Evaluation of Attractant Composition, Application Rate, and Trap Type for Potential Mass Trapping of Ips typographus (L.)
Abstract
1. Introduction
- (1)
- attractant composition,
- (2)
- application rate, and
- (3)
- trap type.
2. Materials and Methods
2.1. Study Site
2.2. Species Identification
2.3. Study Design
2.3.1. General Design
2.3.2. Attractant Composition Trial
| Dispenser | IT Ecolure Classic® | IT Ecolure Extra® | IT Ecolure Mega® | Ipsowit® | Pheroprax® | Typosan® |
|---|---|---|---|---|---|---|
| Duration of attractivity (weeks) | 8–10 | 6–8 | 18–20 | 6–8 | 6–8 | 10 |
| Release rate (mg/d) | 36.5 | 70.4 | 69.5 | 16.2 | 33.8 | 30.0 |
| Composition | (S)-cis-Verbenol | (S)-cis-Verbenol | (S)-cis-Verbenol | S-Ipsdienol, (S)-cis-Verbenol | S-Ipsdienol, (S)-cis-Verbenol 2-Methyl-3-buten-1-ol | (S)-cis-Verbenol, 2-Methyl-3-buten-1-ol |
| Dispenser type | blotter (aluminum foil) | blotter (aluminum foil) | blotter (aluminum foil) | blotter (membrane foil) | ampoule | blotter (membrane foil) |
| Producer | Fytofarm | Fytofarm | Fytofarm | Witasek | BASF | Sintagro AG |
| Country of production | Slovakia | Slovakia | Slovakia | Austria | Germany | Switzerland |
2.3.3. Application Rate Trial
2.3.4. Trap Type Trial
2.4. Data Analysis
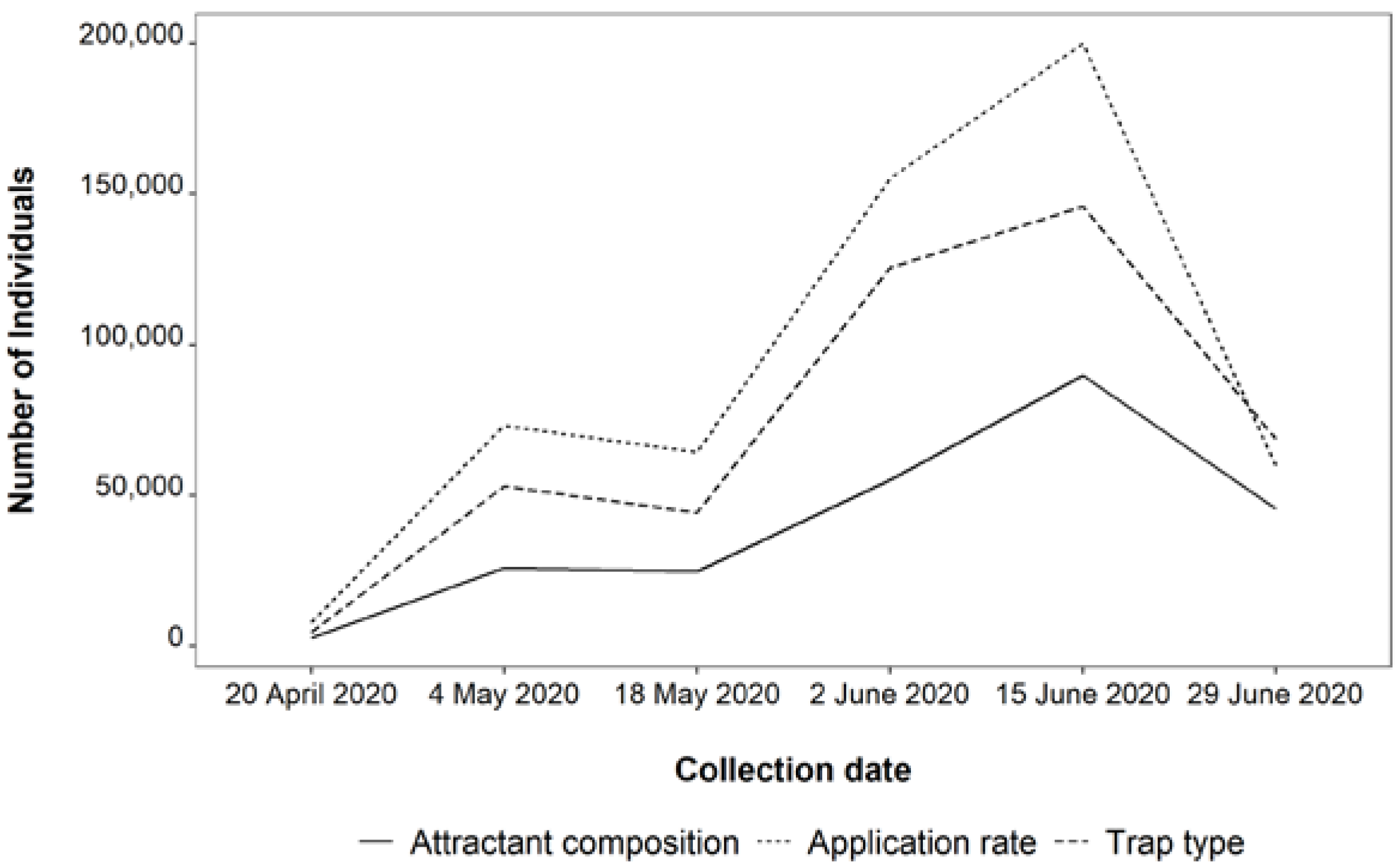
3. Results
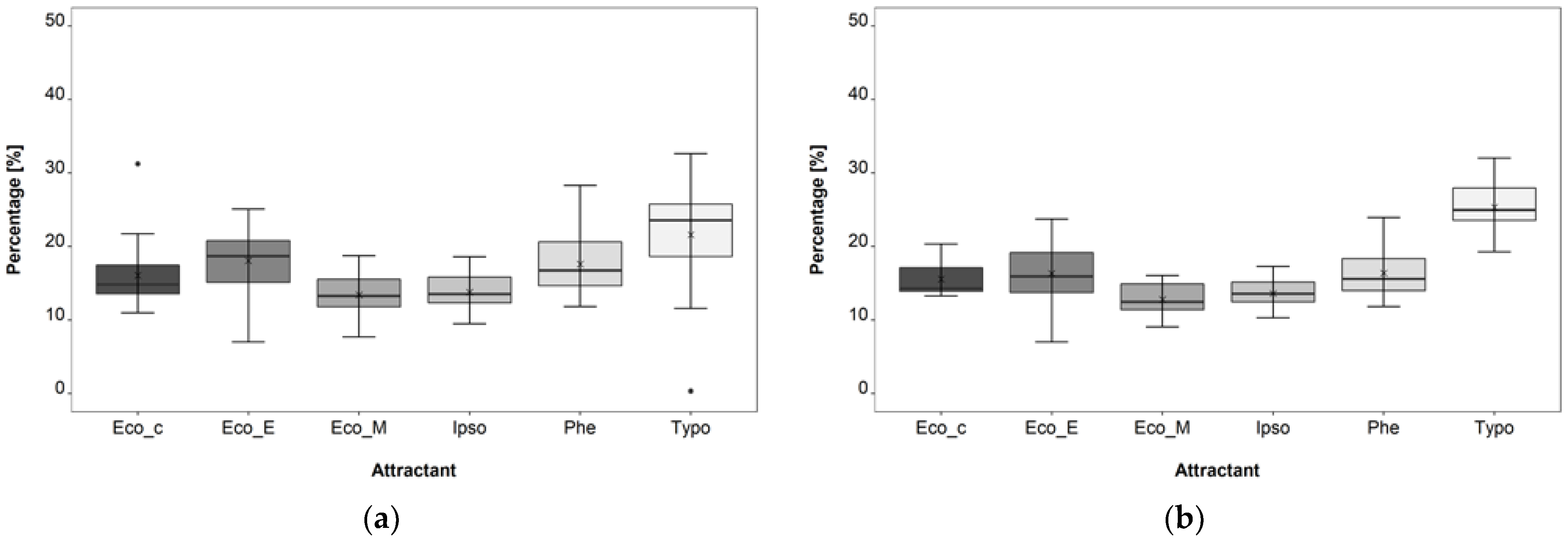
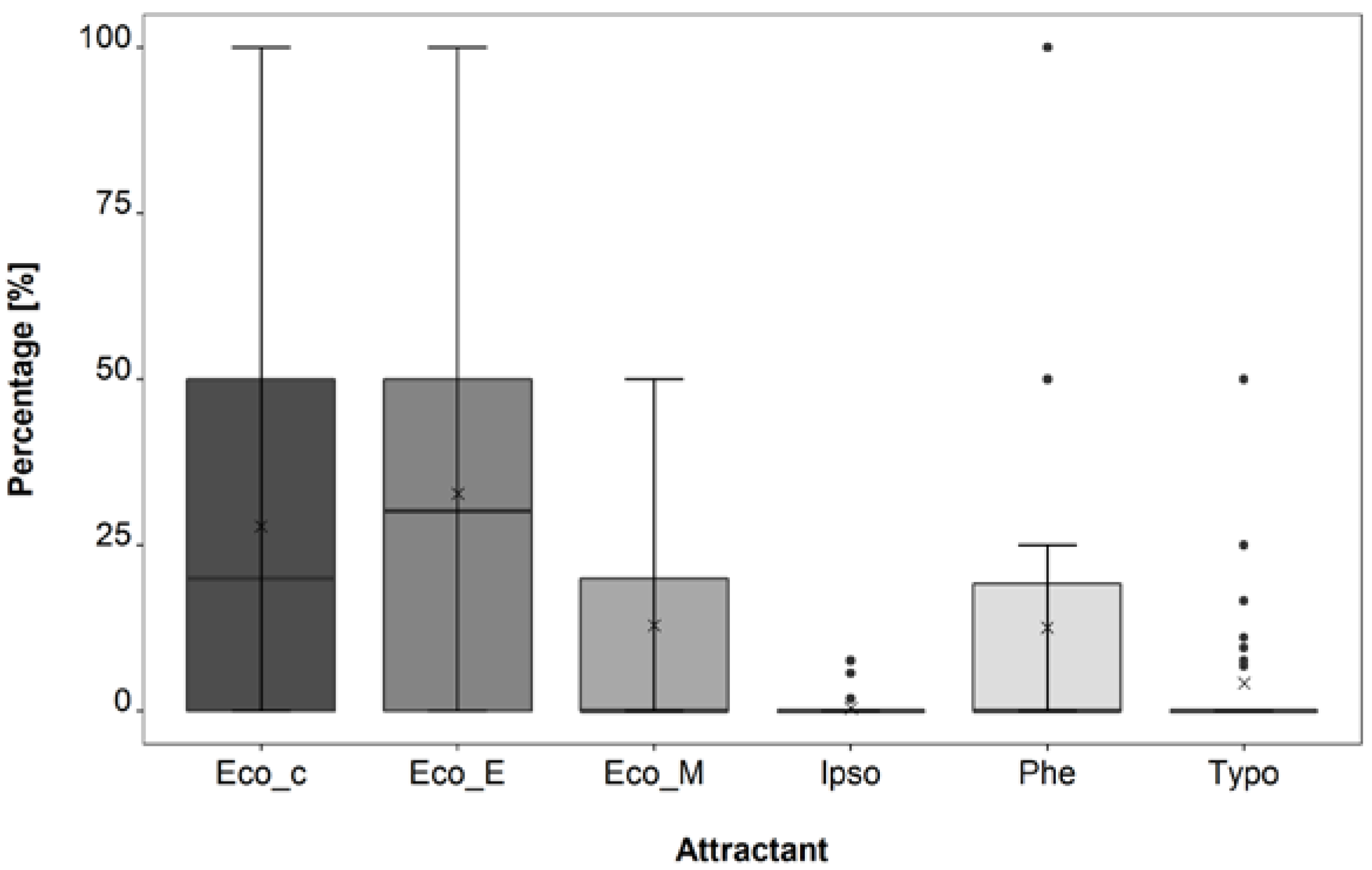
3.1. Attractant Composition Trial
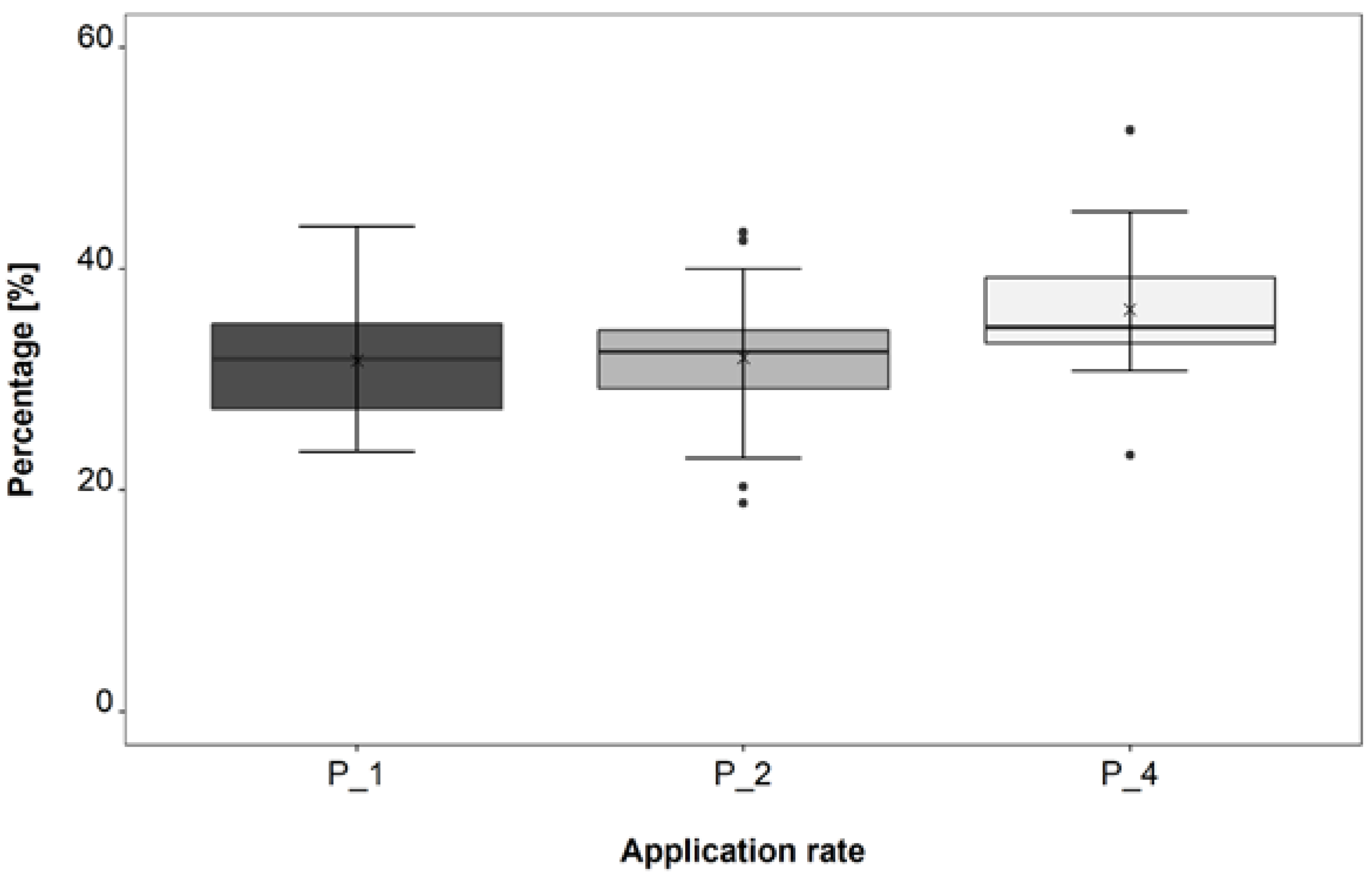
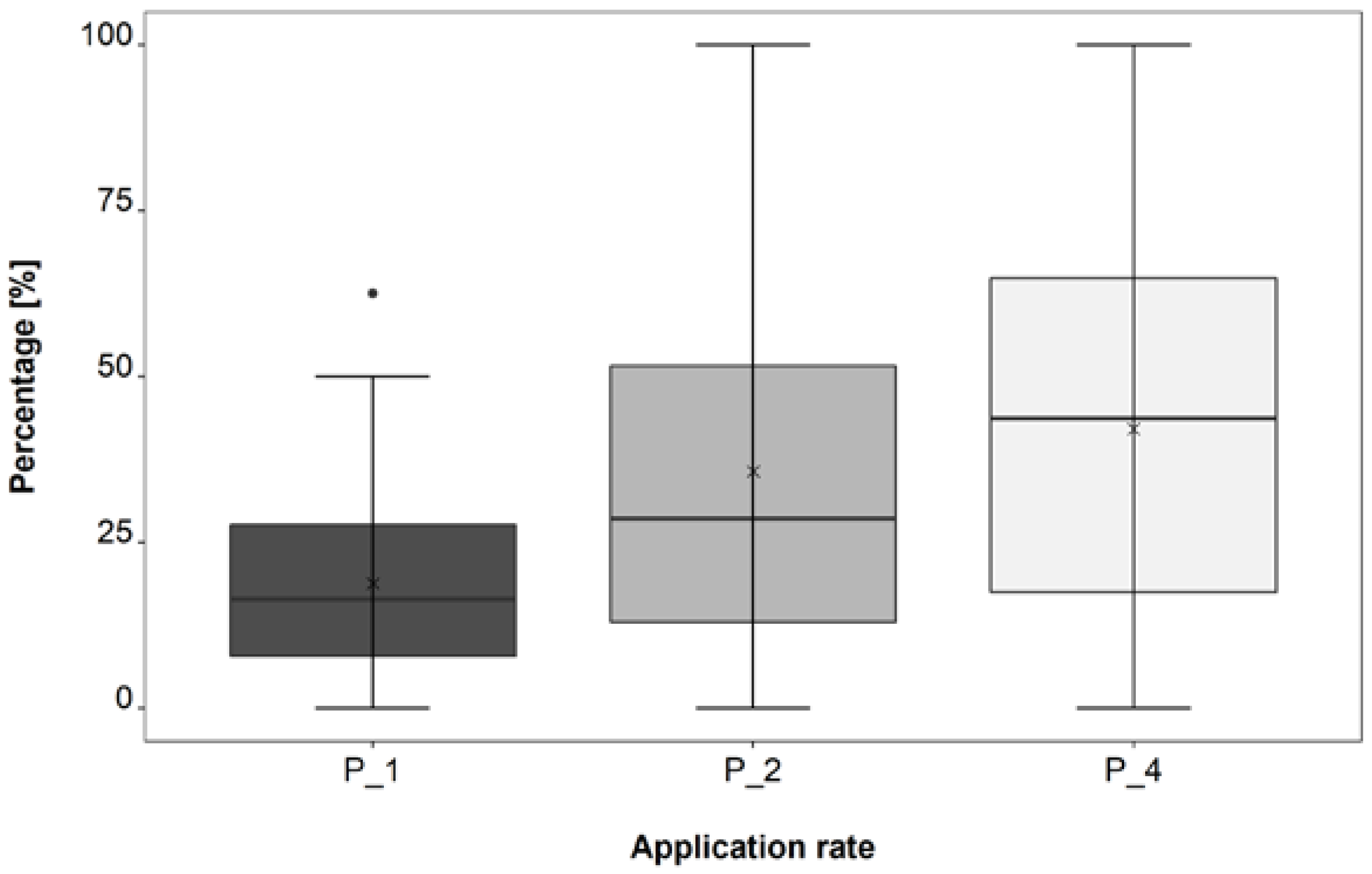
3.2. Application Rate Trial
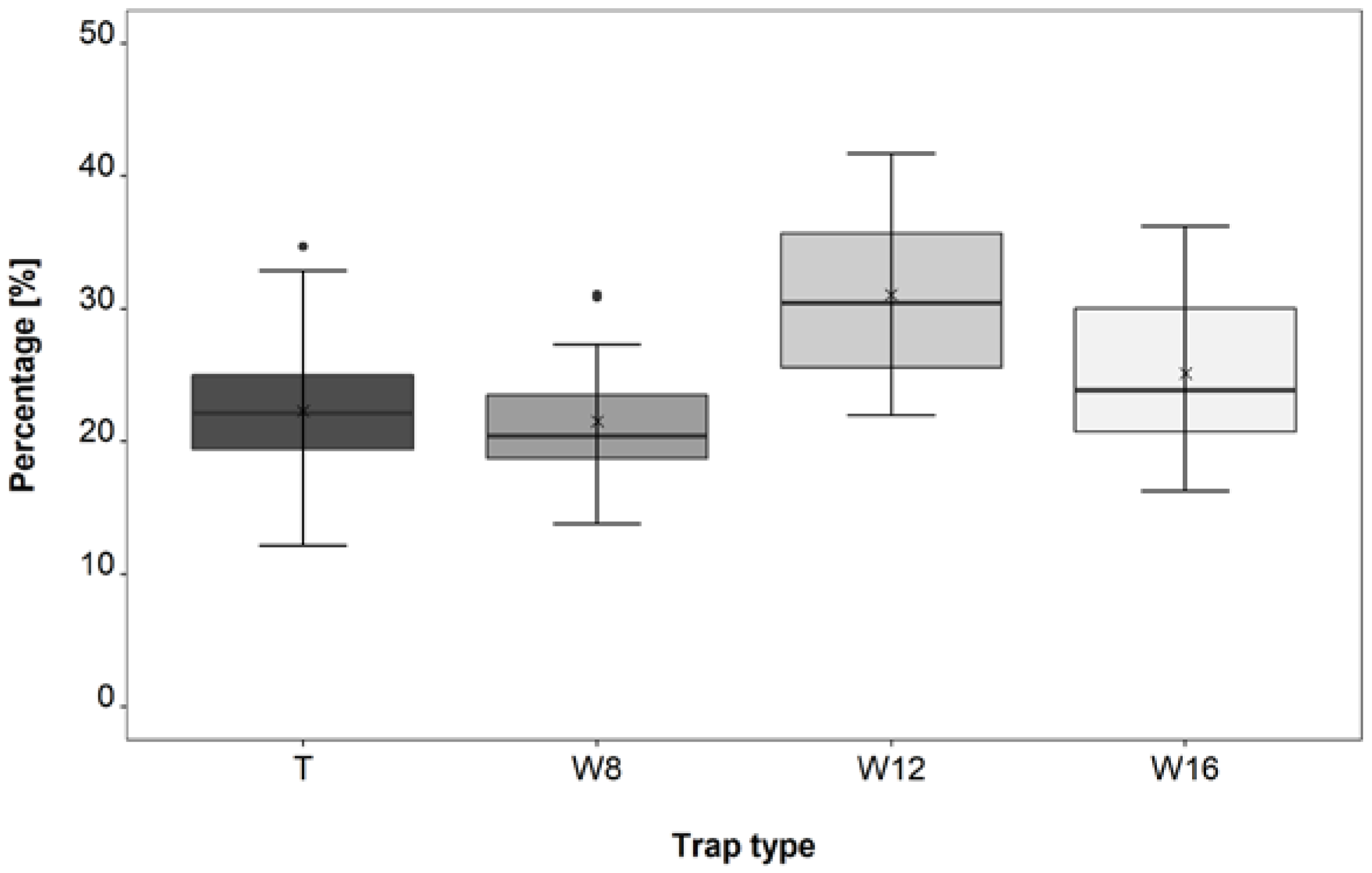
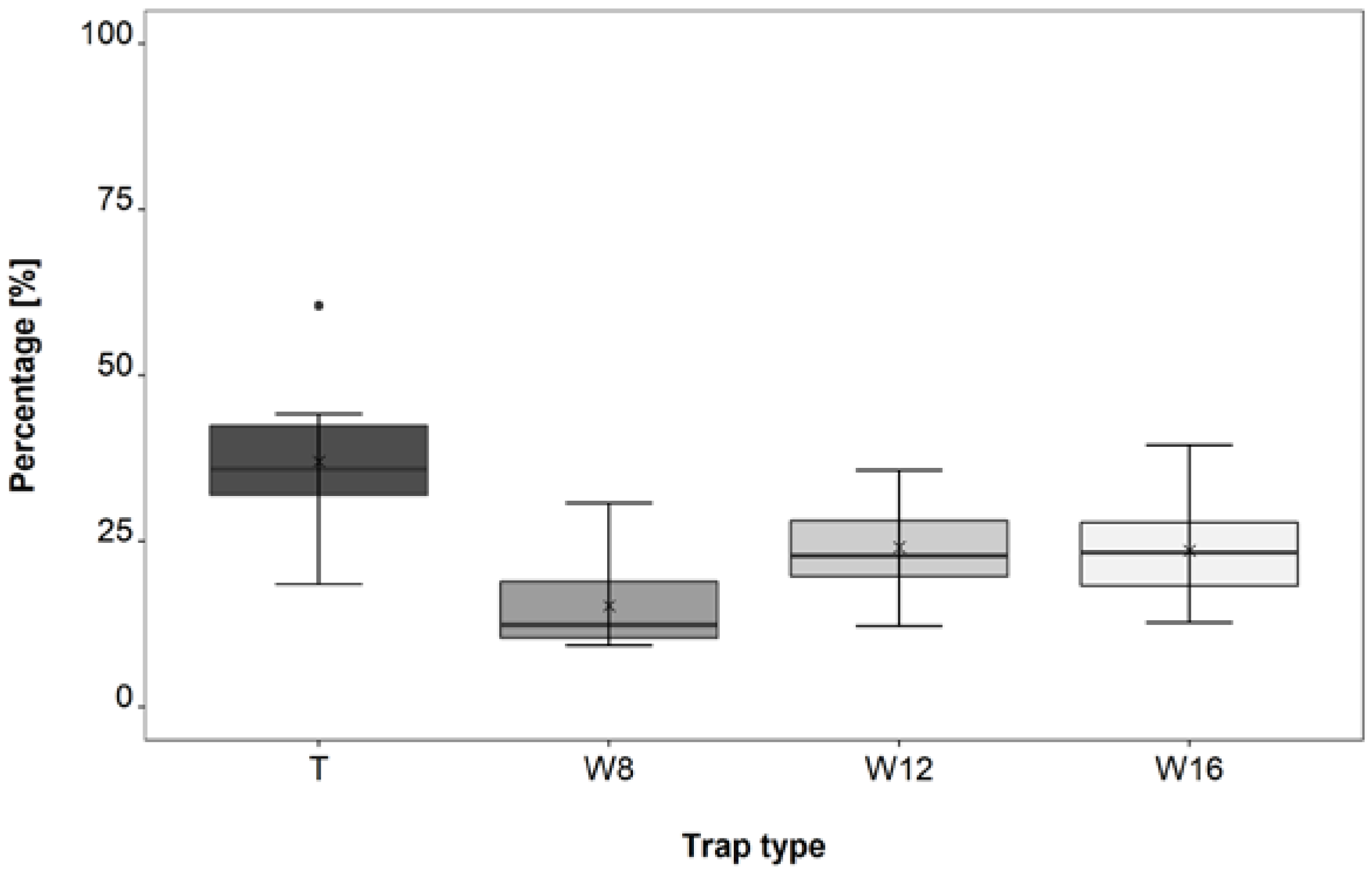
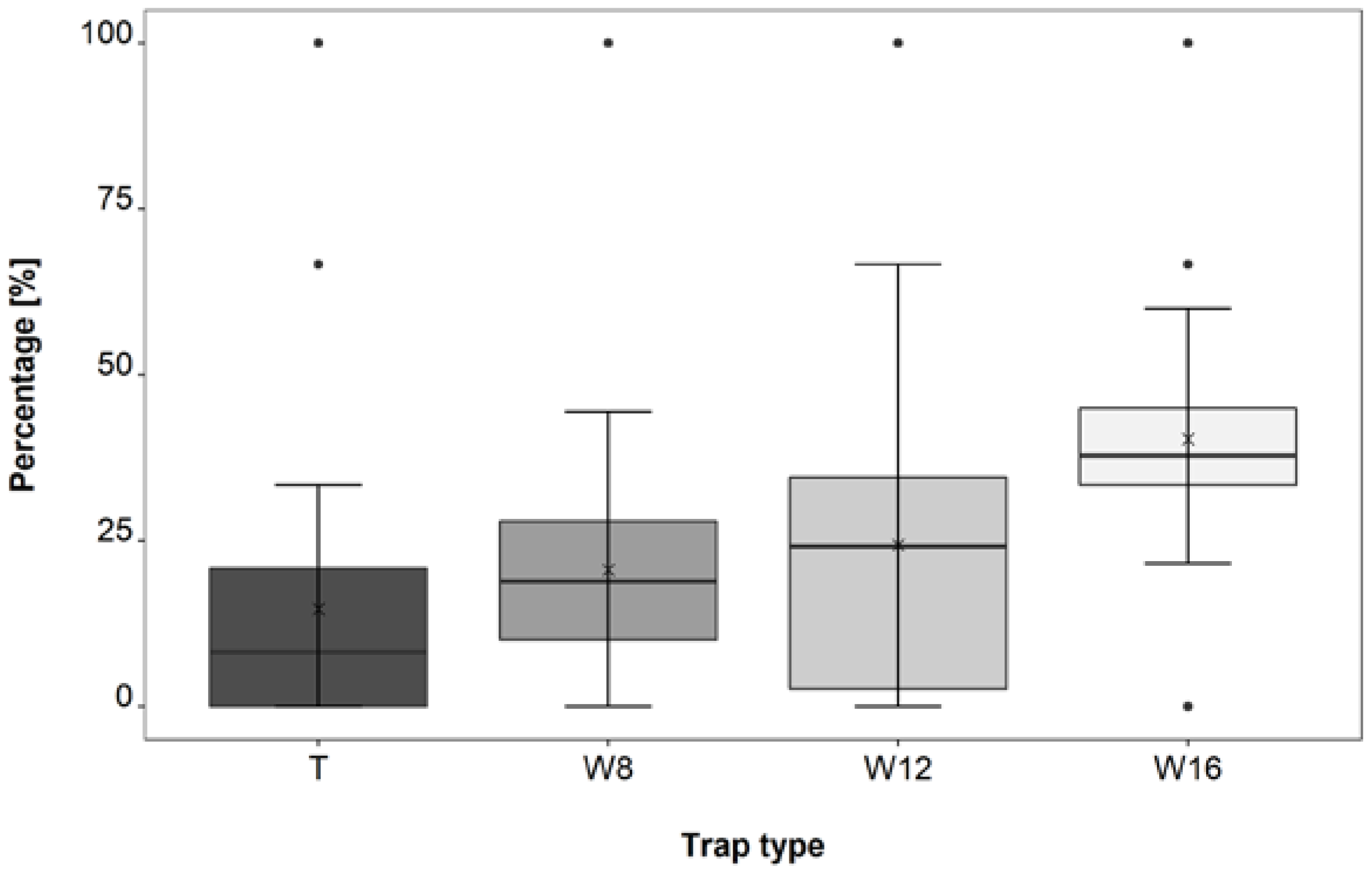
3.3. Trap Type Trial
4. Discussion
4.1. Attractant Composition Trial
4.2. Application Rate Trial
4.3. Trap Type Trial
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Pairwise Comparison | p Value | Significance Code |
|---|---|---|
| Eco_E–Eco_M | 2.5 × 10−5 | *** |
| Eco_E–Ipso | 0.0001 | *** |
| Phe–Eco_M | 0.00045 | *** |
| Phe–Ipso | 0.00156 | ** |
| Typo–Eco_c | 0.00013 | *** |
| Typo–Eco_M | 2.3 × 10−10 | *** |
| Typo–Ipso | 1.4 × 10−9 | *** |
| Typo–Phe | 0.04111 | * |
| Pairwise Comparison | p Value | Significance Code |
|---|---|---|
| Eco_c—Eco_M | 0.0413 | * |
| Eco_E–Eco_M | 0.0098 | ** |
| Phe–Eco_M | 0.0135 | * |
| Typo–Eco_c | 9.4 × 10−6 | *** |
| Typo–Eco_E | 5.9 × 10−5 | *** |
| Typo–Eco_M | 1.0 × 10−11 | *** |
| Typo–Ipso | 5.3 × 10−10 | *** |
| Typo–Phe | 4.0 × 10−5 | *** |
| Pairwise Comparison | p Value | Significance Code |
|---|---|---|
| Eco_E–Eco_M | 0.0052 | ** |
| Eco_E–Ipso | 0.0022 | ** |
| Phe–Ipso | 0.0277 | * |
| Pairwise Comparison | p Value | Significance Code |
|---|---|---|
| Eco_c–Ipso | 1.6 × 10−5 | *** |
| Eco_c–Typo | 0.0013 | ** |
| Eco_E–Ipso | 1.9 × 10−7 | *** |
| Eco_E–Phe | 0.0441 | * |
| Eco_E–Typo | 2.8 × 10−5 | *** |
| Eco_M–Ipso | 0.03 | * |
| Pairwise Comparison | p Value | Significance Code |
|---|---|---|
| P_4–P_1 | 0.0059 | ** |
| P_4–P_2 | 0.0108 | * |
| Pairwise Comparison | p Value | Significance Code |
|---|---|---|
| P_4–P_1 | 0.0052 | ** |
| Pairwise Comparison | p Value | Significance Code |
|---|---|---|
| W12–T | 6.1 × 10−8 | *** |
| W12–W8 | 6.7 × 10−10 | *** |
| W12–W16 | 0.00046 | *** |
| W16–W8 | 0.02047 | * |
