Determination of the Chemical Composition of Eucalyptus spp. for Cellulosic Pulp Production
Abstract
:1. Introduction
2. Materials and Methods
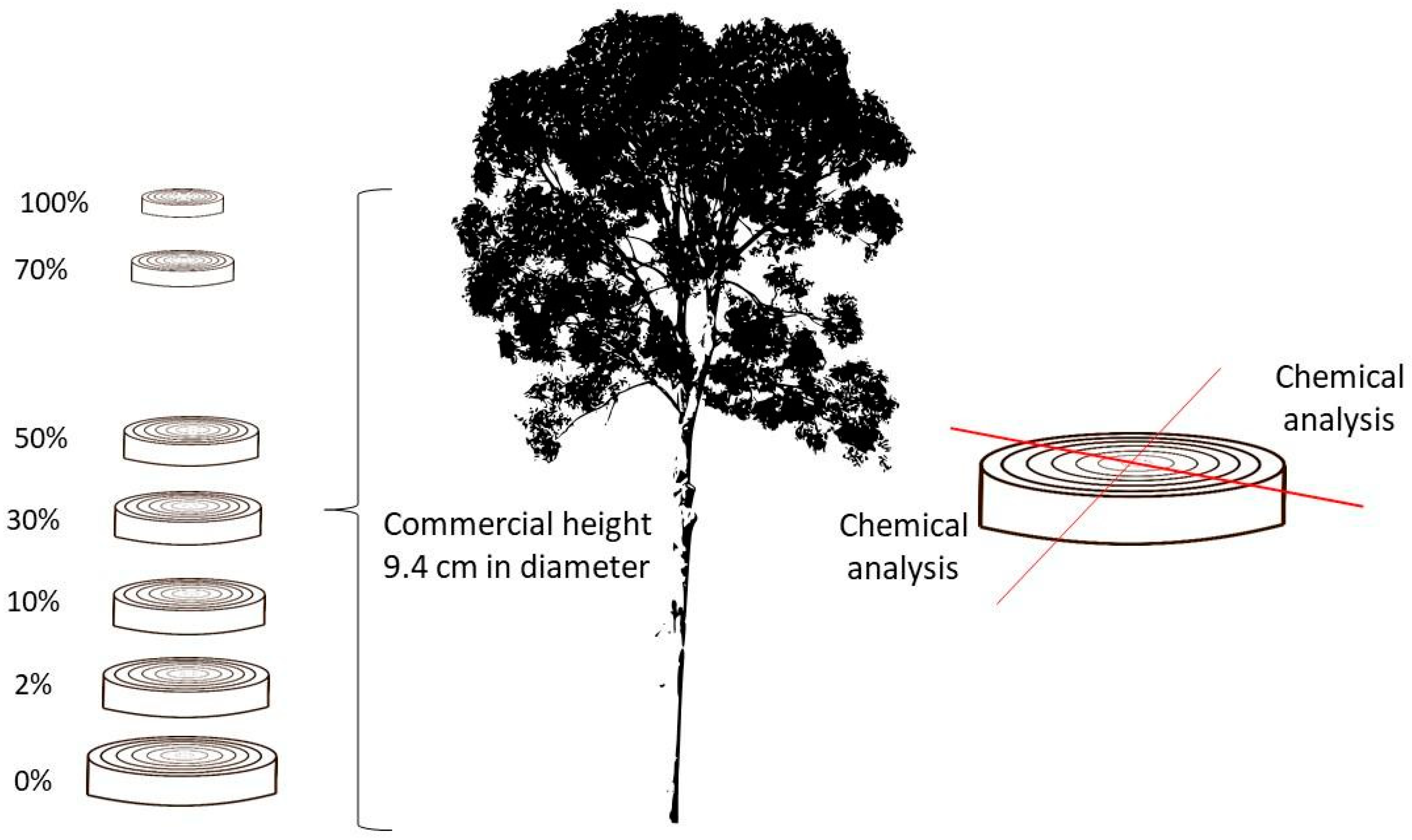
2.1. Description of the Material and Sample Preparation
2.2. Determination of the Amount of Ashes, Extractives and Lignin
2.3. Composition of Monosaccharides, Uronic Acids and Acetates
2.4. Phenolic Content in Polar Extracts
2.5. Lipophilic Composition of Extractives
3. Results
3.1. Ash, Extractive and Lignin Contents
3.2. Composition of Monosaccharides, Uronic Acids and Acetates
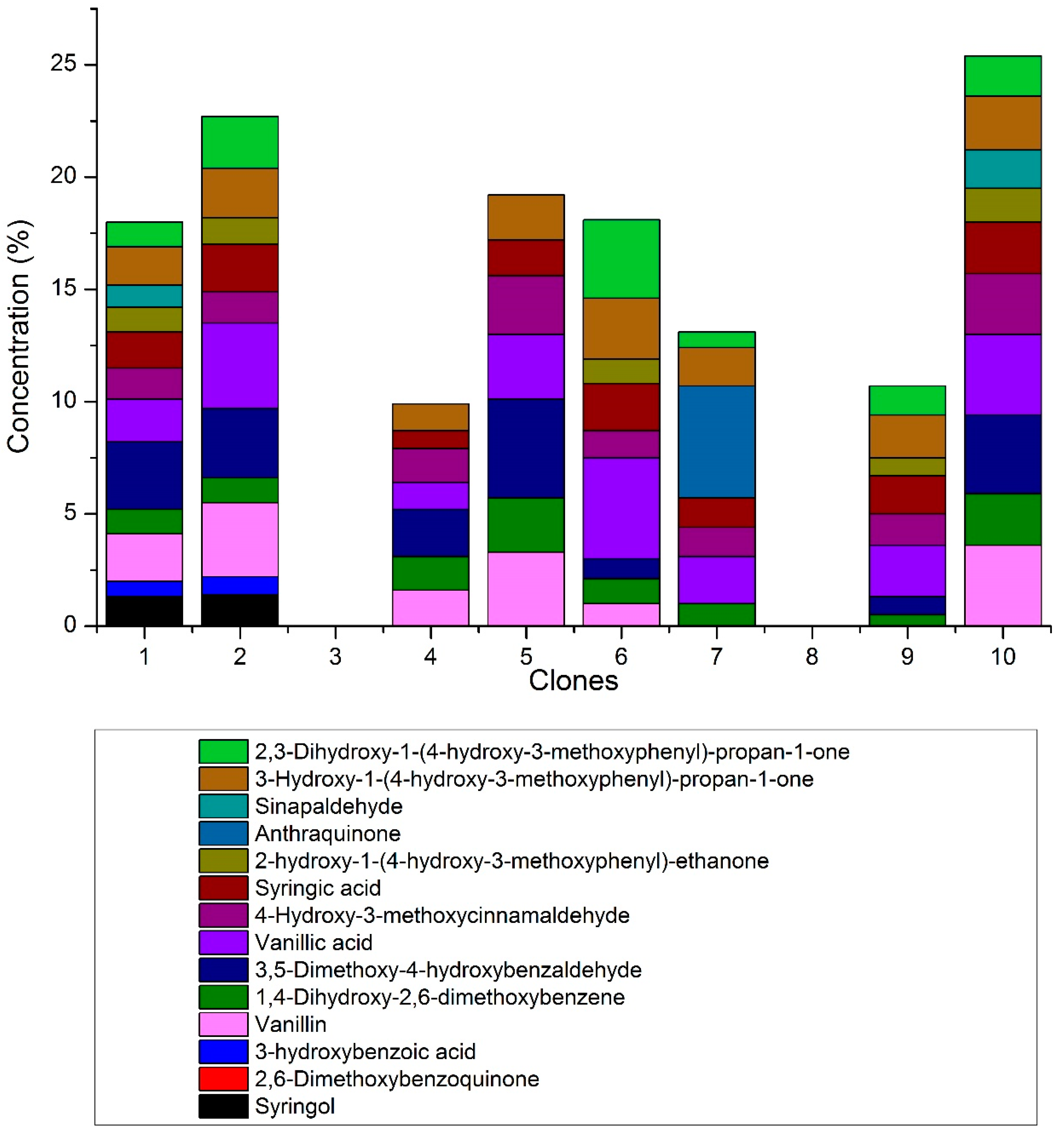
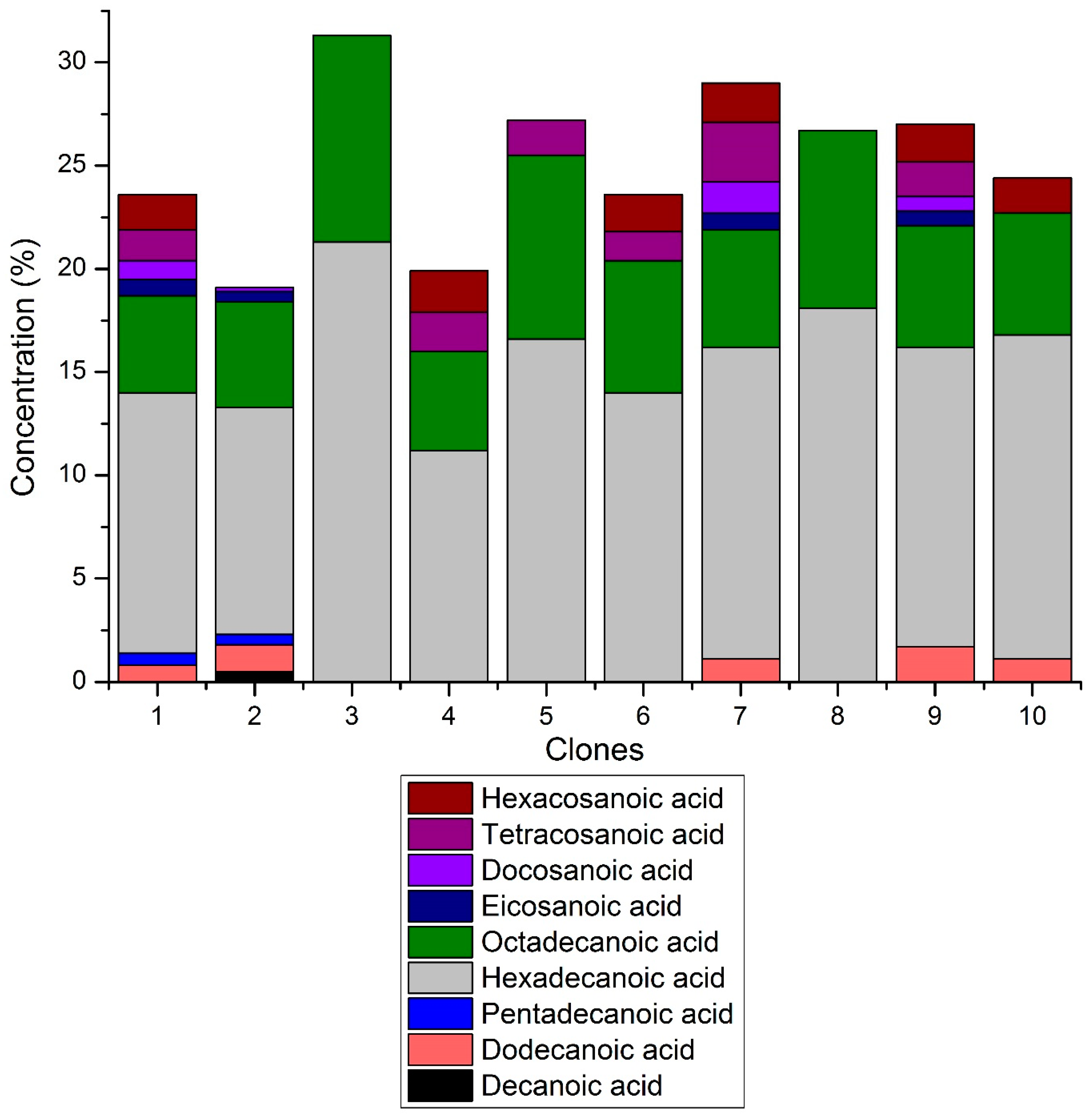
3.3. Composition of Extractives
4. Discussion
4.1. Ash, Extractive and Lignin Contents
4.2. Monosaccharides
4.3. Composition of Wood Extractives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lima, S.R.; Oliveira, G.S.; Morais, S.A.L.; Nascimento, E.A.; Chang, R. Estudo dos constituintes macromoleculares, extrativos voláteis e compostos fenólicos da madeira de candeia–Moquinia polymorpha (LESS.) DC. Ciênc. Florest. 2007, 17, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Mattos, B.D.; Lourençon, T.V.; Gatto, D.A.; Serrano, L.; Labidi, J. Chemical characterization of wood and extractives of fast-growing Schizolobium parahyba and Pinus taeda. Wood Mater Sci Eng. 2016, 11, 209–216. [Google Scholar] [CrossRef]
- Carvalho, S.R.; Holanda, J.S.; Pio, N.S. Análise das propriedades físicas da madeira de Mogno (Swietenia macrophylla King) em floresta plantada na Amazônia Central/Analysis of the physical properties of Mahogany (Swietenia macrophylla King) wood in a planted forest in Central Amazonia. Braz. J. Anim. Environ. Res. 2021, 4, 2749–2763. [Google Scholar] [CrossRef]
- Li, Y. Advances in Composite Materials–Analysis of Natural and Man-Made Materials; InTech Open: London, UK, 2011. [Google Scholar]
- Braz, R.L.; Oliveira, J.T.S.; Arantes, M.D.C.; Rodrigues, B.P. Propriedades físicas e mecânicas da madeira de Toona ciliata em diferentes idades. Floresta 2013, 43, 663. [Google Scholar] [CrossRef] [Green Version]
- Protásio, T.P.; Scatolino, M.V.; de Araújo, A.C.C.; de Oliveira, A.F.C.F.; de Figueiredo, I.C.R.; de Assis, M.R.; Trugilho, P.F. Assessing Proximate Composition, Extractive Concentration, and Lignin Quality to Determine Appropriate Parameters for Selection of Superior Eucalyptus Firewood. Bioenergy Res. 2019, 12, 626–641. [Google Scholar] [CrossRef]
