The Biogeography of Forest Soil Microbial Functional Diversity Responds to Forest Types across Guangxi, Southwest China
Abstract
1. Introduction
2. Materials and Methods
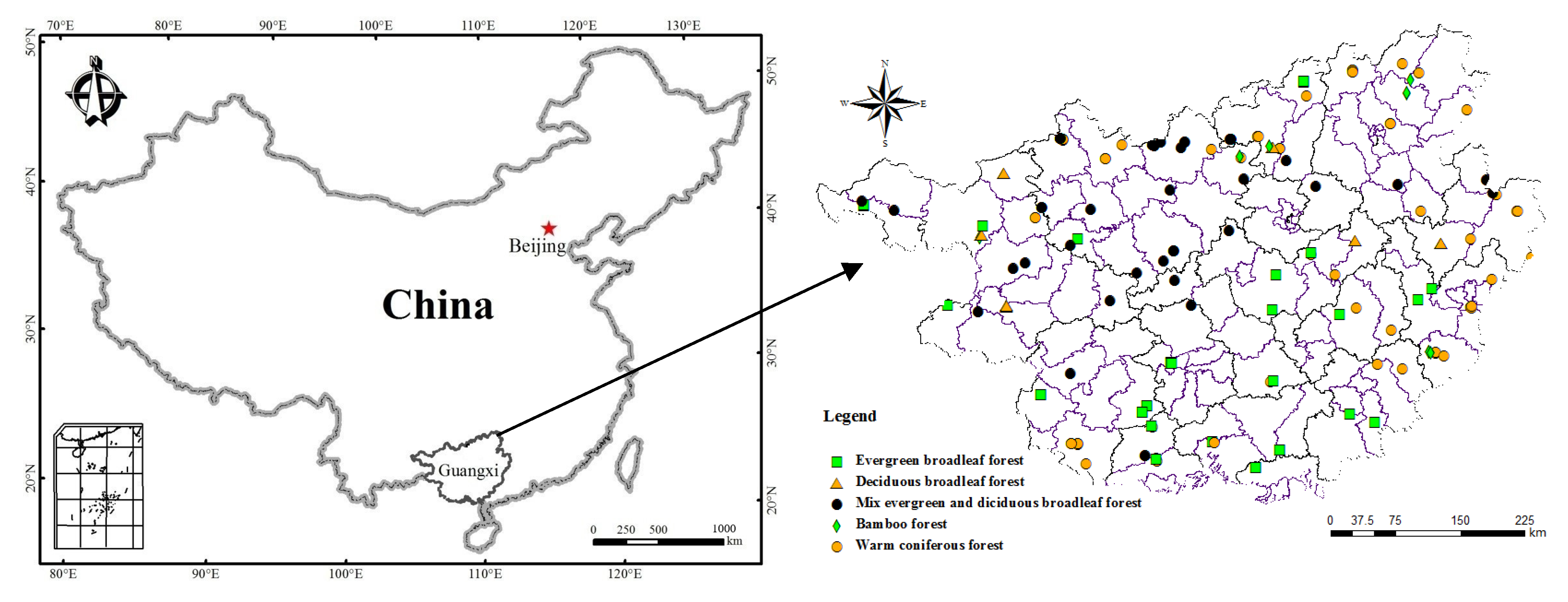
2.1. Site Description
2.2. Field Survey and Soil Collection
2.3. Analyses of Soil Chemical and Physical Properties and Collection of the Climate Data
2.4. Microbial Metabolic Activity Analysis
2.5. Statistical Analyses
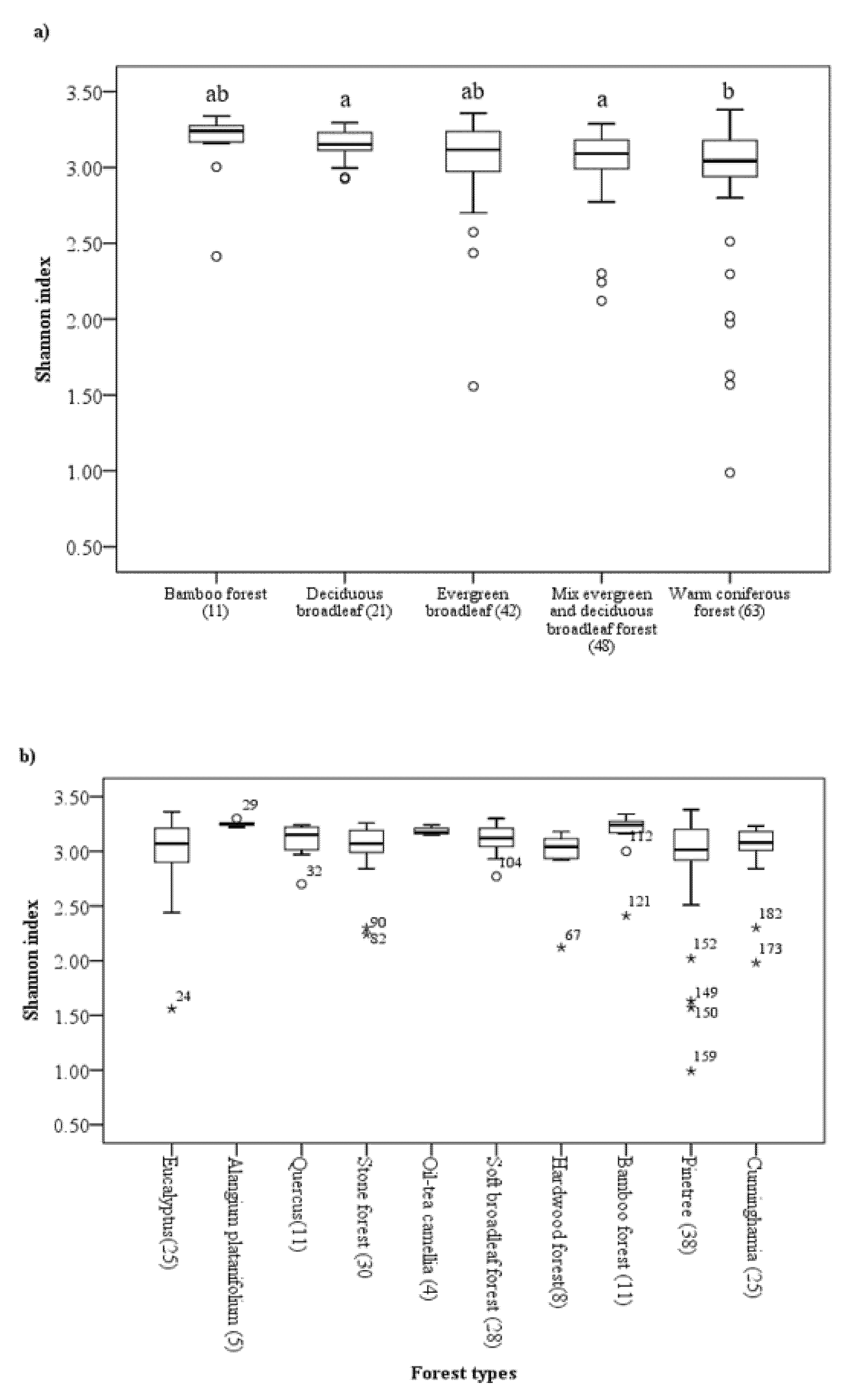
3. Results
3.1. Stand, Environmental, and Soil Characteristics across Guangxi
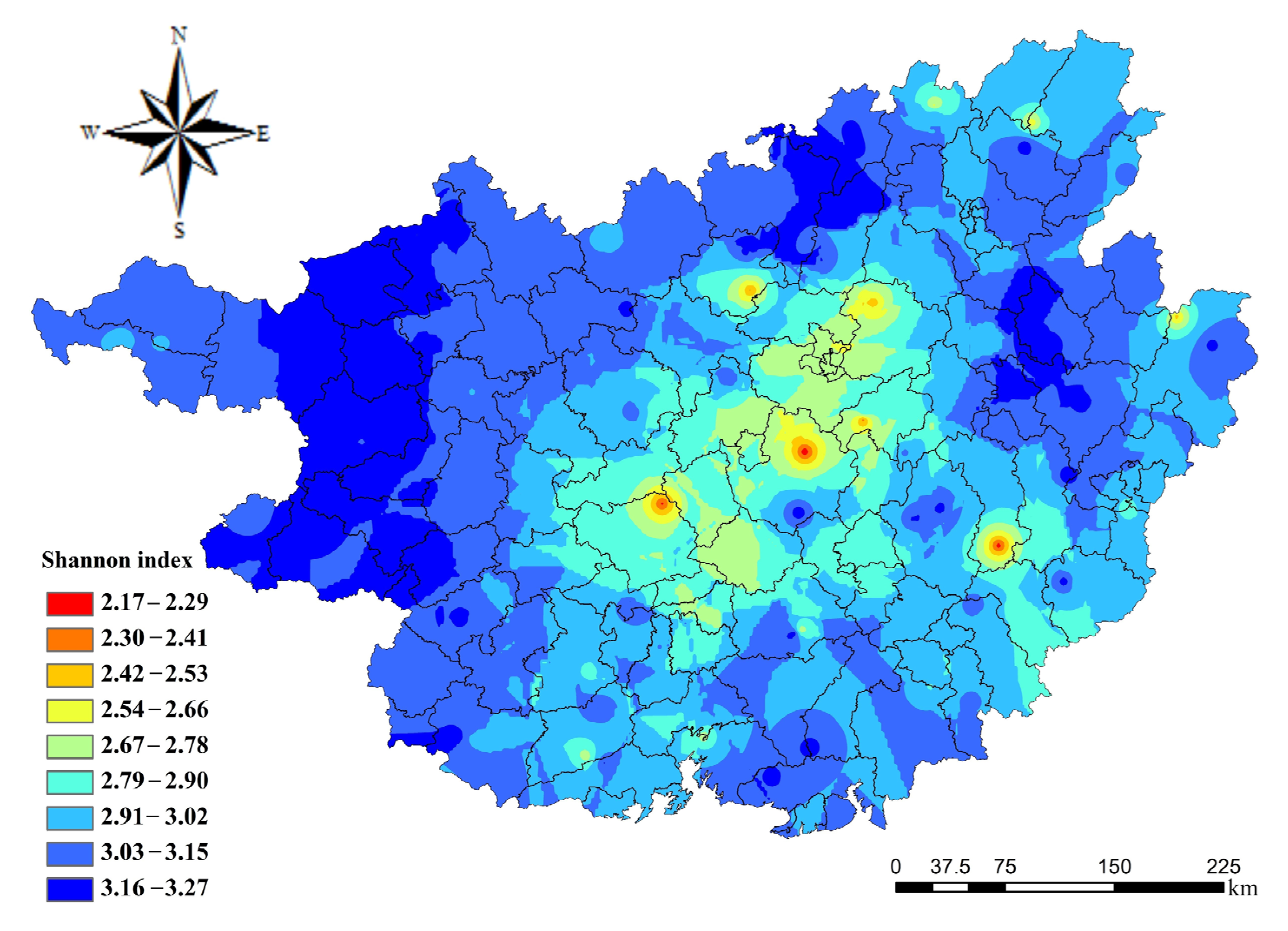
3.2. Spatial Distribution of Microbial Functional Diversity in Guangxi Forest Soils
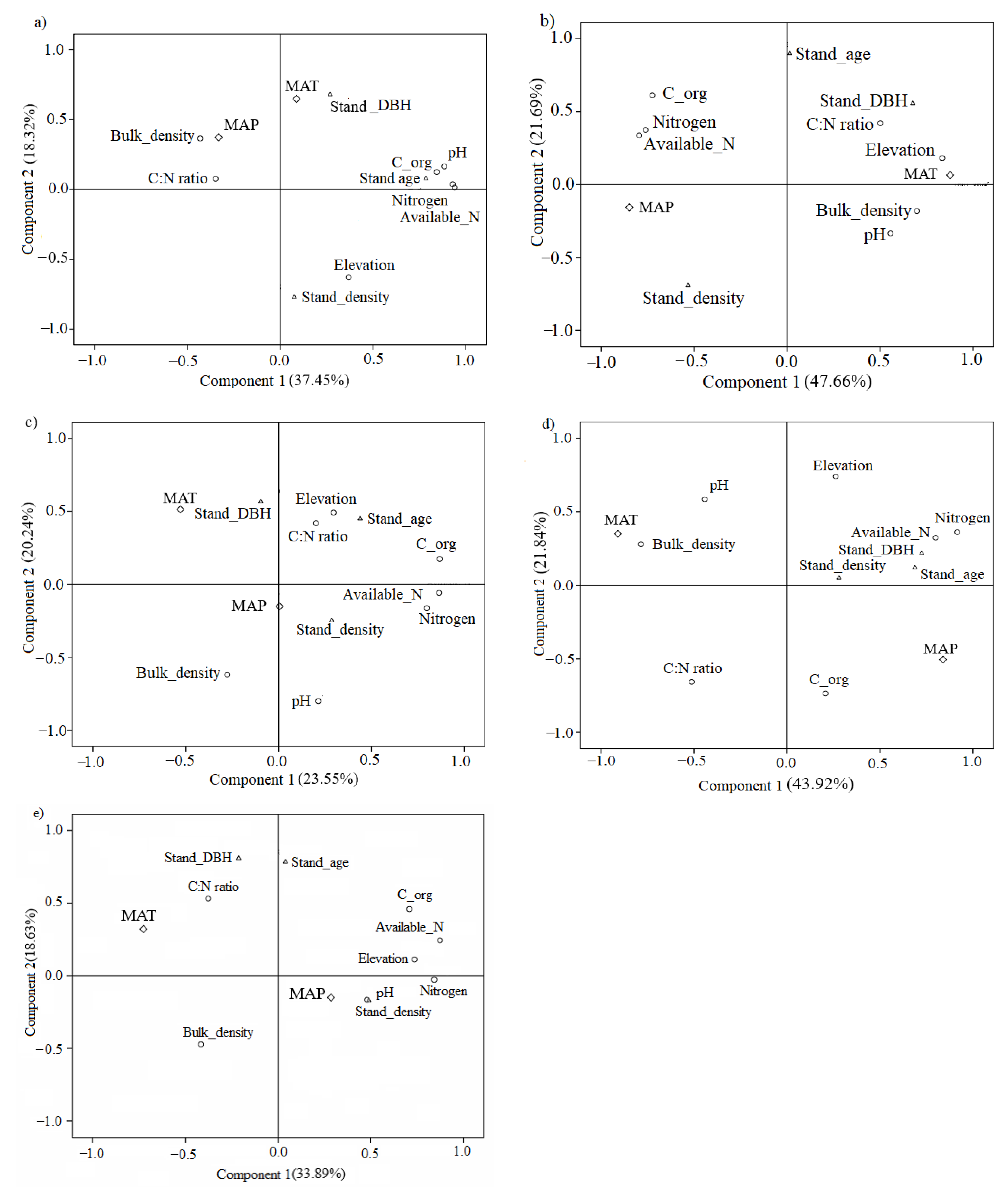
3.3. Relationships between Soil Microbial Functional Diversity and Environmental Factors
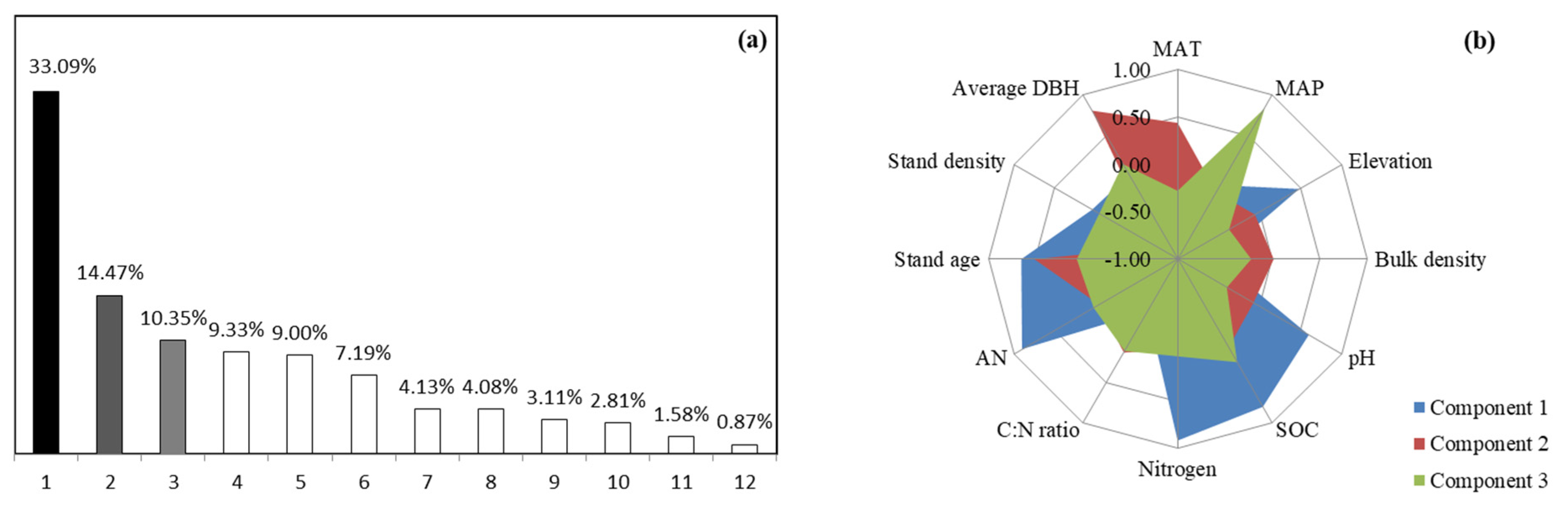
3.4. Drivers of Soil Microbial Functional Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chapin, F.S.; Randerson, J.T.; McGuire, A.D.; Foley, J.A.; Field, C.B. Changing feedbacks in the climate–Biosphere system. Front. Ecol. Environ. 2008, 6, 313–320. [Google Scholar] [CrossRef]
