Growth-Climate Relationships and Long-Term Growth Trends of the Tropical Forest Tree Choerospondias axillaris (Anacardiaceae) in East-Central Thailand
Abstract
:1. Introduction
2. Materials and Methods
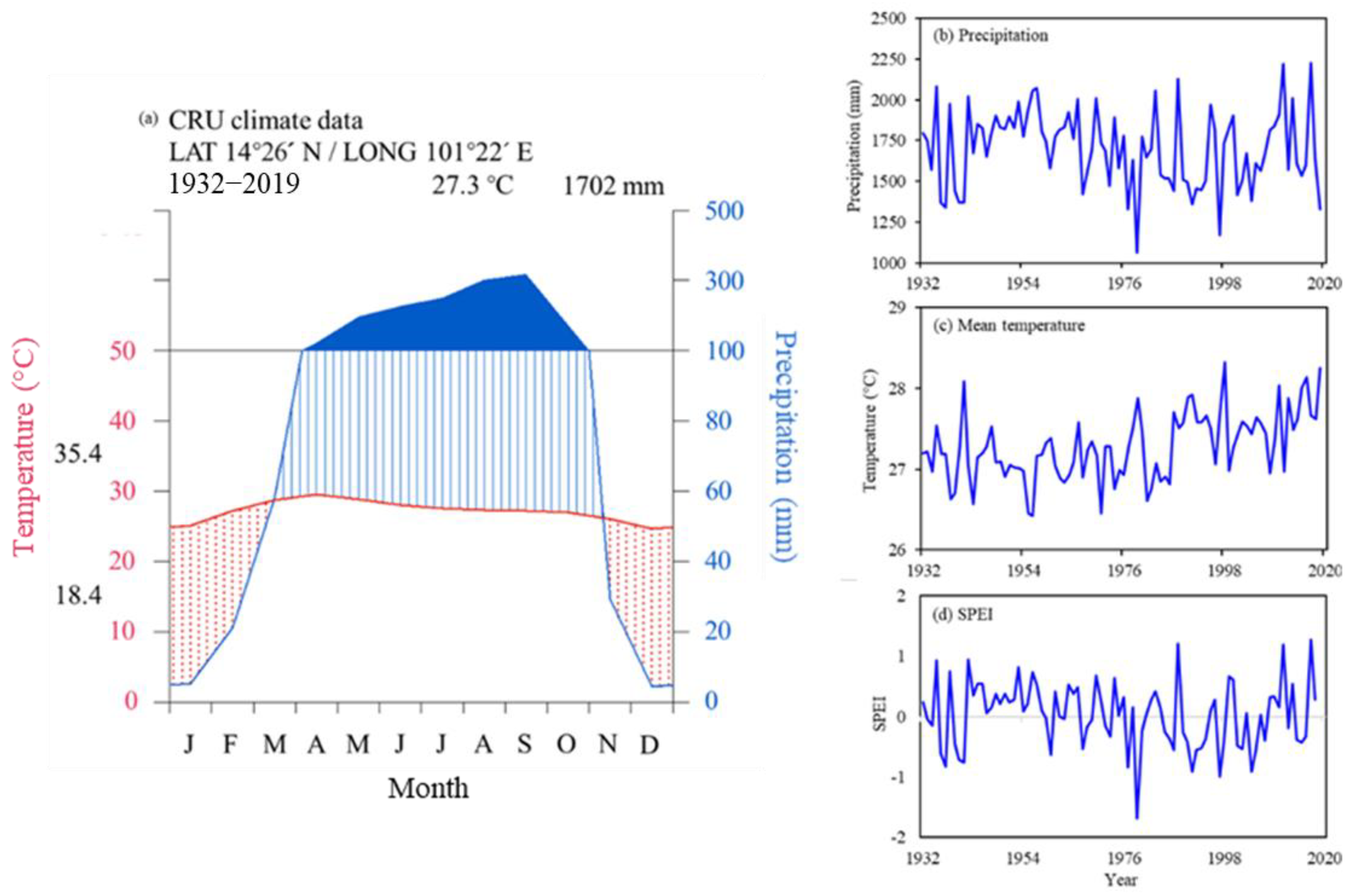
2.1. Study Area and Climate
2.2. Study Species
2.3. Sampling, Sample Preparation, Tree Ring-Width Measurement and Cross-Dating
2.4. Growth-Climate Relationships
2.5. Long-Term Growth Trends
3. Results
3.1. Characteristics Tree-Ring Chronology for Choerospondias axillaris at Mo Singto
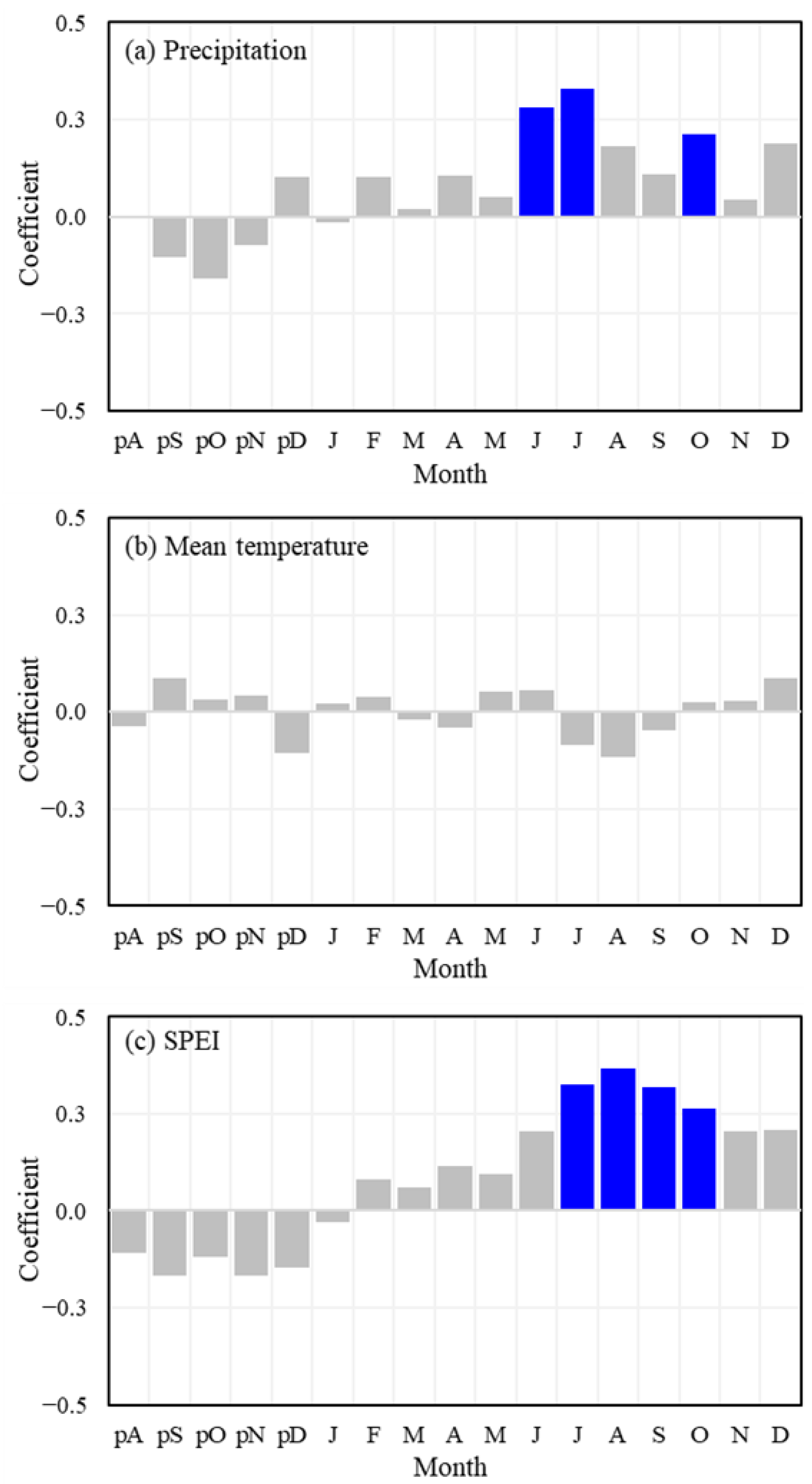
3.2. Growth-Climate Relationships
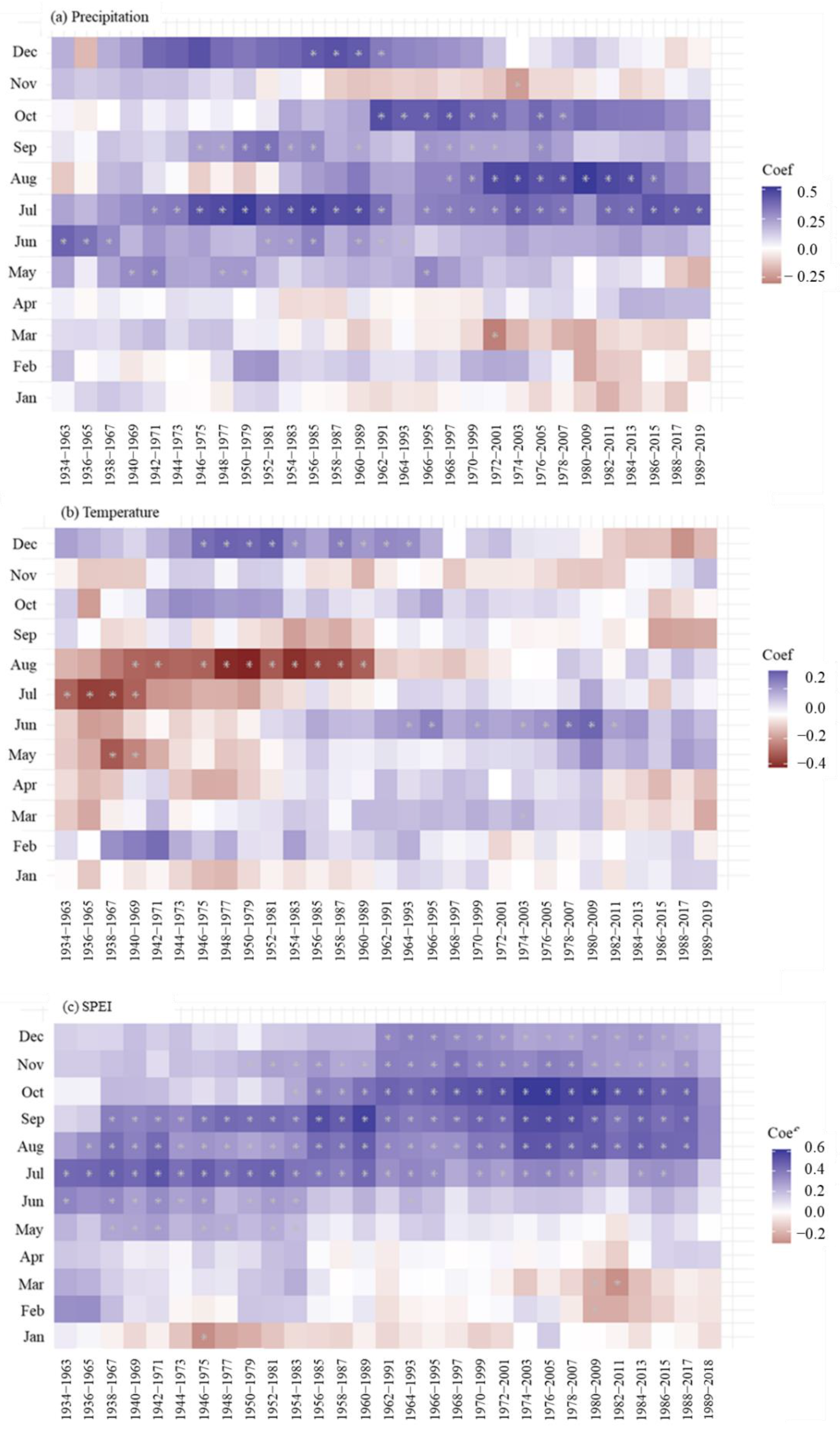
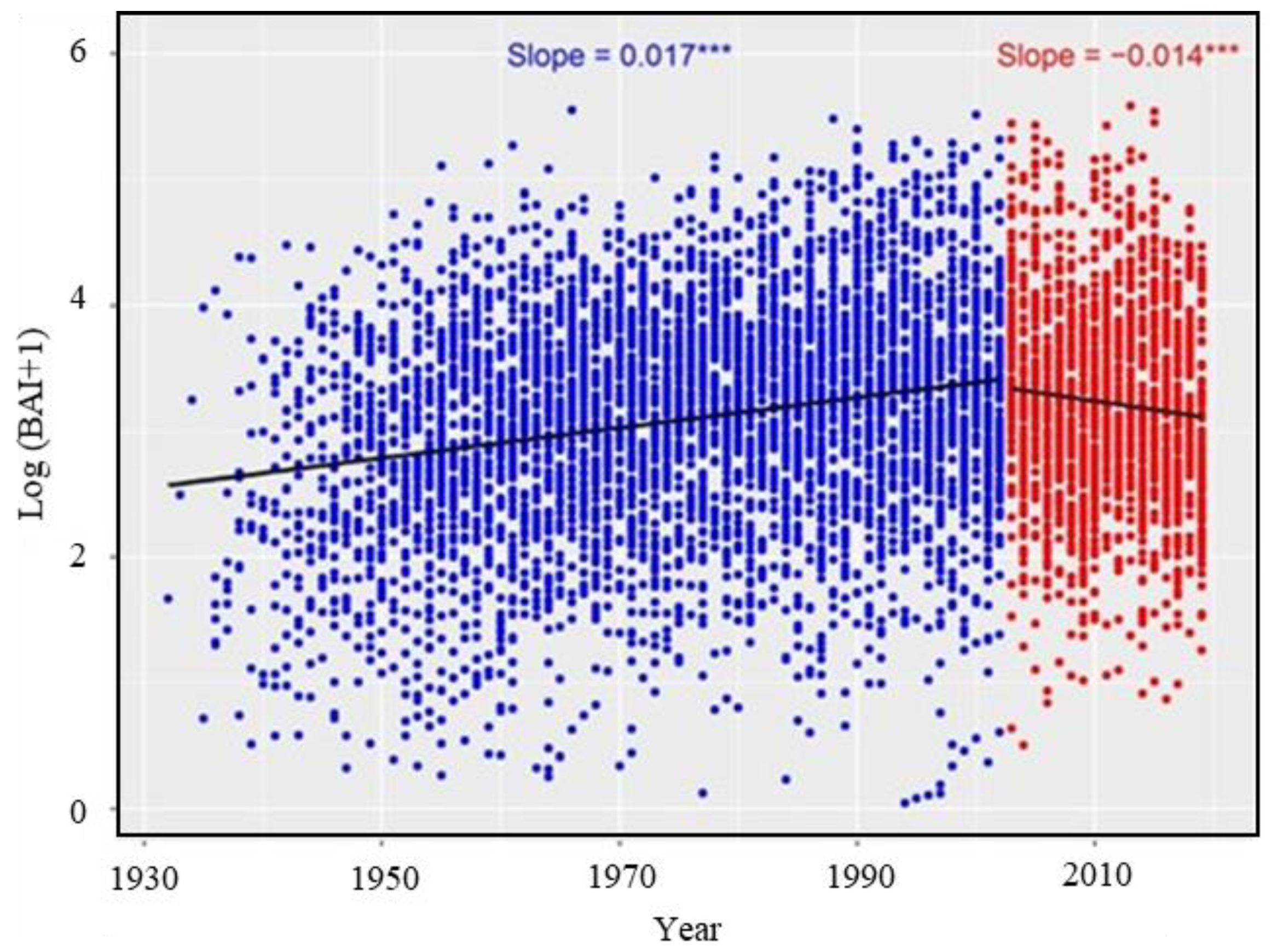
3.3. Long-Term Growth Trend and Climate Responses
4. Discussion
4.1. Choerospondias axillaris Chronology Characteristics
4.2. Growth–Climate Relationships
4.3. Long-Term Growth Trends of Choerospondias axillaris
