Dynamics of Leaf- and Root-Specific Biomarkers during 1-Year of Litter Decomposition
Abstract
:1. Introduction
- (i)
- What is the initial composition of aliphatic biopolyesters in leaf and root litter of the selected tree species?
- (ii)
- Which compounds are suitable as indicator molecules for the distinction of leaf and root material from the respective species?
- (iii)
- What is the degradation pattern of the identified biomarkers during the decomposition of litter and root material?
2. Materials and Methods
2.1. Samples
2.2. Incubation Experiment
2.3. Extraction and Quantification of Root- and Leaf-Derived Biomarkers
2.3.1. Lipid Extraction
2.3.2. Ester-Bound Lipid Extraction and Derivation
2.3.3. Gas Chromatography–Mass Spectrometry (GC-MS)
2.4. Statistical Analyses
3. Results
3.1. Litter Mass Loss
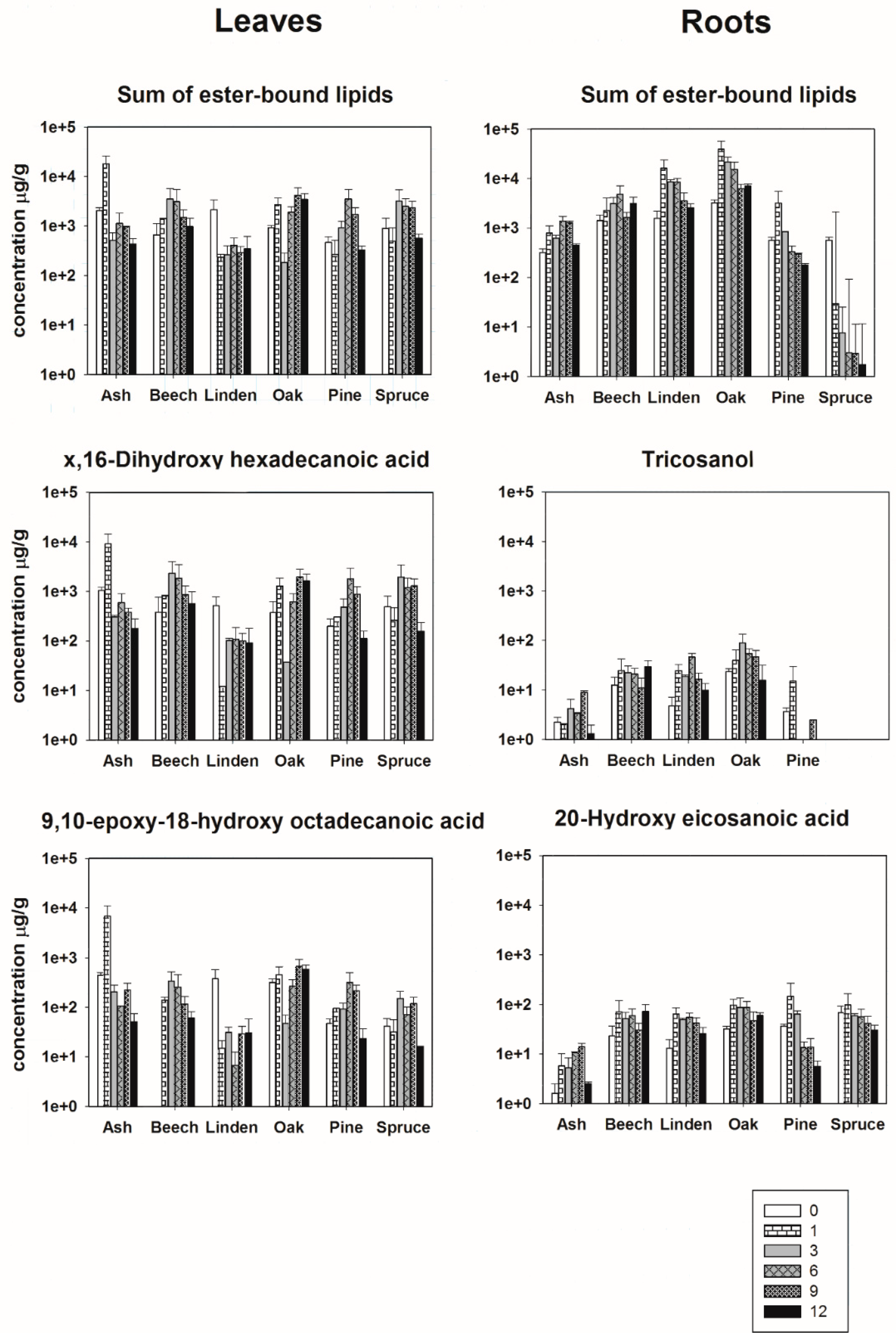
3.2. Initial Molecular Composition of Ester-Bound Lipids in Leaf- and Root-Derived Litter
3.3. Degradation of Ester-Bound Lipids
4. Discussion
4.1. Composition of Ester-Bound Lipids in Leaf- and Root-Derived Litter
4.2. Degradation of Ester-Bound Lipids
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Rasse, D.P.; Rumpel, C.; Dignac, M.-F. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 2005, 269, 341–356. [Google Scholar] [CrossRef]
- Pisani, O.; Lin, L.H.; Lun, O.O.Y.; Lajtha, K.; Nadelhoffer, K.; Simpson, A.J.; Simpson, M. Long-term doubling of litter inputs accelerates soil organic matter degradation and reduces soil carbon stocks. Biogeochemistry 2016, 127, 1–14. [Google Scholar] [CrossRef]
- Lajtha, K.; Bowden, R.D.; Crow, S.; Fekete, I.; Kotroczó, Z.; Plante, A.; Simpson, M.J.; Nadelhoffer, K.J. The detrital input and removal treatment (DIRT) network: Insights into soil carbon stabilization. Sci. Total Environ. 2018, 640, 1112–1120. [Google Scholar] [CrossRef]
- Sokol, N.W.; Bradford, M.A. Microbial formation of stable soil carbon is more efficient from belowground than aboveground input. Nat. Geosci. 2019, 12, 46–53. [Google Scholar] [CrossRef]
- Amelung, W.; Brodowski, S.; Sandhage-Hofmann, A.; Bol, R. Chapter 6 combining biomarker with stable isotope analyses for assessing the transformation and turnover of soil organic matter. Adv. Agron. 2008, 100, 155–250. [Google Scholar] [CrossRef]
- Simoneit, B.R. A review of current applications of mass spectrometry for biomarker/molecular tracer elucidations. Mass Spectrom. Rev. 2005, 24, 719–765. [Google Scholar] [CrossRef]