- Brito, J.O.; Barrichelo, L.E.G.; Seixas, F. Análise da produção energética e de carvão vegetal de espécies de eucalipto. IPEF Piracicaba 1983, 23, 53–56. [Google Scholar]
- Souza Faria, W.; Ribeiro Resende, D.; Luz Guimarães, I.; Protásio, T.D.P.; Guimarães, J.B., Jr. Avaliação das propriedades físico-mecânicas da madeira de Eucalyptus camaldulensis tratado e não tratado com preservativo. In Enciclopédia Biosfera; Centro Científico Conhecer: Goiânia, Brazil, 2015; p. 287. [Google Scholar]
- Indústria Brasileira de Árvores (IBÀ). Relatório Anual. 2019. Available online: https://www.iba.org/datafiles/publicacoes/relatorios/iba-relatorioanual2019.pdf (accessed on 28 January 2021).
- Severo, E.T.D.; Calonego, F.W.; Sansígolo, C.A. Composição química da madeira de Eucalyptus citriodora em função das direcções estruturais. Silva Lusit. 2006, 14, 113–126. [Google Scholar]
- Angelis, M.; Romagnoli, M.; Vek, V.; Poljanšek, I.; Oven, P.; Thaler, N.; Lesar, B.; Kržišnik, D.; Humar, M. Chemical composition and resistance of Italian stone pine (Pinus pinea L.) wood against fungal decay and wetting. Ind. Crops Prod. 2018, 117, 187–196. [Google Scholar] [CrossRef]
- Fengel, D.E.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 1984; 613p. [Google Scholar]
- Sjöström, E. Wood Chemistry: Fundamentals and Application; Academic Press: London, UK, 1993; 293p. [Google Scholar]
- Rowell, R.M.; Pettersen, R.; Han, J.S.; Rowwel, J.S.; Tshabalala, M.A. Handbook of Wood Chemistry and Wood Composites; Rowell, R.M., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 35–74. [Google Scholar]
- Ferreira, C.R.; Fantini, M.; Colodette, J.L.; Gomide, J.L.; Carvalho, A.M.M.L. Avaliação tecnológica de clones de eucalipto: Parte 1–Qualidade da madeira para produção de celulose Kraft. Sci. For. Piracicaba 2006, 70, 161–170. [Google Scholar]
- Zanuncio, A.J.V.; Colodette, J.L. Teores de Lignina e Ácidos Urônicos na Madeira e Polpa Celulósica de Eucalipto. Rev. Árvore 2011, 35, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Segura, T.E.S.; Pimenta, L.R.; Mattiazzo, F.B.; Da Silva, F.M.; Da Cruz, J.A.; Souza, D.O.L. Programa de controle da qualidade da madeira na Eldorado Brasil Celulose. Revista O PAPEL 2017, 78, 82–89. [Google Scholar]
- Rowell, R.M.; Dickerson, J.P. Acetylation of wood. ACS Symp. Ser. 2014, 1158, 301–327. [Google Scholar]
