Abstract
Maritime forests are threatened by sea-level rise, storm surge and encroachment of salt-tolerant species. On barrier islands, these forested communities must withstand the full force of tropical storms, hurricanes and nor’easters while the impact is reduced for mainland forests protected by barrier islands. Geographic position may account for differences in maritime forest resilience to disturbance. In this study, we quantify two geographically distinct maritime forests protected by dunes on Virginia’s Eastern Shore (i.e., mainland and barrier island) at two time points (15 and 21 years apart, respectively) to determine whether the trajectory is successional or presenting evidence of disassembly with sea-level rise and storm exposure. We hypothesize that due to position on the landscape, forest disassembly will be higher on the barrier island than mainland as evidenced by reduction in tree basal area and decreased species richness. Rate of relative sea-level rise in the region was 5.9 ± 0.7 mm yr−1 based on monthly mean sea-level data from 1975 to 2017. Savage Neck Dunes Natural Area Preserve maritime forest was surveyed using the point quarter method in 2003 and 2018. Parramore Island maritime forest was surveyed in 1997 using 32 m diameter circular plots. As the island has been eroding over the past two decades, 2016 Landsat imagery was used to identify remaining forested plots prior to resurveying. In 2018, only plots that remained forested were resurveyed. Lidar was used to quantify elevation of each point/plot surveyed in 2018. Plot elevation at Savage Neck was 1.93 ± 0.02 m above sea level, whereas at Parramore Island, elevation was lower at 1.04 ± 0.08 m. Mainland dominant species, Acer rubrum, Pinus taeda, and Liquidambar styraciflua, remained dominant over the study period, with a 14% reduction in the total number of individuals recorded. Basal area increased by 11%. Conversely, on Parramore Island, 33% of the former forested plots converted to grassland and 33% were lost to erosion and occur as ghost forest on the shore or were lost to the ocean. Of the remaining forested plots surveyed in 2018, dominance switched from Persea palustris and Juniperus virginiana to the shrub Morella cerifera. Only 46% of trees/shrubs remained and basal area was reduced by 84%. Shrub basal area accounted for 66% of the total recorded in 2018. There are alternative paths to maritime forest trajectory which differ for barrier island and mainland. Geographic position relative to disturbance and elevation likely explain the changes in forest community composition over the timeframes studied. Protected mainland forest at Savage Neck occurs at higher mean elevation and indicates natural succession to larger and fewer individuals, with little change in mixed hardwood-pine dominance. The fronting barrier island maritime forest on Parramore Island has undergone rapid change in 21 years, with complete loss of forested communities to ocean or conversion to mesic grassland. Of the forests remaining, dominant evergreen trees are now being replaced with the expanding evergreen shrub, Morella cerifera. Loss of biomass and basal area has been documented in other low elevation coastal forests. Our results indicate that an intermediate shrub state may precede complete loss of woody communities in some coastal communities, providing an alternative mechanism of resilience.
1. Introduction
Forests face multiple abiotic and biotic disturbance events due to climate change. Many forests face new disturbance regimes (e.g., fire, drought, flooding, frost frequency). They also may face invasion by non-native species or encroachment of native species that are more adapted to frequent disturbance. Low-lying coastal forests are most vulnerable to the gradual rise in sea level and storm-related disturbance. If forested communities are unable to adapt quickly enough, the imposed disturbances could lead to a state change. Disturbance is considered a driver of change in community structure in many ecosystems, such as maritime forests [1,2,3,4]. Occurring within a few miles of the coastline and found worldwide, maritime forests often establish behind large dunes, which limit disturbance through the interception of salt spray and preventive flooding of freshwater communities [5]. However, after coastal regions sustain multiple abiotic and biotic disruptions, including coastal storms, saltwater intrusion, flooding from sea-level rise (SLR), and encroachment from more salt-tolerant species [6,7,8,9], maritime forests become more vulnerable to these multiple factors.
The capacity of maritime forests to experience resilience, the recovery of communities or ecosystems to their structure and function after a disturbance [10], may be due to the intensity, frequency and duration of events [11,12]. Community recovery may transition to a community that is functionally similar, but differ in species abundance from the previous community. Communities or ecosystems that exhibit resistance to disturbance are unchanged (e.g., in mortality and growth) [13]. Although there may be shifting in species abundance within a community or ecosystem, the system will be relatively unchanged following a disturbance.
For communities that lack resilience to disturbance, a tipping point may be reached which reduces community capacity to recover, potentially leading to forest disassembly and ecosystem collapse [14,15]. Relative sea-level rise (RSLR) rates and storm frequency/intensity are immediate threats to maritime forests but differ in rates and impact along coastlines [16,17,18]. Disturbances from coastal storms have increased over the last half-century [19]. When long-lived trees succumb to disturbances, the magnitude of disturbance and potential recovery may differ based on many factors including RSLR rates, landscape position sensu of Young et al. [5] and storm frequency/intensity [17,18]. The future trajectory of maritime forests may differ in species diversity and composition [20] as successive storms cause plant communities to reassemble in a new way.
Many forest species exhibit some level of salt tolerance, but due to their protected position on the landscape, may have salt-sensitive species that experience large-scale dieback with saltwater intrusion [6,9,21]. Differences in how woody species respond to salt intrusion can affect distribution patterns within coastal communities [22]. In addition to salinity stress, coastal storms have strong winds that can cause extensive damage to maritime forests [23,24]. As recovery may be delayed due to multiple environmental stressors, it can take many years to fully assess the impact of storms in forested communities [25,26]. Storms impact long-term coastal community composition through multiple mechanisms: decreasing shade-intolerant species [27], decreasing wind-intolerant species [28] or through replacing salt-sensitive species with more salt-tolerant shrubs and herbs [29]. Large infrequent disturbance events can have catastrophic effects on community composition [30]. Equally crucial to plant spatial distributions is the gradual threat of salt intrusion through relative sea-level rise (RSLR).
Plant successional patterns based on vulnerability to salt tolerance may be disrupted as species adapt or disappear to flooding [7]. It is hard to model how coastal plant communities will respond to RSLR due to many coastal processes that differ among coastal communities (e.g., elevation, species salt tolerance, land subsidence). There also may be a sharp distinction between barrier island and mainland coastal forests due to geographic position relative to disturbance. Barrier islands are the first line of defense for mainland communities during storms, buffer coasts and inland areas from the full force of wind, storm surge and wave energy [31,32], which can shift sand and change shorelines through erosion. With increased storms and RSLR, islands may be more vulnerable than the mainland as erosion occurs on all sides of the island, leading to reduced upland area [33].
Vegetative composition and topography affect coastline response to disturbance. Maritime forests enhance physical resistance to storms, providing short-term stability [34], but may increase long-term erosion in areas with high RSLR by limiting movement of sediment [33]. Maritime forests disassemble when they succumb to saltwater inputs from RSLR and storms, and can be replaced by low-lying marsh species that have the potential to migrate to adjacent uplands [35]. Available recruitment space allows marsh species to expand because recruitment of maritime forest species can be impaired by saline conditions (>2–5 ppt), hampering maritime forest reassembly [36]. Forest disassembly (e.g., loss of biodiversity; decrease in species abundance) and shifts in community composition due to RSLR have the potential to alter island response to disturbance and the potential to change coastlines.
