Abstract
Forests are an essential component of the natural environment, as they support biodiversity, sequester carbon, and play a crucial role in biogeochemical cycles—in addition to producing organic matter that is necessary for the function of terrestrial organisms. Forests today are subject to threats ranging from natural occurrences, such as lightning-ignited fires, storms, and some forms of pollution, to those caused by human beings, such as land-use conversion (deforestation or intensive agriculture). In recent years, threats from pests and pathogens, particularly non-native species, have intensified in forests. The damage, decline, and mortality caused by insects, fungi, pathogens, and combinations of pests can lead to sizable ecological, economic, and social losses. To combat forest pests and pathogens, biocontrol may be an effective alternative to chemical pesticides and fertilizers. This review of forest pests and potential adversaries in the natural world highlights microbial inoculants, as well as research efforts to further develop biological control agents against forest pests and pathogens. Recent studies have shown promising results for the application of microbial inoculants as preventive measures. Other studies suggest that these species have potential as fertilizers.
1. Introduction
Forests cover about 30% of the world’s land area [1] and provide an array of ecosystem services and goods that are essential to the functioning of the Earth and human society. For example, they are a vital resource for modulating climate and hydrologic systems. They also contribute directly and indirectly to the world economy by providing multiple products and services that support human health and livelihoods.
As global demand for forest products continues to rise, there is a need to manage forests for greater productivity, with ever-closer attention to the multifaceted services that forests deliver. The forestry profession faces several challenges toward that end. For example, enhancing forest health is an increasingly important goal in managing natural resources, yet the vision of a healthy forest can vary widely between utilitarian and ecosystem-focused perspectives. Historically, forest health has been defined as the forest conditions that directly meet human needs, such as how much merchantable timber is harvested per hectare. An ecosystem-focused perspective characterizes forest health in terms of resilience, persistence, and the biophysical processes that lead to the healthy functioning and sustainability of the ecosystem [2]. Regardless of the definition applied, the assessment and monitoring of the health of forests is of fundamental importance in regards to decisions about forest management and stewardship.
The changing climatic conditions may pose several threats to forest resources that demand the reconsideration of current management strategies. Forest managers respond to these challenges and uncertainties by developing and applying strategies to support economic and ecological benefits [2]. Sound management practices are essential for maintaining the productive and protective functions of forests. Implementation can protect these precious resources against catastrophic loss that results from wildfires and pollution, as well as the damage, decline, and mortality associated with forest pests and pathogens, especially invasive species.
Pests and pathogens damage millions of trees in both natural forests and commercial settings each year. The loss of trees to severe pest outbreaks can be devastating to net primary production and carbon sequestration. Mortality and reduced growth that results from disease-causing microorganisms can inflict substantial ecological and economic damage. When pests and pathogens disrupt a forest ecosystem’s goods and services, the consequences can be long-lasting and far-reaching. Non-native invasive pests pose a particular threat to the world’s forests because they have few or no natural controls in their new location, and a changing climate may exacerbate their spread and establishment [3].
Using chemical agents, such as conventional insecticides and fungicides, to control invasive pathogenic species [2,4] has several drawbacks, such as environmental disturbance, non-targeted effects, and expenses. Biological control strategies can be more cost-effective, efficient, environmentally benign, and sustainable [4]. Therefore, biocontrol of pests and pathogens has become an essential component of forest management practices. Scientists are conducting research and development to evaluate the responses of forests to these practices at different scales, to improve outcomes and reduce inputs (such as phytosanitary products).
This review first reports the impact of pests and pathogens that are involved in invasive processes within forest ecosystems. Then, we describe biological control strategies by discussing the characteristics and activities of organisms that can reduce losses and protect these precious resources. The objective was to show the potential of biocontrol agents and the implementation of biological control initiatives using the plant microbiome, which plays a beneficial role in inhibiting the establishment of pathogens and promoting plant growth. This information is useful for effective forest vegetation management and can generate new insights into targeting efforts when preventing forest diseases.
An Overview of Forest Tree Pests and Pathogens
Forests are routinely exposed to biotic and abiotic disturbances. Abiotic risks (such as fires and deforestation), whether seasonal or sporadic, are tolerated at certain thresholds because they can be incorporated into ecological processes, such as carbon cycling and the regeneration of certain species. For example, although wildfires can cause severe to catastrophic effects on forests, fire-damaged trees in other instances can rebound quickly [4]. However, biotic disturbances can leave even longer-term marks on the landscape, in part by diminishing biodiversity. Examples of biotic threats are insect pests and forest pathogens, which represent taxonomically diverse organisms such as fungi, oomycetes, bacteria, viruses, nematodes, and parasitic plants. Forest pest outbreaks and epidemics can eliminate tree species, and even some genera, forever [5]. In Europe, pest and disease outbreaks have affected cumulatively greater areas than fires [6].
The duration, frequency, and extent of pest- or pathogen-induced decline depend on factors such as the species and age of trees, their geographic location, the soil type, and genetic factors (genomic traits) [7]. Climatic conditions can have a more significant impact on forest infections than other factors [8]. The direct impacts of climate change on forest ecosystems can have severe effects on tree mortality and vegetation composition patterns, and increase the susceptibility of forests to other disturbances. A changing climate can alter the dynamics of disturbances that are caused by insect pest invasions and local forest pathogens, such as facilitating the establishment and spread of introduced pest species. Studies have shown that high temperatures and waves of drought can accelerate the life cycle of insects [4]. In addition, many important diseases result from interactions with exotic insects and pathogens, which have dramatically altered forest ecosystem diversity, function, and productivity [9]. The following sections offer an overview of different types of forest pests and pathogens, as well as their threats.
2. Insect Pests
Insect pests on trees are an aggressive biotic threat. Most of the commonly reported pest species belong to the orders Coleoptera and Lepidoptera. Species in the order of Hemiptera are also a pernicious threat [8]. Insects make their way through the tree to feed and/or build a home. Insects can feed on all parts of a tree. The species which exploit the same class of resources can be classified. This type of grouping draws attention to the ecological functions of insects and especially to their impacts on the forest. Usually, each tree species has a characteristic spectrum of associated insects [10]. For each type of mode of attack, forest insect pests include various subcortical feeders, wood borers, root feeders, twig girdlers, sap feeders, and defoliators.
Subcortical feeders are insects that attack trees by tunneling under the bark. The larvae feed in the cambium and phloem and create an interruption to sap transport between the shoots and roots, disrupting water and nutrient supply [11,12]. Subcortical feeders of the Curculionidae family are primarily represented by the mountain pine beetle (Dendroctonus ponderosae Hopkins), which can kill healthy, unstressed trees and affect a landscape scale (Table S1). Although outbreaks of subcortical feeders are strongly associated with fungal infections of trees, the damage is often an entry for diseases and other pests that attack the tree and can cause rapid and widespread tree mortality [9,13].
Wood borers or xylophagous insects are among the most destructive pests of trees. They tunnel and feed under the bark of living wood, destroying the tissue that conducts water and sap. Many borers that eat healthy wood do not directly digest cellulose but instead employ intestinal symbionts (bacteria, fungi, or protozoa) that provide wood digesting enzymes [14]. In general, borers structurally weaken their hosts and reduce their growth, resulting in the susceptible plants’ decline and eventual death. Infestation sites also provide entry points for other plant pathogens. Examples of these include the Asian long-horned beetle Anoplophora glabripennis Motschulsky (Coleoptera: Cerambycidae) and the emerald ash borer Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), destructive polyphagous insects that attack and kill healthy trees. A native of Asia, they have become serious pests of extensive forests worldwide, after numerous accidental introductions within North America and Europe [14,15].
Shoot borers are insects that bore into young, tender tree shoots and saplings. They feed inside the twigs. Once the twigs are emptied, they fall to the ground. The larvae remain in the fallen twigs, where they will eventually pupate and emerge as adults to repeat the cycle of mating, girdling, and egg-laying [16]. On the other hand, root feeders are insects that spend their larval stage within the soil around the tree. They are among the most formidable enemies of trees, particularly seedlings, due to their habit of severing roots [10].
Sap feeders constitute a relatively small proportion of species and most belong to the order Hemiptera. These insects are equipped with penetrating mouthparts, allowing them to feed on plant parts such as the tender leaves, shoots, fruits, flowers, or seeds by sucking the sap or liquefied tissues [17]. They inject toxic saliva by piercing the plant tissue, causing injury, and frequently serving as a conduit of viruses and disease. By extracting the sap, they deplete resources and weaken trees. Some species release a sugar-rich liquid called honeydew, which serves as a carrier for fungi, fumaginia, that can form crusts on foliage and block photosynthesis [18]. Evidence of attack includes the presence of galls within shoots, discoloration, necrosis and dieback, defoliation, and even tree death. Extensive epidemics of the psyllid Heteropsylla cubana Crawford and conifer aphids Cinara spp. Curtis have occurred across continents, resulting in extensive economic damage to forests [14].
The phyllophages, or defoliators, form an important insect guild within the forest ecosystem, the Lepidoptera being the most prominent (Table S1). Defoliators are insects that damage trees by feeding on their leaves or needles. Damage from defoliating insects varies widely depending on the tree and insect species, feeding intensity, and the time of year when feeding occurs [10]. Defoliation can kill trees directly, or weaken trees and, consequently, predispose them to attacks by other insects or pathogens. Defoliators are primary pests: they attack healthy and vigorous trees, paving the way for secondary pests. In general, high levels of defoliation can occur for several consecutive years before trees are killed. On the other hand, some defoliators are significant forest pests and can kill trees in large landscapes during epidemics. For example, Lymantria dispar L. (Lepidoptera: Lymantriidae) is a destructive defoliator that is classified as a quarantine pest in many parts of the world [19,20].
Insects become particularly damaging when they are introduced into an area outside of their native range. Non-native (sometimes also called exotic or invasive) insects have attracted a great deal of attention due to their devasting impact and rapid spread across continents, as well as their strong competitive ability in novel environments without natural controls. Commercial trade in imported live plants or wood materials is the primary source of introduction for many of these species [21,22]. Insects that are native to Asia have been introduced to Europe; others have migrated from the Western Hemisphere to Europe [21]. In the opposite direction, many European insects have been found in New Zealand [21]. More than 450 non-native insect species have been introduced in the United States [23]. In their native settings, such insects generally colonize only dead or dying trees and are sometimes not recognized as pests. After their accidental introduction into these new ecosystems, these pests become aggressive and particularly harmful, because the predators that have kept them in check in their native habitats may not be present in these newly invaded ecosystems. Non-native insects target healthy trees that typically lack the resistance to these recently arrived species. They are responsible for the mortality of millions of trees in Europe and North America, and their potential for invasion and subsequent spread constitutes a major threat to the health of the world’s forests [23,24,25].
3. Pathogens
3.1. Phytopathogenic Fungi
Fungi are the most common causative agents of tree disease, with various invasion mechanisms and an arsenal of virulence factors [26]. The fungal pathogens are found primarily in the phyla Basidiomycota and Ascomycota [26].
3.2. Root Rot
The Armillaria Staude basidiomycete fungi are a globally distributed group of pathogens that cause root rot disease in a wide variety of hosts, primarily woody forest plants (both coniferous and deciduous species) [27]. Through their rhizomorphs (a collection of intertwining fungal threads) and their bioactive metabolites that facilitate infection, they colonize the roots of living trees and feed on their host, thereby allowing for their long-term persistence on the dead residues of infected plants [28]. In addition to root decay, other severe symptoms include wilting, premature defoliation, and the production of dwarf leaves and fruits. Among the most virulent species are A. mellea, A. ostoyae, and A. luteobubalina. These native components of forest ecosystems can cause wood decay, reduced growth, and even mortality, especially in those trees that are stressed by other factors, or in young trees planted on infected sites. After the host’s death, Armillaria changes from the parasitic phase to the saprophytic phase and persists in the rhizosphere as white, thick mats of mycelium (white rot) under the bark of infected roots [28].
Various Heterobasidion spp. Bref cause another fungal root rot disease. This fungus is widely distributed in the coniferous forests of the Northern Hemisphere, causing infection mainly within roots. Root rot caused by the species H. annosum is among the most destructive diseases of conifers, especially in Europe, North America, China, and Japan [28]. A distinct host preference also characterizes these species. Heterobasidion annosum is associated primarily with pines, particularly the Scots pine (Pinus sylvestris L.), but attacks several other conifers and even deciduous species. Heterobasidion irregulare attacks pines, junipers (Juniperus spp. L), and frankincense cedar (Calocedrus decurrens Florin), while H. occidentale infects a broader range of hosts, mainly Abies Miller, Picea Dietrich, Tsuga Carriere, Pseudotsuga, and Sequoiadendron Buchholz. Relatively mild temperatures are favorable to the production of the spores of Heterobasidion spp. [29]. Climate changes toward warmer winters would increase the sporulation frequency and the incidence rate of infections that are caused by Heterobasidion spp. [29]. These fungi have long-term effects on tree growth and survival, as well as on the overall productivity of forests [28,29].
3.3. Rusts
The most important rusts within forests are the rusts of pine stems and cones [30] which are caused by the Melampsora spp. Castagne, which is a macrocyclic (producing five types of spores during the life cycle) and heteroic (requiring two hosts to complete its life cycle) fungus. These rusts often affect several host species of the genus Populus L. and other trees of the Salicaceae family, including various poplars, aspens, and willows throughout the world. The disease is caused by several species of the genus Melampsora, including M. larici-populina (mainly in Europe), and M. medusa and M. occidentalis (in North America) [30]. Tree pathogens present in the soil also include Rhizoctonia spp. These fungi are distributed worldwide and can produce sclerotia (firm multicellular resting structures) that overwinter in the soil. Members of this genus have significant plant pathogenic potential and a wide host range, including conifers, where the fungus can cause damping-off and root damage [30]. White pine blister rust is another devastating disease, caused by the obligate bio-trophic fungus Cronartium ribicola Fisch, which is widespread in North America and Europe. The development of its life cycle requires the passage of two stages within Pinus spp. and three stages within the alternative host Ribes spp. L. [31].
3.4. Wilt Fungi
Among ascomycetes, the two most pervasive genera are Fusarium Link and Verticillium Nees. The genus Fusarium occurs worldwide and can be found in the soil and aerial parts of plants. It is considered one of the primary pathogens of crop plants [32]. Some species of this genus appear to be adapted to particular climatic regions, while others are unaffected by the climate, occurring in the subtropical and temperate tropics [33]. The Fusarium oxysporum species complex is responsible for Fusarium wilt (vascular wilt disease), which induces systemic infection in economically important crops [33]. The pathogen F. circinatum is the causal agent of pitch canker, an important disease of Pinus spp. that has been particularly damaging worldwide since the 1980s [34]. Fusarium wilt results in reduced growth of mature trees, as well as considerable economic and ecological losses. Verticillium may also be responsible for vascular wilt or other leaf diseases [34]. The soil-borne pathogen V. dahliae attacks more than 200 tree species worldwide; it can survive for years in the soil via germination of its microsclerotia, and can subsequently spread over long distances [34]. Elm (Ulmus spp. L.), cork oak (Quercus suber L.), elderberry (Sambucus spp. L.), maple (Acer spp. L.), oak (Quercus), and walnut (Carya spp. Nuttal) are the forest trees that are most susceptible to the infections caused by V. dahlia [32]. We list other important pathogenic fungi in Table S2.