| Pairwise Comparison | p Value | Significance Code |
|---|---|---|
| T–W8 | 1.0 × 10−5 | *** |
| T–W12 | 0.044 | * |
| T–W16 | 0.028 | * |
| W12–W8 | 0.041 | * |
| Pairwise Comparison | p Value | Significance Code |
|---|---|---|
| W16–T | 1.6 × 10−6 | *** |
| W16–W8 | 0.0009 | *** |
| W16–W12 | 0.0186 | * |
References
- Jakoby, O.; Lischke, H.; Wermelinger, B. Climate change alters elevational phenology patterns of the European spruce bark beetle (Ips typographus). Glob. Chang. Biol. 2019, 25, 4048–4063. [Google Scholar] [CrossRef]
- Dale, V.H.; Joyce, L.A.; McNulty, S.; Neilson, R.P. The interplay between climate change, forests, and disturbances. Sci. Total Environ. 2000, 262, 201–204. [Google Scholar] [CrossRef]
- Millar, C.I.; Stephenson, N.L.; Stephens, S.L. Climate change and forests of the future: Managing in the face of uncertainty. Ecol. Appl. 2007, 17, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- La Porta, N.; Capretti, P.; Thomsen, I.M.; Kasanen, R.; Hietala, A.M.; von Weissenberg, K. Forest pathogens with higher damage potential due to climate change in Europe. Can. J. Plant Pathol. 2008, 30, 177–195. [Google Scholar] [CrossRef]
- Mezei, P.; Jakuš, R.; Pennerstorfer, J.; Havašová, M.; Škvarenina, J.; Ferenčík, J.; Slivinský, J.; Bičárová, S.; Bilčík, D.; Blaženec, M.; et al. Storms, temperature maxima and the Eurasian spruce bark beetle Ips typographus—An infernal trio in Norway spruce forests of the Central European High Tatra Mountains. Agric. For. Meteorol. 2017, 242, 85–95. [Google Scholar] [CrossRef]
- Ferretti, M. Criterion 2: Maintenance of Forest Ecosystem Health and Vitality. In State of Europe’s Forests 2020; Raši, R., Ed.; Ministerial Conference on the Protection of Forests in Europe: Zvolen, Slovakia, 2020; pp. 52–87. [Google Scholar]
- Netherer, S.; Kandasamy, D.; Jirosová, A.; Kalinová, B.; Schebeck, M.; Schlyter, F. Interactions among Norway spruce, the bark beetle Ips typographus and its fungal symbionts in times of drought. J. Pest Sci. 2021, 197, 1142. [Google Scholar] [CrossRef]
- Müller, J.; Bußler, H.; Goßner, M.; Rettelbach, T.; Duelli, P. The European spruce bark beetle Ips typographus in a national park: From pest to keystone species. Biodivers. Conserv. 2008, 17, 2979–3001. [Google Scholar] [CrossRef]
- Toth, D.; Maitah, M.; Maitah, K.; Jarolínová, V. The impacts of calamity logging on the development of spruce wood prices in czech forestry. Forests 2020, 11, 283. [Google Scholar] [CrossRef]
- Sommerfeld, A.; Rammer, W.; Heurich, M.; Hilmers, T.; Müller, J.; Seidl, R. Do bark beetle outbreaks amplify or dampen future bark beetle disturbances in Central Europe? J. Ecol. 2021, 109, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, G.; Bugmann, H.; Meier, F.; Wermelinger, B.; Bigler, C. Effects of salvage logging and sanitation felling on bark beetle (Ips typographus L.) infestations. For. Ecol. Manag. 2013, 305, 273–281. [Google Scholar] [CrossRef]
- Seidl, R.; Müller, J.; Hothorn, T.; Bässler, C.; Heurich, M.; Kautz, M. Small beetle, large-scale drivers: How regional and landscape factors affect outbreaks of the European spruce bark beetle. J. Appl. Ecol. 2015, 53, 530–540. [Google Scholar] [CrossRef]