Virginia barrier islands have experienced dramatic changes in sediment accretion and erosion over the last few decades; however, erosion has exceeded accretion on these islands. Overall island area has decreased for the Virginia barrier island system [37] with shrub expansion dominating the landscape and changing the recruitment environment [36]. This change in recruitment may alter resilience of forested ecosystems by interfering with establishment of shade-intolerant tree species. Decreased recruitment of tree species on barrier islands could limit their capacity to provide protection for mainland communities. Additionally, land subsidence caused by isostatic adjustments post-glaciation, groundwater withdrawals, and increased global sea-level rise has resulted in the southern Chesapeake Bay region having the highest rate of sea-level rise and sediment erosion on the Atlantic Coast [38].
Forest decline is well documented along the mid-Atlantic coast, typically converting to marsh with RSLR [8], but similar effects in maritime forest protected by tall dunes are less studied. It is essential to determine the role of geographic location on the resilience of maritime forests due to climate drivers and to project future community composition during a time of increased RSLR and storm frequency and intensity [39]. The relative extent to which forests on the mainland and barrier islands can be resilient to change after long-term disturbance is unclear. The immediate effect of storm damage on forests is well understood [25]; what is less well recognized is the long-term structural and compositional changes in forests due to combined effects of storms and sea-level rise [40]. Mortality can be delayed, so long-term monitoring of these ecosystems can provide insight into species compositional changes and future community trajectory after a storm event. Here, we fill a knowledge gap by quantifying maritime forest compositional change over a decade using data on the Virginia, USA, mainland (Savage Neck Natural Area Preserve) and a nearby barrier island (Parramore Island). We hypothesize that due to position on the landscape, forest disassembly will be higher on barrier islands than mainland as seen in reduction in tree basal area and species richness.
2. Materials and Methods
2.1. Study Sites
Savage Neck Dunes Natural Area Preserve (37.3279° N, 75.0042° W) is located on the southwest portion of the Eastern Shore of Virginia (Figure 1). The site owned and managed by the Virginia Department of Conservation and Recreation’s Natural Heritage Division serves as an important stopover site for migratory birds. The preserve contains 86 ha of maritime forest, 16 of which was first surveyed in summer 2003 [41]. The maritime forest is located behind a large dune (Supplementary Figure S1). Savage Neck Dunes was hit by Hurricane Isabel (2003) after the initial survey, notably the most destructive hurricane to impact Virginia in recent history [42]. Prior to Hurricane Isabel (2003), the Eastern Shore of Virginia had been hit by multiple infrequent storms. The dominant canopy species in 2003 prior to the storm were Liquidambar styraciflua, Acer rubrum L., and Pinus taeda.
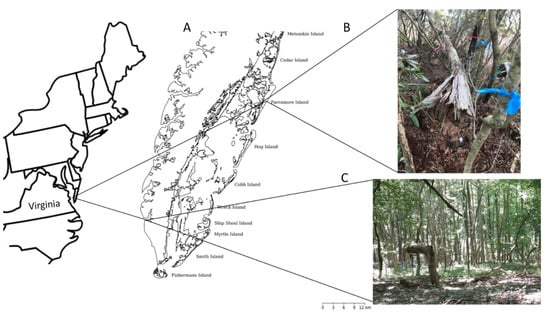
Figure 1.
(A) Delmarva Peninsula of Virginia showing locations of Savage Neck Dunes and Parramore Island; (B) Maritime forest on Parramore Island; (C) Maritime forest on Savage Neck Dunes.
Parramore Island (37.5387° N, 75.6280° W) is one of fourteen islands in the Virginia Coast Reserve Long-Term Ecological Research site, located midway along the Eastern Shore of the Delmarva Peninsula (Figure 1). It is among the largest uninhabited barrier islands in the USA and is managed by The Nature Conservancy. Rapid erosion of sediment on Parramore Island since 1980 may have contributed to vegetation changes such as species loss [43,44]. Uprooted Pinus taeda and Prunus serotina accumulate on the oceanside beach of Parramore Island following erosion [43], creating ghost forests (i.e., snags that were created due to salt intrusion), although intact maritime forest occurs behind well-established interior dunes (Supplementary Figure S1). The dominant canopy species in 1996 prior to Hurricane Isabel in 2003 were Persea palustris, Juniperus virginiana, and Morella cerifera.
These two locations also differ in how they obtain freshwater. Barrier islands such as Parramore Island are unique in that they typically have a freshwater lens, which contains fresh groundwater from rainfall. The freshwater lens sits on top of seawater. As the sea level rises, the freshwater lens is brought closer to the surface. Shrubs also can influence freshwater groundwater through bringing the lens closer to the surface relative to grassland plant communities (Wood and Zinnert, unpublished). Mainland coastlines have additional sources of freshwater (e.g., rivers) but freshwater sources, similar to barrier islands, are becoming more saline as sea level rises [45].
2.2. Sea-Level Rise, Storms, and Elevation
Mean sea level was accessed from NOAA Tides and Currents (https://tidesandcurrents.noaa.gov (accessed on 8 October 2019)) for Chesapeake Bay Bridge Tunnel, VA, over the study period (1997–2018) [46]. Relative sea-level rise at this location is 5.9 ± 0.7 mm yr−1 based on monthly mean sea-level data from 1975 to 2018 (NOAA 2019). Hurricane and tropical storms data were accessed from NOAA Historical Hurricane Tracks (https://oceanservice.noaa.gov/news/historical-hurricanes (accessed on 8 October 2019)) within a 100 mile radius of Parramore Island, VA, between 1997–2018). Elevation of plots was collected using 2015 Eastern Shore, VA Lidar Digital Elevation Model of study areas (https://coast.noaa.gov (accessed on 12 December 2019)). Horizontal accuracy was ±65 cm and vertical accuracy was ±12.5 cm.
2.3. Savage Neck Field Measurements
Following methods from the first observational study [41], we used the point-quarter distance method to quantify the forest overstory in 2018 [47] and compared to the initial summer 2003 data (months prior to Hurricane Isabel). Transects were 30 m apart and positioned in an east-west orientation from the forest edge to the base of the inland dune. Forest on the dune was not included in this survey. We sampled points (n = 266) every 10 m along the transects and set up a quadrat in the four cardinal directions. In each quadrat, we measured the distance from the center to the nearest woody species that was >3 cm diameter at 1.3 m DBH (diameter at breast height) and recorded the species. No shrubs >3 cm cumulative basal stem diameter were recorded. We calculated mean basal area, density and frequency using calculations from [47].