- Evershed, R.P. Organic residue analysis in archaeology: The archaeological biomarker revolution. Archaeometry 2008, 50, 895–924. [Google Scholar] [CrossRef]
- Owens, P.N.; Blake, W.H.; Gaspar, L.; Gateuille, D.; Koiter, A.J.; Lobb, D.A.; Petticrew, E.L.; Reiffarth, D.G.; Smith, H.G.; Woodward, J.C. Fingerprinting and tracing the sources of soils and sediments: Earth and ocean science, geoarchaeological, forensic, and human health applications. Earth-Sci. Rev. 2016, 162, 1–23. [Google Scholar] [CrossRef] [Green Version]
- E Kolattukudy, P. Structure, biosynthesis, and biodegradation of cutin and suberin. Annu. Rev. Plant Physiol. 1981, 32, 539–567. [Google Scholar] [CrossRef]
- Philippe, G.; Sørensen, I.; Jiao, C.; Sun, X.; Fei, Z.; Domozych, D.S.; Rose, J.K. Cutin and suberin: Assembly and origins of specialized lipidic cell wall scaffolds. Curr. Opin. Plant Biol. 2020, 55, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Nierop, K.G.J.; Verstraten, J.M. Rapid molecular assessment of the bioturbation extent in sandy soil horizons under pine using ester-bound lipids by on-line thermally assisted hydrolysis and methylation-gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; Simpson, M.J. Sources and composition of hydrolysable aliphatic lipids and phenols in soils from western Canada. Org. Geochem. 2006, 37, 385–407. [Google Scholar] [CrossRef]
- Graça, J. Suberin: The biopolyester at the frontier of plants. Front. Chem. 2015, 3, 62. [Google Scholar] [CrossRef]
- Angst, G.; John, S.; Mueller, C.W.; Kögel-Knabner, I.; Rethemeyer, J. Tracing the sources and spatial distribution of organic carbon in subsoils using a multi-biomarker approach. Sci. Rep. 2016, 6, 29478. [Google Scholar] [CrossRef] [Green Version]
- Armas-Herrera, C.M.; Dignac, M.-F.; Rumpel, C.; Arbelo, C.D.; Chabbi, A. Management effects on composition and dynamics of cutin and suberin in topsoil under agricultural use. Eur. J. Soil Sci. 2016, 67, 360–373. [Google Scholar] [CrossRef]
- Mendez-Millan, M.; Dignac, M.-F.; Rumpel, C.; Derenne, S. Can cutin and suberin biomarkers be used to trace shoot and root-derived organic matter? A molecular and isotopic approach. Biogeochemistry 2010, 106, 23–38. [Google Scholar] [CrossRef]
- Vidal, A.; Quenea, K.; Alexis, M.; Derenne, S. Molecular fate of root and shoot litter on incorporation and decomposition in earthworm casts. Org. Geochem. 2016, 101, 1–10. [Google Scholar] [CrossRef]
- Klotzbücher, T.; Kaiser, K.; Guggenberger, G.; Gatzek, C.; Kalbitz, K. A new conceptual model for the fate of lignin in decomposing plant litter. Ecology 2011, 92, 1052–1062. [Google Scholar] [CrossRef]
