The Selective Effects of Environmental Change on the Functional Diversity of Soil Decomposers
Abstract
:1. Background and History of the Topic
2. Recent Developments
2.1. Diversity and Decomposition
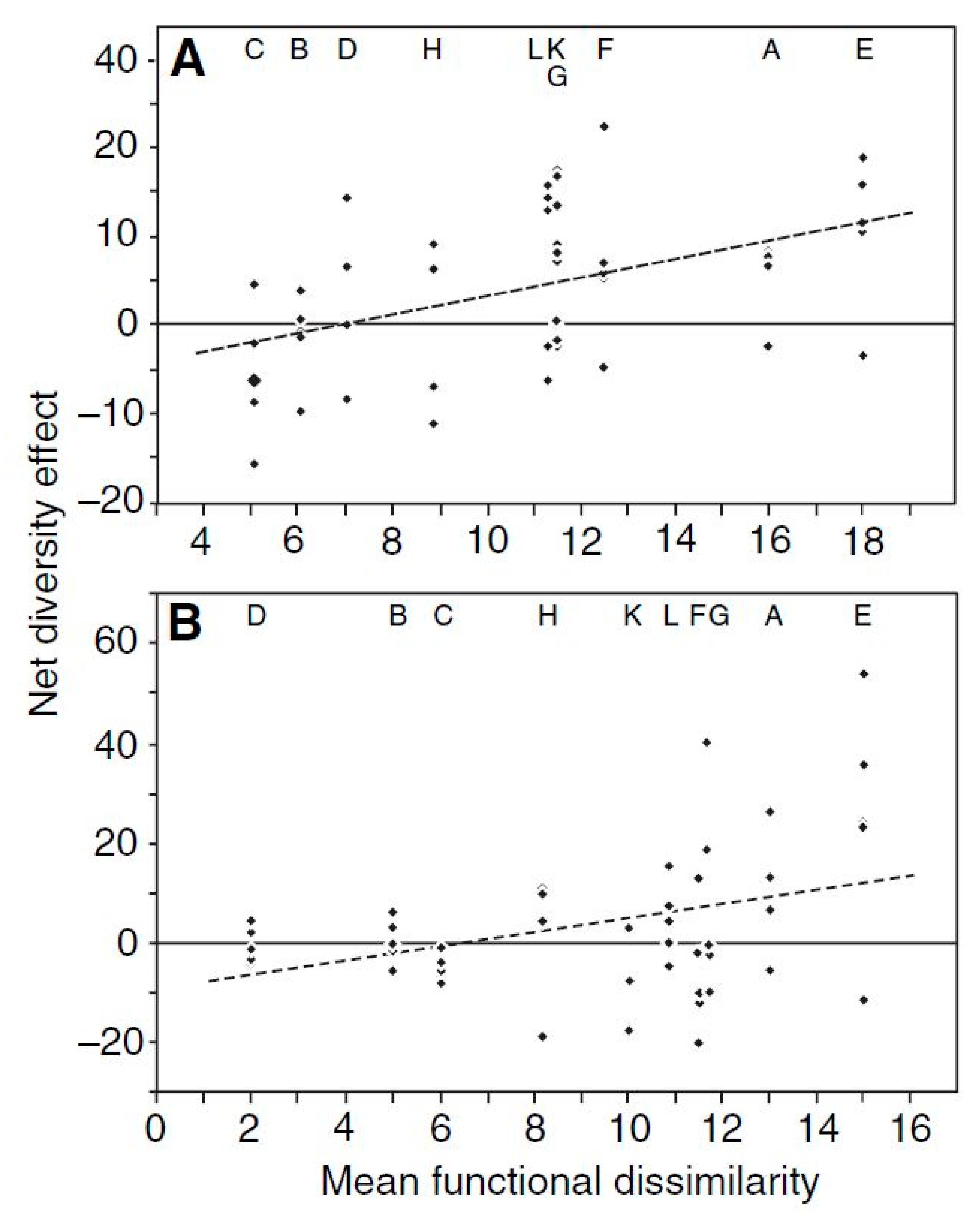
2.2. Functional Diversity
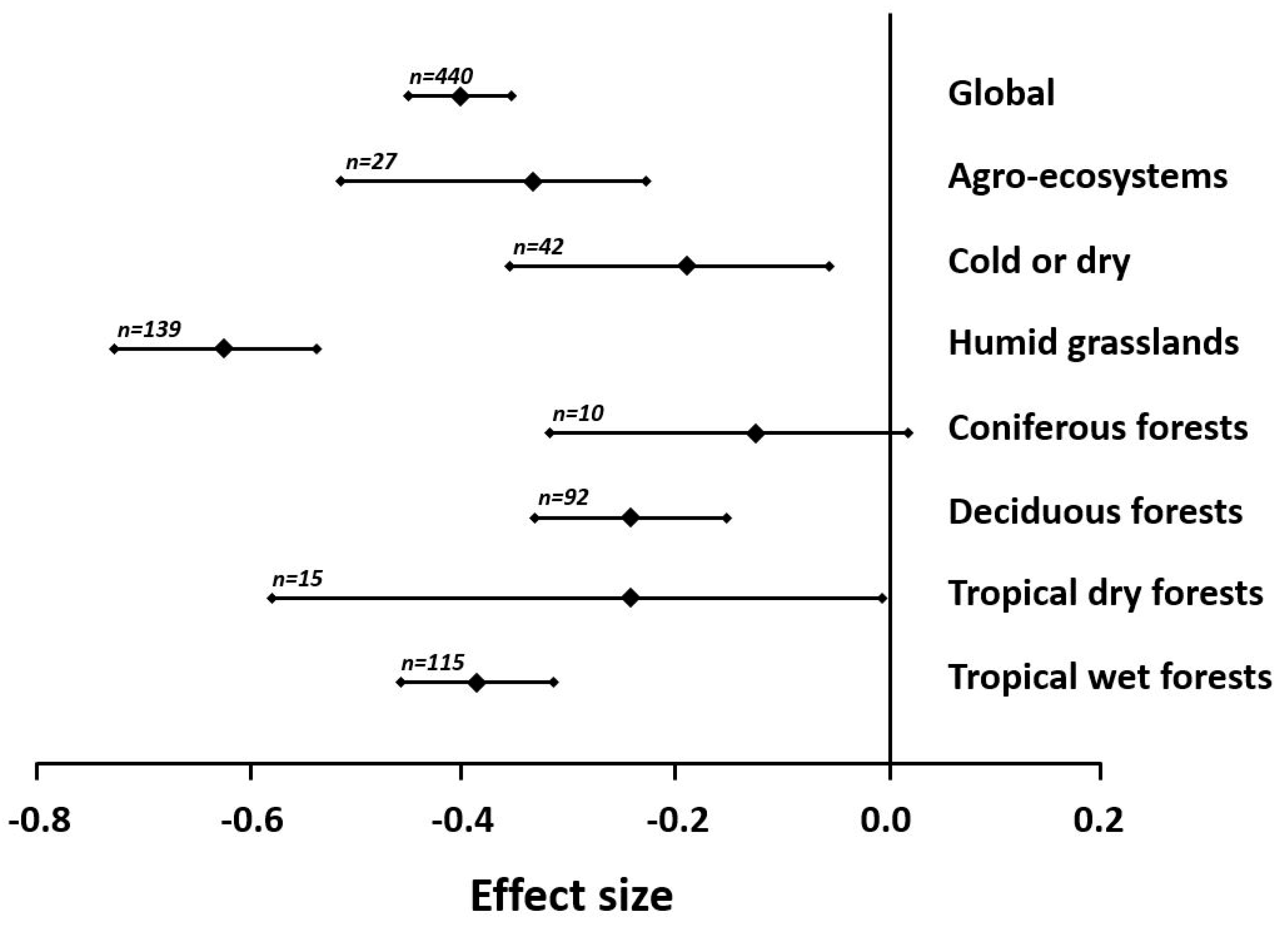
2.3. Environmental Change and Diversity
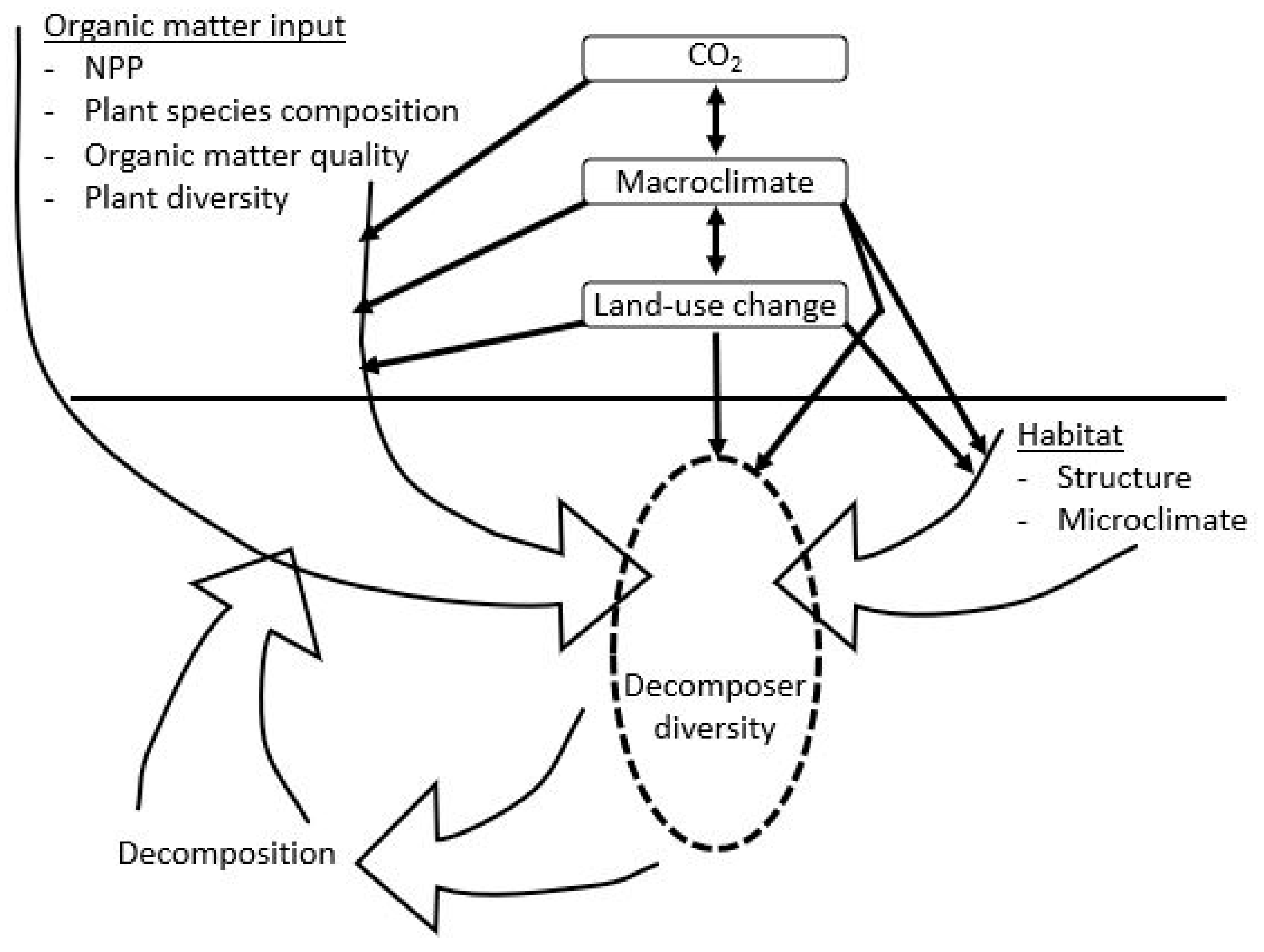
2.4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Dickinson, C.H.; Pugh, G.J.F. (Eds.) Biology of Plant. Litter Decomposition; Academic Press: London, UK; New York, NY, USA, 1974; Volumes 1 and 2. [Google Scholar]
- Anderson, J.M.; Macfadyen, A. (Eds.) The Role of Terrestrial and Aquatic Organisms in Decomposition Processes; Blackwell Scientific Publications: Oxford, UK, 1975. [Google Scholar]
- Fitter, A.H.; Atkinson, D.; Read, D.J.; Usher, M.B. Ecological Interactions in Soil; Plants, Microbes and Animals; Blackwell Scientific Publications: Oxford, UK, 1985. [Google Scholar]
- Teller, A.; Mathy, P.; Jeffers, J.N.R. Responses of Forest Ecosystems to Environmental Changes; Elsevier Applied Science: London, UK, 1992. [Google Scholar]
- Berg, M.P. Decomposition, Nutrient Flow and Food Web Dynamics in a Stratified Pine Forest Soil. Ph.D. Thesis, VU University Amsterdam, Amsterdam, The Netherlands, 1997. [Google Scholar]
- Hunt, H.W.; Coleman, D.C.; Ingham, E.R.; Ingham, R.E.; Elliott, E.T.; Moore, J.C.; Rose, S.L.; Reid, C.P.; Morley, C.R. The detrital food web in a short grass prairie. Biol. Fertil. Soils 1987, 3, 57–68. [Google Scholar]
- De Ruiter, P.C.; Moore, J.C.; Zwart, K.B.; Bouwman, L.A.; Hassink, J.; Bloem, J.; De Vos, J.A.; Marinissen, J.C.Y.; Didden, W.A.M.; Lebrink, G.; et al. Simulation of nitrogen mineralization in the below-ground food webs of two winter wheat fields. J. Appl. Ecol. 1993, 30, 95–106. [Google Scholar] [CrossRef]