- Santos, R.C.; Carneiro, A.C.O.; Vital, B.R.; Castro, R.V.O.; Vidaurre, G.B.; Trugilho, P.F.; Castro, A.F.N.M. Influência das propriedades químicas e da relação siringil/guaiacil da madeira de eucalipto na produção de carvão vegetal. Ciênc. Florest. 2016, 26, 657–669. [Google Scholar] [CrossRef] [Green Version]
- TAPPI. Standard T 211 om-12: Ash in Wood, Pulp, Paper, and Paperboard: Combustion at 525 °C; TAPPI: Atlanta, GA, USA, 2012. [Google Scholar]
- TAPPI. T 207 om-93: Water Solubility of Wood and Pulp; TAPPI: Atlanta, GA, USA, 1994. [Google Scholar]
- TAPPI. Standard T 222 om-11: Acidinsoluble Lignin in Wood and Pulp; TAPPI: Atlanta, GA, USA, 2011. [Google Scholar]
- TAPPI. UM 250: Acid-Soluble Lignin in Wood and Pulp; TAPPI: Atlanta, GA, USA, 1991. [Google Scholar]
- TAPPI. T 249 CM-09 Carbohydrate Ition of Extractive-Free Wood and Wood Pulp by Gas-Liquid Chromatography. TAPPI: Atlanta, GA, USA, 2009; p. 8. [Google Scholar]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Miranda, I.; Lima, L.; Quilhó, T.; Knapic, S.; Pereira, H. The bark of Eucalyptus sideroxylon as a source of phenolic extracts with anti-oxidant properties. Ind. Crops Prod. 2016, 82, 81–87. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Abdalla, S.; Pizzi, A.; Ayed, N.; Bouthoury, F.C.-E.; Charrier, B.; Bahabri, F.; Ganash, A. MALDI-TOF Analysis of Aleppo Pine (Pinus halepensis) Bark Tannin. BioResources 2014, 9, 3396–3406. [Google Scholar] [CrossRef] [Green Version]
- Eglinton, G.; Hunneman, D. Gas chromatographic-mass spectrometric studies of long chain hydroxy acids—I: The constituent cutin acids of apple cuticle. Phytochemistry 1968, 7, 313–322. [Google Scholar] [CrossRef]
- Kolattukudy, P.E.; Agrawal, V.P. Structure and composition of aliphatic constituents of potato tuber skin (suberin). Lipids 1974, 9, 682–691. [Google Scholar] [CrossRef]
- Nosek, R.; Holubcik, M.; Jandacka, J. The Impact of Bark Content of Wood Biomass on Biofuel Properties. BioResources 2015, 11, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Araújo, S.O.; Neiva, D.M.; Carneiro, A.C.; Esteves, B.; Pereira, H. Potential of mild torrefaction for upgrading the wood energy value of different Eucalyptus species. Forests 2018, 9, 535. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Luo, J.; Qin, W.; Tong, Z. Elemental analysis, chemical composition, cellulose crystallinity, and FT-IR spectra of Toona sinensis wood. Mon. Chem. Chem. Mon. 2014, 145, 175–185. [Google Scholar] [CrossRef]