- Zech, M.; Pedentchouk, N.; Buggle, B.; Leiber, K.; Kalbitz, K.; Marković, S.B.; Glaser, B. Effect of leaf litter degradation and seasonality on D/H isotope ratios of n-alkane biomarkers. Geochim. Cosmochim. Acta 2011, 75, 4917–4928. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.L.; Jansen, B.; van Loon, E.E.; Wiesenberg, G.L.B. Transformation of n-alkanes from plant to soil: A review. SOIL Discuss. 2021, 7, 785–809. [Google Scholar] [CrossRef]
- Goñi, M.A.; Hedges, J.I. The diagenetic behavior of cutin acids in buried conifer needles and sediments from a coastal marine environment. Geochim. Cosmochim. Acta 1990, 54, 3083–3093. [Google Scholar] [CrossRef]
- Angst, G.; Heinrich, L.; Kögel-Knabner, I.; Mueller, C.W. The fate of cutin and suberin of decaying leaves, needles and roots–Inferences from the initial decomposition of bound fatty acids. Org. Geochem. 2016, 95, 81–92. [Google Scholar] [CrossRef]
- Opsahl, S.; Benner, R. Early diagenesis of vascular plant tissues: Lignin and cutin decomposition and biogeochemical implications. Geochim. Cosmochim. Acta 1995, 59, 4889–4904. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant Litter, Decomposition, Humus Formation, Carbon Sequestration, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–341. [Google Scholar]
- Langenbruch, C.; Helfrich, M.; Flessa, H. Effects of beech (Fagus sylvatica), ash (Fraxinus excelsior) and lime (Tilia spec.) on soil chemical properties in a mixed deciduous forest. Plant Soil 2012, 352, 389–403. [Google Scholar] [CrossRef] [Green Version]
- WRB. World Reference Base for Soil Resources: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2014. [Google Scholar]
- Nordgren, A. Apparatus for the continuous, long-term monitoring of soil respiration rate in large numbers of samples. Soil Biol. Biochem. 1988, 20, 955–957. [Google Scholar] [CrossRef]
- Jansen, B.; Nierop, K.G.; Hageman, J.A.; Cleef, A.M.; Verstraten, J.M. The straight-chain lipid biomarker composition of plant species responsible for the dominant biomass production along two altitudinal transects in the Ecuadorian Andes. Org. Geochem. 2006, 37, 1514–1536. [Google Scholar] [CrossRef]
- Hunneman, D.; Eglinton, G. The constituent acids of gymnosperm cutins. Phytochemistry 1972, 11, 1989–2001. [Google Scholar] [CrossRef]
- Spielvogel, S.; Prietzel, J.; Leide, J.; Riedel, M.; Zemke, J.; Kögel-Knabner, I. Distribution of cutin and suberin biomarkers under forest trees with different root systems. Plant Soil 2014, 381, 95–110. [Google Scholar] [CrossRef]