- Singh, B.K.; Bardgett, R.D.; Smith, P.; Reay, D.S. Microorganisms and climate change: Terrestrial feedbacks and mitigation options. Nat. Rev. Microbiol. 2010, 8, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Thornton, P.E.; Post, W.M. A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Glob. Ecol. Biogeogr. 2013, 22, 737–749. [Google Scholar] [CrossRef]
- Paul, E.A. Soil Microbiology, Ecology, and Biochemistry, 3rd ed.; Academic Press: Burlington, MA, USA, 2007. [Google Scholar]
- Song, H.; Xu, Y.; Hao, J.; Zhao, B.; Guo, D.; Shao, H. Investigating distribution pattern of species in a warm-temperate conifer-broadleaved-mixed forest in China for sustainably utilizing forest and soils. Sci. Total Environ. 2017, 578, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Hu, F.; Zeng, F.P.; Wang, K.L.; Peng, W.X.; Zhang, H.; Zeng, Z.; Zhang, F.; Song, T. Spatial distribution of tree species in evergreen-deciduous broadleaf karst forests in southwest China. Sci. Rep. 2017, 7, e15664. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a ‘Redfield ratio’ for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Wu, J.; Brookes, P.C. Measuring soil microbial biomass using an automated procedure. Soil Biol. Biochem. 2011, 43, 873–876. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef]