- Vanická, H.; Holuša, J.; Resnerová, K.; Ferenčík, J.; Potterf, M.; Véle, A.; Grodzki, W. Interventions have limited effects on the population dynamics of Ips typographus and its natural enemies in the Western Carpathians (Central Europe). For. Ecol. Manag. 2020, 470–471, 1–16. [Google Scholar] [CrossRef]
- Haeseler, S. Sturmtief HERWART Sorgt am 28./29. Oktober 2017 für Orkanböen über Deutschland. 2017. Available online: https://www.dwd.de/DE/leistungen/besondereereignisse/stuerme/20171030_herwart_europa.pdf?__blob=publicationFile&v=6 (accessed on 11 February 2021).
- Haeseler, S. Sturmtief XAVIER Zieht am 5. Oktober 2017 mit Orkanböen über Deutschland. 2017. Available online: https://www.dwd.de/DE/leistungen/besondereereignisse/stuerme/20171009_sturmtief_xavier_deutschland.pdf?__blob=publicationFile&v=4 (accessed on 11 February 2021).
- Haeseler, S. Orkantief FRIEDERIKE Wütet am 18. Januar 2018 über Europa. 2018. Available online: https://www.dwd.de/DE/leistungen/besondereereignisse/stuerme/20180123_friederike_europa.pdf?__blob=publicationFile&v=9 (accessed on 11 February 2021).
- Haeseler, S.; Bissoli, P.; Dassler, J.; Zins, V.; Kreis, A. Orkantief Sabine Löst am 09./10. Februar 2020 eine Schwere Sturmlage über Europa aus 2020. Available online: https://www.dwd.de/DE/leistungen/besondereereignisse/stuerme/20200213_orkantief_sabine_europa.pdf?__blob=publicationFile&v=3 (accessed on 11 February 2021).
- Haeseler, S.; Bissoli, P.; Lefebvre, C.; Dassler, J.; Zins, V. Serie von Sturmtiefs im März 2019 über Europa mit Orkanböen in Deutschland. 2019. Available online: https://www.dwd.de/DE/leistungen/besondereereignisse/stuerme/20190320_sturmtiefs_europa.pdf?__blob=publicationFile&v=1 (accessed on 11 February 2021).
- Lefebvre, C.; Bissoli, P.; Hafer, M.; Rocek, M. Sturmtief FABIENNE Bringt am 23. September 2018 Sturm und Regen, Deutscher Wetterdienst. 2018. Available online: https://www.dwd.de/DE/leistungen/besondereereignisse/stuerme/20180923_sturm_fabienne.pdf?__blob=publicationFile&v=4 (accessed on 11 February 2021).
- Büntgen, U.; Urban, O.; Krusic, P.J.; Rybníček, M.; Kolář, T.; Kyncl, T.; Ač, A.; Koňasová, E.; Čáslavský, J.; Esper, J.; et al. Recent European drought extremes beyond Common Era background variability. Nat. Geosci. 2021, 14, 190–196. [Google Scholar] [CrossRef]
- Schlyter, P.; Stjernquist, I.; Bärring, L.; Jönsson, A.; Nilsson, C. Assessment of the impacts of climate change and weather extremes on boreal forests in northern Europe, focusing on Norway spruce. Clim. Res. 2006, 31, 75–84. [Google Scholar] [CrossRef]
- Ghimire, R.P.; Kivimäenpää, M.; Blomqvist, M.; Holopainen, T.; Lyytikäinen-Saarenmaa, P.; Holopainen, J.K. Effect of bark beetle (Ips typographus L.) attack on bark VOC emissions of Norway spruce (Picea abies Karst.) trees. Atmos. Environ. 2016, 126, 145–152. [Google Scholar] [CrossRef]
- Hroššo, B.; Mezei, P.; Potterf, M.; Majdák, A.; Blaženec, M.; Korolyova, N.; Jakuš, R. Drivers of spruce bark beetle (Ips typographus) infestations on downed trees after severe windthrow. Forests 2020, 11, 1290. [Google Scholar] [CrossRef]
- Hoch, G.; Steyrer, G.; Netherer, S. The outbreak of Ips typographus in Northern Austria. In Proceedings of the Forests’ Future—Consequences of Bark Beetle Calamity for the Future of Forestry in Central Europe, Strnady, Czech Republic, 23 March 2021. [Google Scholar]