4. Oomycetes
The class of the Oomycetes (phylum Oomycota) contains the genera Phytophthora de Bari and Pythium Pringsh., which are known to be causal agents of certain root rots (Table S3). The most devastating Phytophthora spp. in trees infect many hosts and have been responsible for severe forest epidemics worldwide [35]. These fungi can lead to water and nutrient stress and a change in tree physiology [35], resulting in tree defoliation, chlorosis, and frequent death. Phytophthora cinnamomi has been described as the most destructive pathogen because it can infect almost 5000 host plant species [36]. Scientists classify this species as one of the 100 worst non-native invasive species—one of only three phytopathogens on this list [37]. Its accidental introduction has had disastrous consequences for the overall biodiversity and selected forest ecosystems in Europe [36,37]. It is considered to be one of the primary causes of mortality in holm oak in Europe [37], as well as eucalyptus dieback in Australia [35,37]. Other species of Phytophthora have been the cause of epidemics. Phytophthora ramorum, which causes the disease known as sudden oak death, has led to the death of more than 1 million plants in the family Fagaceae since the mid-1990s, those being primarily oaks and related genera within California and Oregon (United States). Death can strike quickly (less than two years) or take several years. The most vulnerable species are currently the native oaks of the West Coast of the United States and the Japanese larch (Larix kaempferi) in England, all of which suffer high mortality upon infection. A symptom of this disease is stem bleeding, where a dark, sticky fluid oozes from cracks in the tree trunk. Relatively warm and humid winter and spring conditions are ideal for zoospore proliferation and host infection, and in times of drought, the plant’s root system is vulnerable to mortality [38].
Interest in prospecting for Phytophthora in natural ecosystems increased after several Phytophthora spp. were implicated in severe epidemics of forest decline and tree mortality [39]. Additional Phytophthora spp. continue to emerge as important pathogens within agricultural systems, and preventing their movement and establishment is necessary in order to limit their harmful effects. The ecology and pathogenic status of some Phytophthora spp. (such as P. multivora, P. polonica, and P. virginiana) remain uncertain [39,40]; they may yet cause severe damage when introduced into new environments. Studies on the host range and ecological roles of these agents are warranted and suggest that some species will, over time, become as pathogenic as P. cinnamomi and P. ramorum [39].
Like Phytophthora, the genus Pythium is potentially infectious for trees. Pythium spp. are prominent soil pathogens. They cause root rot and damping-off in the Aleppo pine (Pinus halipensis) [41] and Douglas-fir (Pseudotsuga menziesii Franco) [42], as well as seedlings of conifers in the wild and in forest nurseries [41,42]. Typical symptoms of infection by Pythium spp. include soft and rotten seeds before germination, damping-off before or after emergence at the seedling stage, and discoloration of the hypocotyl and root rot in the late-growth stages [43]. The increased prevalence of this seedling disease has been associated with the relative abundance of pathogenic Pythium spp. [43]. Pythium can colonize the plant residues that have been left on the soil by the previous crop, causing an accumulation of inoculum in the seedbed. The repetition of several cycles over time increases the pathogenic Pythium populations within the soil [44], which spread rapidly and lead to severe yield losses.
These oomycetes remain resistant structures in either soil, infected roots, or debris under unfavorable conditions [37], and await suitable biotic and abiotic conditions for germination. The resulting sporangia production and subsequent release of zoospores then infect new root hosts [45]. Other Phytophthora and Pythium spp. have recently emerged as invasive pathogens with the potential to inflict enormous environmental damage.
5. Phytopathogenic Bacteria
Although many taxa of soil bacteria are essential to plant health, bacterial communities may harbor phytopathogenic agents. Bacteria are the causal agents of disease in a wide variety of plants worldwide [44,45] (Table S4). These organisms, known as phytopathogenic bacteria, affect plants by colonizing their surface or tissues [45]. Only a few taxa are directly or indirectly associated with the diseases of forest trees and forest ecosystems [46]. They cause symptoms such as spots, burns, cankers, tissue rot, and hormonal imbalances that lead to plant overgrowth, root branching, and leaf epinasty [45]. Unlike fungi, bacteria cannot directly enter their host. They must enter through natural plant openings or wounds [47]. Upon infecting a plant, bacteria release extracellular enzymes that break down plant cells. Bacteria then invade and colonize the spaces between the plant’s cells. In addition, bacteria produce large amounts of polysaccharides that obstruct the vascular system and reduce water movement through the xylem. Other metabolites interfere with certain essential physiological processes and prevent photosynthesis. Phytopathogenic bacteria survive on trees around perennial cankers, within vascular systems, or in association with the roots [48]. On the other hand, their survival in the soil depends on the species’ ability to persist in a hostile environment, until they infect a new host [47,48]. Along with other plant pathogens, abiotic stressors, climate change, and chemical pollution, bacterial pathogens pose a global threat to plant production [48].
Two bacterial species, Rhizobium radiobacter Beijerinck and van Delden and R. rhizogenes (formerly known as Agrobacterium tumefaciens and A. rhizogenes, respectively), can induce tumor formation within many economically important tree crops [49]. Tumors that form on or near the root crown are characteristic of crown gall, which is primarily a problem in agriculture, often with woody plants. Despite many host species, only a small proportion are susceptible to the development of appreciably sized tumors [49]. Most conifer species are resistant. When the bacteria encounter injured cells, they inject their plasmid, which is subsequently incorporated into the host’s chromosomes. The new genes cause an increased production of hormones, such as auxins and cytokinins, which then stimulate localized growth. The resulting galls provide a nutrient-rich environment for the pathogen. Eventually, the galls break down, and the bacteria return to the soil. In some cases, the pathogen can move up the stem and branches, triggering galls higher in the plant [50].
Bacterial leaf blight caused by Xylella fastidiosa Wells (also known as Pierce’s disease), a bacterium with a wide range of hosts, has been detected in many forest tree species in the United States [51]. Leaf “burns” can occur on elm (Ulmus spp.), maple (Acer spp.), mulberry (Morus spp. L.), oak (Quercus spp.), and sycamore (Platanus spp. L.) [51]. Each host group has specific variants of the pathogen, indicating a highly specialized host–pathogen relationship. Diseased plants express symptoms of marginal burn-necrosis; their leaves turn black with a yellow border, or other discoloration, and curl, followed by plant wilting and mortality. Leaf wilt results from the systemic colonization of plant vessels by the multiplication of bacteria, as well as biofilm production, which blocks xylem circulation and impedes the movement of water and nutrients within plants [52]. Xylem-feeding beetles or leafhoppers carry pathogens. These vectors can transmit the disease effectively in nurseries. Bacterial growth in the biofilm state is also necessary for the insect vector to acquire the bacteria from infected plants. Bacteria are obligate parasites that are difficult to cultivate. They live only in the xylem of infected plants and in the intestines of insect vectors, which transmit them directly to the xylem of host plants [52]. Xylella fastidiosa is a growing problem in agriculture; it is well known in the United States, but this global threat has also been found in Europe and Asia.
Ralstonia solanacearum Smith is also known as a bacterial wilting agent. This soil bacterium attacks plants through their roots, spreads through the vascular system, and eventually causes death. Infection with this bacterium causes the appearance of several symptoms: permanent wilting of the leaves, rotting of the stem, loss of the phelloderm near to the ground, and the discoloration of vascular tissues [53]. Eucalyptus wilting has long been attributed to R. solanacearum and R. pseudosolanacearum. However, data collected by Coutinho and Wingfield [54] suggest that Ralstonia bacteria are opportunistic pathogens that can proliferate when other abiotic stresses weaken tree defenses, but that may not be directly linked to Eucalyptus wilting.
Pseudomonas syringae pv. aesculi Van Hall is a bacterial species that is devastating to trees. It is responsible for the epidemic of bleeding canker among European horse chestnuts (Aesculus hippocastanum L.). First appearing in the aerial parts of the tree, lesions appear on the cortex and the phloem and then spread over the cambium, in the form of a lethal continuous canker [55].
Plant pathogens of the genus Xanthomonas Dowson are of particular concern because they can cause disease in almost all economically important crops [56]. Poplars and willows are particularly susceptible to the cankers caused by X. populi. Cankers can start with small blisters. The foliage of infected branches and stems may have black spots and then die. The mechanical failure of cankers, branch dieback, and even mortality can result [56]. Xanthomonas spp. are found in the United Kingdom, Ireland, and elsewhere in Western Europe.
A recent study has shown that a polymicrobial complex consisting of Brenneria goodwinii Brenner, Gibbsiella quercinecans Gibbs, and Rahnella victoriana Rahn may induce acute oak decline, which is associated with the beetle Agrilus biguttatus Curtis [56,57]. Another study, conducted on trees exhibiting symptoms that were similar to acute oak decline—Quercus castaneifolia (chestnut-leaved oak), Q. brantii (Persian oak), and Carpinus betulus L. (hornbeam)—used genotypic tests to show that B. goodwinii, B. roseae subsp. Roseae, and Rahnella victoriana are linked to the symptoms observed on these trees [57,58].
Downy mildew is an emerging disease of red oaks that is caused by a Kermes scale insect (Allokermes galliformis Riley) which interacts with the bacteria Lonsdalea quercina subsp. quercina Hildebrand and Schroth. The pathogen infects the wounds made by insects within developing acorns. When infected, Quercus spp. exhibit cankers and a dripping bud symptom [58]. For many years this disease seemed to be confined to California (USA). However, it was found in Spain in 2003 [58,59]. In 2010, the pathogen was identified within urban oak trees in Colorado (USA) and California [59]. Its range of hosts has expanded beyond the reported host range of A. galliformis (which includes the range of pin oaks (Q. palustris)) and now encompasses northern red oak (Quercus rubra) and Shumard oak (Q. shumardii) [59,60].
Poplars are also prone to attack by various microbial pathogens. Among them are cankers which are caused by a potentially fatal bacterium, Lonsdalea populi (formerly L. quercina subsp. populi) [61,62]. These diseases, characterized by a canker with white exudates, are confined to the bark of the trunk and branches, primarily in summer [62]. A large percentage of the plantation area of P. × euramericana hybrid poplars in China and Hungary is affected by L. populi, which causes extensive damage to the plantations [63].
6. Phytoplasmas
Phytoplasmas Doi are bacterial plant pathogens belonging to the class Mollicutes. They are assigned to the taxon Candidatus Phytoplasma and are obligate, intracellular parasites of plant phloem. In the host plant, they colonize the nutrient-rich phloem sap, and are transmitted from plant to plant by homopterous insects that feed on phloem (e.g., leafhoppers and psyllids) [64,65]. Phytoplasmas cannot be transmitted mechanically; however, they can spread through the use of infected vegetative propagation material [65,66]. These phytopathogenic bacteria are associated with diseases in over 1000 plant species, including forest trees. Phytoplasmas induce a wide range of symptoms that are either specific (such as virescence, phyllody, witches’ brooms, rosettes, out-of-season growth, and the brown discoloration of phloem tissue) or non-specific (such as yellowing and leaf reddening, small leaves, leaf curl, premature fall color, premature defoliation, reduced growth, dieback, and decline) [66]. However, the expression of symptoms is highly variable depending on the host plant. Most phytoplasma strains have been grouped according to phylogenetic analyses and named according to their symptoms or trees colonized (Table S4). A well-known example is the elm yellows (EY), a disease that results in decline or death that affects several elm species, and is widespread in North America and Europe [67]. In the United States, the disease spreads gradually, causing a considerable loss of native elms.
Alder yellows (ALY), occurring on several species of Alnus Miller (alder), is a disease of decline that is found in several countries. Several Populus spp. are affected by poplar witches’ broom (PopWB). Ash yellows (AshY) is a disease of ash known in North America [65,67]. This disease is associated with the agent AshY “Ca. Phytoplasma fraxini”, a member of the AshY phytoplasma group, subgroup 16SrVII-A [66]. In recent years, phytoplasmas have been detected and identified at the molecular level within several species of conifers. Marcon [67] reported the appearance of a taxon, Ca. Phytoplasma pini, on Pinus sylvestris (Scots pine) and P. halepensis cultivated in Germany and Spain, respectively.
7. Viruses
Viral pathogens are present within plants of every ecosystem and lead to substantial losses worldwide in agriculture, horticulture, and forestry [68]. Viruses have infected trees and caused diseases for centuries before they were detected and identified as the causal agents of these diseases. The symptoms associated with viral diseases can differ substantially from those that are attributed to bacteria and fungi. Some viruses produce distinctive symptoms in plants, while others are more difficult to detect. Viral infections result in extensive tissue damage [68] and can induce necrotic and chlorotic lesions, ring spots, and yellowing. Symptoms are often mistaken for mineral deficiency, ozone damage, or drought. Infected and decaying plants, as well as living root tissue, release viruses into the soil [68,69] (Table S5). Stable viruses (e.g., tobacco mosaic virus) can be spread without vectors. Other viruses require vectors, either biological (e.g., aphids, leafhoppers, fungi, mites, nematodes, beetles) or others (e.g., water, soil, other plants, organic debris). For instance, soil fungi can transmit the tobacco necrosis virus. Some plant genera, such as Carpinus L., are susceptible to only one or two virus species, whereas others, such as Betula L. and Fraxinus L., are hosts for several virus species [68]. The tobacco necrosis virus can infect a variety of hosts, including Populus spp., Pinus sylvestris, and Picea abies [69]. Tomato mosaic virus can also infect willow, causing brown necrotic lesions on the leaves. Viruses recovered from many trees have been identified, but other viruses have not yet been classified, such as maple mosaic virus and oak ring spot virus [69]. European mountain ash ringspot-associated virus (EMARaV) has been described by Mielke and Mühlbach [69] and classified by the International Committee for Taxonomy of Viruses (ICTV) [68,69,70]. This phytopathogen is probably a new kind of virus [69,70]. This genus, Emaravirus, belongs to the family Fimoviridae, which includes several species that are associated with EMARaV. Fimoviruses are transmitted to plants by eriophyid mite vectors, and induce similar characteristic cytopathologies within their host plants [70]. Elucidation of the complex nature of viral infections in trees requires the development of more refined diagnostic techniques, to confirm the classification of known viruses, identify unknown viruses, and increase the understanding of the diversity of arthropod vectors.
8. Nematodes
Although nematodes are natural inhabitants of forest soils, they have received less attention than tree pathogens. Their importance as phytopathogens is difficult to quantify, because the number of nematodes that have been found in soil is highly variable within and across terrestrial biomes, ranging from tens to thousands of individuals per 100 g of soil. Symptoms that are caused by nematode infections are difficult to differentiate from those caused by other pathogens. Symptoms take a long time to appear, slowly worsen, and can lead to death [71]. The pathogenicity of nematodes is largely influenced by the environmental conditions and the host’s susceptibility and resistance (such as the availability of organic matter, soil type, and soil texture). Nematodes are vectors of several phytopathogenic viruses. For example, nematodes belonging to the families Longidoridae and Trichodoridae are known vectors of Nepovirus and Tobravirus, respectively, and make infested trees more vulnerable to secondary infections by other pathogens [71].
Since nematodes are usually found in the soil, their primary target tissue is the root system. The root lesions that are generated differ depending on the causal agent and the host species [71,72]. Some nematodes cause necrotic reactions; others can induce gall formation on the roots, or cause swollen and coarse roots. They induce hyperplasia and hypertrophy, which result in major deformations of the root system. In more severe cases, they cause the total inhibition of root development. Xiphinema americanum Cobb. (Dorylaimida: Longidoridae) infects bay oak (Quercus laurifolia), and manifests itself by surface browning [71,72]. Pratylenchus brachyurus Godfrey (Tylenchida: Pratylenchinae) enters the root tissue, forms aggregates, and induces deeper necrosis within yellow poplar (Liriodendron tulipifera). Generalized cortical necrosis, observed within pine (Pinus elliottii and P. taeda), is caused by Hoplolaimus galeatus Thorne (Tylenchida: Hoplolaimidae) [71,72].