- Griffiths, R.I.; Thomson, B.C.; James, P.; Bell, T.; Bailey, M.; Whiteley, A.S. The bacterial biogeography of British soils. Environ. Microbiol. 2011, 13, 1642–1654. [Google Scholar] [CrossRef]
- Dequiedt, S.; Saby, N.P.A.; Lelievre, M.; Jolivet, C.; Thioulouse, J.; Toutain, B.; Arrouays, D.; Bispo, A.; Lemanceau, P.; Ranjard, L. Biogeographical patterns of soil molecular microbial biomass as influenced by soil characteristics and management. Glob. Ecol. Biogeog. 2011, 20, 641–652. [Google Scholar] [CrossRef]
- Zheng, Y.M.; Cao, P.; Fu, B.J.; Hughes, J.M.; He, J.Z. Ecological drivers of biogeographic patterns of soil archaeal community. PLoS ONE 2013, 8, e63375. [Google Scholar] [CrossRef][Green Version]
- Singh, D.; Shi, L.L.; Adams, J.M. Bacterial diversity in the mountains of south-west China: Climate dominates over soil parameters. J. Microbiol. 2013, 51, 439–447. [Google Scholar] [CrossRef]
- Fierer, N.; Carney, K.M.; Horner-Devine, M.C.; Megonigal, J.P. The Biogeography of Ammonia-oxidizing bacterial communities in soil. Microb. Ecol. 2009, 58, 435–445. [Google Scholar] [CrossRef]
- Hu, Y.J.; Xiang, D.; Veresoglou, S.D.; Chen, F.L.; Chen, Y.L.; Hao, Z.; Zhang, X.; Chen, B. Soil organic carbon and soil structure are driving microbial abundance and community composition across the arid and semi-arid grasslands in northern China. Soil Biol. Biochem. 2014, 77, 51–57. [Google Scholar] [CrossRef]