- Knížek, M.; Liska, J.; Lubojacky, J. Recent spruce bark beetle calamity in Czechia. In Proceedings of the Forests’ Future—Consequences of Bark Beetle Calamity for the Future of Forestry in Central Europe, Strnady, Czech Republic, 23 March 2021. [Google Scholar]
- Sonnemann, S.; Otto, L.F.; Matschulla, F.; Hodel, M. Forest damages in the federal state of Saxony during the extreme years 2018–2019. In Proceedings of the Forests’ Future—Consequences of Bark Beetle Calamity for the Future of Forestry in Central Europe, Strnady, Czech Republic, 23 March 2021. [Google Scholar]
- Mergl, V.; Zemánek, T.; Šušnjar, M.; Klepárník, J. Efficiency of harvester with the debarking head at logging in spruce stands affected by bark beetle outbreak. Forests 2021, 12, 1348. [Google Scholar] [CrossRef]
- Schelhaas, M.-J.; Nabuurs, G.-J.; Schuck, A. Natural disturbances in the European forests in the 19th and 20th centuries. Glob. Chang. Biol. 2003, 9, 1620–1633. [Google Scholar] [CrossRef]
- Gmelin, J.F. Gmelin´s Abhandlung über die Wurmtroknis; Crusius: Leipzig, Germany, 1787. [Google Scholar]
- Pfeffer, A.; Skuhravy, V. Der Buchdrucker (Ips typographus L.) (Col., Scolytidae) und seine Problematik in der Tschechischen Republik. Anz. Schädlkd. Pflanzenschutz Umweltschutz 1995, 68, 151–152. [Google Scholar] [CrossRef]
- Bakke, A. The recent Ips typographus outbreak in Norway—Experiences from a control program. Holarct. Ecol. 1989, 12, 515–519. [Google Scholar] [CrossRef]
- Ozcan, G.E.; Cicek, O.; Enez, K.; Yildiz, M. A new approach to determine the capture conditions of bark beetles in pheromone-baited traps. Biotechnol. Biotechnol. Equip. 2014, 28, 1057–1064. [Google Scholar] [CrossRef]
- Bakke, A. Field response to a new pheromonal compound isolated from Ips typographus. Naturwissenschaften 1977, 64, 98–99. [Google Scholar] [CrossRef]
- Vité, J.P. The European struggle to control Ips typographus—Past, present and future. Ecography 1989, 12, 520–525. [Google Scholar] [CrossRef]
- Weslien, J.; Annila, E.; Bakke, A.; Bejer, B.; Eidmann, H.H.; Narvestad, K.; Nikula, A.; Ravn, H.P. Estimating risks for spruce bark beetle (Ips typographus (L.)) damage using pheromone-baited traps and trees. Scand. J. For. Res. 1989, 4, 87–98. [Google Scholar] [CrossRef]
- Weslien, J. Monitoring Ips typographus (L.) populations and forecasting damage. J. Appl. Entomol. 1992, 114, 338–340. [Google Scholar] [CrossRef]
- Bakke, A. Deploying pheromone-baited traps for monitoring Ips typographus populations1. Z. Für Angew. Entomol. 1985, 99, 33–39. [Google Scholar] [CrossRef]
- Galko, J.; Gubka, A.; Vakula, J.; Brutovský, D. Comparison of catches of the spruce bark beetle (Ips typographus L.) (Coleoptera: Scolytidae) in pheromone traps of Canadian and European production. Cent. Eur. For. J. 2010, 56, 337–347. [Google Scholar] [CrossRef][Green Version]
- Galko, J.; Nikolov, C.; Kunca, A.; Vakula, J.; Gubka, A.; Zubrik, M.; Rell, S.; Konôpka, B. Effectiveness of pheromone traps for the European spruce bark beetle: A comparative study of four commercial products and two new models. Cent. Eur. For. J. 2016, 62, 207–215. [Google Scholar] [CrossRef]
- Abgrall, J.F. Utilisation de phéromones artificielles contre Ips typographus. EPPO Bull. 1986, 16, 633–637. [Google Scholar] [CrossRef]