Bursaphelenchus is classified as a quarantine pest [72]. The migratory endoparasite B. xylophilus Nickle (Aphelenchida: Parasitaphelenchidae) is the causal agent of pine blight disease, a destructive disease of conifers worldwide [72]. This disease threatens coniferous forests’ biodiversity and faunal complexity, and can cause extensive economic loss [73,74]. This devastating agent is native to North America and migrated to Europe after causing widespread damage since its accidental introduction into Asia [73]. Beetles in the genus Monochamus Dejean (Coleoptera, Cerambycidae) transmit the pinewood nematode. When inoculated into healthy branches, it rapidly migrates to the main trunk through the resin channel system. The nematode enters the internal tissues and begins to multiply rapidly, ultimately invading all the tissues of the tree. When the nematode reaches its adult stage and arrives at the xylem, embolisms occur due to damage to the water-conducting channel. Thus, photosynthesis is blocked, manifested by a browning or reddening of the needles, and the tree dies quickly [73]. Bursaphelenchus xylophilus feeds on the tissues of the tree, as do the the fungi that colonize it, in response to production of these extracellular peptidases [74] and various other proteins such as cellulases, calreticulin [75], expansins, and pectate lyase [76]. Bacteria that are associated with B. xylophilus can increase the virulence of this nematode [77], and researchers have concluded that the nematode injects these phytotoxin-producing bacteria into the tree [76,77]. This process of co-action has not been well documented, but there is some evidence that the aseptic nematode is incapable of causing disease [76,77,78]. The characteristics of the nematode and the various interactions among the beetle vector, host tree, and the colonizing fungi determine its pathogenicity and disease establishment [78].
The root-knot nematodes, Meloidogyne, are ubiquitous plant pests that parasitize almost all vascular plant species. These nematodes have a set of cellulolytic and pectolytic enzymes that allow them to degrade the root wall and penetrate it. During their second stage, they induce hypertrophy and hyperplasia following the formation of coarse multinucleate cells, which feed on the root and form a pear-shaped structure that is characteristic of infection [75,76,77,78]. In addition to the typical symptom of root deformation, other symptoms include stunted growth, wilting, and leaf discoloration [78].
Meloidogyne incognita Kofoid and White (Tylenchida: Heteroderidae) infects Acacia spp., flowering dogwood (Cornus florida L.), and kadam (Neolamarckia cadamba Roxb.), while Meloidogyne ovalis has been reported as a pathogen of sugar maple (Acer saccharum) and white ash (Fraxinus americana). Meloidodera floridensis Chitwood; Hannon and Esser (Tylenchida: Heteroderidae) is another species that infects pine trees. Some research did not report observations of gall formation, but did note the presence of hypertrophy and hyperplasia [72,73,74,75,76,77,78] (Table S6).
9. Biological Control Strategies against Phytopathogenic Agents
For decades, people have relied on chemical pesticides to combat the persistent threat of pests and pathogens to plants, in general, and to forests in particular. Scientific research has brought to light the harmful effects of pesticides on the natural environment and human health. In addition to killing target species, pesticides may leave residues which are toxic to non-target species, and contaminate soils by persisting within them for years [79]. These residues can exhibit “cumulative toxicity”, such that upon entering the hydrologic system, they can reach concentrations that are dangerous or lethal to terrestrial and aquatic organisms [80]. A study carried out in 2018 indicated the presence of 260 pesticide residues in honeybee samples, collected from several apiaries in Spain [81]. In addition to residues, the products of decomposition or other chemical transformations of compounds in pesticides can be harmful to the environment, often persisting for a long time in nature [79,80,81]. The risk from pesticides in forests is two-pronged: persistent airborne organic pollutants can be transferred to the soil. For example, studies in fir forests have found organochlorine pesticides originating as airborne pollutants, that are then deposited in the soil [82]. In addition to environmental and ecological hazards, pesticides can endanger human health. They can be potent carcinogens, due to their effects on the endocrine and immune systems and their potential cumulative effect on the human body [83].
Given the enormity of pest threats and the limitations of alternative control methods, researchers have looked to biological control methods for solutions. Demonstrations of biological control as a promising approach for pest control date back at least to the 19th century. The earliest documented use of a biocontrol agent was in 1889, when the American entomologist Riley used the entomophagous ladybug Rodolia cardinalis Mulsant. (vedalia beetle) to fight against the exotic scale insect pest Icerya purchasi Maskell, which attacks citrus trees. It took only two years to dramatically reduce the population of pests and their damage [84]. Since then, there has been a steady stream of reports of the successful use of biological control agents, and biological control has assumed a prominent place in the pest control toolbox. Biological control, or biocontrol, is defined as “the use of living organisms to reduce or prevent damage caused by pests,” according to the International Organization for the Biological Control of Pests and Animals. This classic and broad definition, which is limited to living organisms, has undergone many changes. According to the Food and Agriculture Organization of the United Nations [85], biological control is any use of biological agents (insects, microorganisms, microbial metabolites) to control mites, pests, phytopathogens, or spoilage organisms. The International Standard for Phytosanitary Measures in 2005 has introduced auxiliaries, antagonists, competitors, insects, and other agents into the biocontrol strategy. Most types of biocontrol agents are described in the following summary (Figure 1).
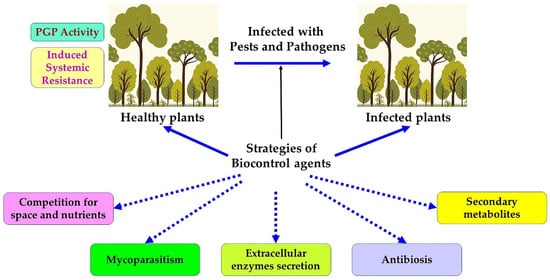
Figure 1.
Different strategies of biocontrol agents.
10. Entomopathogenic Fungi
Entomopathogenic fungi (EPF) are among the natural alternatives to chemical insecticides, due to their ability to invade a wide range of insect pests, as well as their various mechanisms for evading the immune system of their hosts [85,86]. Among the most widely described potential EPFs are the Ascomycete species Beauveria and Metarhizium. These are increasingly being used on a commercial scale in many countries [85].
Several studies have shown that Beauveria (Bals.) Vuill. (Ascomycota: Hypocreales) is one of the most important entomopathogenic fungi that is distributed around the world [86]. Beauveria bassiana is a cosmopolitan, soil-dwelling, hyphomycete fungus. Its host range extends to insects of all kinds, and it can also inhibit the growth of other microorganisms [87]. It is highly pathogenic and causes considerable mortality in adult insects that encounter its infectious propagules, or conidia. Beauveria bassiana is known to produce cyclic peptides that are cytotoxic and immunosuppressive [86,87]. This fungus also produces various secondary metabolites, including non-peptide pigments and polyketides, such as oosporein, peptides that are synthesized non-ribosomally, such as beauvericin, and secreted metabolites that play a role in pathogenesis and virulence [88] in addition to their insecticidal properties. Beauveria bassiana causes white muscardine within host arthropods when the host comes into contact with the fungus’ conidia. These conidia adhere to the host’s body and penetrate the cuticle, where they proliferate and produce beauvericin. This peptide induces the progressive degeneration of the host’s tissues and obstructs their hemolymph circulation, leading to asphyxia and, a few days later, death. The fungus continues to grow saprophytically, and, under favorable conditions, the hyphae emerge from the intersegmental areas of the insect’s corpse, producing conidia.
Beauveria bassiana has a great potential to be an ecological and sustainable alternative to control important insect pests, by parasitizing insects such as Xylotrechus rusticus L. [88], spruce beetle (Dendroctonus rufipennis Kirby), Ips sexdentatus Börner., and I. typographus L. [89,90]. Mortality of 100% was observed in I. sexdentatus and I. typographus within 5 and 7 days, respectively, after treatment with different concentrations of B. bassiana to determine its virulence [88]. In addition, B. bassiana controls several life stages of the pine moth, Panolis flammea Denis & Schiffermüller [91]. In Europe, the most widespread natural pathogen of Melolontha spp. is Beauveria brongniartii (Saccardo), which infects all stages of development of these pests. Due to the ability of B. brongniartii to specifically infect and kill insects, several strains have been tested and used commercially as biocontrol agents against beetle larvae in various European countries [88,89,90,91]. The most commonly described EPF application strategy is spraying spore suspensions directly onto an infected tree trunk. This application resulted in adult beetle mortality and reduced reproduction and emergence; in addition, these conidia can persist for 90 days in the soil, providing long-lasting protection [92]. Beauveria bassiana thrives in the undisturbed soils of humid forest habitats; it is rather sensitive to mechanical soil manipulation, high temperatures, drought, and UV radiation [92].
The genus Metarhizium Sorokin was isolated and identified by Sorokin almost 140 years ago. Since then, it has been considered one of the most important entomopathogenic fungi, and is used for the biological control of insects, fungi, bacteria, and viruses [93]. It is responsible for green muscardine disease, a fungal infection of insects [94]. Metarhizium anisopliae is a soil fungus that consists of several genotypes that are distributed from the Arctic to the tropics. It has a wide range of host species, however the individual strains and some genotypes are generally more specific. However, its host range is narrower than that of B. bassiana. Most of its hosts are soil-inhabiting insect pests and termites, such as beetles; infections in Diptera and Hymenoptera are rare [93,94].
The infection process of M. anisopliae is similar to that of other entomopathogenic fungi. Usually, the fungus penetrates its host insect through the outer cuticle, particularly along the intersegmental folds, such as the joints between segments or around the mouthparts. After successfully penetrating the host insect, the fungus produces blastospores or hyphal bodies, which are passively distributed into the hemolymph, allowing the fungus to invade other tissues of the host insect through extensive vegetative growth. The fungus depletes the nutrients of the hemolymph and the fatty body, and produces a wide range of enzymes, secondary metabolites, and toxins, those primarily being destrixins. Death of the insect ensues, and the pathogenic process ends. The incubation period depends on the host, temperature, and virulence of the fungal strain. After the host’s death, and under humid conditions, the fungus begins its saprophytic growth outside the body. Conidia are produced on the outside of the dead insect, and the cycle starts anew. The secondary compounds have several different biological activities. For example, cyclic hexadepsipeptide destrixins [93] exhibit a range of effects, including antiviral, antitumor, insecticidal, cytotoxic, immunosuppressive, phytotoxic, and anti-proliferative [93,94]. Metarhizium anisopliae has shown significant insecticidal activity on both pine sawyer (Monochamus galloprovincialis) adults and larvae in vitro. In vivo tests where a conidia suspension was sprayed into the larval attack holes on pine confirmed the insecticidal activity [94].
Apart from Beauveria spp. and Metarhizium spp., EPF products that use Isaria fumosorosea Wise (formerly Paecilomyces fumosoroseus) and Lecanicillium spp. Zimm. are available, marketed based on their myco-insecticidal and myco-acaricidal properties [93,94]. Another promising tool in the management of insects is entomopathogenic nematodes (EPN). Steinernema carpocapsae Weiser and Heterorhabditis downesi Poinar are both candidates for suppressing the pine weevil [95].
Furthermore, the application of a combination of EPF and other biocontrol agents has successfully controlled pathogen and insect populations. For example, a suspension of conidiophores from the EPF strains B. bassiana, B. caledonica, and M. brunneum with EPN Steinernema carpocapsae and Heterorhabditis downesi was found to be effective against the large pine weevil (Hylobius abietis L.). In field tests, the application of this suspension to the soil reduced the emergence of the pathogen [85]. A similar approach was adopted against the red palm weevil (Rhynchophorus ferrugineus Olivier.). In an effort to reduce the cost of palm management, and to minimize the damage to the environment, a recent study proposed the combination of EPF (B. bassiana) with insecticides of the genus Nitenpyram at low doses. This combination was fatal to red palm weevil larvae and adults [96]. Further proteomic data on the effect of applying a culture filtrate of three EPFs combined with the yeast Candida albicans Berkhout for the co-injection of pine weevil larvae revealed a profound alteration of the larval metabolic systems, which induced significant mortality [85].
To meet the increasing demand for EPFs as effective biocontrol agents, and to improve their efficacy, the development of genetically engineered EPFs has become an important area of research. Researchers have incorporated an Archean photo-reactivation system and pigment synthesis pathways from non-EPF, as ways to boost EPF virulence and enhance EPF resistance to insecticidal peptides and proteins [97]. Colonization of the host by EPF requires the ability to cope with the host’s immune defenses and to extract nutrients from the host [98,99], which is achieved through immune evasion by cryptic forms (genetically different species), or by modulation of the immune system by the action of secreted molecules [93].
Other fungi also exhibit antagonistic potential and are considered to be effective biological control agents against infection by such fungi as Fusarium spp., Rhizoctonia solani Kühn., Botrytis cinerea Pers., Colletotrichum spp. Corda, Phytophthora spp., and Alternaria spp. Fries; these species are effective against bacteria such as Xanthomonas spp. and Pseudomonas syringae, and even by viruses such as the cucumber mosaic virus. Several studies have demonstrated an effect of Trichoderma spp. on the systemic activation of resistance mechanisms in plants against pathogenic fungi. These fungi have advantageously modified the response of several plants following infections by phytopathogens [98]. The genus Trichoderma acts by a synergistic action between its lytic enzymes, its competing iron siderophores, and its peptaibols (antibiotic peptides capable of destroying the fungal wall), and inhibits the mycelial growth of Fusarium oxysporum [99]. The Trichoderma antagonists of Armillaria have several strategies for attacking the fungus. They can inhibit rhizomorph formation by producing volatile compounds and by penetrating the mycelium inside the rhizomorphs, thus causing lysis and degeneration of the rhizomorphic tissue [98,99]. Alternatively, they may also act via the production of extracellular enzymes, siderophores, and indole acetic acid for a combined effect of eliminating the pathogen and promoting tree growth [99]. A strain of Trichoderma koningiopsis was examined against the boxwood blight agent Calonectria pseudonaviculata Henricot. The diffusible antifungal substances were reported to inhibit the mycelial growth of the pathogen by more than 80% in vitro, while its in vivo application, as a preventive measure, significantly reduced infection and induced resistance in boxwood (Buxus spp. L.) [100].
Esteya vermicola Liou (Ophiostomataceae) is an endo-parasitic fungus and is the only nematophagous fungus known to have potential as a biocontrol agent against the pine tree nematode, Bursaphelenchus xylophilus, which is responsible for the pine blight disease [101]. Esteya vermicola conidia that has been sprayed on artificial wounds of pine seedlings, has been shown to control this disease effectively. Six strains of E. vermicola have been identified worldwide, and isolation substrates indicate that they not only live saprophytically but can also infect nematodes [102].