- Song, M.; Peng, W.X.; Zeng, F.P.; Du, H.; Peng, Q.; Xu, Q.; Chen, L.; Zhang, F. Spatial patterns and drivers of microbial taxa in a karst broadleaf forest. Front. Microbiol. 2018, 9, 1691. [Google Scholar] [CrossRef]
- Peng, W.X.; Zhu, Y.F.; Song, M.; Du, H.; Song, T.Q.; Zeng, F.P.; Zhang, F.; Wang, K.; Luo, Y.; Lan, X.; et al. The spatial distribution and drivers of soil microbial richness and diversity in a karst forest. For. Ecol. Manag. 2019, 499, e117241. [Google Scholar] [CrossRef]
- Regan, K.M.; Nunan, N.; Boeddinghaus, R.S.; Baumgartner, V.; Berner, D.; Boch, S.; Oelmann, Y.; Overmann, J.; Prati, D.; Schloter, M.; et al. Seasonal controls on grassland microbial biogeography: Are they governed by plants, abiotic properties or both? Soil Biol. Biochem. 2014, 71, 21–30. [Google Scholar] [CrossRef]
- Dixon, R.K.; Brown, S.; Houghton, R.A.; Solomon, A.M.; Trexler, M.C.; Wisniewski, J. Carbon pools and flux of global forest ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Carbon Sequestration in Forest Ecosystems; Springer: Berlin, Germany, 2010. [Google Scholar]
- Song, T.Q.; Wang, K.L.; Zeng, F.P.; Peng, W.X.; Du, H. Plants and Environment in Karst Areas of Southwest China; China Science Press: Beijing, China, 2015. (In Chinese) [Google Scholar]