- Faccoli, M.; Stergulc, F. Ips typographus (L.) pheromone trapping in south Alps: Spring catches determine damage thresholds. J. Appl. Entomol. 2004, 128, 307–311. [Google Scholar] [CrossRef]
- Faccoli, M.; Stergulc, F. Damage reduction and performance of mass trapping devices for forest protection against the spruce bark beetle, Ips typographus (Coleoptera Curculionidae Scolytinae). Ann. For. Sci. 2008, 65, 1–9. [Google Scholar] [CrossRef]
- Grégoire, J.C.; Raty, L.; Drumont, A.; de Windt, N. Pheromone mass trapping: Does it protect windfalls from attack by Ips typographus L. (Coleoptera: Scolytidae). In Integrating Cultural Tactics into the Management of Bark Beetle and Reforestation Pests; USDA Forest Service General Technical Report NE-236; USDA: Vallombrosa, Italy, 1997; pp. 1–8. [Google Scholar]
- Jakuš, R. A method for the protection of spruce stands against Ips typographus by the use of barriers of pheromone traps in north-eastern Slovakia. Anz. Schädlkd. Pflanzenschutz Umweltschutz 1998, 71, 152–158. [Google Scholar] [CrossRef]
- Weslien, J. Effects of mass trapping on Ips typographus (L.) populations. J. Appl. Entomol. 1992, 114, 228–232. [Google Scholar] [CrossRef]
- Dimitri, L.; Gebauer, U.; Lösekrug, R.; Vaupel, O. Influence of mass trapping on the population dynamic and damage-effect of bark beetles. J. Appl. Entomol. 1992, 114, 103–109. [Google Scholar] [CrossRef]
- Pfister, A.; Hueber, W. Eignung von Pheromonfallen zur Borkenkäferbekämpfung im fünfjährigen Dauerversuch. Forstschutz Aktuell 2008, 43, 7–11. [Google Scholar]
- Matyjaszczyk, E.; Karmilowicz, E.; Skrzecz, I. How European Union accession and implementation of obligatory integrated pest management influenced forest protection against harmful insects: A case study from Poland. For. Ecol. Manag. 2019, 433, 146–152. [Google Scholar] [CrossRef]
- Weslien, J.; Regnander, J. The influence of natural enemies on brood production in Ips typographus (Col. scolytidae) with special reference to egg-laying and predation by Thanasimus formicarius (Col: Cleridae). Entomophaga 1992, 37, 333–342. [Google Scholar] [CrossRef]
- Hulcr, J.; Ubik, K.; Vrkoc, J. The role of semiochemicals in tritrophic interactions between the spruce bark beetle Ips typographus, its predators and infested spruce. J. Appl. Entomol. 2006, 130, 275–283. [Google Scholar] [CrossRef]
- Göthlin, E.; Schroeder, L.M.; Lindelöw, A. Attacks by Ips typographus and Pityogenes chalcographus on windthrown spruces (Picea abies) during the two years following a storm felling. Scand. J. For. Res. 2000, 15, 542–549. [Google Scholar] [CrossRef]
- Byers, J.A. Orientation of bark beetles Pityogenes chalcographus and Ips typographus to pheromonebaited puddle traps placed in grids: A new trap for control of scolytids. J. Chem. Ecol. 1993, 19, 2297–2316. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Byers, J.A. Avoidance of competition by spruce bark beetles, Ips typographus and Pityogenes chalcographus. Experientia 1993, 49, 272–275. [Google Scholar] [CrossRef]
- Lompe, A. Die Käfer Mitteleuropas. Ein Bestimmungswerk im Internet: Gattung: Ips De Geer. 2020. Available online: http://coleonet.de/coleo/texte/ips.htm (accessed on 30 January 2021).