The fungal endophytes from the roots of several host plants, in particular conifers, have been isolated and tested for their biocontrol capability. Fungal endophytes can decrease pathogen infections in their host roots, as found in a study that showed that the strains of Phialocephala subalbina Grünig and Sieber could reduce the severity of disease caused by the two oomycete root rot pathogens, Elongisporangium undulatum Petersen and Phytophthora plurivora, in seedlings of Norway spruce (Picea abies) [103]. In addition, metabolites extracted from the liquid cultures of the root endophytes, Phialocephala sphareoides, and Cryptosporiopsis spp., inhibit the growth of the phytopathogens Heterobasidion annosum, H. parviporum, Phytophthora pini, and Botrytis cinerea [104]. Moreover, the compounds extracted from Phialocephala europaea, identified as sclerin and sclerotinin A, considerably reduce the growth of Phytophthora citricola [105]. The pathosystem Phlebiopsis gigantea, a saprotrophic fungus, and the pathogen Heterobasidion spp. exhibit another mechanism of antagonism. Considered in terms of competition for space and nutrients, P. gigantea competes with Heterobasidion spp. following its primary colonization of the freshly cut stumps of P. abies and P. sylvestris [106]. A hypothesis is that the application of P. gigantea spores to the cut surfaces of trees may have the potential to restrict Heterobasidion’s ability to penetrate root systems, thereby reducing its ability to cause secondary infections on host trees. An in vitro application of a mycelial suspension of P. gigantea on freshly cut P. sylvestris stumps has been shown to have a restrictive effect on colonization by Heterobasidion spp. A protective preparation of P. gigantea spores has subsequently been developed on a commercial scale [106] and is considered to be economically advantageous on various tree species (mainly P. abies and P. sylvestris) in many European countries [106].
11. Biological Control Using Bacteria
11.1. Bacillus and Pseudomonas
Most of the bacteria that are used in biological control belong to the genera Bacillus Cohn and Pseudomonas Migula, and are generally endophytes or isolated from the rhizosphere of plants [107]. Assisted laser desorption/ionization mass spectrometry (MALDI-MS) studies have identified the metabolites that are produced by the bacterial strains B. amyloliquefaciens and P. aeruginosa, when inoculated with Fusarium oxysporum f.sp. conglutinans (Foc). The strain of B. amyloliquefaciens produces lipopeptides and bacillibactin E fungicidal siderophores; P. aeruginosa possesses pyoverdine and pseudobactin siderophores. The siderophores of both bacteria are involved in mutualistic competition, and suppress the pathogen’s antibacterial compounds. Bacillus amyloliquefaciens also produces peptaibols. Peptaibols are characterized by the presence of an unusual amino acid, alpha-aminoisobutyric acid, and a C-terminal hydroxylated amino acid. Peptaibols exhibit antibiotic activity against bacteria and fungi [101]. Bovolini et al. [108] reported the successful application of a strain of Bacillus, as a preventive and curative spray, on eucalyptus seedlings for the control of the fungus Oidium eucalypti Rostr. Furthermore, another strain of Bacillus (UnB1366) is highly effective against the phytopathogenic fungus Lasiodiplodia theobromae Grifon and Maubl., the causative agent of teak canker disease and a fungus that has not responded to other control methods. In vitro testing of the Bacillus treatment resulted in the complete inhibition of the mycelial growth of the fungus, and these results were confirmed by an in vivo test, where the preventive application of the biocontrol agent inhibited L. theobromae at 50% of its mycelial growth [109].
Moreover, Bacillus strains that are known to promote plant growth are potent biocontrol agents. A strain of Bacillus that was applied in vivo significantly reduced the incidence of diseases caused by Botrytis cinerea and Calonectria gracilis De Not.; this result was attributed to the various volatile compounds that are produced by the Bacillus [108,109]. Bacillus velezensis, associated with the root microbiota of several plants, can produce a cascade of metabolites that have antagonist properties against Fusarium and Phytophthora, as well as promote plant growth [109].
Bacillus thuringiensis sets the bar for biocontrol agents as the most used globally, and best documented. Bacillus thuringiensis, besides its great ability to effectively control harmful bacteria and fungi through its production of various bacteriocins, fengycins, chitinases, and other cell wall-degrading enzymes, has an arsenal of insecticidal proteins, mainly Cry (parasporal crystal protein) and Cyt (cytolytic) toxins. These compounds are synthesized during the stationary growth phase in the form of crystalline parasporal inclusions, and are active against a wide range of insects [110,111]. This bacterium also synthesizes other proteins during its vegetative growth, which are then secreted into the culture medium. These proteins are vegetative insecticidal proteins (Vip) and secreted insecticidal proteins (Sip) [110,111], and these exhibit insecticidal activity against members of Coleoptera, Lepidoptera, and Hemiptera [111]. The insecticidal proteins of B. thuringiensis are very host-specific, have global significance as biological insecticides, and are already being used successfully in crop protection and vector control programs worldwide. Bacillus thuringiensis is the most effective and most widely used biocontrol agent, due to its wide range of target organisms (insects, mites, nematodes), the variety and selectivity of its toxins, its toxins’ speed of action, and their low cost [110,111]. More than 700 Cry toxins and more than 100 Vip toxins have been identified and characterized in a dedicated B. thuringiensis toxin database [111]. The application of B. thuringiensis is generally through the spraying of crops or by genetic engineering (transgenic plants) [111]. Cry and Cyt toxins work by forming pores in the epithelial cells of the midgut of insects, which eventually lyse [111].
11.2. Streptomyces
Among the actinomycetes, members of Streptomyces Waskman are well known for their extraordinary ability to produce a wide variety of secondary metabolites, namely antibiotics, as well as enzymes capable of lysing fungal walls such as chitinases, cellulases, and hemicellulases [109,110,111]. This capacity makes them effective in biocontrol against a wide range of pathogens. The strains S. atratus, S. tsukiyonensis, and Streptomyces spp. were examined against four wood rot fungi in conifers: Gloeophyllum trabeum Murrill., Donkioporia expansa Desm., Trametes versicolor Lloyd, and Schizophyllum Verhoeff. The strains showed good inhibitory activity of the mycelial growth of the fungi. Streptomyces atratus and S. tsukiyonensis showed the best inhibitory activity, and their inoculation resulted in less rot and the development of wood with better rigidity. The results of this study suggest the potential for Streptomyces strains to protect various woods against rot agents [112].
The genus Streptomyces also exerts nematicidal activity against parasitic nematodes. Nematicidal metabolites that are produced by Streptomyces avermitilis are identified as avermectins, which are macrocyclic polyketides [112,113] that exhibit a broad spectrum of activities against a variety of nematodes [113]. Avermectin and its derivative, abamectin, are used together as a trunk injection agent to control the pine tree nematode Bursaphelenchus xylophilus. Nematicides inhibit the reproduction of adult nematodes and cause tissue destruction by vacuolation and severe morphological alterations. Their injection into infected pine trees effectively suppressed the disease [113,114].
11.3. Myxobacteria
Other bacteria may have important antagonistic activities, such as Myxobacteria, which exhibit remarkable antifungal properties. Myxobacteria have been isolated from the soil of different forest trees: Pinus sylvestris, Betula pendula, Alnus glutinosa, and Quercus robur. They can be used as a biological control agent against certain pathogenic fungi such as Rhizoctonia solani. Myxobacteria have the well-known ability to lyse other microorganisms. They attack them in their natural habitats, releasing antibiotic compounds and enzymes that degrade cellular biopolymers and cause the lysis of host cells [113].
12. Biological Control Using Viruses
The persistence of viruses in the environment, the high mortality rates of the targeted pests, and their host specificity make them successful in controlling the most destructive forest pests. Among the most efficacious viruses are entomopathogenic viruses, which cause fatal, natural, epizootic diseases in insects. Examples include members of the Baculoviridae family, particularly the genera Alphabaculovirus and Betabaculovirus, which are widely used in biological control [114]. Alphabaculoviruses or Nucleopolyhedroviruses (NPV), which are specific to Lepidoptera, can cause a rapidly progressive lethal infection in the Douglas-fir tussock moth. These viruses are ingested by the insect with food and begin their replication in the midgut. Infected cells subsequently lyse, and, after death, high levels of virions are released on the leaves and trunks of trees and the ground. This viral spread increases the incidence of the disease. These NPV can be used for controlling the Douglas-fir tussock moth in forestry applications [115].
The major families of mycoviruses (viruses that infect fungi) applied in forest operations, as well as in recent research on application strategies, are well known [115]. While the effects of mycoviruses on their hosts are highly variable, the course of their interaction to reduce pathogenicity in the plant is known as hypovirulence [115,116]. The use of the Cryphonectria hypovirus for the control of Cryphonectria parasitica, the causal agent of chestnut blight, is a model strategy for viral biocontrol. A study where a Cryphonectria hypovirus 1 (CH1) strain reduced mycelial growth and canker development on chestnut stems [116] demonstrated an example of a successful application. In combatting Heterobasidion, which is destructive to boreal forests and is responsible for the wood rot (white rot) of conifers, infection with Heterobasidion annosum partitivirus 13 (HetPV13-an1) can alter the growth and spread of the fungus in the field, even though the fungus is difficult to eradicate by conventional methods [117]. In addition, Schoebel et al. [118] identified a new member of the mycoviruses that infect the invasive fungus Hymenoscyphus, a severe dieback agent of ash trees in Europe.
The development of sequencing tools has made it possible to study the mycoviruses that are associated with the Fusarium species commonly found in phyto-infections, which cause hypovirulence and latent infections. This biotechnology subdiscipline, known informally as omics, which analyses structure and function at a variety of levels (molecular, gene, protein, metabolic), has shed light on the mechanisms involved in fungal–virus interactions that enhance viral biocontrol [115,116,117,118].
13. Biological Control Using Insects and Nematodes
While some insects and nematodes can cause problems for forests, others are useful and can counteract many pests. Entomopathogenic nematodes harbor symbiotic bacteria that have a crucial role in biocontrol. The injection of these bacteria into the hemolymph, and successive bacterial regeneration, leads to the insect’s death; the corpse then serves as food for the nematode [117,118]. Moreover, the nematode–bacteria complex weakens the insect’s immune response, making it more vulnerable to treatment [117,118]. The symbiotic bacteria release volatile and non-volatile exudates that can have a deleterious effect against phytopathogenic fungi, suggesting that entomopathogenic nematodes may be used in the natural regulation of insect and pest fungi populations. Many nematodes are used in tree crops as biocontrol agents [116,117,118]. The parasitic association between the nematode Bursaphelenchus fraudulentus and the fungus Armillaria ostoyae helps to maintain Armillaria populations at sublethal levels [119].
Among insects, parasitoid wasps are widely used in forest ecosystems to control harmful arthropods. Many agencies and companies are making large investments in developing biocontrol technologies which use parasitoid wasps, as an alternative to chemical pesticide use [4]. In forest ecosystems, parasitoid wasps of the genus Sclerodermus Latreille (Hymenoptera: Bethylidae) are currently the most important natural enemies of wood borers. The efficacy of the control of bethylid parasitoids in suppressing wood borers has been studied in several wasp host systems [120,121,122,123]. Parasitoid wasps produce several factors that are responsible for their mechanism of action, including venom [122], symbiotic viruses (polydnaviruses) [123], and specialized cells (teratocytes) [124]. These factors cause changes in the development, behavior, physiology, and morphology of the host and promote the survival of parasitoid larvae. With the advent of high-throughput sequencing technologies, the compounds that are present in parasitoid wasp venom, as well as polydnaviruses, have been identified as host modulatory factors, particularly in suppressing host immune responses [125,126].
14. Role of Forest Soil Microbiota in Tree Health
Many microorganisms (viruses, fungi, bacteria, nematodes, archaea, and protozoa) develop symbiotic associations with various forest habitats [127,128,129]. All the communities of these organisms that are associated with plants, whether mutualists, endophytes, pathogens, or commensals, are collectively called plant microbiota [130]. The tree microbiota is the result of millions of years of evolution [131]. Whether internal (endophytes) or external (soil, rhizosphere, and phyllosphere microbiota), the microbiota confers resistance to different abiotic and biotic stresses that are inflicted on the tree [132]. The host organism and its microbiota constitute a meta-organism [133]. They are in continuous communication, and exchange signals and metabolites on a permanent basis [133], hence the concept of the microbiota as the “second brain” of its host [134]. The plant microbiome has been shown to play a beneficial role in biocontrol, by inhibiting the establishment of pathogens and promoting plant growth; disruption of the microbiome leads to degradation of the health and physiological condition of its host [135]. The plants release signals that affect the microorganisms around it during attack or biotic threats [136].
New genomic technologies have shown that the entire microbial community does not provide the molecular mechanisms contributing to the overall health of trees. Instead, these mechanisms can be attributed to a few individuals, or synergistic actions, among a few microbial community members [137]. Researchers can select those members that perform these beneficial functions, convert them into synthetic communities, and subsequently manipulate them through microbiome engineering for their use in various agricultural applications [135]. The synthetic microbiome is a consortium of targeted and specific bacterial or fungal strains, whose functions are controlled through gene expression. This microbiome is used to study the plant–microbe interactions at the community and individual level, as well as the potential elimination of phytopathogens. Scientists believe that these synthetic consortia are promising biocontrol products [138] (Figure 2).
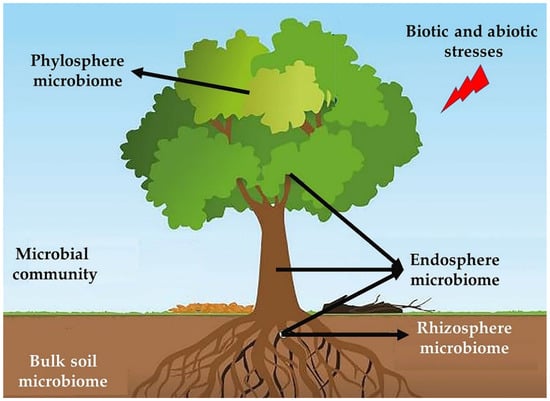
Figure 2.
Different microbial communities consisting of biological activities against biotic and abiotic stresses.
Metagenomic approaches have made it possible to identify the genes of interest, link phylogenetic profiles to their functions, and sequence the genetic material within the samples [138]. The study of the plant microbiome has become a popular field of research in response to the advances in high-throughput sequencing technologies and “omics” analyses, microbiome engineering technologies, the rapid evolution of synthetic biology, and the availability of bioinformatics resources and data. These advances allow in-depth and large-scale studies of these microorganisms which are unfettered by the limited information that is provided by the classical amplification of a single gene by the polymerase chain reaction technique (PCR). In addition, meta “omics” analyses and functional genomics have made it possible to elucidate the metabolic and physiological potentials, and the phylogenetic diversity, of these members, up to the architecture of their communities and their changes over time [139,140].
15. Soil Microbiota
Soil is an integral part of the forest, supporting complex microbial communities. The characteristics of forest soils differ greatly from those of agricultural soils. Forest soils generally receive few, if any, amendments and are not intensively managed in the same way as agricultural lands. Forest soils are poorer in nutrients but richer in organic matter than agricultural soils [141]. Up to 33% to 50% of the carbon that is fixed by trees is allocated to the soil by their roots [141], while tree litter and deadwood are important sources of persistent organic matter. Research indicates that soil is an important reservoir of microorganisms [139]. Forest soils are among the most diverse microbial habitats on Earth, and bacteria are the most abundant of soil microorganisms. There are 107 to 109 bacterial cells per gram of forest soil and 0.2 to 0.7 milligrams of fungal mycelium per gram of soil. The soil bacterial community directly mediates the functioning, health, and resistance of this ecosystem [140], maintains the biogeochemical cycles (nitrogen and carbon cycle) [140,141], protects plants from climatic disturbances [142,143], and affects the rate of pathogen invasion and plant responses to these infections [127].
The biggest obstacle to studying forest soil biodiversity is the heterogeneity of the soil environment and the abundance of a limited number of taxa [142,144]. The study of the structural and functional diversity of the soil microbiota has moved from traditional cultivation methods to techniques for amplifying small RNA amplicons by PCR, to methods for sequencing complete genomes, by use of high-throughput sequencing technologies and analyses that are provided by metagenome clustering algorithms [145,146,147]. Despite the enormous progress in high-throughput sequencing technologies, the most beneficial soil microorganisms remain unknown.