2.4. Parramore Island
2.4.1. Landsat Imagery
In order to assess long-term fate of woody vegetation due to known erosion on Parramore Island [48], we used Landsat TM5 satellite imagery accessed through USGS Global Visualization Viewer. Images were those used in a recent study [33] and were chosen based upon similar growing seasons, cloud-free conditions, and tide levels. Dates chosen were 12 September 1998 and 12 September 2016. Using ENVI 4.7, the Landsat multi-spectral files were calibrated and radiometrically corrected. QUick Atmospheric Correction (ENVI QUAC), based upon scene statistics, was applied to correct pixel spectra at water bands. ENVI QUAC was used to remove atmospheric effects via atmospheric compensation [49,50]. Each Landsat image was classified via vegetation cover (including woody, grassland, sand/bare, water, and marsh) and exported to ArcGIS 10.4.1 for vegetative cover area analysis [33]. Island-wide analysis was made to compare the area change woody cover over time. Identification of individual plot change was accomplished by overlaying all permanent plots onto classified images.
2.4.2. Field Measurements
Plots for this study were selected from the 32 m (diameter) circular, permanent plots established in 1996 on Parramore Island, VA, and surveyed in 1997. DBH for trees and basal stem diameter (BSD) for shrubs were collected for all woody species that were >3 cm. Many of these permanent plots have been undisturbed by direct human contact since the recording of the geographic coordinates of the plots in 2002. Only plots with woody vegetation were selected for the 2018 survey. Due to forest decline and accessibility, only 9 plots were re-surveyed in 2018 from the original set of plots from 1997.
2.5. Statistical Analysis
Importance values were calculated by adding the relative density, relative basal area, and relative frequency of sampled species. Importance values were used to identify the ecological importance and dominance of each species throughout both years of the survey [51]. The Hutcheson t-test was used to compare Shannon Weiner diversity indices [52]. Nonmetric Multidimensional Scaling (NMDS, PC-ORD software version 7.06) based on basal area of species and incorporating Sorenson’s distance measure was used to determine the composition of each forest over the two timeframes [53]. The resulting NMS over the two timeframes were compared using multiple response permutation procedures (MRPP). Indicator species analyses identified species characteristic of each forest over the two timeframes (PC-ORD software version 7.06). Indicator species analysis was performed to determine the extent to which species composition had changed in each community. Kolmogorov–Smirnov analyses were performed to determine significant differences between species distributions at each site during the two timeframes (JMP Pro 13, SAS Institute).
3. Results
3.1. Sea-Level Rise, Storm Events, and Elevation
Based on the rate of 5.92 mm yr−1 (NOAA, 2019) total sea-level rise was ~124.32 mm from 1997 to 2018. Since 2003, there were 21 tropical storms and hurricanes that came within a 100 mi radius that had the potential to impact Savage Neck Dunes and Parramore Island, VA (Figure 2). Plot elevation at Savage Neck was 1.93 ± 0.02 m and 1.04 ± 0.08 m on Parramore Island.
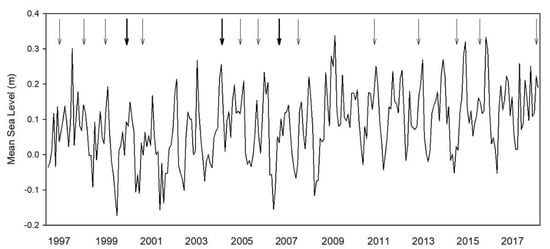
Figure 2.
Mean sea level over the time period from 1997 to 2018 from Chesapeake Bay Bridge Tunnel, VA. Hurricanes and tropical storms that came within a 100 mi radius of Parramore Island are indicated by arrows. Bold arrows indicate more than one storm within that year.
3.2. Species Change
All Savage Neck Dunes plots surveyed in 2003 remained forested in 2018. Shannon–Weiner diversity indices were not significantly different for the two time periods (2003 = 1.9, 2018 = 1.7; t = 0.00005, p = 0.99). The top three canopy species in 2003 based on importance values were Liquidambar styraciflua L. (78%), Acer rubrum L. (70%), and Pinus taeda (70%) (Supplementary Table S1, Figure 3). In 2018, the top three dominants remained the same, although the ranking among the three species changed, A. rubrum (87%), P. taeda (84%), and L. styraciflua (60%). All three species increased in relative abundance and relative frequency from 2003 to 2018 (Supplementary Table S1). There were no shrubs present in the canopy. This community appeared resilient to disturbance during the 15-year timespan based on growth and species richness. The total number of individuals recorded in 2018 was 86% of those recorded in 2003. Three species identified in 2018 were not found in 2003, Robinia pseudoacacia, Celtis occidentalis, and Quercus stellata. Three species were lost in the interim, Carya tomentosa, Cercis canadensis, and Cornus florida, all low in 2003 abundance.
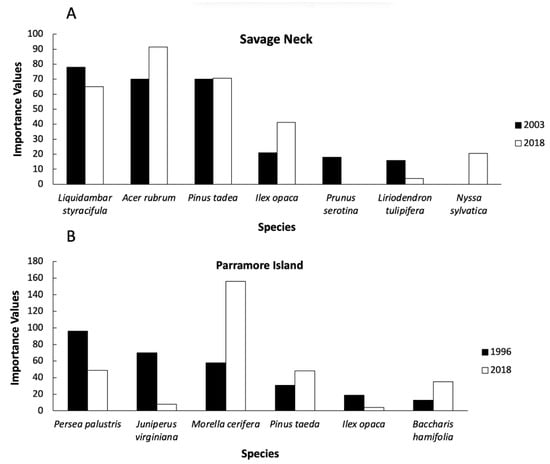
Figure 3.
Importance values for Savage Neck (A) and Parramore Island (B). At Savage Neck, species have similar distribution from 2003 and 2018. On Parramore Island, there is a change in cover from tree to shrub dominance from 1997 to 2018. Only the top six species are shown in this graph for each site for each time period.
During the study timeframe, Parramore Island upland was reduced in size by 9% (from 1199 ha to 1092 ha [33]). Of permanent forested plots surveyed in 1997, 33% converted to grassland and 33% were lost to erosion and now occur as ghost forest on the shore or lost to the ocean (Figure 4). Substantial change in community composition was observed in the remaining forested plots but Shannon–Wiener diversity did not change (1996 = 0.99, 2018 = 0.97; t = 0.44, p = 0.66). The forest once dominated by evergreen trees (i.e., Persea palustris and J. virginiana; Supplementary Table S2) was converted to dominance primarily by Morella cerifera, although Persea palustris and Pinus taeda remained in the top three species based on importance value (Supplementary Table S2; Figure 3). Juniperus virginiana was lost as a dominant species with only 2 individuals recorded in 2018. Total number of individuals recorded in 2018 was 46% of those in 1997. Species richness was reduced by 33%. Three infrequent 1997 species were lost (Prunus serotina, Celtis laevigata, and Zanthoxylum clava-herculis).
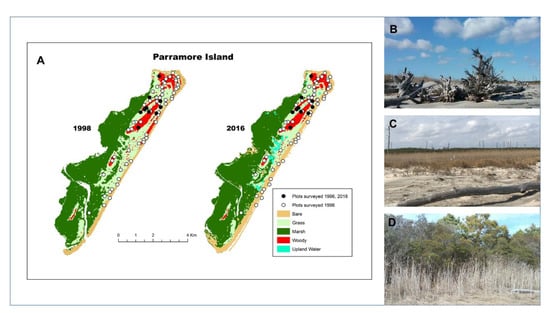
Figure 4.