- Jardim, J.M.; Gomes, J.B.F.; Colodette, J.L.; Brahim, B.P. Avaliação da qualidade e desempenho de clones de eucalipto na produção de celulose. Revista O PAPEL 2017, 78, 122–129. [Google Scholar]
- Costa, A.C.S.; Leal, C.S.; Santos, L.C.; Carvalho, A.M.M.L.; Oliveira, A.C.; Pereira, B.L.C. Propriedades da madeira de cerne e alburno de Eucalyptus camaldulensis. Rev. Ciênc. Madeira–RCM 2017, 8, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Moore, R.K.; Smaglick, J.; Leitch, E.A.M. The effect of polarity of extractives on the durability of wood. In Proceedings of the 18th ISWFPC (International Symposium on Wood, Fibre, and Pulp Chemistry), Vienna, Austria, 8–11 September 2015. [Google Scholar]
- Lovaglio, T.; D’Auria, M.; Rita, A.; Todaro, L. Compositions of compounds extracted from thermo-treated wood using solvents of different polarities. iForest Biogeosci. For. 2017, 10, 824–828. [Google Scholar] [CrossRef]
- Herrera, M.; Thitiwutthisakul, K.; Yang, X.; Rujitanaroj, P.-O.; Rojas, R.; Berglund, L. Preparation and evaluation of high-lignin content cellulose nanofibrils from eucalyptus pulp. Cellulose 2018, 25, 3121–3133. [Google Scholar] [CrossRef] [Green Version]
- Jankowska, A.; Boruszewski, P.; Kaczmarczyk, A. The Role of Extractives and Wood Anatomy in the Wettability and Free Surface Energy of Hardwoods. BioResources 2018, 13, 3082–3097. [Google Scholar] [CrossRef] [Green Version]
- Arantes, M.D.C.; Trugilho, P.F.; Lima, J.T.; Carneiro, A.D.C.O.; Alves, E.; Guerreiro, M.C. Longitudinal and radial variation of extractives and total lignin contents in a clone of Eucalyptus grandis W.Hill ex Maiden x Eucalyptus urophylla ST Blake. Cerne 2011, 17, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Gomide, J.L.; Neto, H.F.; Regazzi, A.J. Análise de critérios de qualidade da madeira de eucalipto para produção de celulose kraft. Rev. Árvore 2010, 34, 339–344. [Google Scholar] [CrossRef]
- Antes, R.; Joutsimo, O.P. Effect of Modified Cooking on Chemical Composition of Pulps from Eucalyptus globulus and Eucalyptus nitens. BioResources 2014, 10, 210–226. [Google Scholar] [CrossRef]
- Schlemmer, W.; Egger, M.; Dachert, M.; Lahti, J.; Gschiel, M.; Walzl, A.; Leitner, E.; Spirk, S.; Hirn, U. Rapid Separation and Quantitative Analysis of Complex Lipophilic Wood Pulp Extractive Mixtures Based on 2D Thin Layer Chromatography. ACS Sustain. Chem. Eng. 2020, 8, 12534–12541. [Google Scholar] [CrossRef]