Current research is examining the microbial taxonomic diversity of Harvard Forest (Massachusetts, USA) soil. Alteio et al. [148] employed mini metagenomics (an analysis involving cell sorting coupled with shotgun sequencing) followed by bulk metagenomics. This approach was intended to fill the gaps in global metagenomics, which carries the risk of ignoring rare taxa and the exogenous DNA from dead cells that may be relatively abundant. These high-throughput analyses allowed total-community comparative research into the under-studied clades and revealed the relative abundance of Bacteroides, Alphaproteobacteria, Gammaproteobacteria, and some Archaean taxa (Thaumarchaeota and Euryarchaeota).
Some soils, called disease suppressive soils, can suppress pathogens, a biological function that is attributed to the action of microbial communities in the soil [136]. There are two types of disease suppression: general and specific. General disease suppression results from the activity of all the microorganisms in the soil. Information is scarce on the microbes and the mechanisms involved in general control, especially in forest soils. Despite being slower, this strategy appears to be more effective than the specific version. Specific suppressive soils can retain their suppression characteristics for long periods and can be transferred to other soils by transplanting [149]. What is evident is that pathogen eradication is the result of the combined action of fungi and bacteria through the production of antifungal components, competition for carbon sources, and the attainment of induced systemic resistance (ISR) [149,150]. Bioengineering approaches can enrich soils with a consortium of specific microorganisms and activate the genes responsible for their antagonistic effects [149]. Studies on the suppressing power of soils have been carried out, particularly on Fusarium wilt disease [149]. However, additional studies on other tree crops are needed to determine the merit of transplanting suppressive soils as a biocontrol strategy. Introducing microorganisms that are isolated from suppressive soils into suitable new soils does not necessarily provide conclusive information on their contribution to soil suppression. Introduced populations are unlikely to replicate the microbial community structure and interactions that occur naturally in suppressive soils.
16. Rhizosphere
The rhizosphere is the narrow zone of the soil where plants grow. The presence of root secretions (exudates and rhizodeposits) in this area makes it the most complex and diverse habitat in nature; 1 g of rhizospheric soil can contain up to 1011 microbial cells [135,136].
Plants and microorganisms interact by signaling via root exudates. The composition of root exudates varies among plant species [150], and this variability plays an important role in establishing the plant–rhizosphere microbial communities [150,151]. This microecosystem is the main region where chemical communications and the exchange of compounds and nutrients occur between soil microorganisms and the plant [150,151].
The role of bacteria in the forest ecosystem has recently been described in greater detail as analytical methods have become more sophisticated. The composition of the bacterial community is affected by the organic matter content, nutrient availability, climatic conditions, biotic interactions, and soil pH, the last of which appears to be the most important factor [151]. Five phyla—including Acidobacteria, Actinobacteria, Proteobacteria, Bacteroidetes, and Firmicutes—appear to be abundant in most soils [150,151]. The rhizosphere is considered to be a subset of the bulk soil microbiome. However, differences in the metabolic processes between these phyla allow some to dominate in the soil. Research indicates that each niche has specific properties, and, therefore, a specific bacterial community, which can be enriched by members of Proteobacteria, Actinobacteria, and Bacteroidetes [126,127]. The dominance of Alphaproteobacteria, Betaproteobacteria, Actinobacteria, and Bacteroides has been observed in the rhizospheres of beeches (Fagus spp.) in a mountain forest [151]. These observations suggest the enrichment by heterotrophic and fast-growing bacterial taxa [151]. The rhizosphere and the adjacent soil are “war zones” for microorganisms, and competition is vigorous for the niche and for nutrients. The microorganisms that survive in such a biotope and successfully colonize the roots appear to be highly competitive and promising as biocontrol agents.
Strategies which utilize rhizosphere bacteria to improve plant health and development include the selecting and modifying of the rhizosphere microbiome [149]. These bacteria can eliminate soil-transmitted pathogens by competition for nutrients, by producing antimicrobial molecules and lytic enzymes, and by consuming pathogen-stimulating compounds [151]. The prevailing strategy is to trigger ISR, thereby enhancing and accelerating the tree’s immune responses [152]. When exposed to phytopathogens, plants recruit beneficial bacteria from the rhizosphere through root exudates [151,152]. In a recent study on the phylogenic diversity of the Caucasian or Nordmann fir (Abies nordmanniana Steven.) and its antioxidant enzyme profile, Garcia-Lemos et al. [152] found a strong correlation between the rhizospheric bacterial communities and the oxidative defenses of the tree. Furthermore, they confirmed that the microbiota influences the metabolism of the tree when it is under stress.
Microorganisms can develop forced symbioses with their hosts [152]. Several types of trees can develop symbiotic associations with mycorrhizal fungi, some forming ectomycorrhizas (EM), others forming arbuscular mycorrhizas (AM), and some forming both types of associations (as in poplar) [128,153]. Mycorrhizal fungi that reside in the rhizosphere contribute to tree resistance through their ability to produce a wide range of extracellular enzymes [153,154,155]. They also help induce plant ISR. Further, due to their significant mycelial growth, they have the unique ability to form a signal transduction network between plants to warn them of an attack [156,157]. Mycorrhizal fungi play a central role in the mobilization and sequestration of nitrogen and phosphorus in the forest soil and are also responsible for significant carbon transport in the soil [158]. In mycoforestry, this important symbiosis between mycorrhizal fungi and trees is used to preserve and maintain forest ecosystems and their biodiversity due to their ability to recycle debris, boosting forests to recover while strengthening trees during their replanting [159]. However, the roles of bacteria and fungi should not be viewed as separate. The high abundance of fungal biomass in forest soils has multiple consequences for bacteria, including creating specific niches in soil patches which are colonized by mycorrhizal fungi (the mycorrhizosphere) [158]. Enrichment of the mycorrhizosphere by Proteobacteria, such as Burkholderia, Rhizobium, and Pseudomonas, and Actinobacteria, such as Streptomyces, has been reported, based on culture-dependent studies [158,160]. A recent molecular study of Scots pine mycorrhizospheres found that the composition of the community is much more complex than previously believed, and includes both copiotrophic and oligotrophic bacteria, as well as soil mycelial mats [160].
The plant microbiome includes bacterial communities and other organisms, such as nematodes, which have important effects on the biology of the host. Several beneficial nematodes inhabit the rhizosphere, such as entomopathogenic nematodes, which harbor endosymbiotic bacteria. These species contribute to the regulation of organic matter and the regulation of bacteria and pests, maintaining pathogens and pests at non-hazardous levels [128]. Many entomopathogenic nematodes are marketed as biocontrol agents and applied to tree plantations [158].
17. Phyllosphere
The phyllosphere consists of the surface and the interior of the leaves, and is colonized by various microorganisms, most of which are bacteria. Little research is devoted to the phyllosphere as a microbial habitat. However, culture-independent techniques have revealed the predominance of Proteobacteria (Alphaproteobacteria and Gammaproteobacteria), gathered as aggregates [158], such that fungi and archaea are less abundant [160,161]. The role of the phyllosphere microbiota in biological control has been widely covered [135]. Metaproteomics and community profiling-based studies have provided insight into the crucial role of native phyllosphere microbes in host health. The mechanisms by which these microorganisms improve tree health include atmospheric nitrogen fixation and the production of plant hormones. The primary hormone that is produced is indole-3-acetic acid, which, in addition to its role in colonization, can affect the release of saccharides by the plant cell wall [162]. Saccharide release leads to increased nutrient availability [162] and the suppression of the proliferation of pathogens via competition for nutrients and space, the production of antibiotics, and the signaling of systemic host responses [161,162]. Peñuelas and Terradas [162] stated that the leaf microbiota may be the best alternative to pesticides and chemical fertilizers; because the leaf microbiota is present on the aerial part of the tree, they are exposed to various changes in environmental factors that can alter its composition and the abundance of the individuals that compose it [162]. This variation has made it challenging to isolate specific combinations and effects. As a result, studies on the bioengineering of the leaf microbiome are limited.
18. Endophytes
The internal tissues of the tree represent a large habitat, abundant in various microorganisms. These microbial communities are the least studied of the plant microbiome. The diversity of endophytes and their adaptation to various plant habitats represent a new secondary metabolite source [152].
The first description of endophytic communities dates back to the 19th century, when the German botanist Heinrich Freidrich Link (1809) reported that any parasitic fungus that lives inside plants is an endophyte. The definition of endophytes was linked to fungal parasitism until Galippe [163] stated that even bacteria that migrate from the soil could colonize and play a beneficial role in plant tissue. These definitions have changed considerably in recent decades, with many ramifications. Endophytes are commonly defined in the recent literature as microorganisms that can colonize the interior tissues of plants without causing damage or disease [164]. The endosphere is the internal environment of plants in which endophytes, including bacteria, archaea, fungi, and viruses, live, colonize, and survive without harming the plant [165]. The formation of endophyte communities may be affected by the environment, geographic location, plant species, and genotype [166]. The endosphere microbiome may be a subset of the rhizosphere microbiome [165,166]. Colonization of internal plant tissues by endophytes occurs through various entry points. The rhizosphere is an important region for the entry of microorganisms into the plant, due to its richness in nutrients that is derived from root exudates and its high concentration of microorganisms [167]. Other entry areas include lenticels, stomata, wounds, ruptures, and nodules. Endophytes can also be transmitted vertically through seeds [162].
The plant microbiome allows plants to survive and gives them the capacity to tolerate various biotic or abiotic stresses. To improve or activate the defense systems of other plant species (heterologous hosts) which are sensitive to certain pathogens or abiotic factors [129,151], it is necessary to transfer such capabilities to the plants that lack them [151,168]. These capabilities are required if the endophyte–host relationship is to develop efficient physiological processes that modify the microbiome, such that it is stable and beneficial in the host microenvironment [168,169]. Promising endophytes are active organisms that modify plant physiology and development, as well as induce pathogen resistance systems and tolerance mechanisms to various types of stress [162,169,170]. A potentially effective strategy is to select highly competitive organisms with colonization capabilities [171] that can have additive or synergistic effects [171,172]. By transferring these microbial species, a greater amount of genetic material or number of genes can be transferred [173], thus simultaneously improving several plant growth- and health-related functions. Plant growth-promoting (PGP) microorganisms that perform multiple direct and indirect growth promotion activities are beneficial, and exhibit the desired characteristics of a microbiome [162,173].
The plant endosphere is a composite microecosystem, where different types of microorganisms can occupy different niches. Consequently, low specificity between the host and the endophyte often exists. Little is known about bacterial endophyte communities in different plant species’ tissues [174]. Despite the diversity of endophytes, only certain types have been studied [174].
Beneficial endophytic root fungi live in the intercellular or intracellular spaces of plant tissues, resulting in a symbiotic association with the host plants [175]. Endophytic fungi may be constitutive systemic mutualists, which are transmitted vertically through seeds and infected grasses exclusively, or they may be inducible mutualists, which are non-systemic, taxonomically diverse, are transmitted horizontally from plant to plant, and capable of colonizing all plants of the ecosystem [176]. Plants use these fungal endophytes to escape different stress conditions, both biotic (pathogenic fungi, oomycetes, bacteria, and nematodes, as well as herbivores) and abiotic (oxidation, salt, drought, water, heavy metals) [177].
Among these non-systemic, endophytic fungi, Piriformospora indica Verma is one of the most important and extensively studied taxa. Piriformospora indica is a unique fungus that is capable of colonizing the roots of many plant species, and thus establishing symbiotic relationships. The fungus lacks host specificity and is cosmopolitan in nature. It has already been applied in agroforestry and arboriculture on bryophytes, pteridophytes (slender brake fern or silver lace fern; Pteris ensiormis), gymnosperms (Aleppo pine), and angiosperms [177]. Various studies have reported the potential for P. indica as a biocontrol agent against several fungal pests such as Alternaria brassicae, Botrytis cinerea, Verticillium, Fusarium, and Rhizoctonia [178]. In addition, studies have shown that P. indica can improve stress resistance, photosynthesis, and germination and that it exhibits other activities which are related to the promotion of plant growth [179].
Other root endophytic fungi that have been observed belong to the group of dark septate endophytes (DSE). These fungi can be found in terrestrial plants worldwide. This type of endophyte must be able to form specialized structures (microsclerotia) in the host’s roots, as well as asexual, septate, and melanized hyphae [179]. They often coexist with ectomycorrhizal fungi at the tips of tree roots [130]. It has been estimated that DSE fungi may be more abundant in forest ecosystems than mycorrhizae [179].
Arbuscular mycorrhizae have been identified in several species of herbaceous and woody plants [179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200]. After colonization of the roots, they form arbuscules that mediate an exchange between the fungus and the tree [179,180,181]. Ectomycorrhizae that are associated with some woody plants are characterized by the formation of a hyphal network that surrounds the roots [180]. Mycorrhizal fungi improve mineral absorption and allow their hosts to adapt to abiotic stresses, such as heavy metal pollutants [179]. The fungus Trichoderma can also colonize the stems, branches, roots, and epiphytes of tree foliage and leaves, and persist there for a long time [179,181].
Despite the scarcity of practical endophyte-based applications that can protect trees of great economic and ecological importance [181], the potential for endophyte biocontrol has been demonstrated experimentally by numerous studies. Fungal endophytes can effectively control the dieback of ash (Fraxinus excelsior) caused by Hymenoscyphus fraxineus [181]. Many endophytes contribute to resistance to necrosis caused by Gremmeniella abietina Morelet. on the Aleppo pine. Isolates of fungal endophytes from the date palm are important inhibitors of several bacterial and fungal pathogens [182,183]. The endophyte Hypoxylon rubiginosum is a promising protector against ash dieback [184]. EPFs such as Beauveria and Metarhizium are also able to colonize plants and exist as endophytes. There is the potential of EPFs to exert adverse effects on insect pests, resulting in a reduced rate of insect development, retarded insect growth, and reduced survival and oviposition. In addition, other roles have been reported for EPFs [185], which can act as plant disease antagonists, rhizosphere colonizers and promoters of plant growth. This symbiosis can have a profound influence on the function and stabilization of forest ecosystems [186].
Bacterial endophytes are found in all types of plant tissues of all major plant lineages. Bacteria which are associated with plants provide many benefits to host plants. These interactions between plants and endophytic bacteria can significantly influence the general vigor of plants, and thus improve net productivity. For instance, nitrogen-fixing bacteria benefit their hosts by providing nitrogen and stimulating plant growth [162]. Burkholderiales and Rhizobiales include nitrogen-fixing bacteria and are among the most abundant root-associated bacterial orders for a wide range of plant hosts [187,188]. Although Rhizobiaceae are closely related to legumes, their presence in forest soils has been reported [189]. By producing various antibiotic molecules, siderophores, and hydrogen cyanide [189], Rhizobia can effectively control the spread of soil-borne pathogens in forests. Diazotrophic bacteria (N2-fixing bacteria) are ubiquitous inside the tissues of plants. The presence of these bacteria, which include Burkholderia, Rahnella, Sphingomonas, and Acinetobacter spp., in the plant tissues of poplar and willow, has been reported by Doty et al. [189]. Bacterial endophytes are a permanent and stable source of nitrogen. Pyrosequencing of the 16S-rRNA gene from two conifers demonstrated that N2-binding endophytes dominated 14% to 53% of the sequences; this beneficial function allows for conifers to grow in nitrogen-limited soils [190]. Gram-positive, filamentous bacteria of the genus Frankia (Frankiaceae, Actinobacteria) can colonize the roots of woody trees called actinorhizal plants and form nodules there; these bacteria are known for their substantial N2-fixing capacity [190]. In addition, several trees which are colonized by Frankia can also form mycorrhizal symbioses in a double association that gives the host tree a greater resistance to unfavorable conditions, such as drought and salinity [190].