- Berg, M.P.; de Ruiter, P.C.; Didden, W.A.M.; Janssen, M.P.M.; Schouten, A.J.; Verhoef, H.A. Community food web, decomposition and nitrogen mineralisation in a stratified Scots pine forest soil. Oikos 2001, 94, 130–142. [Google Scholar] [CrossRef]
- Berg, M.P. Spatio-temporal structure in soil communities and ecosystem processes. In Community Ecology; Processes, Models, and Applications; Verhoef, H.A., Morin, P.J., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 69–80. [Google Scholar]
- Gessner, M.O.; Swan, C.M.; Dang, C.K.; McKie, B.G.; Bardgett, R.D.; Wall, D.H.; Hättenschwiller, S. Diversity meets decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Schleuter, D.; Daufresne, M.; Masson, F.; Argillier, C. A user’s guide to functional diversity indices. Ecol. Monogr. 2010, 80, 469–484. [Google Scholar] [CrossRef] [Green Version]
- Hedde, M.; Bureau, F.; Chauvat, M.; Decaëns, T. Patterns and mechanisms responsible for the relationship between the diversity of litter macro-invertebrates and leaf degradation. Basic Appl. Ecol. 2010, 11, 35–44. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional diversity: Back to basics and looking forward. Ecol. Lett. 2006, 9, 741–758. [Google Scholar] [CrossRef]
- Heemsbergen, D.A.; Berg, M.P.; Loreau, M.; van Hal, J.R.; Faber, J.H.; Verhoef, H.A. Biodiversity Effects on Soil Processes Explained by Interspecific Functional Dissimilarity. Science 2004, 306, 1019–1020. [Google Scholar] [CrossRef] [Green Version]
- Botta-Dukát, Z. Rao’s quadratic entropy as a measure of functional diversity based on multiple traits. J. Veg. Sci. 2005, 16, 533–540. [Google Scholar] [CrossRef]
- Coulis, M.; Fromin, N.; David, J.-F.; Gavinet, J.; Cleft, A.; Devidal, S.; Roy, J.; Hättenschwiler, S. Functional dissimilarity across trophic levels as a driver of soil processes in a Mediterranean decomposer system exposed to two moisture levels. Oikos 2015, 124, 1304–1316. [Google Scholar] [CrossRef]