- Lompe, A. Die Käfer Mitteleuropas. Ein Bestimmungswerk im Internet: Gattung Pityogenes. 2021. Available online: http://coleonet.de/coleo/texte/pityogenes.htm (accessed on 4 February 2021).
- Thomaes, A.; Drumont, A.; Warzee, N.; Grégoire, J.-C.; Stassen, E.; Crevecoeur, L.; Berckvens, N.; Casteels, H.; van de Vijver, D.; Raemdonck, H. Ecology and distribution of Thanasimus formicarius (Linnaeus, 1758) and the newly discovered Thanasimus femoralis (Zetterstedt, 1828) in Belgium. Bull. Ann. Soc. R. Belge Entomolog. 2017, 153, 206–214. [Google Scholar]
- Grüne, S. Handbuch zur Bestimmung der Europäischen Borkenkäfer: Brief Illustrated Key to European Bark Beetles; M. & H. Schaper: Hannover, Germany, 1979. [Google Scholar]
- Wehnert, M.; Müller, M. ‘Allochthonous Kairomones’ in stands of European beech (Fagus sylvatica)—Approach for nature-based bark beetle management with clerid beetles (Thanasimus spp.). Biol. Control 2012, 62, 16–23. [Google Scholar] [CrossRef]
- Wehnert, M. Analyse und Olfaktorische Steuerung Bast- und Holzbesiedelnder Sowie Diese Natürlich Regulierender Zoophager Insekten an Laubbäumen als Grundlage für Ein Zukunftsfähiges und Nachhaltiges Risikomanagement. Ph.D. Thesis, Technische Universität Dresden, Dresden, Germany, 24 February 2014. [Google Scholar]
- Helbig, C.; Heber, T. Chemical sabotage in forest health management: Effects of Allium ursinum L. based semiochemicals on the catches of bark beetles and their antagonists in stands of European ash (Fraxinus excelsior L.). Mitt. Dtsch. Ges. Allg. Angew. Entomol. 2020, 22, 269–272. [Google Scholar]
- Schlyter, F.; Byers, J.A.; Löfqvist, J. Attraction to pheromone sources of different quantity, quality, and spacing: Density-regulation mechanisms in bark beetle Ips typographus. J. Chem. Ecol. 1987, 13, 1503–1523. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Crowe, C.M.; Barnes, B.F.; Gandhi, K.J.K.; Duerr, D.A. Attaching lures to multiple-funnel traps targeting saproxylic beetles (Coleoptera) in pine stands: Inside or outside funnels? J. Econ. Entomol. 2013, 106, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Zumr, V. Effect of synthetic pheromones Pheroprax on the coleopterous predators of the spruce bark beetle Ips typographus (L.). J. Appl. Entomol. 1983, 95, 47–50. [Google Scholar] [CrossRef]
- Franklin, A.J.; Grégoire, J.C. Dose-dependent response and preliminary observations on attraction range of Ips typographus to pheromones at low release rates. J. Chem. Ecol. 2001, 27, 2425–2435. [Google Scholar] [CrossRef] [PubMed]
- Hrasovec, B.; Kasumovic, L.; Franjevic, M. Overwintering of eight toothed spruce bark beetle (Ips typographus) in spruce forests of North Velebit. Croat. J. For. Eng. 2011, 32, 211–222. [Google Scholar]
- Nakládal, O.; Sova, J. Comparison of two types of ECOLURE lure on Ips typographus (L.) (Coleoptera: Scolytidae). J. For. Sci. 2010, 56, 609–613. [Google Scholar] [CrossRef]
- Jakuš, R.; Simko, J. The use of dispensers with different release rates at pheromone trap barriers for Ips typographus. Anz. Schädlkd. Pflanzenschutz Umweltschutz 2000, 73, 33–36. [Google Scholar]
- Pfister, A. Borkenkäferpheromontests 1997. Report 1997. Available online: https://bfw.ac.at/rz/bfwcms.web?dok=6463 (accessed on 20 February 2020).