(A) Over the 21-year period, >60% of the forested plots on Parramore Island, VA exhibited transition to other states: 31% woody vegetation converted to grassland and 33% were lost to non-vegetative beach or ocean as a result of erosion. Only 33% of the surveyed plots remained as woody vegetation; (B) Ghost forest on the oceanside beach (denoted as Bare); (C) Dead tree trunks in a grassland area (denoted as Grass); (D) Maritime forest with encroaching M. cerifera shrub (denoted as Woody).
3.3. Basal Area and DBH Distributions
At Savage Neck, there was a modest increase in total basal area (9%) as the total number of individuals declined. Kolmogorov–Smirnov analysis of the top four species indicated that the distributions of Acer rubrum (p = 0.0091), Pinus taeda (p < 0.0001), Liquidambar styraciflua (p = 0.0014), and L. tulipifera (p = 0.0016) were different from one another during the two timeframes, indicating succession into larger stage classes for the dominant species (Figure 5). Acer rubrum increased in basal area by 32% and P. taeda increased by 31% (Supplementary Table S1). Although L. styraciflua individuals were reduced in number by 52%, the trees that remained increased in basal area (Supplementary Table S1, Figure 5). Understory sub-shrub Ilex opaca experienced the greatest gain in basal area (65%), primarily due to a greater number of individuals (Supplementary Table S1).
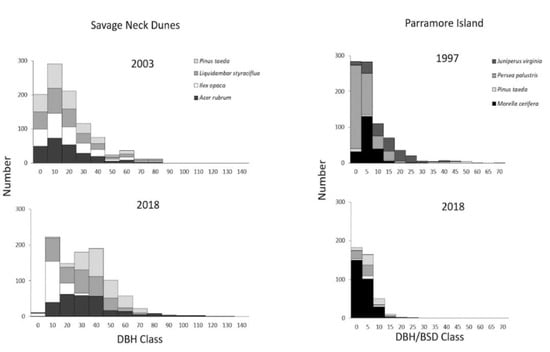
Figure 5.
Frequency distributions of diameter at breast height (DBH) or basal area diameter (BSD) size classes for selected species in the maritime forest at Savage Neck between 2003 and 2018, and Parramore Island between 1997 and 2018.
Conversely, on Parramore Island, basal area was drastically reduced to 16% of 1997 values, with the majority of 2018 basal area comprised of the shrub, Morella cerifera (Supplementary Table S2). Kolmogorov–Smirnov analysis indicated that three of the top four species (Pinus taeda, p < 0.0001; Persea palustris, p = 0.017; M. cerifera, p < 0.0001) differed in distribution from 1997 to 2018. Pinus taeda increased to larger-size classes while Persea palustris and M. cerifera shifted to higher frequency in lower-size classes (Figure 5). No trees were identified >35 cm DBH on Parramore Island in 2018 and there was no notable evidence of tree recruitment in the understory (personal observation).
3.4. Long-Term Shifts in Community Composition
NMS analysis of species basal area at Savage Neck resulted in a final solution with three axes (final stress = 12.75). Convex hulls of points from each year show overlap (Figure 6), although there were significant differences between 2003 and 2018 based on MRPP (T = −2.659, A = 0.003, p = 0.024). Liquidambar styraciflua and Pinus taeda were the dominant species associated with axis 1 (r = −0.54, r = 0.748, respectively, Supplementary Table S1). Acer rubrum was most strongly correlated with axis 2 (r = 0.54, Supplementary Table S1). Conversely, at Parramore Island there was modest overlap between the two years (Figure 6) with tight clustering of most plots in 2018 (final stress = 3.16). There were significant differences between 1997 and 2018 based on MRPP (T = −4.668, A = 0.169, p = 0.004). Pinus taeda was the dominant species associated with axis 1 (r = −0.995, Supplementary Table S2). Juniperus virginiana was most strongly correlated with axis 2 (r = 0.918, Supplementary Table S2).
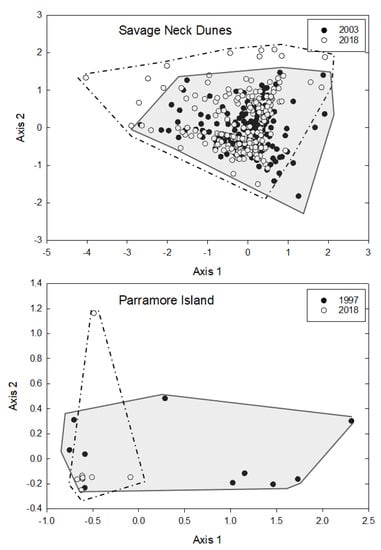
Figure 6.
NMS of basal area at Savage Neck in 2003 and 2018, and Parramore Island in 1997 and 2018. Degree of overlap between the two years is highlighted with convex hulls (light gray = 2003 or 1997; dark gray = 2018).
4. Discussion
The extent to which forested communities of similar species are resilient or disassembled may depend on the intensity and frequency of the disturbance. Coastal communities are threatened by multiple disturbances, largely episodic storms and chronic RSLR. Storms can temporarily alter sea level and often result in saltwater intrusion that can result in forest species loss [29,54]. Our study documents resistance and resilience to disturbance (i.e., storms and RSLR) along the protected mainland maritime forest community of Savage Neck Dunes. Resistance is seen in the three dominant species, Liquidambar styraciflua, Pinus taeda, and Acer rubrum remaining the same and increased basal area during a time with multiple storm events. Geographic position increases likelihood of protection from the full force of storms through position on the bayside and large intact dunes. Resilience is seen in that the community was able to recover after Hurricane Isabel and several tropical storms, thereafter. It typically takes a decade or more for communities to recover after a storm [25] and this community appears to be undergoing a typical successional pattern. Conversely, Parramore Island is directly exposed to disturbance effects, and we document both alternative resilience and lack of resilience in maritime forests based on landscape position, similar to forest islands along the Florida Gulf Coast [29].
The alternative form of resilience on Parramore Island is shown by the increase of a woody presence. Woody species functionally have the same response to island erosion, as they are disturbance-resisting species in sensu [11] and will impact island migration in a similar way, creating a temporary form of resilience. Considering the loss of most of its long-lived species, this study demonstrates that forest disassembly (e.g., loss of biodiversity; decrease in species abundance) is taking place on the island. Recruitment of forested species may also be impeded by high saline conditions [36].
Based on our data and ongoing research in forest to marsh transitions, we synthesize the possible trajectories for maritime forest due to changes in RSLR and storm disturbance (Figure 7). If salinity and flooding is not sustained in the long term following storm (or other) disturbance, forests may recover and regenerate. Forests near the shoreline are typically lost through erosion processes as documented on Parramore Island (33% maritime forest lost to ocean). In forests experiencing high rates of RSLR or sustained saline flooding through exposure, conversion to salt marsh dominates (i.e., [9]). In our study, we document an alternative form of resilience through conversion of forest to salt-sensitive shrub dominance (primarily Morella cerifera). We suggest an alternative form of resilience through maintaining a woody-dominated state, rather than complete loss of woody biomass that is typically seen in forest to marsh transition and erosion.