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. The physical science basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, A.P., Pirani, S.L., Connors, C., Péan, S., Berger, N., Caud, Y., Chen, L., Goldfarb, M.I., Gomis, M., et al., Eds.; Cambridge University: Cambridge, UK, 2021. [Google Scholar]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Zuidema, P.; Groenendijk, P.; Trouet, V.; Babst, F. Sensitivity of tropical tree growth to climatic variation: A global meta-analysis of tree-ring data. In EGU General Assembly Conference Abstracts; EGU: Munich, Germany, 2020; p. 7770. [Google Scholar]
- Van Der Sleen, P.; Groenendijk, P.; Vlam, M.; Anten, N.P.R.; Boom, A.; Bongers, F.J.J.M.; Pons, T.L.; Terburg, G.; Zuidema, P. No growth stimulation of tropical trees by 150 years of CO2 fertilization but water-use efficiency increased. Nat. Geosci. 2015, 8, 24–28. [Google Scholar] [CrossRef]
- Panthi, S.; Bräuning, A.; Zhou, Z.-K.; Fan, Z.-X. Tree rings reveal recent intensified spring drought in the central Himalaya, Nepal. Glob. Planet. Chang. 2017, 157, 26–34. [Google Scholar] [CrossRef]
- Panthi, S.; Fan, Z.; Van Der Sleen, P.; Zuidema, P.A. Long-term physiological and growth responses of Himalayan fir to environmental change are mediated by mean climate. Glob. Chang. Biol. 2019, 26, 1778–1794. [Google Scholar] [CrossRef] [PubMed]
- Carrer, M.; Urbinati, C. Long-term change in the sensitivity of tree-ring growth to climate forcing in Larix decidua. New Phytol. 2006, 170, 861–872. [Google Scholar] [CrossRef]
- Debel, A.; Meier, W.J.-H.; Bräuning, A. Climate signals for growth variations of F. sylvatica, P. abies, and P. sylvestris in Southeast Germany over the past 50 years. Forests 2021, 12, 1433. [Google Scholar] [CrossRef]
- Elias, S.A.; Bradley, R.S.; Oakes, J.; Ogilvie, A.E.J.; Van Woert, M.L. Paleoclimatology: Reconstructing climates of the quaternary. Arctic Antarct. Alp. Res. 1999, 31, 329. [Google Scholar] [CrossRef]
- Speer, J. The Fundamentals of Tree-Ring Research; The University of Arizona Press: Tucson, Arizona, 2012. [Google Scholar]