- Pfister, A. Borkenkäferpheromontests 1998. Report 1998. Available online: https://bfw.ac.at/rz/bfwcms.web?dok=6473 (accessed on 20 February 2020).
- Otto, L.F. Untersuchungen zur Fangleistung Verschiedener Dispenser für den Buchdrucker und Kupferstecher. Report 2005. Available online: https://docplayer.org/161657598-Untersuchungen-zur-fangleistung-verschiedener-dispenser-fuer-den-buchdrucker-und-kupferstecher-html (accessed on 20 February 2020).
- Šramel, N.; Kavčič, A.; Kolšek, M.; Groot, M. Estimating the most effective and economical pheromone for monitoring the European spruce bark beetle. J. Appl. Entomol. 2021, 145, 312–325. [Google Scholar] [CrossRef]
- Thüringen Forst. Bericht über Ergebnisse zur Anlockung des Buchdruckers (Ips typographus) mit Verschiedenen Lockstoffdispensern im Jahr 2008. Report 2008.
- Harper, J.K.; Arif, A.M.; Grant, D.M. cis-Verbenol. Acta Crystallogr. C Cryst. Struct. Commun. 2000, 56, 451–452. [Google Scholar] [CrossRef]
- Lindgren, B.S. A multiple funnel trap for scolytid beetles (Coleoptera). Can. Entomol. 1983, 115, 299–302. [Google Scholar] [CrossRef]
- Niemeyer, H.; Schröder, T.; Watzek, G. Eine neue Lockstoff-Falle zur Bekämpfung von rinden- und holzbrütenden Borkenkäfern. Forst-Holzwirt 1983, 38, 105–112. [Google Scholar]
- Wickham, H. The split-apply-combine strategy for data analysis. J. Stat. Softw. 2011, 40, 1–29. [Google Scholar] [CrossRef]
- Wickham, H. Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1–6. [Google Scholar] [CrossRef]
- Kassambara, A. ggpubr: “ggplot2” Based Publication Ready Plots. R Package Version 0.4.0. 2020. Available online: https://joss.theoj.org/papers/10.21105/joss.01686 (accessed on 29 October 2021).
- Giraudoux, P. pgirmess: Spatial Analysis and Data Mining for Field Ecologists. R Package Version 1.7.0. 2021. Available online: https://cran.r-project.org/web/packages/pgirmess/index.html (accessed on 29 October 2021).
- Pohlert, T. PMCMRplus: Calculate Pairwise Multiple Comparisons of Mean Rank Sums Extended. R Package Version 1.9.0. 2021. Available online: https://cran.r-project.org/web/packages/PMCMRplus/index.html (accessed on 29 October 2021).
- Wickham, H.; Bryan, J. readxl: Read Excel Files. R Package Version 1.3.1. 2019. Available online: https://cran.r-project.org/web/packages/readxl/index.html (accessed on 29 October 2021).
- Wood, D.L.; Vité, J.P. Studies on the host selection behavior of Ips confusus (LeConte) (Coleoptera: Scolytidae) attacking Pinus ponderosa. Contr. Boyce. Thompson Inst. Pl. Res. 1961, 21, 79–95. [Google Scholar]
- Wood, D.L.; Bushing, R.W. The olfactory response of Ips confusus (LeConte) (Coleoptera: Scolytidae) to the secondary attraction in the laboratory. Can. Entomol. 1963, 95, 1066–1078. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Rodin, J.O.; Wood, D.L. Sex attractants in frass produced by male Ips confusus in Ponderosa Pine. Science 1966, 154, 509–510. [Google Scholar] [CrossRef]
- Wood, D.L.; Browne, L.E.; Bedard, W.D.; Tilden, P.E.; Silverstein, R.M.; Rodin, J.O. Response of Ips confusus to synthetic sex pheromones in nature. Science 1967, 159, 1373–1374. [Google Scholar] [CrossRef] [PubMed]
- Bakke, A. Evidence of a population aggregating pheromone in Ips typographus (Coleoptera: Scolytidae). Contr. Boyce. Thompson Inst. Pl. Res. 1970, 24, 309–310. [Google Scholar]
- Vité, J.P.; Bakke, A.; Renwick, J.A.A. Pheromones in Ips (Coleoptera: Scolytidae): Occurrence and production. Can. Entomol. 1972, 104, 1967–1975. [Google Scholar] [CrossRef]
- Rudinsky, J.A.; Novák, V.; Švihra, P. Attraction of the bark beetle Ips typographus L. to terpenes and a male-produced pheromone. J. Appl. Entomol. 1971, 67, 179–188. [Google Scholar] [CrossRef]
- Vité, J.P.; Francke, W. The aggregation pheromones of bark beetles: Progress and problems. Naturwissenschaften 1976, 63, 550–555. [Google Scholar] [CrossRef]
- Voolma, K. Comparison of two traps used for monitoring large pine weevil populations, Hylobius abietis L. (Coleoptera: Curculionidae). Trans. Est. Agric. Univ. Agron. 2000, 209, 233–235. [Google Scholar]
- Schlyter, F.; Löfqvist, J.; Byers, J.A. Behavioural sequence in the attraction of the bark beetle Ips typographus to pheromone sources. Physiol. Entomol. 1987, 12, 185–196. [Google Scholar] [CrossRef]
- Andersson, M.N.; Larsson, M.C.; Schlyter, F. Specificity and redundancy in the olfactory system of the bark beetle Ips typographus: Single-cell responses to ecologically relevant odors. J. Insect. Physiol. 2009, 55, 556–567. [Google Scholar] [CrossRef]
- Sun, X.; Yang, Q.; Sweeney, J.D.; Gao, C. A review chemical ecology of Ips typographus (Coleoptera, Scolytidae). J. For. Res. 2006, 17, 65–70. [Google Scholar] [CrossRef]
- Schlyter, F.; Birgersson, G.; Byers, J.A.; Löfqvist, J.; Bergström, G. Field response of spruce bark beetle, Ips typographus, to aggregation pheromone candidates. J. Chem. Ecol. 1987, 13, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Dickens, J.C. Behavioural and electrophysiological responses of the bark beetle, Ips typographus, to potential pheromone components. Physiol. Entomol. 1981, 6, 251–261. [Google Scholar] [CrossRef]
- Olenici, N.; Duduman, M.-L.; Olenici, V. Inhibitory Effect of (-)Alpha Pinene High Release Rates on Ips typographus (L.) Response to its Aggregation Pheromone. An. ICAS 2007, 50, 203–212. [Google Scholar]
- Jönsson, A.M.; Schroeder, L.M.; Lagergren, F.; Anderbrant, O.; Smith, B. Guess the impact of Ips typographus—An ecosystem modelling approach for simulating spruce bark beetle outbreaks. Agric. For. Meteorol. 2012, 166–167, 188–200. [Google Scholar] [CrossRef]
- Berec, L.; Doležal, P.; Hais, M. Population dynamics of Ips typographus in the Bohemian Forest (Czech Republic): Validation of the phenology model PHENIPS and impacts of climate change. For. Ecol. Manag. 2013, 292, 1–9. [Google Scholar] [CrossRef]
- Wermelinger, B.; Seifert, M. Analysis of the temperature dependent development of the spruce bark beetle Ips typographus (L) (Col., Scolytidae). J. Appl. Entomol. 1998, 122, 185–191. [Google Scholar] [CrossRef]
- Baier, P.; Pennerstorfer, J.; Schopf, A. PHENIPS—A comprehensive phenology model of Ips typographus (L.) (Col., Scolytinae) as a tool for hazard rating of bark beetle infestation. For. Ecol. Manag. 2007, 249, 171–186. [Google Scholar] [CrossRef]
- Niemeyer, H.; Watzek, G. Test von Monoterpenen als Zusatz zu Pheroprax® bzw. Chalcoprax® in Pheromonfallen zum Fang des Buchdruckers, Ips typographus L. bzw. des Kupferstechers, Pityogenes chalcographus L. (Col., Scolytidae). Anz. Schädlkd. Pflanzenschutz Umweltschutz 1996, 69, 109–110. [Google Scholar] [CrossRef]
- Birgersson, G.; Schlyter, F.; Löfqvist, J.; Bergström, G. Quantitative variation of pheromone components in the spruce bark beetle Ips typographus from different attack phases. J. Chem. Ecol. 1984, 10, 1029–1055. [Google Scholar] [CrossRef]