- Frainer, A.; Moretti, M.S.; Xu, W.; Gessner, M.O. No evidence for leaf-trait dissimilarity effects on litter decomposition, fungal decomposers, and nutrient dynamics. Ecology 2015, 96, 550–561. [Google Scholar] [CrossRef] [Green Version]
- FAO; ITPS; GSBI; SCBD; EC. State of Knowledge of Soil Biodiversity—Status, Challenges and Potentialities; Report; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Bradford, M.A.; Jones, T.H.; Bardgett, R.D.; Black, H.I.J.; Boag, B.; Bonkowski, M.; Cook, R.; Eggers, T.; Gange, A.C.; Grayson, S.J.; et al. Impacts of Soil Faunal Community Composition on model Grassland Ecosystems. Science 2002, 298, 615–618. [Google Scholar] [CrossRef] [Green Version]
- Eisenhauer, N.; Hörsch, V.; Moeser, J.; Scheu, S. Synergistic effects of microbial and animal decomposers on plant and herbivore performance. Basic Appl. Ecol. 2010, 11, 23–34. [Google Scholar] [CrossRef]
- Wardle, D.A. Do experiments exploring plant diversity-ecosystem functioning relationships inform how biodiversity loss impacts natural ecosystems? J. Veg. Sci. 2016, 27, 646–653. [Google Scholar] [CrossRef]
- Beaumelle, L.; De Leander, F.; Eisenhauer, N. Biodiversity mediates the effects of stressors but not nutrients on litter decomposition. eLife 2020, 9, e55659. [Google Scholar] [CrossRef]
- Bradford, M.A.; Berg, B.; Maynard, D.S.; Wieder, W.R.; Wood, S.A. Understanding the dominant controls on litter decomposition. J. Ecol. 2016, 104, 229–238. [Google Scholar] [CrossRef]
- Berg, B.; Berg, M.P.; Bottner, P.; Box, E.; Breymeyer, A.; Deanta, R.C.; Couteaux, M.-M.; Escudero, A.; Gallardo, A.; Kratz, W.; et al. Litter mass loss rates in pine forests of Europe and Eastern United States: Some relationships with climate and litter quality. Biogeochemistry 1993, 20, 127–159. [Google Scholar] [CrossRef]