- Wang, C.; Fu, B.J.; Zhang, L.; Xu, Z.H. Soil moisture-plant interactions: An ecohydrological review. J. Soil Sediment 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Wen, Z.; Zheng, H.; Smith, J.R.; Ouyang, Z.W. Plant functional diversity mediated indirect effects of land-use intensity on soil water conservation in the dry season of tropical areas. For. Ecol. Manag. 2019, 480, 118646. [Google Scholar] [CrossRef]
- Qiu, H.; Ge, T.; Liu, J.; Chen, X.; Hu, Y.; Wu, J.; Su, Y.; Kuzyakov, Y. Effects of biotic and abiotic factors of soil organic matter mineralization: Experiment and structural modeling analysis. Eur. J. Soil Biol. 2018, 84, 27–34. [Google Scholar] [CrossRef]
- Insam, H. A New Set of Substrates Proposed for Community Characterization in Environmental Samples. In Microbial Communities. Functional Versus Structural Approaches; Insam, H., Rangger, A., Eds.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 260–261. [Google Scholar]
- State Soil Survey Service of China. China Soil; China Agricultural Press: Beijing, China, 1998. (In Chinese) [Google Scholar]
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Garland, J.L.; Mills, A.L. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microb. 1991, 57, 2351–2359. [Google Scholar] [CrossRef]