- Brienen, R.J.W.; Schöngart, J.; Zuidema, P.A. Tree rings in the tropics: Insights into the ecology and climate sensitivity of tropical trees. In Tree Physiology; Springer: Singapore, 2016; Volume 6, pp. 439–461. [Google Scholar]
- Pumijumnong, N. Tree-Ring and Applications in Thailand; Amarin Printing & Publishing Public Company Limited: Bangkok, Thailand, 2013. [Google Scholar]
- Hughes, A.C. Understanding the drivers of Southeast Asian biodiversity loss. Ecosphere 2017, 8, e01624. [Google Scholar] [CrossRef]
- Simonsen, D. The Importance of Tropical Forests: Why We Should Conserve Them and How They Affect the Rest of the World. Available online: https://www.oxfordstudent.com/2018/10/16/the-importance-of-tropical-forests-why-we-should-conserve-them-and-how-they-affect-the-rest-of-the-world/ (accessed on 21 September 2021).
- Tansley, A.G.; Douglass, A.E. Climatic cycles and tree-growth: A study of the annual rings of trees in relation to climate and solar activity. J. Ecol. 1920, 8, 62. [Google Scholar] [CrossRef]
- Rozendaal, D.M.A.; Zuidema, P. Dendroecology in the tropics: A review. Trees 2010, 25, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Sarutanon, S.; Boonchirdchoo, S.; Arrigo, R.; Watanasak, M.; Barbetti, M.; Buckley, B. Dendrochronological investigations in Thailand. IAWA J. 1995, 16, 393–409. [Google Scholar]
- Worbes, M. One hundred years of tree-ring research in the tropics–a brief history and an outlook to future challenges. Dendrochronologia 2002, 20, 217–231. [Google Scholar] [CrossRef] [Green Version]
- Fichtler, E. Dendroclimatology using tropical broad-leaved tree species–a review. Erdkunde 2017, 71, 5–22. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Baas, P. Wood anatomy of trees and shrubs from China. V. Anacardiaceae. IAWA J. 1993, 14, 87–102. [Google Scholar] [CrossRef]
- Min, T.-L.; Barford, A. Choerospondias axillaris. Flora of China. Missouri Botanical Garden, St. Louis, MO & Harvard University Herbaria, Cambridge, MA, USA. Available online: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200012681 (accessed on 25 November 2021).