- Järvinen, R.; Silvestre, A.J.; Holopainen, U.; Kaimainen, M.; Nyyssölä, A.; Gil, A.M.; Neto, C.P.; Lehtinen, P.; Buchert, J.; Kallio, H. Suberin of potato (Solanum tuberosum Var. Nikola): Comparison of the effect of cutinase CcCut1 with chemical depolymerization. J. Agric. Food Chem. 2009, 57, 9016–9027. [Google Scholar] [CrossRef] [Green Version]
- Olson, J.S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef] [Green Version]
- Goñi, M.A.; Hedges, J.I. Cutin-derived CuO reaction products from purified cuticles and tree leaves. Geochim. Cosmochim. Acta 1990, 54, 3065–3072. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Ziegler, F.; Riederer, M.; Zech, W. Distribution and decomposition pattern of cutin and suberin in forest soils. J. Plant Nutr. Soil Sci. 1989, 152, 409–413. [Google Scholar] [CrossRef]
- Wang, J.-J.; Bowden, R.D.; Lajtha, K.; Washko, S.; Wurzbacher, S.J.; Simpson, M.J. Long-term nitrogen addition suppresses microbial degradation, enhances soil carbon storage, and alters the molecular composition of soil organic matter. Biogeochemistry 2019, 142, 299–313. [Google Scholar] [CrossRef] [Green Version]
- Hamer, U.; Rumpel, C.; Dignac, M.-F. Cutin and suberin biomarkers as tracers for the turnover of shoot and root derived organic matter along a chronosequence of Ecuadorian pasture soils. Eur. J. Soil Sci. 2012, 63, 808–819. [Google Scholar] [CrossRef]
- Nierop, K.G.; Naafs, D.F.; Verstraten, J.M. Occurrence and distribution of ester-bound lipids in Dutch coastal dune soils along a pH gradient. Org. Geochem. 2003, 34, 719–729. [Google Scholar] [CrossRef]
- Holloway, P.J. Some variations in the composition of suberin from the cork layers of higher plants. Phytochemistry 1983, 22, 495–502. [Google Scholar] [CrossRef]
- Matzke, K.; Riederer, M. A comparative study into the chemical constitution of cutins and suberins from Picea abies (L.) Karst., Quercus robur L., and Fagus sylvatica L. Planta 1991, 185, 233–245. [Google Scholar] [CrossRef]
- Graça, J.; Pereira, H. Suberin structure in potato periderm: Glycerol, long-chain monomers, and glyceryl and feruloyl dimers. J. Agric. Food Chem. 2000, 48, 5476–5483. [Google Scholar] [CrossRef]
- Crow, S.E.; Lajtha, K.; Filley, T.R.; Swanston, C.W.; Bowden, R.D.; Caldwell, B.A. Sources of plant-derived carbon and stability of organic matter in soil: Implications for global change. Glob. Chang. Biol. 2009, 15, 2003–2019. [Google Scholar] [CrossRef]