- Swift, M.J.; Heal, O.W.; Anderson, J.M. Decomposition in Terrestrial Ecosystems. In Studies in Ecology; Blackwell Scientific Publications: Oxford, UK, 1979. [Google Scholar]
- Moore, T.R.; Trofymow, J.A.; Taylor, B.; Prescott, C.; Camire, C.; Duschene, L.; Fyles, J.; Kozak, L.; Kranabetter, M.; Morrison, I.; et al. Litter decomposition rates in Canadian forests. Glob. Change Biol. 1999, 5, 75–82. [Google Scholar] [CrossRef]
- Wall, D.H.; Bradford, M.A.; St. John, M.G.; Trofymow, J.A.; Behan-Pelletier, V.A.; Bignell, D.E.; Dangerfield, J.M.; Parton, W.J.; Rusek, J.; Voigt, W.; et al. Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Glob. Chang. Biol. 2008, 14, 2661–2677. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Palacios, P.; Maestre, F.T.; Kattge, J.; Wall, D.H. Climate and litter quality differently modulate the effects of soil fauna on litter decomposition across biomes. Ecol. Lett. 2013, 16, 1045–1053. [Google Scholar] [CrossRef] [Green Version]
- Verhoef, H.A. The role of soil microcosms in the study of ecosystem processes. Ecology 1996, 77, 685–690. [Google Scholar] [CrossRef]
- Waring, B.; Adams, R.; Branco, S.; Powers, J.S. Scale-dependent variation in nitrogen cycling and soil fungal communities along gradients of forest composition and age in regenerating tropical dry forests. New Phytol. 2015, 209, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Luan, J.; Liu, S.; Lee, S.; Whalen, J.K.; Wang, Y.; Wang, J.; Liu, Y.; Dong, W.; Chang, S.X. Functional diversity of decomposers modulates litter decomposition affected by plant invasion along a climate gradient. J. Ecol. 2021, 109, 1236–1249. [Google Scholar] [CrossRef]