- Shrestha, N. An analysis of production and sales of Choerospondias axillaris. Int. J. Hortic. Agric. Food Sci. 2020, 4, 10–13. [Google Scholar]
- McConkey, K.R.; Brockelman, W.Y.; Saralamba, C.; Nathalang, A.; Appendix, A. Percentage of Garcinia benthamii trees (n = 10) with fruit from 2003 to 2010 on the Mo Singto Forest Dynamics Plot, Khao Yai National Park, Thailand. In 2007 All Adult G. Benthamii Trees (n = 54) on the Plot Were Checked for Fruit and 45% Were Fruiting; Hence, Figures from 10 Trees May not Be Representative of Overall Fruit Availability; Figshare: London, UK, 2016. [Google Scholar]
- Harris, I.; Osborn, T.J.; Jones, P.; Lister, D. Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci. Data 2020, 7, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Chayamarit, K. Preliminary Checklist of the Family Anacardiaceae in Thailand. Thai For. Bull. Bot. 1994, 22, 1–25. [Google Scholar]
- Van Sam, H.; Nanthavong, K.; Kessler, P. Trees of Laos and Vietnam: A Field Guide to 100 Economically or Ecologically Important Species. Blumea-Biodivers. Evol. Biogeogr. Plants 2004, 49, 201–349. [Google Scholar] [CrossRef] [Green Version]
- Chanthorn, W.; Brockelman, W. Seed dispersal and seedling recuitment in the light-demanding tree Choerospondias axillaris in old-growth forest in Thailand. Sci. Asia 2008, 34, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Pumijumnong, N.; Buajan, S. Seasonal cambial activity of five tropical tree species in central Thailand. Trees 2013, 27, 409–417. [Google Scholar] [CrossRef]
- Elliott, S.; Navakitbumrung, P.; Kuarak, C.; Zangkum, S.; Anusarnsunthorn, V.; Blakesley, D. Selecting framework tree species for restoring seasonally dry tropical forests in northern Thailand based on field performance. For. Ecol. Manag. 2003, 184, 177–191. [Google Scholar] [CrossRef]
- Cook, E.R.; Kariukstis, L.A. Methods of Dendrochronology: Applications in the Environmental Sciences; Springer: Dordrecht, The Netherlands, 1990; p. 394. [Google Scholar]
- Holmes, R.L. Computer-Assisted quality control in Tree-Ring dating and measurement. Tree-Ring Bull. 1983, 44, 69–75. [Google Scholar]
- Cook, E.R.; Peters, K. Calculating unbiased tree-ring indices for the study of climatic and environmental change. Holocene 1997, 7, 361–370. [Google Scholar] [CrossRef]
- Bunn, A. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 26 May 2021).
- Fritts, H. Tree Rings and Climate; Serbiula (Sistema Librum 2.0); Elsevier: Amsterdam, The Netherlands, 1976. [Google Scholar]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Clim. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Briffa, K.R. Interpreting High-Resolution proxy climate data—The example of dendroclimatology. In Analysis of Climate Variability: Applications of Statistical Techniques; von Storch, H., Navarra, A., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 77–94. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, 25.0. Available online: https://www.ibm.com/analytics/spss-statistics-software (accessed on 21 November 2021).