- Wermelinger, B. Ecology and management of the spruce bark beetle Ips typographus—A review of recent research. For. Ecol. Manag. 2004, 202, 67–82. [Google Scholar] [CrossRef]
- Bakke, A.; Kvamme, T. Kairomone response in Thanasimus predators to pheromone components of Ips typographus. J. Chem. Ecol. 1981, 7, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Tømmerås, B. Specialization of the olfactory receptor cells in the bark beetle Ips typographus and its predator Thanasimus formicarius to bark beetle pheromones and host tree volatiles. J. Comp. Physiol. 1985, 157, 335–341. [Google Scholar] [CrossRef]
- Hansen, K. Reception of bark beetle pheromone in the predaceous clerid beetle, Thanasimus formicarius (Coleoptera: Cleridae). J. Comp. Physiol. 1983, 150, 371–378. [Google Scholar] [CrossRef]
- Warzee, N.; Grégoire, J.-C. Thanasimus formicarius (Coleoptera: Cleridae): Why a large range of prey for a specialized predator? In Proceedings of the IUFRO Kanazawa 2003 “Forest Insect Population Dynamics and Host Influences”, Kanazawa, Japan, 14–19 September 2003; pp. 16–18. [Google Scholar]
- Zahradník, P.; Zahradníková, M. Evaluation of the efficacy duration of different types of pheromone dispensers to lure Ips typographus (L.) (Coleoptera: Curculionidae: Scolytinae). J. For. Sci. 2014, 60, 456–463. [Google Scholar] [CrossRef]
- Nakládal, O.; Senfeld, P.; Franjevic, M.; Uhlikova, H. Comparison of all season and standard type of ECOLURE® dispenser efficacy in trap catches of Europaean spruce bark beetle (Ips typographus L.). J. For. Soc. Croatia 2013, 137, 395–400. [Google Scholar]
- Ditz, H.M.; Nieder, A. Numerosity representations in crows obey the Weber-Fechner law. Proc. Biol. Sci. 2016, 283, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Müller, M. Untersuchungen zur Attraktion und Repulsion des Großen Braunen Rüsselkäfers, Hylobius abietis L. (Coleoptera, Curculionidae). Ph.D. Thesis, Technische Universität Dresden, Dresden, Germany, 23 June 1993. [Google Scholar]
- Park, J.; Reid, M.L. Distribution of a bark beetle, Trypodendron lineatum, in a harvested landscape. For. Ecol. Manag. 2007, 242, 236–242. [Google Scholar] [CrossRef]
- Wright, R.H. Some quantitative aspects of insect attraction. Can. Entomol. 1966, 98, 1114–1117. [Google Scholar] [CrossRef]
- Altenkirch, W.; Bogenschütz, H. Waldschutz auf Ökologischer Grundlage: 86 Tabellen; Ulmer: Stuttgart (Hohenheim), Germany, 2002. [Google Scholar]
- Helmund, M. Aggregation von Borkenkäferprädatoren unter Ausnutzung des Prinzips der Allochthonen Kairomone in Ausgewählten Nadelwaldhabitaten unter Besonderer Berücksichtigung der Ameisenbuntkäfer. Ph.D. Thesis, Technische Universität Dresden, Dresden, Germany, 8 April 2013. [Google Scholar]
- Schumacher, J.; Pohris, V. Der Kleine Buchenborkenkäfer als relevanter Rindenbrüter in Schwarzerlen-Beständen. Allg Forst Jagdztg 2000, 14, 760–763. [Google Scholar]
- Flechtmann, C.A.H.; Ottati, A.L.T.; Berisford, C.W. Comparison of four trap types for ambrosia beetles (Coleoptera, Scolytidae) in Brazilian eucalyptus stands. J. Econ. Entomol. 2000, 93, 1701–1707. [Google Scholar] [CrossRef]
- Miller, D.R.; Crowe, C.M. Relative performance of Lindgren multiple-funnel, intercept panel, and colossus pipe traps in catching Cerambycidae and associated species in the southeastern United States. J. Econ. Entomol. 2011, 104, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Petrice, T.; Haack, R.; Poland, T. Evaluation of three trap types and five lures for monitoring Hylurgus ligniperda (Coleoptera: Scolytidae) and other local scolytids in New York. Great Lakes Entomol. 2018, 37, 1–9. [Google Scholar]
- Dodds, K.J.; Dubois, G.D.; Hoebeke, E.R. Trap type, lure placement, and habitat effects on Cerambycidae and Scolytinae (Coleoptera) catches in the Northeastern United States. J. Econ. Entomol. 2010, 103, 698–707. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, R.L.; Katinic, P.J.; Allison, J.D.; Borden, J.H.; Downey, D.L. Comparative efficacy of five types of trap for woodborers in the Cerambycidae, Buprestidae and Siricidae. Agric. For. Entomol. 2001, 3, 113–120. [Google Scholar] [CrossRef]
- Lindgren, B.S.; Fraser, R.G. Control of ambrosia beetle damage by mass trapping at a dryland log sorting area in British Columbia. For. Chron. 1994, 70, 159–163. [Google Scholar] [CrossRef]
- Zumr, V. Dispersal of the spruce bark beetle Ips typographus (L.) (Col., Scolytidae) in spruce woods. J. Appl. Entomol. 1992, 114, 348–352. [Google Scholar] [CrossRef]
- Goyer, R.A.; Lenhard, G.J.; Strom, B.L. The influence of silhouette color and orientation on arrival and emergence of Ips pine engravers and their predators in loblolly pine. For. Ecol. Manag. 2004, 191, 147–155. [Google Scholar] [CrossRef]
- Zumr, V.; Soldan, T. Reproductive cycles of Ips typographus, Ips amitinus and Pityogenes chalcographus (Coleoptera, Scolytidae). Acta Entomol. Bohemoslov. 1981, 78, 280–289. [Google Scholar]
- McCravy, K.W.; Nowak, J.T.; Douce, G.K.; Berisford, C.W. Evaluation of multiple-funnel and slot traps for collection of southern pine bark beetles and predators. J. Entomol. Sci. 2000, 35, 77–82. [Google Scholar] [CrossRef]
- Martín, A.; Etxebeste, I.; Pérez, G.; Álvarez, G.; Sánchez, E.; Pajares, J. Modified pheromone traps help reduce bycatch of bark-beetle natural enemies. Agric. For. Entomol. 2013, 15, 86–97. [Google Scholar] [CrossRef]
- Mills, N.J. Some observations on the role of predation in the natural regulation of Ips typographus populations. J. Appl. Entomol. 1985, 99, 209–215. [Google Scholar] [CrossRef]
- Raty, L.; Drumont, A.; de Windt, N.; Grégoire, J.C. Mass trapping of the spruce bark beetle Ips typographus L.: Traps or trap trees? For. Ecol. Manag. 1995, 78, 191–205. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heber, T.; Helbig, C.E.; Osmers, S.; Müller, M.G. Evaluation of Attractant Composition, Application Rate, and Trap Type for Potential Mass Trapping of Ips typographus (L.). Forests 2021, 12, 1727. https://doi.org/10.3390/f12121727
Heber T, Helbig CE, Osmers S, Müller MG. Evaluation of Attractant Composition, Application Rate, and Trap Type for Potential Mass Trapping of Ips typographus (L.). Forests. 2021; 12(12):1727. https://doi.org/10.3390/f12121727
Chicago/Turabian StyleHeber, Tobias, Christiane E. Helbig, Sören Osmers, and Michael G. Müller. 2021. "Evaluation of Attractant Composition, Application Rate, and Trap Type for Potential Mass Trapping of Ips typographus (L.)" Forests 12, no. 12: 1727. https://doi.org/10.3390/f12121727
APA StyleHeber, T., Helbig, C. E., Osmers, S., & Müller, M. G. (2021). Evaluation of Attractant Composition, Application Rate, and Trap Type for Potential Mass Trapping of Ips typographus (L.). Forests, 12(12), 1727. https://doi.org/10.3390/f12121727






