pak. J. Biol. Sci. 2004, 7, 1640–1642. [Google Scholar] [CrossRef]
- Castell, C.; Terradas, J. Effects of water and nutrient availability on water relations, gas exchange and growth rate of mature plants and resprouts of Arbutus unedo L. Ann. Bot. 1994, 73, 595–602. [Google Scholar] [CrossRef]
- Raimondo, F.; Trifilò, P.; Lo Gullo, M.A.; Andri, S.; Savi, T.; Nardini, A. Plant performance on Mediterranean green roofs: Interaction of species-specific hydraulic strategies and substrate water relations. AOB Plants 2015, 7, plv007. [Google Scholar] [CrossRef] [PubMed]
- Vasques, A.; Chirino, E.; Vilagrosa, A.; Vallejo, V.R.; Keizer, J.J. The role of seed provenance in the early development of Arbutus unedo seedlings under contrasting watering conditions. Environ. Exp. Bot. 2013, 96, 11–19. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).