- Viana, B.N.; Pontes, K.V. Virtual analyzer of extractive content in Eucalyptus wood based on hybrid modeling approach for the pulp and paper industry. Wood Sci. Technol. 2021, 55, 1–19. [Google Scholar] [CrossRef]
- Sitholé, B.; Shirin, S.; Ambayec, B. Analysis and Fate of Lipophilic Extractives in Sulphite Pulps. J. Wood Chem. Technol. 2010, 30, 31–47. [Google Scholar] [CrossRef]
- Gominho, J.; Lourenço, A.; Marques, A.V.; Pereira, H. An extensive study on the chemical diversity of lipophilic extractives from Eucalyptus globulus wood. Phytochemistry 2020, 180, 112520. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Wei, L.; Fang, G.; Xu, F.; Deng, Y.; Shen, K.; Tian, Q.; Wu, T.; Zhu, B. Prediction of holocellulose and lignin content of pulp wood feedstock using near infrared spectroscopy and variable selection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 225, 117515. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Ahmed, S.; Ray, S.K.; Islam, M.S.; Quadery, A.H.; Jahan, M.S. Method for predicting lignocellulose components in jute by transformed FT-NIR spectroscopic data and chemometrics. Nord. Pulp Pap. Res. J. 2019, 34, 1–9. [Google Scholar] [CrossRef]
- Silva, V.L.; Jameel, H.; Gomes, F.J.B.; Batalha, L.; Coura, M.R.; Colodette, J.L. Effect of lignin carbohydrate complexes of hardwood hybrids on the kraft pulping process. J. Wood Chem. Technol. 2016, 37, 1–10. [Google Scholar] [CrossRef]
- Neiva, D.; Fernandes, L.; Araújo, S.; Lourenço, A.; Gominho, J.; Simoes, R.; Pereira, H. Chemical composition and kraft pulping potential of 12 eucalypt species. Ind. Crops Prod. 2015, 66, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.C.; Mendonça, M.C.; Damásio, R.A.P.; Zidanes, U.L.; Mori, F.A.; Ferreira, S.R.; Tonoli, G.H. Influence of hemicellulose content of Eucalyptus and Pinus fibers on the grinding process for obtaining cellulose micro/nanofibrils. Holzforschung 2019, 73, 1035–1046. [Google Scholar] [CrossRef]
- Wan, J.; Wang, Y.; Xiao, Q. Effects of hemicellulose removal on cellulose fiber structure and recycling characteristics of Eucalyptus pulp. Bioresour. Technol. 2010, 101, 4577–4583. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, A.; Gominho, J.; Pereira, H. Pulping and delignification of sapwood and heartwood from Eucalyptus globulus. J. Pulp Pap. Sci. 2010, 36, 85–90. [Google Scholar]