Endophytes have many strategies that confer biocontrol potential. They may affect the health of their hosts either directly, by promoting their growth via the production of plant hormones (phytohormones) and by improving nutritional status through the provision of minerals, or indirectly, by eliminating pathogens [130,191]. Endophytes tend to eliminate pathogens in one of three ways: by competition for the niche and nutrients [187]; by the production of various antimicrobial metabolites, such as alkaloids, flavonoids, phenols, terpenoids, and xanthones [191,192]; by activating the ISR system of the tree [193]. Compounds involved in ISR activation include lipopolysaccharides (LPS) and siderophores [193,194]. Phytohormones can stimulate the differentiation of plant tissues as well as fulfill other ecological roles [194]. Indole-3-acetic acid is involved in colonization and root elongation [194], leading to the increased absorption of nutrients. Aminocyclopropane-1-carboxylate deaminase can alleviate the stress induced by ethylene, a growth inhibitor for trees [194]. Volatile compounds have a role in triggering immune responses and stimulating growth in the host. Other phytohormones, such as gibberellins and cytokinins, are produced by a few specific endophytes [194,195].
In addition, endophytes are more beneficial than the external microbiota because they, and their metabolites, are influenced neither by environmental conditions nor by predators, which strongly suggests that their communication and exchanges within the plant are of a higher priority than those outside [162,195]. In addition, researchers have proposed that the metabolites that are secreted by bacterial endophytes may have a greater effect on the plant than those that are secreted in an open environment, such as the rhizosphere, where biotic and abiotic factors can counteract and minimize their effect [162].
Since research has confirmed that the endophytic genome is inheritable from one generation to another, the engineering of the internal microbiota has attracted increased attention from researchers [135]. Most endophytes are applied by seed inoculation, atomization of a few tissues (flowers or seeds) [196], or by injection into tissues or wounds [197].
19. Gaps and Opportunities in Forest Microbiota Research
Despite enormous progress toward understanding the plant microbiome, our knowledge of the composition, diversity, dynamics, and evolution of microbial communities that are associated with plants is still limited. Furthermore, most contemporary studies have focused on the microbiota of certain herbaceous species, such as wheat and rice, because of their high economic value. Consequently, the forest microbiota has received less attention.
Though new methods of studying microbial diversity have been developed, research on forest microbial ecology faces obstacles. Forest habitats are extraordinarily complex and heterogeneous; even within a single forest ecosystem, the differences in microsite topography, climate, and other conditions create a highly varied environment. Some microbiomes, regardless of variations that are present at the forest level, have not been sufficiently explored, such as dead wood, rocky surfaces, and soil vegetation [127].
To date, much of the research has focused on the study of fungal or bacterial communities. Other communities such as viruses, algae, and protists are poorly understood. Although found in forests [197,198], their functional potential and ecological role have not been elucidated. Moreover, microbial biomass is rarely established even for the most studied groups, such as bacteria [199].
“Omics” technologies are being developed rapidly, but they do not provide a comprehensive view of microbial diversity (taxonomic or functional). Errors in the markers used [200] can ignore diverse taxa and information from relatively dead cells. In addition, progress is still to be made in functional metagenomics, because the analyses are incomplete. Finally, these approaches may not consider how the microbiota acts as a single organism, rather than as individual cells [201].
Researchers can address these shortcomings in the near future by:
- -
- providing other analytical tools to complement those in use;
- -
- investigating the factors that help establish symbiotic relationships between microbes and their biological processes within an ecosystem from gene to population;
- -
- conducting in-depth multidisciplinary studies of the ecosystem associated with studies of the microbiota, and establishing the relationships between the biocenosis and the biotope (or biosphere).
20. Biofertilizer Perspectives of Microbial Inoculants
As we confront the challenges of global climate change, the degradation of agricultural land, and pollution, it is vital to direct research toward finding sustainable and, as much as possible, natural options for agricultural and forest management. One alternative is the deployment of rhizosphere microbes and upper soil layers as inoculants. These fertilizers positively impact resident microbial communities in the soil, affecting their dynamics and biodiversity [201].
Properly applied, inoculants based on PGP microorganisms can constitute a technology that is respectful of nature while improving the growth and yield of plants (biofertilizers) and for biocontrol [202]. A biofertilizer is any microorganism which is capable of improving mineral transport and providing nutrients to the plant [200].
Microorganisms in forest soils have been increasingly recognized to be as promising as those from tree plantations. A recent study showed that the microbiome of the soils which surround tree plantations could be used as a biofertilizer in a sustainable management approach [203]. Jeong et al. [204] isolated a new strain of Bacillus from a forest soil with significant keratinolytic, antifungal, and plant growth-promoting activities (hydrolytic enzymes, indoleacetic acid, phosphate solubilization, and antimicrobial activities), and suggested that this strain should be used for its potential in biocontrol and biofertilization [204]. This research demonstrated that tropical forest soil is rich in beneficial, functional microbial groups that exhibit highly efficient cellulolytic, nitrogen-fixing, phosphate-solubilizing, and indoleacetic acid-producing activities. Managing these microbial communities is a promising strategy for increasing soil fertility and improving crop growth [205]. Greenhouse trials testing bacterial inoculants with PGP activities on Pinaceae species showed that these PGP bacteria are crucial for the growth of these trees under unfavorable conditions [206].
The use of biofertilizers in agroforestry systems is not well documented. Most studies that have been conducted in vivo (in greenhouse or culture chambers) were carried out on limited tree species or limited areas. A research gap is in the application of microbial inoculants on a forest scale. The impact of the introduction of inoculants on beneficial resident microbial communities also needs to be carefully analyzed.
There is an extensive record of the applications of inoculants based on PGP microorganisms. Several bacterial species have been tested for their fertilizing power, such as Azospirillum brasilense, which increased the biomass of Casuarina cunninghamiana during its application in the greenhouse [202]. Pseudomonas spp. have been applied to apple (Malus spp.) trees in fields; in addition to improving growth, these microorganisms have controlled certain pathogenic fungi [206,207]. Other greenhouse studies have used strains of Bacillus polymyxa and Pseudomonas fluorescens, which improve the size and weight of roots and seedlings of the Douglas-fir, lodgepole pine (Pinus contorta), white spruce (Picea glaucus), western hemlock (Tsuga heterophylla), torch pine (loblolly pine; Pinus taeda), and Elliot pine (slash pine; Pinus elliottii) [207].
One of the most widely used microbial groups as a biofertilizer in forestry are the mycorrhizae. Their resistance to high concentrations of salinity, acidity, toxic metals, large variations in temperature and pH, and lack of water has been widely demonstrated [206,207]. However, despite these successful applications, very little field research has occurred. Currently, there is growing evidence that the efficacy of microbial inoculants is limited mainly by the specificity of action (interspecific and intraspecific) [205,206,207] and by various biotic and abiotic conditions [207], such as the climatic conditions, soil type, host species, and genotype, as well as the ability to compete with native microbes [205,206,207].
The first use of microbial inoculants and biocontrol dates back several centuries, with the application of mycorrhizae on a forest scale in a nursery. Despite the constraints that are associated with the contamination of seedlings by other harmful fungi, these mycorrhizae could survive, resist hostile soil conditions, and remain functional even after their transport and implantation in fields. This application was described as successful and revolutionized the agroforestry system [207]. In addition, mycorrhizae can develop synergistic symbioses with bacteria which have PGP activities, which are referred to as MHB (mycorrhiza helper bacteria). These associated bacteria provide nitrogen for the fungus and the tree [196], enhance the tree–fungus interactions, produce growth factors and phytohormones that enhance mycelial growth and spore germination, and accelerate root colonization [207]. This capability indicates that the polymicrobial application of synthetic communities of PGP could help to replace chemical fertilizers, thereby eliminating a threat to the environment. Integrating these organic practices into forest (and agricultural) management could contribute to a more sustainable approach to ecosystem management.
21. Conclusions
Forest insects, fungi, and other organisms pose both a danger and an opportunity for natural resource managers. Pests and pathogens kill or otherwise impact millions of trees each year. Insects such as defoliators and borers can severely weaken tree vigor and impair health to the point of killing large swaths of trees across a landscape during epidemic situations. Frequently, insect populations get their start in fallen slash or other dead trees, then achieve a population density that enables them to attack living, healthy trees. Beyond the natural dynamics of native species, international trade and inadequate biosanitation practices have resulted in the transport of exotic insects to new locations where they have no natural predators, emerald ash borer being only the most recent example of these invaders.
Phytopathogenic fungi are another source of tree mortality, whether they have been locally transmitted from the soil to root systems, or spreading their spores via the air. Often, humans aid this transmission by unwittingly bringing the fungi to new regions, thus accelerating their spread. The infamous case of chestnut blight in North America is but one example. Phytopathogenic bacteria and viruses are further potential sources of harm to a forest ecosystem. Although they are more frequently studied in agricultural systems, nematodes can cause considerable damage to trees, particularly younger ones.
As forests, similar to agricultural systems, become more monospecific, their vulnerability to these pests increases. With larger areas of vulnerable ecosystems, the likelihood of reaching critically lethal population levels rises, as demonstrated by our earlier discussion of the mountain pine beetle in Canada and the U.S. Traditional forestry treatments of these harmful attackers have paralleled similar actions in agriculture. Chemical treatments often damage the desirable biotic members of the forest community along with the undesirable ones. Intensifying the stress that forests are exposed to is the result of changing climate. Trees may suffer stress due to a higher temperature and higher vapor pressure deficit, resulting in droughty conditions. Moreover, a changing climate may also extend the growing season for these pests, allowing them to achieve critical levels more easily over a given period of time.
Since the role of soil trees microbiota in forest health is undoubted, more complex and comparable research should shed the light on field application of beneficial microbial communities as BCAs and intensive efforts should be held, in depth, to establish a network between governments and forest epidemiologists, to materialize rapidly sustainable management tools for forest ecosystems.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/f12111579/s1, Table S1: Most prevalent insect pest species in forest trees and their characteristics. Table S2: Major Fungal causal agents of forest tree diseases. Table S3: Major oomycete causal agents of forest tree diseases. Table S4: Major bacterial causal agents of forest tree diseases. Table S5: Major viral causal agents of forest tree diseases. Table S6: Major parasitic nematodes of forest trees.
Author Contributions
Conceptualization, H.C.-S. and L.B.; Methodology, H.C.-S., A.C.B., A.B. and L.B.; Software, H.C.-S., A.C.B., A.B. and L.B.; Validation, A.S., H.C.-S., A.B. and L.B.; Formal analysis, H.C.-S., A.B., A.C.B. and L.B.; Investigation, H.C.-S., A.B. and L.B.; Resources, T.O. and J.A.N.; Data curation, A.B., H.C.-S., F.B. and L.B.; Writing—original draft preparation, A.B., H.C.-S. and L.B.; Writing—review and editing, H.C.-S., A.C.B., A.S., W.K.M. and L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Ministry of Science and Higher Education through a Forest Research Institute statutory activity no. 240327.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Keenan, R.J.; Reams, G.A.; Achard, F.; de Freitas, J.V.; Grainger, A.; Lindquist, E. Dynamics of global forest area: Results from the FAO Global Forest Resources Assessment 2015. Forest Ecol. Manag. 2015, 352, 9–20. [Google Scholar] [CrossRef]
- Klapwijk, M.J.; Bylund, H.; Schroeder, M.; Björkman, C. Forest management and natural biocontrol of insect pests. Forestry 2016, 89, 253–262. [Google Scholar] [CrossRef]
- Prospero, S.; Botella, L.; Santini, A.; Robin, C. Biological control of emerging forest diseases: How can we move from dreams to reality? For. Ecol. Manag. 2021, 496, 119377. [Google Scholar] [CrossRef]