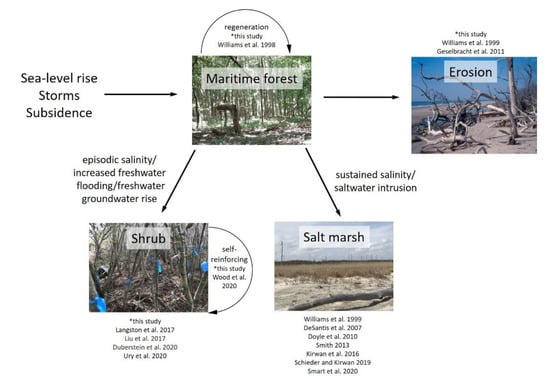
Figure 7.
External drivers that alter salinity, sediment, and flooding dynamics in coastal systems lead to alternative pathways for maritime forest. Depending on elevation and geographic position, maritime forest can regenerate if salinity and flooding pressures are not sustained (i.e., after a storm disturbance): it can be converted to salt marsh due to sustained salinity pressure, it can convert to a shrub-dominated stage through increased freshwater flooding/intermittent saline flooding, or it can be directly lost due to coastline erosion. A sustained woody state provides an alternative form of maritime forest resilience.
Unlike coastal forests on the mainland, barrier island forests are often discontinuous due to topography, development, heterogenous distribution of resources, and island size. Coastal topography is particularly sensitive to changes in erosion and accretion from changes in RSLR and coastal storms [12,55], potentially leading to higher rates of forest disassembly. Collectively, biotic and abiotic factors make barrier island maritime forests more susceptible to environmental perturbations affecting coastline dynamics. Although Virginia does not have frequent hurricanes that make landfall, the effect of hurricanes, nor’easters or tropical storms can be expansive and affect forested communities for hundreds of miles outside of the landfall area. Plant communities on barrier islands endure the full force of wind, increased wave height and energy, and salt inundation relative to mainland coastal communities due to their geographic position [56]. Species have differential responses to infrequent disturbances and not all communities may recover to their pre-disturbance state [28]. On Parramore Island, Persea palustris and Juniperus virginiana were lost as dominant species, but Pinus taeda appeared resilient to disturbance as seen in increased number, relative basal area, and importance value over the time period. Juniperus virginiana stands are susceptible to storms and drought, and they have been shown to use less fresh ground when sea level has risen [40], potentially leading to their decline. Pinus taeda germinates at higher salinities compared to many coastal tree species [36], providing an advantage for these conifers on barrier islands. Dispersal of woody species that are tolerant of salinity and flooding is necessary for regeneration of maritime forest.
As maritime forest on Parramore Island is declining, shrubs are expanding, which is a phenomenon seen worldwide [57,58]. It is unclear if a threshold has been reached that would implicate a tipping point to continual disassembly of the maritime forest community. Conversion to shrub dominance is occurring elsewhere along the mid-Atlantic and Gulf coasts and is well documented on the Virginia barrier islands [37,48]. Hog Island, VA, had extensive maritime forest ~100 years ago on the southern end which later experienced substantial erosion and loss of forest. Since 1984, there has been a 400% increase in the shrub, Morella cerifera, [48] and no remaining maritime forest or evidence of new maritime forest forming [59]. We quantified a similar trajectory on Parramore Island as M. cerifera increased over the twenty-one-year timeframe and all maritime forest tree species decreased in the study plots. With the expansion of M. cerifera, typical successional patterns are interrupted. Conversion to shrubs may be accelerated as documented elsewhere along the Atlantic coast following salt intrusion due to coastal storms and sea-level rise [60,61]. Additional climate change factors, such as warming winter temperatures, favor M. cerifera expansion along with microclimate modification, creating a positive feedback to maintain a shrub state [62,63]. As M. cerifera takes up more recruitment space and limits forest seedling recruitment below the canopy, tree species may no longer find habitable locations for germination. This may be a mechanism for disassembly of maritime forest on barrier islands in areas where woody species can survive.
RSLR may be another factor preventing recovery of forested plots on Parramore Island. High elevation fronting dunes and woody species create resistance; however, over time, these have contributed to higher rates of erosion on the shoreface compared to low-lying elevation areas that allow for landward island migration [33]. As sea level rises, shoreline erosion affects communities inland with increased exposure to winds, salt spray and overwash. Trees that were once within 20 m from the ocean become closer to the ocean [64]. As woody species limit sediment movement into the island [33,65], maritime forest disassembles due to salt exposure, forming ghost forests and reshaping coastlines. When forests dieback, marsh expands into adjacent available habitat [29,35]. Lower elevation on Parramore Island may allow for interior saltwater intrusion during storm events.
In the short term, conversion to a shrub state may provide protection to the mainland similar to maritime forest during storm events [11,34]. Morella cerifera is also known to exhibit high annual net primary productivity [66], which may partially compensate for biomass lost through forest decline. However, position on barrier islands with high RSLR, creates temporary alternative resilience paths. Over time, barrier islands will migrate landward or erode if sediment is blocked [33], thus, exposing and affecting all communities that lay beyond. The loss of function would place mainland coastal communities on the forefront of impending storms.
5. Conclusions
In order to enhance or support resilience of ecosystems, it is important to know which factors contribute to their maintenance. Geographic location of coastal and maritime forests coupled with elevation may be critical factors that determine how ecosystems will respond to RSLR and may be a significant form of maintenance for maritime forests [33,67,68,69,70,71]. Savage Neck Dunes forest is protected from the full force of storms through position on the bayside and large intact dunes. Fifteen years following Hurricane Isabel, there are no obvious signs of previous disturbance, and it exhibits signs of traditional successional trajectory through increased basal area and reduction in number of trees. Degradation of barrier islands may change the extent to which the coastal mainland is affected by storm disturbance. Even though barrier islands, as well as mainland coastal communities, are typically found behind large dunes, barrier islands are at the forefront of impact by RSLR and storm surge. The disassembly of maritime forest at the forefront of coastal storms on a Virginia barrier island is complex and attributed to disturbance and other climate change factors. Parramore Island has experienced significant loss of dune habitat and maritime forest through loss of land (i.e., erosion into the ocean) due to RSLR and conversion to mesic grassland. Remaining woody vegetation is now dominated by the shrub Morella cerifera, which is expanding due to warming climate. The maintenance of woody dominance through shrub expansion may indicate a temporary, alternative path of resilience as maritime forest is lost.
The current study shows a direct comparison of how two coastal ecosystems that are geographically different recover from natural disturbance over the long term. This is important because as islands experience fragmentation, they will not be present to protect the mainland and many mainland communities may be in danger of forest disassembly similar to what the current study has documented on Parramore Island. This is especially important considering the extent to which sea-level rise is contaminating freshwater along coastlines [45].