- Bonaventure, G.; Beisson, F.; Ohlrogge, J.; Pollard, M. Analysis of the aliphatic monomer composition of polyesters associated with Arabidopsis epidermis: Occurrence of octadeca-cis-6, cis-9-diene-1,18-dioate as the major component. Plant J. 2004, 40, 920–930. [Google Scholar] [CrossRef]
- Franke, R.; Briesen, I.; Wojciechowski, T.; Faust, A.; Yephremov, A.; Nawrath, C.; Schreiber, L. Apoplastic polyesters in Arabidopsis surface tissues–A typical suberin and a particular cutin. Phytochemistry 2005, 66, 2643–2658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, B.B.; Kolattukudy, P.E. Synthesis of suberin during wound-healing in jade leaves, tomato fruit, and bean pods’. Plant Physiol. 1976, 58, 411–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espelie, K.E.; Davis, R.W.; Kolattukudy, P.E. Composition, ultrastructure and function of the cutin- and suberin-containing layers in the leaf, fruit peel, juice-sac and inner seed coat of grapefruit (Citrus paradisi Macfed.). Planta 1980, 149, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; Shunthirasingham, C.; Simpson, M.J. A comparison of plant and microbial biomarkers in grassland soils from the Prairie Ecozone of Canada. Org. Geochem. 2005, 36, 425–448. [Google Scholar] [CrossRef]
- Kallio, H.; Nieminen, R.; Tuomasjukka, S.; Hakala, M. Cutin composition of five finnish berries. J. Agric. Food Chem. 2006, 54, 457–462. [Google Scholar] [CrossRef]
- Holloway, P.J.; Deas, A.H.B. Epoxyoctadecanoic acids in plant cutins and suberins. Phytochemistry 1973, 12, 1721–1735. [Google Scholar] [CrossRef]
- Viikari, L.; Kantelinen, A.; Buchert, J.; Puls, J. Enzymatic accessibility of xylans in lignocellulosic materials. Appl. Microbiol. Biot. 1994, 41, 124–129. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Kim, S.-U.; Kim, K.-R.; Kim, J.-W.; Kim, S.; Kwon, Y.-U.; Oh, D.-K.; Park, J.-B. Microbial synthesis of plant oxylipins from γ-linolenic acid through designed biotransformation pathways. J. Agric. Food Chem. 2015, 63, 2773–2781. [Google Scholar] [CrossRef]
- Van Bogaert, I.N.A.; Groeneboer, S.; Saerens, K.; Soetaert, W. The role of cytochrome P450 monooxygenases in microbial fatty acid metabolism. FEBS J. 2010, 278, 206–221. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [Green Version]
- Mansfield, S.; Mooney, C.; Saddler, J.N. Substrate and enzyme characteristics that limit cellulose hydrolysis. Biotechnol. Prog. 1999, 15, 804–816. [Google Scholar] [CrossRef]
- Buléon, A.; Colonna, P.; Planchot, V.; Ball, S. Starch granules: Structure and biosynthesis. Int. J. Biol. Macromol. 1998, 23, 85–112. [Google Scholar] [CrossRef] [Green Version]