- Trumbore, S.; Brando, P.; Hartmann, H. Forest health and global change. For. Health 2015, 349, 814–818. [Google Scholar] [CrossRef]
- Cobb, R.C.; Eviner, V.T.; Rizzo, D.M. Mortality and community changes drive sudden oak death impacts on litterfall and soil nitrogen cycling. New Phytol. 2013, 200, 422–431. [Google Scholar] [CrossRef]
- Heinzelmann, R.; Rigling, D.; Prospero, S. Population genetics of the wood-rotting basidiomycete Armillaria cepistipes in a fragmented forest landscape. Fung. Biol. 2012, 116, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Marini, L.; Økland, B.; Jönsson, A.M.; Bentz, B.; Carroll, A.; Forster, B.; Grégoire, J.-C.; Hurling, R.; Nageleisen, L.M.; Netherer, S.; et al. Climate drivers of bark beetle outbreak dynamics in Norway spruce forests. Ecography 2017, 49, 1426–1435. [Google Scholar] [CrossRef]
- Björkman, C.; Bylund, H.; Nilsson, U.; Nordlander, G.; Schroeder, M. Effects of new forest management on insect damage risk in a changing climate. In Climate Change and Insect Pests; Björkman, C., Niemelä, P., Eds.; CABI International: Wallingford, UK, 2015; pp. 248–266. [Google Scholar]
- Linnakoski, R.; Forbes, K.M. Pathogens—The hidden face of wood-boring forest pest invasions. Front. Plant Sci. 2018, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Nair, K. Tropical Forest Insect Pests: Ecology, Impact, and Management; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar] [CrossRef]
- Flower, C.E.; Knight, K.S.; Rebbeck, J.; Gonzalez-Meler, M.A. The relationship between the emerald ash borer (Agrilus planipennis) and ash (Fraxinus spp.) tree decline: Using visual canopy condition assessments and leaf isotope measurements to assess pest damage. For. Ecol. Manag. 2013, 303, 143–147. [Google Scholar] [CrossRef]
- Nowakowska, J.A.; Hsiang, T.; Patynek, P.; Stereńczak, K.; Olejarski, I.; Oszako, T. Health assessment and genetic structure of monumental Norway spruce trees during a bark beetle (Ips typographus L.) outbreak in the Białowieża Forest District, Poland. Forests 2020, 11, 647. [Google Scholar] [CrossRef]
- Goczał, J.; Oleksa, A.; Rossa, R.; Chybicki, I.; Meyza, K.; Plewa, R.; Landvik, M.; Gobbi, M.; Hoch, G.; Tamutis, V.; et al. Climatic oscillations in Quaternary have shaped the co-evolutionary patterns between the Norway spruce and its host-associated herbivore. Sci. Rep. 2020, 10, 16524. [Google Scholar] [CrossRef]
- Meng, P.S.; Hoover, K.; Keena, M.A. Asian long horned beetle (Coleoptera: Cerambycidae), an introduced pest of maple and other hardwood trees in North America and Europe. J. Integr. Pest Manag. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Valenta, V.; Moser, D.; Kapeller, S.; Essl, F. A new forest pest in Europe: A review of emerald ash borer (Agrilus planipennis) invasion. J. Appl. Entomol. 2017, 141, 507–526. [Google Scholar] [CrossRef]
- Coppedge, B.R. Twig morphology and host effects on reproductive success of the twig girdler Oncideres cingulata (Say) (Coleoptera: Cerambycidae). Coleopt. Bull. 2011, 65, 405–410. [Google Scholar] [CrossRef]
- Pincebourde, S.; Ngao, J. The impact of phloem feeding insects on leaf ecophysiology varies with leaf age. Front. Plant Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zvereva, E.L.; Lanta, V.; Kozlov, M.V. Effects of sap-feeding insect herbivores on growth and reproduction of woody plants: A meta-analysis of experimental studies. Oecologia 2010, 163, 949–960. [Google Scholar] [CrossRef]
- McManus, M.; Csóka, G. History and impact of gypsy moth in North America and comparison to the recent outbreaks in Europe. Acta Silv. Lignaria Hung. 2007, 3, 47–64. [Google Scholar]
- Paini, D.R.; Mwebaze, P.; Kuhnert, P.M.; Kriticos, D.J. Global establishment threat from a major forest pest via international shipping: Lymantria dispar. Sci. Rep. 2018, 8, 13723. [Google Scholar] [CrossRef]
- Lovett, G.M.; Weiss, M.; Liebhold, A.M.; Holmes, T.P.; Leung, B.; Lambert, K.F.; Orwig, D.A.; Campbell, F.T.; Rosenthal, J.; McCullough, D.G.; et al. Nonnative forest insects and pathogens in the United States: Impacts and policy options. Ecol. Appl. 2016, 26, 1437–1455. [Google Scholar] [CrossRef] [PubMed]
- Volney, W.J.A.; Fleming, R.A. Climate change and impacts of boreal forest insects. Agr. Ecosyst. Environ. 2000, 82, 283–294. [Google Scholar] [CrossRef]
- Aukema, J.E.; Leung, B.; Kovacs, K.; Chivers, C.; Britton, K.O.; Englin, J.; Frankel, S.J.; Haight, R.G.; Holmes, T.P.; Liebhold, A.M.; et al. Economic impacts of non-native forest insects in the continental United States. PLoS ONE 2011, 6, e24587. [Google Scholar] [CrossRef]
- Hogg, E.H.; Brandt, J.P.; Michaelian, M. Impacts of a regional drought on the productivity, dieback, and biomass of western Canadian aspen forests. Can. J. For. Res. 2008, 38, 1373–1384. [Google Scholar] [CrossRef]
- Dara, S.K.; Montalva, C.; Barta, M. Microbial control of invasive forest pests with entomopathogenic fungi: A review of the current situation. Insects 2019, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Doehlemann, G.; Ökmen, B.; Zhu, W.; Sharon, A. Plant pathogenic fungi. In The Fungal Kingdom; Heitman, J., Howlett, B., Crous, P., Stukenbrock, E., James, T., Gow, N., Eds.; ASM Press: Washington, DC, USA, 2017; pp. 703–726. [Google Scholar]
- Cromey, M.G.; Drakulic, J.; Beal, E.J.; Waghorn, I.A.G.; Perry, J.N.; Clover, G.R.G. Susceptibility of garden trees and shrubs to Armillaria root rot. Plant Dis. 2020, 104, 483–492. [Google Scholar] [CrossRef]
- Worrall, J.J.; Harrington, T.C.; Blodgett, J.T.; Conklin, D.A.; Fairweather, M.L. Heterobasidion annosum and H. parviporum in the southern Rocky Mountains and adjoining states. Plant Dis. 2010, 94, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Mattila, U.; Nuutinen, T. Assessing the incidence of butt rot in Norway spruce in southern Finland. Silva Fenn. 2007, 41, 29–43. [Google Scholar] [CrossRef]
- Guinet, C.; Boutigny, A.L.; Vialle, A.; Hamelin, R.C.; Frey, P.; Ioos, R. Simultaneous monitoring and quantification of Melampsora allii-populina and Melampsora larici-populina on infected poplar leaves using a duplex real-time PCR assay. Plant Pathol. 2016, 65, 380–391. [Google Scholar] [CrossRef]
- Liu, J.J.; Chan, D.; Xiang, Y.; Williams, H.; Li, X.R.; Sniezko, R.A.; Sturrock, R.N. Characterization of five novel mitoviruses in the white pine blister rust fungus Cronartium ribicola. PLoS ONE 2016, 11, e0154267. [Google Scholar] [CrossRef][Green Version]
- Cobo-Díaz, J.F.; Baroncelli, R.; Le Floch, G.; Picot, A. A novel metabarcoding approach to investigate Fusarium species composition in soil and plant samples. FEMS Microbiol. Ecol. 2019, 95, fiz084. [Google Scholar] [CrossRef]
- Gordon, T.R. Fusarium oxysporum and the Fusarium wilt syndrome. Annu. Rev. Phytopathol. 2017, 55, 23–39. [Google Scholar] [CrossRef]
- Kombrink, A.; Rovenich, H.; Shi-Kunne, X.; Rojas-Padilla, E.; van den Berg, G.C.M.; Domazakis, E.; de Jonge, R.; Valkenburg, D.-J.; Sánchez-Vallet, A.; Seidl, M.F.; et al. Verticillium dahliae LysM effectors differentially contribute to virulence on plant hosts. Mol. Plant Pathol. 2017, 18, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cuesta, R.; Navarro-Cerrillo, R.M.; Quero, J.L.; Ruiz-Gómez, F.J. Small-scale abiotic factors influencing the spatial distribution of Phytophthora cinnamomi under declining Quercus ilex trees. Forests 2020, 11, 375. [Google Scholar] [CrossRef]
- Jung, T.; Vettraino, A.M.; Cech, T.L.; Vannini, A. The impact of invasive Phytophthora species on European forests. In Phytophthora: A Global Perspective; Lamour, K., Ed.; CABI: Wallingford, UK, 2013; pp. 146–158. [Google Scholar]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; Invasive Species Specialist Group (ISSG): Auckland, New Zealand, 2000. [Google Scholar]
- Desprez-Loustau, M.L.; Marçais, B.; Nageleisen, L.M.; Piou, D.; Vannini, A. Interactive effects of drought and pathogens in forest trees. Ann. For. Sci. 2006, 63, 597–612. [Google Scholar] [CrossRef]
- Migliorini, D.; Ghelardini, L.; Luchi, N.; Capretti, P.; Onorari, M.; Santini, A. Temporal patterns of airborne Phytophthora spp. in a woody plant nursery area detected using real-time PCR. Aerobiologia 2019, 35, 201–214. [Google Scholar] [CrossRef]
- Belbahri, L.; Moralejo, E.; Calmin, G.; Oszako, T.; García, J.A.; Descals, E.; Lefort, F. Phytophthora polonica, a new species isolated from declining Alnus glutinosa stands in Poland. FEMS Microbiol. Lett. 2006, 261, 165–174. [Google Scholar] [CrossRef]
- Lazreg, F.; Belabid, L.; Sanchez, J.; Gallego, E.; Garrido-Cardenas, J.A.; Elhaitoum, A. First report of Globisporangium ultimum causing Pythium damping-off on Aleppo pine in Algeria, Africa, and the Mediterranean region. Plant Dis. 2013, 97, 1111. [Google Scholar] [CrossRef] [PubMed]
- Weiland, J.E.; Beck, B.R.; Davis, A. Pathogenicity and virulence of Pythium species obtained from forest nursery soils on Douglas-fir seedlings. Plant Dis. 2013, 97, 744–748. [Google Scholar] [CrossRef]
- Rosso, M.L.; Rupe, J.C.; Chen, P.; Mozzoni, L.A. Inheritance and genetic mapping of resistance to Pythium damping-off caused by Pythium aphanidermatum in ‘Archer’ soybean. Crop Sci. 2008, 48, 2215–2222. [Google Scholar] [CrossRef]
- Pankhurst, C.E.; McDonald, H.J.; Hawke, B.G. Influence of tillage and crop rotation on the epidemiology of Pythium infections of wheat in a red-brown earth of South Australia. Soil Biol. Biochem. 1995, 27, 1065–1073. [Google Scholar] [CrossRef]
- Kannan, V.R.; Bastas, K.K.; Devi, R.S. 20 Scientific and economic impact of plant pathogenic bacteria. In Sustainable Approaches to Controlling Plant Pathogenic Bacteria; Kannan, V.R., Bastas, K.K., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 369–392. [Google Scholar]
- Griffiths, H.M. Forest diseases caused by prokaryotes: Phytoplasmal and bacterial diseases. In Infectious Forest Diseases; Gonthier, P., Nicolotti, G., Eds.; CABI: Wallingford, UK, 2013; pp. 76–96. [Google Scholar]
- Melotto, M.; Underwood, W.; He, S.Y. Role of stomata in plant innate immunity and foliar bacterial diseases. Annu. Rev. Phytopathol. 2008, 46, 101–122. [Google Scholar] [CrossRef]
- Martins, P.M.; Merfa, M.V.; Takita, M.A.; De Souza, A.A. Persistence in phytopathogenic bacteria: Do we know enough? Front. Microbiol. 2018, 9, 1099. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.E.; Wang, M.B.; Bravo, J.E.; Banta, L.M. Unmasking host and microbial strategies in the Agrobacterium-plant defense tango. Front. Plant Sci. 2015, 6, 200. [Google Scholar] [CrossRef] [PubMed]
- Kado, C.I. Crown gall. In The Plant Health Instructor; American Phytopathological Society: St. Paul, MN, USA, 2002. [Google Scholar] [CrossRef]
- Rapicavoli, J.; Ingel, B.; Blanco-Ulate, B.; Cantu, D.; Roper, C. Xylella fastidiosa: An examination of a re-emerging plant pathogen. Mol. Plant Pathol. 2018, 19, 786–800. [Google Scholar] [CrossRef]
- Chatterjee, S.; Newman, K.L.; Lindow, S.E. Cell-to-cell signaling in Xylella fastidiosa suppresses movement and xylem vessel colonization in grape. Mol. Plant Microbe Int. 2008, 21, 1309–1315. [Google Scholar] [CrossRef]
- Tebaldi, N.D.; Leite, L.N.; Marque, J.M.D.; Furlanetto, M.C.A.; Mota, L.C.B.M. Occurrence of Ralstonia solanacearum on olive tree in Brazil. Summa. Phytopathol. 2014, 40, 185. [Google Scholar] [CrossRef][Green Version]
- Coutinho, T.A.; Wingfield, M.J. Ralstonia solanacearum and R. pseudosolanacearum on Eucalyptus: Opportunists or primary pathogens? Front. Plant Sci. 2017, 8, 761. [Google Scholar] [CrossRef]
- Steele, H.; Laue, B.E.; MacAskill, G.A.; Hendry, S.J.; Green, S. Analysis of the natural infection of European horse chestnut (Aesculus hippocastanum) by Pseudomonas syringae pv. aesculi. Plant Pathol. 2010, 59, 1005–1013. [Google Scholar] [CrossRef]
- Denman, S.; Doonan, J.; Ransom-Jones, E.; Broberg, M.; Plummer, S.; Kirk, S.; Scarlett, K.; Griffiths, A.R.; Kaczmarek, M.; Forster, J.; et al. Microbiome and infectivity studies reveal complex polyspecies tree disease in Acute Oak Decline. ISME J. 2018, 12, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Moradi-Amirabad, Y.; Rahimian, H.; Babaeizad, V.; Denman, S. Brenneria spp. and Rahnellavictoriana associated with acute oak decline symptoms on oak and hornbeam in Iran. For. Pathol. 2019, 49, e12535. [Google Scholar] [CrossRef]
- Snelling, J.; Tisserat, N.A.; Cranshaw, W. Kermes scale (Allokermes sp.) and the drippy nut pathogen (Brenneria quercina) associated with a decline of red oak species in Colorado. Phytopathology 2011, 101, S168. [Google Scholar]
- Sitz, R.A.; Zerillo, M.M.; Snelling, J.; Ibarra Caballero, J.; Alexander, K.; Nash, K.; Tisserat, N.A.; Cranshaw, W.S.; Stewart, J.E. Drippy blight, a disease of red oaks in Colorado, US, produced from the combined effect of the scale insect Allokermes galliformis and the bacterium Lonsdalea quercina subsp. quercina. Arboricu. Urban For. 2018, 43, 146–153. [Google Scholar]
- Sitz, R.A.; Aquino, V.M.; Tisserat, N.; Cranshaw, W.; Stewart, J.E. Insects visiting drippy blight diseased red oak trees are contaminated with the pathogenic bacterium Lonsdalea quercina. Plant Dis. 2019, 103, 1940–1946. [Google Scholar] [CrossRef]
- Li, Y.; He, W.; Ren, F.; Guo, L.; Chang, J.; Cleenwerck, I.; Ma, Y.; Wang, H. A canker disease of Populus × euramericana in China caused by Lonsdalea quercina subsp. populi. Plant Dis. 2014, 98, 368–378. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zlatković, M.; Tenorio-Baigorria, I.; Lakatos, T.; Tóth, T.; Koltay, A.; Pap, P.; Marković, M.; Orlović, S. Bacterial canker disease on Populus × euramericana caused by Lonsdalea populi in Serbia. Forests 2020, 11, 1080. [Google Scholar] [CrossRef]
- Li, A.; He, W. Molecular aspects of an emerging poplar canker caused by Lonsdalea populi. Front. Microbiol. 2019, 10, 2496. [Google Scholar] [CrossRef]
- Weintraub, P.G.; Beanland, L. Insect vectors of phytoplasmas. Annu. Rev. Entomol. 2006, 51, 91–111. [Google Scholar] [CrossRef]
- Lee, I.M.; Davis, R.E.; Gundersen-Rindal, D.E. Phytoplasma: Phytopathogenic mollicutes. Ann. Rev. Microbiol. 2000, 54, 221–255. [Google Scholar] [CrossRef] [PubMed]
- Bertaccini, A.; Duduk, B.; Paltrinieri, S.; Contaldo, N. Phytoplasmas, and phytoplasma diseases: A severe threat to agriculture. Am. J. Plant Sci. 2014, 5, 1763–1788. [Google Scholar] [CrossRef]
- Marcone, C. Elm yellows: A phytoplasma disease of concern in forest and landscape ecosystems. For. Pathol. 2017, 47, e12324. [Google Scholar] [CrossRef]