This study also highlights that shrubs can be self-reinforcing and rapidly expanding in areas where trees are decreasing. Future long-term studies should focus on monitoring shrubs over time to examine their capacity to be resilient to environmental perturbations relative to other species. Shrub expansion depends on the capacity of the shrubs to overcome abiotic and biotic stressors. Future studies should also focus on factors that examine the mechanism by which shrubs can encroach across barrier islands into multiple vegetative communities (i.e., grasslands). These studies would provide a greater understanding of future trajectories for barrier islands.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/f12081063/s1, Figure S1: Digital elevation model of the two sites from the 2015 Eastern Shore, VA Lidar, Table S1: Point quarter plot summary table of the canopy layer of maritime forest Savage Neck, Table S2: Fixed radial plot summary table of the canopy layer of maritime forest on Parramore Island, VA.
Author Contributions
Conceptualization, J.C.Z.; methodology, J.C.Z. and P.A.T.; software, P.A.T.; validation, N.N.W., P.A.T. and J.C.Z.; formal analysis, N.N.W., P.A.T. and J.C.Z.; investigation, N.N.W., P.A.T. and J.C.Z.; resources, N.N.W. and J.C.Z.; writing—original draft preparation, N.N.W.; writing—review and editing, N.N.W. and J.C.Z.; supervision, N.N.W. and J.C.Z.; funding acquisition, J.C.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Science Foundation Long-Term Ecological Research grants DEB-1237733 and DEB-1832221, the Virginia Commonwealth University Presidential Research Quest and Dean funds to J.C. Zinnert, and Ford Foundation Fellowship to N.N. Woods.
Data Availability Statement
Data associated with this research are available at: Richardson, D.L. 1999. Parramore Island of the Virginia Coast Reserve Permanent Plot Resurvey: Plot data 1996. Virginia Coast Reserve Long-Term Ecological Research Project Data Publication knb-lter-vcr.107.19 (doi:10.6073/pasta/7c5547d1f778e102fd4d05bd3c53d451). Richardson, D.L. 1999. Parramore Island of the Virginia Coast Reserve Permanent Plot Resurvey: Tree data 1997. Virginia Coast Reserve Long-Term Ecological Research Project Data Publication knb-lter-vcr.102.18 (doi:10.6073/pasta/7ade4c94f6a6be7f239b873e4fd87ce4). Richardson, D.L. 1999. Parramore Island of the Virginia Coast Reserve Permanent Plot Resurvey: Shrub data 1996. Virginia Coast Reserve Long-Term Ecological Research Project Data Publication knb-lter-vcr.99.18 (doi:10.6073/pasta/0382ee52fbd0485325e8d052d3587399). Other data are available upon request from the corresponding author.
Acknowledgments
We thank the Virginia Coast Reserve staff for logistical support, Dot Field with the Virginia Department of Conservation and Recreation for permitting access to collect data on the Eastern Shore of Virginia, and Joseph Brown, Michael Sinclair, Audrey Kirschner, Lauren Wood, Caitlin Bishop, Edward Long, Caroline Baucom, and Spencer Bissett for field and technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mitchell, R.M.; Bakker, J.D.; Vincent, J.B.; Davies, G.M. Relative importance of abiotic, biotic, and disturbance drivers of plant community structure in the sagebrush steppe. Ecol. Appl. 2017, 27, 756–768. [Google Scholar] [CrossRef] [PubMed]
- Lucash, M.S.; Scheller, R.M.; Sturtevant, B.R.; Gustafson, E.J.; Kretchun, A.M.; Foster, J.R. More than the sum of its parts: How disturbance interactions shape forest dynamics under climate change. Ecosphere 2018, 9, e02293. [Google Scholar] [CrossRef]
- Park, I.W.; Hooper, J.; Flegal, J.M.; Jenerette, G.D. Impacts of climate, disturbance and topography on distribution of herbaceous cover in Southern California chaparral: Insights from a remote-sensing method. Divers. Distrib. 2018, 24, 497–508. [Google Scholar] [CrossRef]
- Gaiser, E.E.; Bell, D.M.; Castorani, M.C.; Childers, D.L.; Groffman, P.M.; Jackson, C.R.; Kominoski, J.S.; Peters, D.P.; Pickett, S.T.; Ripplinger, J.; et al. Long-term ecological research and evolving frameworks of disturbance ecology. BioScience 2020, 70, 141–156. [Google Scholar] [CrossRef]
- Young, D.R.; Brantley, S.T.; Zinnert, J.C.; Vick, J.K. Landscape position and habitat polygons in a dynamic coastal environment. Ecosphere 2011, 2, 1–15. [Google Scholar] [CrossRef]
- Saha, A.K.; Saha, S.; Sadle, J.; Jiang, J.; Ross, M.S.; Price, R.M.; Sternberg, L.S.L.O.; Wendelberger, K.S. Sea level rise and South Florida coastal forests. Clim. Chang. 2011, 107, 81–108. [Google Scholar] [CrossRef]
- Liu, X.; Conner, W.H.; Song, B.; Jayakaran, A.D. Forest composition and growth in a freshwater forested wetland community across a salinity gradient in South Carolina, USA. For. Ecol. Manag. 2017, 389, 211–219. [Google Scholar] [CrossRef]
- Schieder, N.W.; Kirwan, M.K. Sea-level driven acceleration in coastal forest retreat. Geology 2019, 47, 1151–1155. [Google Scholar] [CrossRef]
- Kirwan, M.L.; Gedan, K.B. Sea-level driven land conversion and the formation of ghost forests. Nat. Clim. Chang. 2019, 9, 450–457. [Google Scholar] [CrossRef]
- Brooks, M.L.; Brown, C.S.; Chambers, J.C.; D’Antonio, C.M.; Keeley, J.E.; Belnap, J. Exotic annual Bromus invasions: Comparisons among species and ecoregions in the western United States. In Exotic Brome-Grasses in Arid and Semiarid Ecosystems of the Western US; Springer: Cham, Switzerland, 2016; pp. 11–60. [Google Scholar]
- Zinnert, J.C.; Stallins, J.A.; Brantley, S.T.; Young, D.R. Crossing scales: Complexity of barrier island processes for predicting future change. Bioscience 2017, 67, 39–52. [Google Scholar] [CrossRef]
- Stallins, J.A.; Corenblit, D. Interdependence of geomorphic and ecologic resilience properties in a geographic context. Geomorphology 2018, 305, 76–93. [Google Scholar] [CrossRef]
- Holling, C.S. Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef]
- Reyer, C.P.; Brouwers, N.; Rammig, A.; Brook, B.W.; Epila, J.; Grant, R.F.; Holmgren, M.; Langerwisch, F.; Leuzinger, S.; Lucht, W.; et al. Forest resilience and tipping points at different spatio-temporal scales: Approaches and challenges. J. Ecol. 2015, 103, 5–15. [Google Scholar] [CrossRef]
- Matusick, G.; Ruthrof, K.X.; Brouwers, N.C.; Dell, B.; Hardy, G.S.J. Sudden forest canopy collapse corresponding with extreme drought and heat in a mediterranean-type eucalypt forest in southwestern Australia. Eur. J. For. Res. 2013, 132, 497–510. [Google Scholar] [CrossRef]