- Zang, C.; Biondi, F. Treeclim: An R package for the numerical calibration of proxy-climate relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- Bunn, A. Statistical and visual crossdating in R using the dplR library. Dendrochronologia 2010, 28, 251–258. [Google Scholar] [CrossRef]
- Guada, G.; Camarero, J.; Sanchez-Salguero, R.; Navarro Cerrillo, R. Limited growth recovery after drought-induced forest dieback in very defoliated trees of two pine species. Front. Plant Sci. 2016, 7, 418. [Google Scholar] [CrossRef]
- Muggeo, V.M. Interval estimation for the breakpoint in segmented regression: A smoothed score-based approach. Aust. N. Z. J. Stat. 2017, 59, 311–322. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. arXiv 2015, arXiv:1406.5823. [Google Scholar]
- Zaw, Z.; Fan, Z.-X.; Bräuning, A.; Liu, W.; Gaire, N.P.; Than, K.Z.; Panthi, S. Monsoon precipitation variations in Myanmar since AD 1770: Linkage to tropical ocean-atmospheric circulations. Clim. Dyn. 2021, 56, 3337–3352. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, R.; Islam, M. Long-term growth decline in Toona ciliata in a moist tropical forest in Bangladesh: Impact of global warming. Acta Oecol. 2017, 80, 8–17. [Google Scholar] [CrossRef]
- Pumijumnong, N. Teak tree ring widths: Ecology and climatology research in Northwest Thailand. Sci. Tech. Dev. 2012, 31, 165–174. [Google Scholar]
- Vlam, M.V.; Baker, P.; Bunyavejchewin, S.; Zuidema, P. Temperature and rainfall strongly drive temporal growth variation in Asian tropical forest trees. Oecologia 2014, 174, 1449–1461. [Google Scholar] [CrossRef]
- Mund, M.; Herbst, M.; Knohl, A.; Matthäus, B.; Schumacher, J.; Schall, P.; Siebicke, L.; Tamrakar, R.; Ammer, C. It is not just a ‘trade-off’ indications for sink and source limitation to vegetative and regenerative growth in an old growth beech forest. New Phytol. 2020, 226, 111–125. [Google Scholar] [CrossRef]
- Knops, J.M.H.; Koenig, W.; Carmen, W.J. Negative correlation does not imply a tradeoff between growth and reproduction in California oaks. Proc. Natl. Acad. Sci. USA 2007, 104, 16982–16985. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Fan, Z. Stem radial growth in response to microclimate in an Asian tropical dry karst forest. Acta Ecol. Sin. 2016, 36, 401–409. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, M.; Wernicke, J.; Bräuning, A. Changes in sensitivity of Tree-Ring widths to climate in a tropical moist forest tree in Bangladesh. Forests 2018, 9, 761. [Google Scholar] [CrossRef] [Green Version]
- Snyder, R.L.; Moratiel, R.; Zhenwei, S.; Swelam, A.; Jomaa, I.; Shapland, T. Evapotranspiraiton response to climate change. Acta Hortic 2011, 922, 91–98. [Google Scholar] [CrossRef]
- Rakthai, S.; Fu, P.-L.; Fan, Z.-X.; Gaire, N.; Pumijumnong, N.; Eiadthong, W.; Tangmitcharoen, S. Increased drought sensitivity results in a declining tree growth of Pinus latteri in Northeastern Thailand. Forest 2020, 11, 361. [Google Scholar] [CrossRef] [Green Version]