- Hedges, J.I.; Cowie, G.L.; Ertel, J.R.; Barbour, R.J.; Hatcher, P.G. Degradation of carbohydrates and lignins in buried woods. Geochim. Cosmochim. Acta 1985, 49, 701–711. [Google Scholar] [CrossRef]
- Lynd, L.R.; Weimer, P.J.; van Zyl, W.H.; Pretorius, I.S. Microbial cellulose utilization: Fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 2002, 66, 506–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puls, J. Chemistry and biochemistry of hemicelluloses: Relationship between hemicellulose structure and enzymes required for hydrolysis. Macromol. Symp. 1997, 120, 183–196. [Google Scholar] [CrossRef]
- Feng, X.; Xu, Y.; Jaffé, R.; Schlesinger, W.H.; Simpson, M. Turnover rates of hydrolysable aliphatic lipids in Duke Forest soils determined by compound specific 13C isotopic analysis. Org. Geochem. 2010, 41, 573–579. [Google Scholar] [CrossRef]
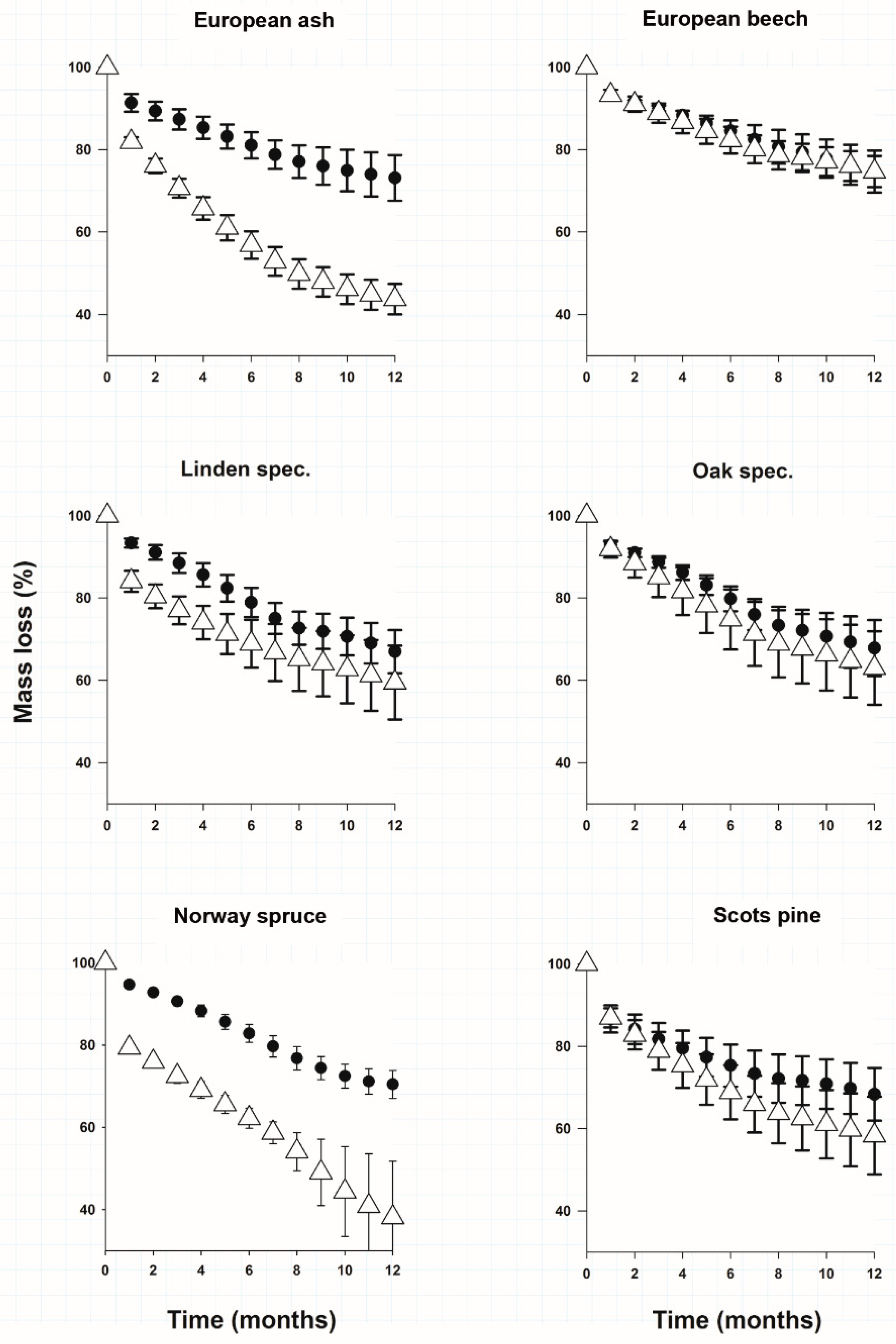
| Species | Plant Part | k-Values | R2 |
|---|---|---|---|
| European Ash | root | 0.024 (0.0016) a | 0.9363 |
| leaves | 0.0753 (0.0053) c | 0.9523 | |
| European Beech | root | 0.0227 (0.001) a | 0.9510 |
| leaves | 0.0227 (0.0013) a | 0.9597 | |
| Linden spec. | root | 0.031 (0.0013) a | 0.9843 |
| leaves | 0.0383 (0.0037) a,b | 0.8990 | |
| Oak spec. | root | 0.032 (0.0016) a | 0.9750 |
| leaves | 0.0373 (0.0017) a,b | 0.9777 | |
| Norway spruce | root | 0.023 (0.002) a | 0.9403 |
| leaves | 0.0683 (0.0063) b,c | 0.9153 | |
| Scots pine | root | 0.027 (0.003) a | 0.8810 |
| leaves | 0.0517 (0.004) a,b,c | 0.9300 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altmann, J.G.; Jansen, B.; Jungkunst, H.F.; Kalbitz, K. Dynamics of Leaf- and Root-Specific Biomarkers during 1-Year of Litter Decomposition. Forests 2021, 12, 1732. https://doi.org/10.3390/f12121732
Altmann JG, Jansen B, Jungkunst HF, Kalbitz K. Dynamics of Leaf- and Root-Specific Biomarkers during 1-Year of Litter Decomposition. Forests. 2021; 12(12):1732. https://doi.org/10.3390/f12121732
Chicago/Turabian StyleAltmann, Jens G., Boris Jansen, Hermann F. Jungkunst, and Karsten Kalbitz. 2021. "Dynamics of Leaf- and Root-Specific Biomarkers during 1-Year of Litter Decomposition" Forests 12, no. 12: 1732. https://doi.org/10.3390/f12121732
APA StyleAltmann, J. G., Jansen, B., Jungkunst, H. F., & Kalbitz, K. (2021). Dynamics of Leaf- and Root-Specific Biomarkers during 1-Year of Litter Decomposition. Forests, 12(12), 1732. https://doi.org/10.3390/f12121732