- Sallenger, A.H.; Doran, K.S.; Howd, P.A. Hotspot of accelerated sea-level rise on the Atlantic coast of North America. Nat. Clim. Chang. 2012, 2, 884–888. [Google Scholar] [CrossRef]
- Garner, K.L.; Chang, M.Y.; Fulda, M.T.; Berlin, J.A.; Freed, R.E.; Soo-Hoo, M.M.; Revell, D.L.; Ikegami, M.; Flint, L.E.; Flint, A.L.; et al. Impacts of sea level rise and climate change on coastal plant species in the central California coast. PeerJ 2015, 3, e958. [Google Scholar] [CrossRef]
- Fagherazzi, S.; Anisfield, S.C.; Blum, L.K.; Long, E.V.; Feagin, R.A.; Fernandes, A.; Kearney, W.S.; Williams, K. Sea level rise and the dynamics of the marsh-upland boundary. Front. Environ. Sci. 2019, 7, 25. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. Climate Change 2014: Synthesis report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Szwitzerland, 2014; p. 151. [Google Scholar]
- Uriarte, M.; Canham, C.D.; Thompson, J.; Zimmerman, J.K.; Murphy, L.; Sabat, A.M.; Fetcher, N.; Haines, B.L. Natural disturbance and human land use as determinants of tropical forest dynamics: Results from a forest simulator. Ecol. Monogr. 2009, 79, 423–443. [Google Scholar] [CrossRef]
- Desantis, L.R.; Bhotika, S.; Williams, K.; Putz, F.E. Sea-level rise and drought interactions accelerate forest decline on the Gulf Coast of Florida, USA. Glob. Chang. Biol. 2007, 13, 2349–2360. [Google Scholar] [CrossRef]
- Tolliver, K.S.; Martin, D.W.; Young, D.R. Freshwater and saltwater flooding response for woody species common to barrier island swales. Wetlands 1997, 17, 10–18. [Google Scholar] [CrossRef]
- Conner, W.H.; Mixon II, W.D.; Wood, G.W. Maritime forest habitat dynamics on Bulls Island, Cape Romain National Wildlife Refuge, SC, following Hurricane Hugo. For. Ecol. Manag. 2005, 212, 127–134. [Google Scholar] [CrossRef]
- Wang, W.; Qu, J.J.; Hao, X.; Liu, Y.; Stanturf, J.A. Post-hurricane forest damage assessment using satellite remote sensing. Agric. For. Meteorol. 2010, 150, 122–132. [Google Scholar] [CrossRef]
- Lugo, A.E. Visible and invisible effects of hurricanes on forest ecosystems: An international review. Austral Ecol. 2008, 33, 368–398. [Google Scholar] [CrossRef]
- Scalley, T.H.; Scatena, F.N.; Lugo, A.E.; Moya, S.; Ruiz, C.R.E. Changes in structure, composition, and nutrients during 15 yrs. of hurricane-induced succession in a subtropical wet forest in Puerto Rico. Biotropica 2010, 42, 455–463. [Google Scholar] [CrossRef]
- Xi, W.; Peet, R.K.; Lee, M.T.; Urban, D.L. Hurricane disturbances tree diversity, and succession in North Carolina Piedmont forest, USA. J. For. Res. 2019, 30, 219–231. [Google Scholar] [CrossRef]
- Busing, R.; White, T.R.D.; Harmon, M.E.; White, P.S. Hurricane disturbance in a temperate deciduous forest: Patch dynamics, tree mortality, and coarse woody detritus. Plant Ecol. 2009, 201, 351–363. [Google Scholar] [CrossRef]
- Langston, A.K.; Kaplan, D.A.; Putz, F.E. A casualty of climate change? Loss of freshwater forest islands on Florida’s Gulf Coast. Glob. Chang. Biol. 2017, 23, 5383–5397. [Google Scholar] [CrossRef]
- Turner, M.G.; Baker, W.L.; Peterson, C.J.; Peet, R.K. Factors influencing succession: Lessons from large, infrequent natural disturbances. Ecosystems 1998, 1, 511–523. [Google Scholar] [CrossRef]
- Stutz, M.L.; Pilkey, O.H. Open-ocean barrier islands: Global influence of climatic, oceanographic, and depositional settings. J. Coast. Res. 2011, 27, 207–222. [Google Scholar] [CrossRef]
- Spalding, M.D.; Ruffo, S.; Lacambra, C.; Meliane, I.; Hale, L.Z.; Shepard, C.C.; Beck, M.W. The role of ecosystems in coastal protection: Adapting to climate change and coastal hazards. Ocean. Coast. Manag. 2014, 90, 50–57. [Google Scholar] [CrossRef]
- Zinnert, J.C.; Via, S.M.; Nettleton, B.P.; Tuley, P.A.; Moore, L.J.; Stallins, J.A. Connectivity in coastal systems: Barrier island vegetation influences upland migration in a changing climate. Glob. Chang. Biol. 2019, 25, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- US Army Corps of Engineers. Coastal Risk Reduction and Resilience; US Army Corps of Engineers Civil Works Directorate: Washington, DC, USA, 2013. [Google Scholar]
- Kirwan, M.L.; Temmerman, S.; Skeehan, E.E.; Guntenspergen, G.R.; Fagherazzi, S. Overestimation of marsh vulnerability to sea level rise. Nat. Clim. Chang. 2016, 6, 253. [Google Scholar] [CrossRef]
- Woods, N.N.; Swall, J.L.; Zinnert, J.C. Soil salinity impacts future community composition of coastal forests. Wetlands 2020, 40, 1495–1503. [Google Scholar] [CrossRef]
- Young, D.R.; Porter, J.H.; Bachmann, C.M.; Shao, G.; Fusina, R.A.; Bowles, J.H.; Korwan, D.; Donato, T.F. Cross-scale patterns in shrub thicket dynamics in the Virginia barrier complex. Ecosystems 2007, 10, 854–863. [Google Scholar] [CrossRef]
- Eggleston, J.; Pope, J. Land subsidence and relative sea-level rise in the southern Chesapeake Bay region. US Geol. Surv. Circ. 2013, 1392, 30. [Google Scholar]
- Lentz, E.E.; Zeigler, S.L.; Thieler, E.R.; Plant, N.G. Probabilistic patterns of inundation and biogeomorphic changes due to sea-level rise along the northeastern US Atlantic coast. Landsc. Ecol. 2021, 36, 223–241. [Google Scholar] [CrossRef]
- Williams, K.; MacDonald, M.; Lda Sternberg, S.L. Interactions of storm, drought, and sea-level rise on coastal forest: A case study. J. Coast. Res. 2013, 19, 1116–1121. [Google Scholar]
- Naumann, J.C.; Young, D.R. Relationship between community structure and seed bank to describe successional dynamics of an Atlantic Coast maritime forest. J. Torrey Bot. Soc. 2007, 134, 89–98. [Google Scholar] [CrossRef]
- Prengaman, K.A.; Kribel, J.R.G.; Ware, S. Effects of Hurricane Isabel on a maturing hardwood forest in the Virginia Coastal Plain. J. Torrey Bot. Soc. 2008, 135, 360–366. [Google Scholar] [CrossRef]
- Levy, G.F. A study of vegetational dynamics on Parramore Island, Virginia. Castanea 1983, 48, 32–36. [Google Scholar]
- Raff, J.L.; Shawler, J.L.; Ciarletta, D.J.; Hein, E.A.; Lorenzo-Trueba, J.; Hein, C. Insights into barrier-island stability derived from transgressive/regressive state changes of Parramore Island, Virginia. Mar. Geol. 2018, 403, 1–19. [Google Scholar] [CrossRef]
- Michael, H.A.; Post, V.E.; Wilson, A.M.; Werner, A.D. Science, society, and the coastal groundwater squeeze. Water Resour. Res. 2017, 53, 2610–2617. [Google Scholar] [CrossRef]
- NOAA. Sea Level Trends: Chesapeake Bay Bridge Tunnel, VA. Available online: https://tidesandcurrents.noaa.gov/sltrends/sltrends.html (accessed on 8 October 2019).