- Zanuncio, A.J.V.; Colodette, J.L.; Gomes, J.B.; Carneiro, A.C.O.; Vital, B.R. Composição química da madeira de eucalipto com diferentes níveis de desbaste. Ciênc. Florest. 2013, 23, 755–760. [Google Scholar] [CrossRef] [Green Version]
- Sá, V.A. Influência da Madeira de Clones de Eucalyptus spp. na Deslignificação e Qualidade da Polpa Kraft. Ph.D. Thesis, Universidade Federal de Lavras, Lavras, Brazil, 2014. [Google Scholar]
- Lopes, O.R.; Magaton, A.S.; Morais, P.H.D.; Colodette, J.L.; Veloso, D.P. Caracterização dos ácidos urônicos da madeira de Eucalyptus urograndis. In Proceedings of the Congresso Brasileiro de Química, Salvador, Brazil, 25–29 September 2006. [Google Scholar]
- Gomide, J.L.; Colodette, J.L.; De Oliveira, R.C.; Silva, C.M. Caracterização tecnológica, para produção de celulose, da nova geração de clones de Eucalyptus do Brasil. Rev. Árvore 2005, 29, 129–137. [Google Scholar] [CrossRef]
- Gomes, F.J.B.; Colodette, J.L.; Burnet, A.; Batalha, L.; Santos, F.A.; Demuner, I.F. Thorough Characterization of Brazilian New Generation of Eucalypt Clones and Grass for Pulp Production. Int. J. For. Res. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Jardim, J.M.; Jardim, C.M.; Colodette, J.L. Understanding the pulping and bleaching performances of eucalyptus woods affected by physiological disturbance. TAPPI J. 2018, 17, 633–642. [Google Scholar] [CrossRef]
- Zanão, M.; Colodette, J.L.; Oliveira, R.C.; Almeida, D.P.; Gomes, F.J.B.; De Carvalho, D.M. Evaluation of Kraft-PS Cooking for Eucalypt and Pine Wood Chip Mixtures. J. Wood Chem. Technol. 2018, 39, 149–165. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, D.M.; Silva, M.R.; Colodette, J.L. Efeito da qualidade da madeira no desempenho da polpação Kraft. Ciênc. Florest. 2014, 24, 677–684. [Google Scholar] [CrossRef] [Green Version]
- Raza, W.; Lee, J.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J. Removal of phenolic compounds from industrial waste water based on membrane-based technologies. J. Ind. Eng. Chem. 2019, 71, 1–18. [Google Scholar] [CrossRef]
- Freire, C.; Silvestre, A.; Neto, C.; Cavaleiro, J. Lipophilic Extractives of the Inner and Outer Barks of Eucalyptus globulus. Holzforschung 2002, 56, 372–379. [Google Scholar] [CrossRef]
- Neiva, D.M.; Araújo, S.; Gominho, J.; Carneiro, A.D.C.; Pereira, H. An integrated characterization of Picea abies industrial bark regarding chemical composition, thermal properties and polar extracts activity. PLoS ONE 2018, 13, e0208270. [Google Scholar] [CrossRef]