- Sensuła, B.; Wilczyński, S.; Opała, M. Tree growth and climate relationship: Dynamics of scots pine (Pinus Sylvestris L.) growing in the near-source region of the combined heat and power plant during the development of the pro-ecological strategy in Poland. Water Air Soil Pollut. 2015, 226, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, G.P.; Chen, Y.T.; Yu, Y.H.; Liu, H.T.; Cai, H.M.; Chen, Y.Z. Study on early growth characteristics of Choerospondias axillaris plantation and effect of Choerospondias axillaris and Cunninghamia lanceolata mixed stand. For. Res. 2004, 17, 206–212. [Google Scholar]
- Groenendijk, P. Long-Term Trends in Tropical Tree Growth: A Pantropical Study. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2015. [Google Scholar]
- Somogyi, Z. Recent trends of tree growth in relation to climate change in Hungary. Acta Silv. Lignaria Hung. 2008, 4, 17–27. [Google Scholar]
- Battipaglia, G.; Zalloni, E.; Castaldi, S.; Marzaioli, F.; Gatti, R.C.; Lasserre, B.; Tognetti, R.; Marchetti, M.; Valentini, R. Long Tree-Ring chronologies provide evidence of recent tree growth decrease in a central african tropical forest. PLoS ONE 2015, 10, e0120962. [Google Scholar] [CrossRef]
- Brienen, R.J.W.; Gloor, E.; Zuidema, P.A. Detecting evidence for CO2 fertilization from tree ring studies: The potential role of sampling biases. Glob. Biogeochem. Cycles 2012, 26, 26. [Google Scholar] [CrossRef]

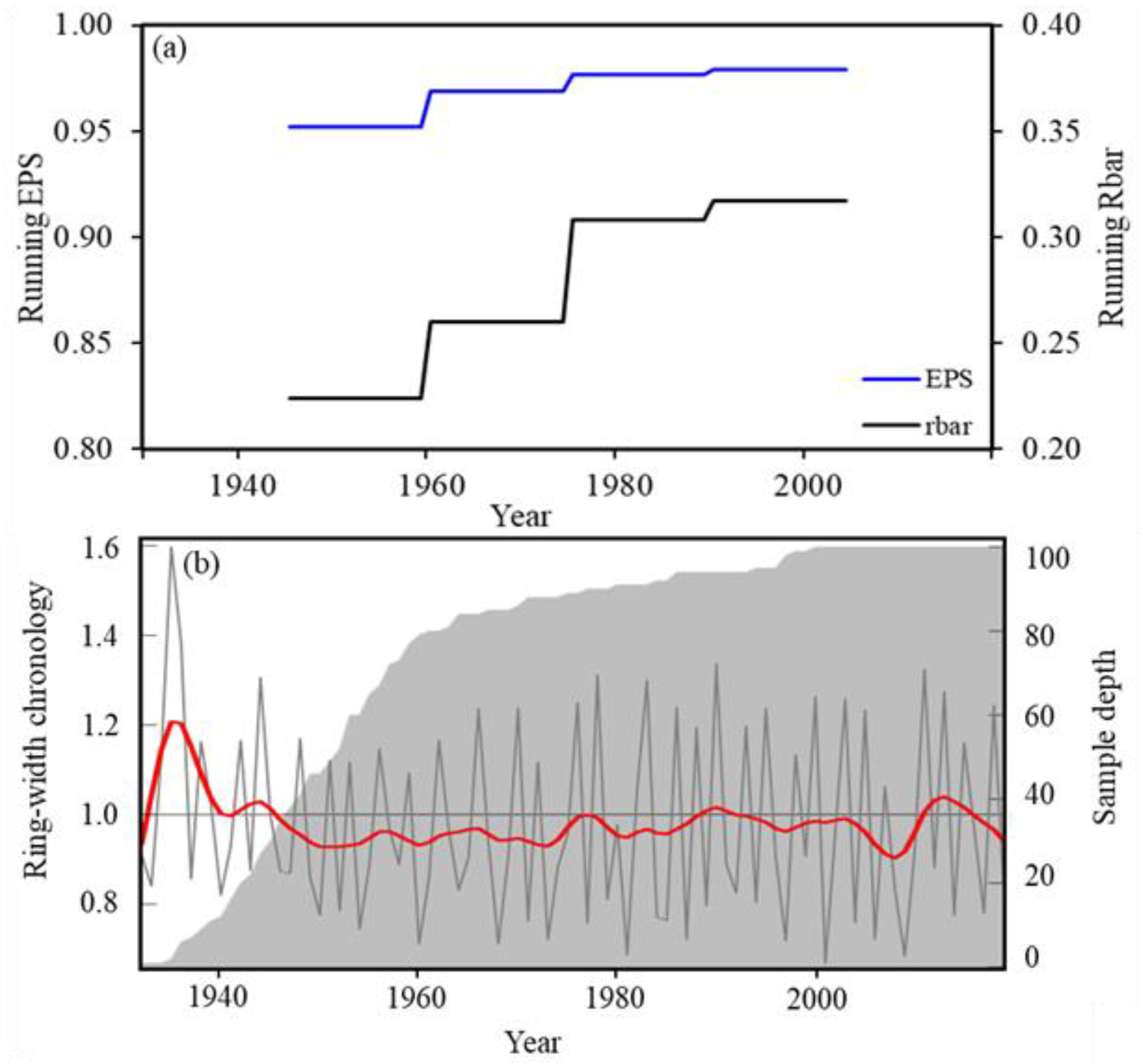
| Latitude (°N) | Longitude (°E) | Elevation (m asl) | Cores/ Trees | Time Span | AGR (mm year−1) | MSL (yr) | MS | Rbar | EPS |
|---|---|---|---|---|---|---|---|---|---|
| 14.26 | 101.22 | 725−815 | 100/56 | 1932−2019 | 3.79 | 65 | 0.412 | 0.297 | 0.968 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surayothee, W.; Buajan, S.; Fu, P.; Pumijumnong, N.; Fan, Z.; Panthi, S.; Finnegan, P.M.; Zhang, Y.; Chen, Y.; Tor-ngern, P.; et al. Growth-Climate Relationships and Long-Term Growth Trends of the Tropical Forest Tree Choerospondias axillaris (Anacardiaceae) in East-Central Thailand. Forests 2021, 12, 1655. https://doi.org/10.3390/f12121655
Surayothee W, Buajan S, Fu P, Pumijumnong N, Fan Z, Panthi S, Finnegan PM, Zhang Y, Chen Y, Tor-ngern P, et al. Growth-Climate Relationships and Long-Term Growth Trends of the Tropical Forest Tree Choerospondias axillaris (Anacardiaceae) in East-Central Thailand. Forests. 2021; 12(12):1655. https://doi.org/10.3390/f12121655
Chicago/Turabian StyleSurayothee, Wisawakorn, Supaporn Buajan, Peili Fu, Nathsuda Pumijumnong, Zexin Fan, Shankar Panthi, Patrick M. Finnegan, Yongjiang Zhang, Yajun Chen, Pantana Tor-ngern, and et al. 2021. "Growth-Climate Relationships and Long-Term Growth Trends of the Tropical Forest Tree Choerospondias axillaris (Anacardiaceae) in East-Central Thailand" Forests 12, no. 12: 1655. https://doi.org/10.3390/f12121655
APA StyleSurayothee, W., Buajan, S., Fu, P., Pumijumnong, N., Fan, Z., Panthi, S., Finnegan, P. M., Zhang, Y., Chen, Y., Tor-ngern, P., Chanthorn, W., Nathalang, A., & Brockelman, W. Y. (2021). Growth-Climate Relationships and Long-Term Growth Trends of the Tropical Forest Tree Choerospondias axillaris (Anacardiaceae) in East-Central Thailand. Forests, 12(12), 1655. https://doi.org/10.3390/f12121655