- Cottam, G.; Curtis, J.T. The use of distance measures in phytosociological sampling. Ecology 1956, 37, 451–460. [Google Scholar] [CrossRef]
- Zinnert, J.C.; Shiflett, S.A.; Via, S.; Bissett, S.; Dows, B.; Manley, P.; Young, D.R. Spatial–temporal dynamics in barrier island upland vegetation: The overlooked coastal landscape. Ecosystems 2016, 19, 685–697. [Google Scholar] [CrossRef]
- Agrawal, G.; Sarup, J.; Bhopal, M. Comparison of QUAC and FLAASH atmospheric correction modules on EO-1 Hyperion data of Sanchi, international. J. Adv. Eng. Sci. Technol. 2011, 4, 178–186. [Google Scholar]
- Bernstein, L.S.; Jin, X.; Gregor, B.; Adler-Golden, S.M. Quick atmospheric correction code: Algorithm description and recent upgrades. Opt. Eng. 2012, 51, 111719. [Google Scholar] [CrossRef]
- Curtis, J.T.; McIntosh, R.P. The interrelations of certain analytic and synthetic phytosociological characters. Ecology 1950, 31, 434–455. [Google Scholar] [CrossRef]
- Hutcheson, K. A test for comparing diversities based on the Shannon formula. J. Theor. Biol. 1970, 29, 151–154. [Google Scholar] [CrossRef]
- McCune, B.; Grace, J.B.; Urban, D.L. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Kearney, W.S.; Fernandes, A.; Fagherazzi, S. Sea-level rise and storm surges structure coastal forests into persistence and regeneration niches. PLoS ONE 2019, 14, e0215977. [Google Scholar] [CrossRef]
- Miller, T.E. Effects of disturbance on vegetation by sand accretion and erosion across coastal dune habitats on a barrier island. AoB Plants 2015, 7, plv003. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Dynamics and processes of barrier-island vegetation. Rev. Aquat. Sci. 1990, 2, 437–480. [Google Scholar]
- Sala, O.E.; Maestre, F.T. Grass–woodland transitions: Determinants and consequences for ecosystem functioning and provisioning of services. J. Ecol. 2014, 102, 1357–1362. [Google Scholar] [CrossRef]
- Archer, S.R.; Andersen, E.M.; Predick, K.I.; Schwinning, S.; Steidl, R.J.; Woods, S.R. Woody plant encroachment: Causes and consequences. In Rangeland Systems; Springer: Cham, Switzerland, 2017; pp. 25–84. [Google Scholar]
- Bissett, S.N.; Zinnert, J.C.; Young, D.R. Woody expansion facilitates liana expansion and affects physical structure in temperate coastal communities. Ecosphere 2016, 7, e01383. [Google Scholar] [CrossRef]
- Duberstein, J.A.; Krauss, K.W.; Baldwin, M.J.; Allen, S.T.; Conner, W.H.; Salter, J.S.; Miloshis, M. Small gradients in salinity have large effects on stand water use in freshwater wetland forests. For. Ecol. Manag. 2020, 473, 118308. [Google Scholar] [CrossRef]
- Ury, E.A.; Anderson, S.M.; Peet, R.K.; Bernhardt, E.S.; Wright, J.P. Succession, regression and loss: Does evidence of saltwater exposure explain recent changes in the tree communities of North Carolina’s Coastal Plain? Ann. Bot. 2020, 125, 255–264. [Google Scholar] [CrossRef]
- Huang, H.; Zinnert, J.C.; Wood, L.K.; Young, D.R.; D’Odorico, P. Non-linear shift from grassland to shrubland in temperate barrier islands. Ecology 2018, 99, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.K.; Hays, S.; Zinnert, J.C. Decreased temperature variance associated with biotic composition enhances coastal shrub encroachment. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Johnson, S.R.; Young, D.R. Variation in tree ring width in relation to storm activity for mid-Atlantic barrier island populations of Pinus taeda. J. Coast. Res. 1992, 8, 99–104. [Google Scholar]
- Claudino-Sales, V.; Wang, P.; Horwitz, M.H. Factors controlling the survival of coastal dunes during multiple hurricane impacts in 2004 and 2005: Santa Rosa barrier island, Florida. Geomorphology 2008, 95, 295–315. [Google Scholar] [CrossRef]
- Knapp, A.K.; Briggs, J.M.; Collins, S.L.; Archer, S.R.; Bret-Harte, M.S.; Ewers, B.E.; Peters, D.P.; Young, D.R.; Shaver, G.R.; Pendall, E.; et al. Shrub encroachment in North American grasslands: Shifts in growth form dominance rapidly alters control of ecosystem carbon inputs. Glob. Chang. Biol. 2008, 14, 615–623. [Google Scholar] [CrossRef]
- Doyle, T.W.; Krauss, K.W.; Conner, W.H.; From, A.S. Predicting the retreat and migration of tidal forests along the northern Gulf of Mexico under sea-level rise. For. Ecol. Manag. 2010, 259, 770–777. [Google Scholar] [CrossRef]
- Geselbracht, L.; Freeman, K.; Kelly, E.; Gordon, D.R.; Putz, F.E. Retrospective and prospective model simulations of sea level rise impacts on Gulf of Mexico coastal marshes and forests in Waccasassa Bay, Florida. Clim. Chang. 2011, 107, 35–57. [Google Scholar] [CrossRef][Green Version]
- Smart, L.S.; Taillie, P.J.; Poulter, B.; Vukomanovic, J.; Singh, K.K.; Swenson, J.J.; Mitasova, H.; Smith, J.W.; Meentemeyer, R.K. Aboveground carbon loss associated with the spread of ghost forests as sea levels rise. Environ. Res. Lett. 2020, 15, 104028. [Google Scholar] [CrossRef]
- Williams, K.; Meads, M.V.; Sauerbrey, D.A. The roles of seedling salt tolerance and resprouting in forest zonation on the west coast of Florida, USA. Am. J. Bot. 1998, 85, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Pinzon, Z.; Stumpf, R.P.; Raabe, E.A. Sea-level rise and coastal forests on the Gulf of Mexico. US Geol. Surv. 1999, 1500, 20910. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).