- Lagerlöf, J.; Adolfsson, L.; Börjesson, G.; Ehlers, K.; Vinyoles, G.P.; Sundh, I. Land-use intensification and agroforestry in the Kenyan Highland: Impacts on soil microbial community composition and functional capacity. Appl. Soil Ecol. 2014, 82, 93–99. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1949. [Google Scholar]
- Fortin, M.J.; Dale, M.R.T. Spatial Analysis: A Guide for Ecologists; Cambridge University Press: Cambridge, UK; New York, NY, USA; Melbourne, Australia, 2005. [Google Scholar]
- Li, F.; Liu, M.; Li, Z.; Jiang, C.; Han, F.; Che, Y. Changes in soil microbial biomass and functional diversity with a nitrogen gradient in soil columns. Appl. Soil Ecol. 2013, 64, 1–6. [Google Scholar] [CrossRef]
- The R Development Core Team. R: A Language and Environment for Statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 1 March 2018).
- Ripley, B. Support Functions and Datasets for Venables and Ripley’s MASS [R Package MASS Version 7.3-53]; R Package Version. 2015. Available online: https://www.semanticscholar.org/paper/Support-Functions-and-Datasets-for-Venables-and-Ripley/53690bf36f2aa0d982dcabb181886c458aa7e26f (accessed on 1 March 2018).