- Blankinship, J.C.; Niklaus, P.A.; Hungate, B.A. A meta-analysis of responses of soil biota to global change. Oecologia 2011, 165, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Faber, J.H.; Verhoef, H.A. Functional differences between closely related soil arthropods with respect to decomposition and nitrogen mobilization in a pine forest. Soil Biol. Biochem. 1991, 23, 15–23. [Google Scholar] [CrossRef]
- Wardle, D.A.; Verhoef, H.A.; Clarholm, M. Trophic relationships in the soil microfood-web: Predicting the responses to a changing global environment. Glob. Chang. Biol. 1998, 4, 713–727. [Google Scholar] [CrossRef]
- Hedlund, K.; Griffiths, B.; Christensen, S.; Scheu, S.; Setälä, H.; Tscharntke, T.; Verhoef, H.A. Trophic interactions in changing landscapes: Responses of soil food webs. Basic Appl. Ecol. 2004, 5, 495–503. [Google Scholar] [CrossRef]
- Berg, M.P.; Kiers, E.T.; Driessen, G.; van der Heijden, M.G.A.; Kooi, B.W.; Kuenen, F.J.A.; Liefting, M.; Verhoef, H.A.; Ellers, J. Adapt or disperse: Understanding species persistence in a changing world. Glob. Chang. Biol. 2010, 16, 587–598. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verhoef, H.A. The Selective Effects of Environmental Change on the Functional Diversity of Soil Decomposers. Forests 2021, 12, 1650. https://doi.org/10.3390/f12121650
Verhoef HA. The Selective Effects of Environmental Change on the Functional Diversity of Soil Decomposers. Forests. 2021; 12(12):1650. https://doi.org/10.3390/f12121650
Chicago/Turabian StyleVerhoef, Herman A. 2021. "The Selective Effects of Environmental Change on the Functional Diversity of Soil Decomposers" Forests 12, no. 12: 1650. https://doi.org/10.3390/f12121650
APA StyleVerhoef, H. A. (2021). The Selective Effects of Environmental Change on the Functional Diversity of Soil Decomposers. Forests, 12(12), 1650. https://doi.org/10.3390/f12121650