- Sartori, C.J.; Mota, G.S.; Miranda, I.; Mori, F.A.; Pereira, H. Tannin extraction and characterization of polar extracts from the barks of two Eucalyptus urophylla hybrids. BioResources 2018, 13, 4820–4831. [Google Scholar]
- Santos, S.A.O.; Freire, C.S.R.; Domingues, M.R.M.; Silvestre, A.J.D.; Neto, C.P. Characterization of Phenolic Components in Polar Extracts of Eucalyptus globulus Labill. Bark by High-Performance Liquid Chromatography–Mass Spectrometry. J. Agric. Food Chem. 2011, 59, 9386–9393. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, G.; Fontenla, E.; Santos, J.; Freire, M.S.; González-Álvarez, J.; Antorrena, G. Antioxidant activity and phenolic content of chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts. Ind. Crops Prod. 2008, 28, 279–285. [Google Scholar] [CrossRef]
- Puttaswamy, N.Y.; Gunashekara, D.R.; Ahmed, F.; Urooj, A. Phytochemical Composition and In vitro Anti-Hyperglycemic Potency of Eucalyptus tereticornis Bark. Indian J. Nutr. 2014, 1, 1–6. [Google Scholar]
- Luís, Â.; Neiva, D.; Pereira, H.; Gominho, J.; Domingues, F.; Duarte, A.P. Stumps of Eucalyptus globulus as a Source of Antioxidant and Antimicrobial Polyphenols. Molecules 2014, 19, 16428–16446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, M.P.; Barbosa, L.C.A.; Maltha, C.R.A.; Gomide, J.L.; Milanez, A.F. Caracterização química do “pitch” em indústria de celulose e papel de Eucalyptus. Quim. Nova 2006, 29, 459–466. [Google Scholar] [CrossRef]
- Dunlop-Jones, J.N.; Huang, J.L.; Allen, L.H. An analysis of the acetone extractives of the wood and bark from fresh trembling aspen [Populus tremuloides]: Implications for deresination and pitch control. J. Pulp Pap. Sci. 1991, 17, 60–66. [Google Scholar]
- Orozco, S.E.; Zeitlinger, P.; Fackler, K.; Bischof, R.H.; Potthast, A. A solid-phase extraction method that eliminates matrix effects of complex pulp mill effluents for the analysis of lipophilic wood extractives. Nord. Pulp Pap. Res. J. 2020, 35, 577–588. [Google Scholar] [CrossRef]
- Orozco, S.E.; Bischof, R.H.; Barbini, S.; Sriranganadane, D.; Fackler, K.; Potthast, A. Fate of Lipophilic Wood Extractives in Oxygen-Based Cellulose Bleaching. ACS Sustain. Chem. Eng. 2021, 9, 4840–4849. [Google Scholar] [CrossRef]
- Gorgich, M.; Mata, T.M.; Martins, A.A.; Branco-Vieira, M.; Caetano, N.S. Comparison of different lipid extraction procedures applied to three microalgal species. Energy Rep. 2020, 6, 477–482. [Google Scholar] [CrossRef]