- Stefanowicz, A.M.; Niklińska, M.; Kapusta, P.; Szarek-Lukaszewska, G. Pine forest and grassland differently influence the response of soil microbial communities to metal contamination. Sci. Total Environ. 2010, 408, 6134–6141. [Google Scholar] [CrossRef]
- Saul-Tcherkas, V.; Steinberger, Y. Substrate utilization patterns of desert soil microbial communities in response to xeric and mesic conditions. Soil Biol. Biochem. 2009, 41, 1882–1893. [Google Scholar] [CrossRef]
- Pignataro, A.; Moscatelli, M.C.; Mocali, S.; Grego, S.; Benedetti, A. Assessment of soil microbial functional diversity in a coppiced forest system. Appl. Soil Ecol. 2012, 62, 115–123. [Google Scholar] [CrossRef]
- Martiny, J.B.H.; Bohannan, B.J.M.; Brown, J.H.; Colwell, F.K.; Fuhrman, J.A.; Green, J.L.; Horner-Devine, M.C.; Kane, M.; Krumins, J.A.; Kuske, C.R.; et al. Microbial biogeography: Putting microorganisms on the map. Nat. Rev. Microbiol. 2006, 4, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Brookes, P.C.; Bååth, E. The microbial PLFA composition as affected by pH in an arable soil. Soil Biol. Biochem. 2010, 42, 183–520. [Google Scholar] [CrossRef]
- Lan, X.; Du, H.; Peng, W.X.; Liu, Y.X.; Fang, Z.L.; Song, T.Q. Functional diversity of the soil culturable microbial community in Eucalytus plantations of different ages in Guangxi, South China. Forests 2019, 10, 1083. [Google Scholar] [CrossRef]
- Wang, Y.; Ouyang, Z.Y.; Zheng, H.; Wang, X.K.; Chen, F.L.; Zeng, J.; Chen, S.B. Relationships between carbon source utilization of soil microbial communities and environmental factors in natural secondary forest in subtropical area, China. Acta Ecol. Sin. 2012, 32, 1839–1845, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Chen, L.; Xiang, W.H.; Wu, H.L.; Ouyang, S.; Zhou, B.; Zeng, Y.; Chen, Y.; Kuzyakov, Y. Tree species identity surpasses richness in affecting soil microbial richness and community composition in subtropical forest. Soil Biol. Biochem. 2019, 130, 113–121. [Google Scholar] [CrossRef]
- Yang, C.M.; Wang, Y.L.; Wang, M.M.; Chen, H.Y. Plant species influence metabolic activity and butachlor biodegradation in a riparian soil from Chongmin Island, China. Geoderma 2013, 193–194, 165–171. [Google Scholar] [CrossRef]
- Leckie, S.E.; Prescott, C.E.; Grayston, S.J.; Neufeld, J.D.; Mohn, W.W. Comparison of chloroform fumigation-extraction, phospholipid fatty acid, and DNA methods to determine microbial biomass in forest humus. Soil Biol. Biochem. 2004, 36, 529–532. [Google Scholar] [CrossRef]
- De Boer, W.; Folman, L.B.; Summerbell, R.C.; Boddy, L. Living in a fungal world: Impact of fungi on soil bacterial niche development. FEMS Microb. Rev. 2005, 29, 795–811. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F.S.; Ewel, A.; Hector, P.; Inchausti, S.; Lavorel, J.H.; Lawton, D.M.; Lodge, M.; Loreau, S.; Naeem, B.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Waldrop, M.P.; Zak, D.R.; Blackwood, C.B.; Curtis, C.D.; Tilman, D. Resource availability controls fungal diversity across a plant diversity gradient. Ecol. Lett. 2006, 9, 1127–1135. [Google Scholar] [CrossRef]
- Chen, F.L.; Zheng, H.; Ouyang, Z.Y.; Zhang, K.; Tu, N.M. Responses of microbial community structure to the leaf litter composition. Acta Pedol. Sin. 2011, 48, 603–611. [Google Scholar]
- Bending, G.D.; Turner, M.K.; Jones, E.J. Interactions between crop residue and soil organic matter quality and the functional diversity of soil microbial communities. Soil Biol. Biochem. 2002, 34, 1073–1082. [Google Scholar] [CrossRef]
- Springob, G.; Kirchmann, H. Bulk soil C to N ratio as a simple measure of net N mineralization from stabilized soil organic matter in sandy arable soils. Soil Biol. Biochem. 2003, 35, 629–632. [Google Scholar] [CrossRef]
- Ren, C.J.; Liu, W.C.; Zhan, F.Z.; Zhong, Z.K.; Deng, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Soil bacterial and fungal diversity and compositions respond differently to forest development. Catena 2019, 181, 104071. [Google Scholar] [CrossRef]
- Stephanie, A.; Mona, N. Soil bacteria and archaea change rapidly in the first century of fennoscandian boreal forest development. Soil Biol. Biochem. 2017, 114, 160–167. [Google Scholar]
- Grządziel, J.; Gałązka, A. Microplot long-term experiment reveals strong soil type influence on bacteria composition and its functional diversity. Appl. Soil Ecol. 2018, 124, 117–123. [Google Scholar] [CrossRef]
- Grządziel, J.; Furtak, K.; Gałązka, A. Community-level physiological profiles of microorganisms from different types of soil that are characteristic to Poland—A long-term microplot experiment. Sustainability 2019, 11, 56. [Google Scholar] [CrossRef]
- Chanthorn, W.; Hartig, F.; Brockelman, W.Y. Structure and community composition in a tropical forest suggest a change of ecological processes during stand development. For. Ecol. Manag. 2017, 404, 100–107. [Google Scholar] [CrossRef]
- Hume, A.; Chen, H.Y.H.; Taylor, A.R.; Kayahara, G.J.; Man, R. Soil C:N:P dynamics during secondary succession following fire in the boreal forest of Central Canada. For. Ecol. Manag. 2016, 369, 1–9. [Google Scholar] [CrossRef]
- Baldrian, P. Microbial activity and the dynamics of ecosystem processes in forest soils. Curr. Opin. Microbiol. 2017, 37, 128–134. [Google Scholar] [CrossRef] [PubMed]
| Variables | Minimum | Maximum | Mean | Standard Error | Coefficient of Variation (%) | |
|---|---|---|---|---|---|---|
| Climate factors | MAT (°C) | 19.5 | 23.1 | 21.6 | 0.7 | 4.5 |
| MAP (mm) | 1059.1 | 247.3 | 1413.7 | 24.4 | 23.1 | |
| Elevation | Elevation (m) | 24.2 | 1441.0 | 441.8 | 25.0 | 75.7 |
| Soil factors | Bulk density | 0.4 | 1.667 | 1.0 | 0.0 | 22.1 |
| pH | 3.3 | 8.0 | 4.8 | 0.1 | 21.7 | |
| SOC (g·kg−1) | 4.8 | 85.8 | 31.1 | 1.1 | 46.7 | |
| TN (g·kg−1) | 0.3 | 8.6 | 2.3 | 0.1 | 60.2 | |
| TP (g·kg−1) | 0.1 | 1.7 | 0.5 | 0.3 | 60.6 | |
| TK (g·kg−1) | 0.4 | 32.1 | 11.2 | 0.5 | 56.6 | |
| C:N ratio | 8.6 | 137.7 | 16.6 | 0.9 | 71.7 | |
| AN (mg·kg−1) | 34.7 | 634.7 | 212.0 | 8.7 | 54.6 | |
| Plant factors | Stand age (a) | 1.0 | 120.0 | 29.8 | 1.9 | 84.9 |
| Stand density (stems·ha−1) | 1700.0 | 46,400.0 | 13,749.0 | 6.2 | 59.8 | |
| DBH (cm) | 1.1 | 31.9 | 13.2 | 0.4 | 44.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, W.; Wang, F.; Song, T.; Tan, Q.; Du, H.; Zeng, F.; Wang, K.; Zhang, H.; Zeng, Z. The Biogeography of Forest Soil Microbial Functional Diversity Responds to Forest Types across Guangxi, Southwest China. Forests 2021, 12, 1578. https://doi.org/10.3390/f12111578
Peng W, Wang F, Song T, Tan Q, Du H, Zeng F, Wang K, Zhang H, Zeng Z. The Biogeography of Forest Soil Microbial Functional Diversity Responds to Forest Types across Guangxi, Southwest China. Forests. 2021; 12(11):1578. https://doi.org/10.3390/f12111578
Chicago/Turabian StylePeng, Wanxia, Feng Wang, Tongqing Song, Qiujin Tan, Hu Du, Fuping Zeng, Kelin Wang, Hao Zhang, and Zhaoxia Zeng. 2021. "The Biogeography of Forest Soil Microbial Functional Diversity Responds to Forest Types across Guangxi, Southwest China" Forests 12, no. 11: 1578. https://doi.org/10.3390/f12111578
APA StylePeng, W., Wang, F., Song, T., Tan, Q., Du, H., Zeng, F., Wang, K., Zhang, H., & Zeng, Z. (2021). The Biogeography of Forest Soil Microbial Functional Diversity Responds to Forest Types across Guangxi, Southwest China. Forests, 12(11), 1578. https://doi.org/10.3390/f12111578