- Branco-Vieira, M.; Martin, S.S.; Agurto, C.; Martins, A.A.; Caetano, N. Phaeodactylum tricornutum derived biosilica purification for energy applications. Energy Procedia 2018, 153, 279–283. [Google Scholar] [CrossRef]




| Clone | Crossing | |
|---|---|---|
| 1 | E. camaldulensis × E. grandis | E. urophylla × E. grandis |
| 2 | E. urophylla × E. grandis | E. camaldulensis × E. grandis |
| 3 | E. camaldulensis × E. grandis | E. urophylla × E. grandis |
| 4 | E. urophylla × E. grandis | E. camaldulensis × E. grandis |
| 5 | E. urophylla × E. grandis | E. camaldulensis × E. grandis |
| 6 | E. urophylla × E. grandis | E. pelita |
| 7 | E. urophylla × E. grandis | E. camaldulensis v E. grandis |
| Clones | Extraction Yield 1 | Total Phenolic Content 2 | Flavonoids 3 | Tannins 3 |
|---|---|---|---|---|
| 1 | 2.6 | 471.6 | 118.5 | 33 |
| 2 | 2.2 | 367.6 | 175.7 | 56.9 |
| 3 | 2.3 | 423.8 | 256.8 | 33.7 |
| 4 | 2.5 | 379.3 | 148.2 | 28.9 |
| 5 | 1.8 | 349 | 143.8 | 40.3 |
| 6 | 2.6 | 360.2 | 221.7 | 65.1 |
| 7 | 1.9 | 447.4 | 110.5 | 37.4 |
| 8 | 1.7 | 586.6 | 236.5 | 60.6 |
| 9 | 2.7 | 321.4 | 73.6 | 28.1 |
| 10 | 1.5 | 541.4 | 112 | 53.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, T.A.S.; Arriel, T.G.; Zanuncio, A.J.V.; Carvalho, A.G.; Branco-Vieira, M.; Carabineiro, S.A.C.; Trugilho, P.F. Determination of the Chemical Composition of Eucalyptus spp. for Cellulosic Pulp Production. Forests 2021, 12, 1649. https://doi.org/10.3390/f12121649
Vieira TAS, Arriel TG, Zanuncio AJV, Carvalho AG, Branco-Vieira M, Carabineiro SAC, Trugilho PF. Determination of the Chemical Composition of Eucalyptus spp. for Cellulosic Pulp Production. Forests. 2021; 12(12):1649. https://doi.org/10.3390/f12121649
Chicago/Turabian StyleVieira, Túlio Anselmo Sacramento, Taiana Guimarães Arriel, Antônio José Vinha Zanuncio, Amélia Guimarães Carvalho, Monique Branco-Vieira, Sónia Alexandra Correia Carabineiro, and Paulo Fernando Trugilho. 2021. "Determination of the Chemical Composition of Eucalyptus spp. for Cellulosic Pulp Production" Forests 12, no. 12: 1649. https://doi.org/10.3390/f12121649
APA StyleVieira, T. A. S., Arriel, T. G., Zanuncio, A. J. V., Carvalho, A. G., Branco-Vieira, M., Carabineiro, S. A. C., & Trugilho, P. F. (2021). Determination of the Chemical Composition of Eucalyptus spp. for Cellulosic Pulp Production. Forests, 12(12), 1649. https://doi.org/10.3390/f12121649