- Büttner, C.; von Bargen, S.; Bandte, M.; Mühlbach, H.P. Forest diseases caused by viruses. In Infectious Forest Diseases; Gonthier, P., Nicolotti, G., Eds.; CABI: Wallingford, UK, 2013; pp. 50–75. [Google Scholar]
- Mühlbach, H.P.; Mielke-Ehret, N. Emaravirus. In Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M., Lefkowitz, E., Adams, M.J., Carstens, E.B., Eds.; Elsevier: Dordrecht, The Netherlands, 2011; pp. 767–770. [Google Scholar]
- Elbeaino, T.; Digiaro, M.; Mielke-Ehret, N.; Muehlbach, H.P.; Martelli, G.P. ICTV virus taxonomy profile: Fimoviridae. J. Gen. Virol. 2018, 99, 1478–1479. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R. Nematodes, an emerging threat to global forests: Assessment and management. Plant Pathol. J. 2012, 11, 99–113. [Google Scholar] [CrossRef]
- Vicente, C.S.; Nascimento, F.; Espada, M.; Barbosa, P.; Mota, M.; Glick, B.R.; Oliveira, S. Characterization of bacteria associated with pinewood nematode Bursaphelenchus xylophilus. PLoS ONE 2012, 7, e46661. [Google Scholar] [CrossRef]
- Umebayashi, T.; Fukuda, K.; Haishi, T.; Sotooka, R.; Zuhair, S.; Otsuki, K. The developmental process of xylem embolisms in pine wilt disease monitored by multipoint imaging using compact magnetic resonance imaging. Plant Physiol. 2011, 156, 943–951. [Google Scholar] [CrossRef]
- Cardoso, J.M.S.; Anjo, S.I.; Fonseca, L.; Egas, C.; Manadas, B.; Abrantes, I. Bursaphelenchus xylophilus and B. mucronatus secretomes: A comparative proteomic analysis. Sci. Rep. 2016, 6, 39007. [Google Scholar] [CrossRef]
- Li, X.; Zhuo, K.; Luo, M.; Sun, L.; Liao, J. Molecular cloning and characterization of a calreticulin cDNA from the pinewood nematode Bursaphelenchus xylophilus. Exp. Parasitol. 2011, 128, 121–126. [Google Scholar] [CrossRef]
- Kikuchi, T.; Shibuya, H.; Aikawa, T.; Jones, J.T. Cloning and characterization of pectate lyases expressed in the esophageal gland of the pine wood nematode Bursaphelenchus xylophilus. Mol. Plant Microbe Int. 2006, 19, 280–287. [Google Scholar] [CrossRef]
- Futai, K. Pine wood nematode, Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissena, V. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Tennekes, H.A. Time-cumulative toxicity of neonicotinoids: Experimental evidence and implications for environmental risk assessments. Int. J. Environ. Res. Public Health 2020, 17, 1629. [Google Scholar] [CrossRef] [PubMed]
- Gil García, M.D.; Martínez Galera, M.; Uclés, S.; Lozano, A.; Fernández-Alba, A.R. Ultrasound-assisted extraction based on QuEChERS of pesticide residues in honeybees and determination by LC-MS/MS and GC-MS/MS. Anal. Bioanal. Chem. 2018, 410, 5195–5210. [Google Scholar] [CrossRef]
- Neves, A.P.; Colabuono, F.I.; Ferreira, P.A.L.; Kawakami, S.K.; Taniguchi Rubens, C.L.; Figueira, S.; Mahiques, M.M.; Montone, R.C.; Bícego, M.C. Depositional history of polychlorinated biphenyls (PCBs), organochlorine pesticides (OCPs) and polycyclic aromatic hydrocarbons (PAHs) in an Amazon estuary during the last century. Sci. Total Environ. 2018, 615, 1262–1270. [Google Scholar] [CrossRef]
- Brasil, V.L.M.; Ramos Pinto, M.B.; Bonan, R.F.; Kowalski, L.P.; da Cruz Perez, D.E. Pesticides as risk factors for head and neck cancer: A review. J. Oral Pathol. Med. 2018, 47, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Sorenson, W.C.; Smith, E.H.; Smith, J.R. Charles Valentine Riley: Founder of Modern Entomology; With Contributions by D.C. Weber; University of Alabama Press: Tuscaloosa, AL, USA, 2019; 214p. [Google Scholar]
- Mc Namara, L.; Griffin, C.T.; Fitzpatrick, D.; Kavanagh, K.; Carolan, J.C. The effect of entomopathogenic fungal culture filtrate on the immune response and haemolymph proteome of the large pine weevil, Hylobius abietis. Insect Biochem. Mol. Biol. 2018, 101, 1–13. [Google Scholar]
- Imoulan, A.; Hussain, M.; Kirk, P.M.; El Meziane, A.; Yao, Y.J. Entomopathogenic fungus Beauveria: Host specificity, ecology and significance of morpho-molecular characterization in accurate taxonomic classification. J. Asia Pac. Entomol. 2017, 20, 1204–1212. [Google Scholar] [CrossRef]
- Van der Weerden, N.L.; Bleackley, M.R.; Anderson, M.A. Properties and mechanisms of action of naturally occurring antifungal peptides. Cell. Mol. Life Sci. 2013, 70, 3545–3570. [Google Scholar] [CrossRef]
- Xiao, G.; Ying, S.H.; Zheng, P.; Wang, Z.-L.; Zhang, S.; Xie, X.-Q.; Shang, Y.; St. Leger, R.J.; Zhao, G.-P.; Wang, C.; et al. Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci. Rep. 2012, 2, 483. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Liu, Y.Q.; Min, S.H.I.; Huang, J.H.; Chen, X.X. Parasitoid wasps as effective biological control agents. J. Integr. Agr. 2019, 18, 705–715. [Google Scholar] [CrossRef]
- Barta, M.; Kautmanová, I.; Čičková, H.; Ferenčík, J.; Florián, S.; Novotný, J.; Kozánek, M. Hypocrealean fungi associated with populations of Ips typographus in West Carpathians and selection of local Beauveria strains for effective bark beetle control. Biologia 2018, 73, 53–65. [Google Scholar]
- Mayerhofer, J.; Enkerli, J.; Zelger, R.; Strasser, H. Biological control of the European cockchafer: Persistence of Beauveria brongniartii after long-term applications in the Euroregion Tyrol. BioControl 2015, 60, 617–629. [Google Scholar] [CrossRef]
- Davis, T.S.; Mann, A.J.; Malesky, D.; Jankowski, E.; Bradley, C. Laboratory and field evaluation of the entomopathogenic fungus Beauveria bassiana (Deuteromycotina: Hyphomycetes) for population management of spruce beetle, Dendroctonus rufipennis (Coleoptera: Scolytinae), in felled trees and factors limiting pathogen success. Environ. Entomol. 2018, 47, 594–602. [Google Scholar]
- Schrank, A.; Vainstein, M.H. Metarhizium anisopliae enzymes and toxins. Toxicon 2010, 56, 1267–1274. [Google Scholar]
- Kim, J.C.; Baek, S.; Park, S.E.; Kim, S.; Lee, M.R.; Jo, M.; Im, J.S.; Ha, P.; Kim, J.S.; Shin, T.Y. Colonization of Metarhizium anisopliae on the surface of pine tree logs: A promising biocontrol strategy for the Japanese pine sawyer, Monochamus alternatus. Fungal Biol. 2019, 124, 125–134. [Google Scholar] [CrossRef]
- Williams, C.D.; Dillon, A.B.; Harvey, C.D.; Hennessy, R.; Namara, L.M.; Griffin, C.T. Control of a major pest of forestry, Hylobius abietis, with entomopathogenic nematodes and fungi using eradicant and prophylactic strategies. For. Ecol. Manag. 2013, 305, 212–222. [Google Scholar] [CrossRef]
- Qayyum, M.A.; Saleem, M.A.; Saeed, S.; Wakil, W.; Ishtiaq, M.; Ashraf, W.; Ahmed, N.; Ali, M.; Muhammad Ikram, R.; Yasin, M.; et al. Integration of entomopathogenic fungi and eco-friendly insecticides for management of red palm weevil, Rhynchophorus ferrugineus (Olivier). Saudi J. Biol. Sci. 2020, 27, 1811–1817. [Google Scholar] [CrossRef]
- Zhao, H.; Lovett, B.; Fang, W. Genetically engineering entomopathogenic fungi. Adv. Genet. 2016, 94, 137–163. [Google Scholar]
- Palyzová, A.; Svobodová, K.; Sokolová, L.; Novák, J.; Novotný, Č. Metabolic profiling of Fusarium oxysporum f. sp. conglutinans race 2 in dual cultures with biocontrol agents Bacillus amyloliquefaciens, Pseudomonas aeruginosa, and Trichoderma harzianum. Folia Microbiol. (Praha) 2019, 64, 779–787. [Google Scholar] [PubMed]
- Chen, L.; Bóka, B.; Kedves, O.; Nagy, D.; Szucs, A.; Champramary, S.; Roszik, R.; Patocskai, Z.; Münsterkötter, M.; Huynh, T.; et al. Towards the biological control of devastating forest pathogens from the genus Armillaria. Forests 2019, 10, 1013. [Google Scholar] [CrossRef]
- Kong, P.; Hong, C. Biocontrol of boxwood blight by Trichoderma koningiopsis Mb2. Crop Protect. 2017, 98, 124–127. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Wang, L.; Zhou, L.; Song, J. Study of the departure of pine wood nematode, Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae) from Monochamus alternatus (Coleoptera: Cerambycidae). J. Asia Pac. Entomol. 2020, 23, 981–987. [Google Scholar] [CrossRef]
- Chu, W.H.; Dou, Q.; Chu, H.L.; Wang, H.H.; Sung, C.K.; Wang, C.Y. Research advance on Esteya vermicola, a high potential biocontrol agent of pine wilt disease. Mycol. Prog. 2015, 14, 115. [Google Scholar] [CrossRef]
- Tellenbach, C.; Sieber, T.N. Do colonization by dark septate endophytes and elevated temperature affect pathogenicity of oomycetes? FEMS Microbiol. Ecol. 2012, 82, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Terhonen, E.; Sipari, N.; Asiegbu, F.O. Inhibition of phytopathogens by fungal root endophytes of Norway spruce. Biol. Control 2016, 99, 53–63. [Google Scholar] [CrossRef]
- Tellenbach, C.; Sumarah, M.W.; Grünig, C.R.; Miller, J.D. Inhibition of Phytophthora species by secondary metabolites produced by the dark septate endophyte Phialocephalaeuropaea. Fungal Ecol. 2013, 6, 12–18. [Google Scholar] [CrossRef]
- Oliva, J.; Messal, M.; Wendt, L.; Elfstrand, M. Quantitative interactions between the biocontrol fungus Phlebiopsis gigantea, the forest pathogen Heterobasidion annosum and the fungal community inhabiting Norway spruce stumps. For. Ecol. Manag. 2017, 402, 253–264. [Google Scholar] [CrossRef]
- Paz, I.C.P.; Santin, R.C.M.; Guimarães, A.M.; Pereira da Rosa, O.P.; Quecine, M.C.; Silva, M.C.P.; Azevedo, J.L.; Santos Matsumura, A.T. Biocontrol of Botrytis cinerea and Calonectria gracilis by eucalypts growth promoters Bacillus spp. Microb. Pathog. 2018, 121, 106–109. [Google Scholar] [CrossRef]
- Bovolini, M.P.; Lazarotto, M.; Gonzatto, M.P.; Campos de Sá, L.; Borges Junior, N. Preventive and curative control of Oidium eucalypti in Eucalyptus benthamii clonal seedlings. Revista Árvore 2018, 42, 420–504. [Google Scholar] [CrossRef]
- Cheffi, M.; Chenari Bouket, A.; Alenezi, F.N.; Luptakova, L.; Belka, M.; Vallat, A.; Rateb, M.E.; Tounsi, S.; Triki, M.A.; Belbahri, L. Olea europaea L. root endophyte Bacillus velezensis OEE1 counteracts oomycete and fungal harmful pathogens and harbours a large repertoire of secreted and volatile metabolites and beneficial functional genes. Microorganisms 2019, 7, 314. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Crickmore, N.; Berry, C.; Panneerselvam, S.; Mishra, R.; Connor, T.R.; Bonning, B.C. A structure-based nomenclature for Bacillus thuringiensis and other bacteria-derived pesticidal proteins. J. Invertebr. Pathol. 2020, 107438. [Google Scholar] [CrossRef]
- Jung, S.J.; Kim, N.K.; Lee, D.-H.; Hong, S.-I.; Lee, J.K. Screening and evaluation of Streptomyces species as a potential biocontrol agent against a wood decay fungus, Gloeophyllum trabeum. Mycobiology 2018, 46, 138–146. [Google Scholar] [CrossRef]
- Dahm, H.; Brzezińska, A.J.; Wrótniak-Drzewiecka, W.; Golińska, P.; Różycki, H.; Rai, M. Myxobacteria as a potential biocontrol agent effective against pathogenic fungi of economically important forest trees. Dendrobiologie 2015, 74, 13–24. [Google Scholar] [CrossRef]
- Masson, T.; Fabre, M.L.; Ferrelli, M.L.; Pidre, M.L.; Romanowski, V. Protein composition of the occlusion bodies of Epinotia aporema granulovirus. PLoS ONE 2019, 14, e0207735. [Google Scholar] [CrossRef]
- Muñoz-Adalia, E.J.; Fernández, M.M.; Diez, J.J. The use of mycoviruses in the control of forest diseases. Biocontrol. Sci. Technol. 2016, 26, 577–604. [Google Scholar] [CrossRef]
- Krstin, L.; Katanić, Z.; Ježić, M.; Poljak, I.; Nuskern, L.; Matković, I.; Idžojtić, M.; Ćurković-Perica, M. Biological control of chestnut blight in Croatia: An interaction between host sweet chestnut, its pathogen Cryphonectria parasitica and the biocontrol agent Cryphonectria hypovirus 1. Pest Manag. Sci. 2016, 73, 582–589. [Google Scholar] [CrossRef]
- Vainio, E.J.; Jurvansuu, J.; Hyder, R.; Kashif, M.; Piri, T.; Tuomivirta, T.; Poimala, A.; Xu, P.; Mäkelä, S.; Nitisa, D.; et al. Heterobasidion Partitivirus 13 mediates severe growth debilitation and major alterations in the gene expression of a fungal forest pathogen. J. Virol. 2018, 92, e01744-17. [Google Scholar] [CrossRef]
- Schoebel, C.N.; Zoller, S.; Rigling, D. Detection and genetic characterization of a novel mycovirus in Hymenoscyphus fraxineus, the causal agent of ash dieback. Infect. Genet. Evol. 2014, 28, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Tomalak, M. Parasitic association of the mycetophagous wood nematode, Bursaphelenchus fraudulentus with the honey fungus Armillaria ostoyae. For. Pathol. 2017, 47, e12325. [Google Scholar] [CrossRef]
- Gao, S.K.; Wei, K.; Tang, Y.L.; Wang, X.Y.; Yang, Z.Q. Effect of parasitoid density on the timing of parasitism and development duration of progeny in Sclerodermus pupariae (Hymenoptera: Bethylidae). Biol. Control 2016, 97, 57–62. [Google Scholar] [CrossRef]
- Wang, X.; Wei, K.; Yang, Z.; Jennings, D.E.; Duan, J.J. Effects of biotic and abiotic factors on phenotypic partitioning of wing morphology and development in Sclerodermus pupariae (Hymenoptera: Bethylidae). Sci. Rep. 2016, 6, 26408. [Google Scholar] [CrossRef]
- Wei, K.; Wang, X.Y.; Yang, Z.Q. Effects of supplementary nutrition on parasitism ability and developmental process of a gregarious parasitoid, Sclerodermus pupariae (Hymenoptera: Bethylidae). For. Res. 2016, 29, 369–376. [Google Scholar]
- Wei, K.; Gao, S.K.; Tang, Y.L.; Wang, X.Y.; Yang, Z.Q. Determination of the optimal parasitoid-to-host ratio for efficient mass-rearing of the parasitoid, Sclerodermus pupariae (Hymenoptera: Bethylidae). J. Appl. Entomol. 2017, 141, 181–188. [Google Scholar] [CrossRef]
- Asgari, S.; Rivers, D.B. Venom proteins from endoparasitoid wasps and their role in host-parasite interactions. Ann. Rev. Entomol. 2011, 56, 313–335. [Google Scholar] [CrossRef] [PubMed]
- Strand, M.R.; Burke, G.R. Polydnaviruses: Nature’s genetic engineers. Annu. Rev. Virol. 2014, 1, 333–354. [Google Scholar] [CrossRef]
- Strand, M.R. Teratocytes and their functions in parasitoids. Curr. Opin. Insect Sci. 2014, 6, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P. Forest microbiome: Diversity, complexity and dynamics. FEMS Microbiol. Rev. 2017, 41, 109–130. [Google Scholar] [CrossRef]
- Mercado-Blanco, J.; Abrantes, I.; Barra Caracciolo, A.; Bevivino, A.; Ciancio, A.; Grenni, P.; Hrynkiewicz, K.; Kredics, L.; Proença, D.N. Belowground microbiota and the health of tree crops. Front. Microbiol. 2018, 9, 1006. [Google Scholar] [CrossRef]
- Mueller, U.G.; Sachs, J.L. Engineering microbiomes to improve plant and animal health. Trends Microbiol. 2015, 23, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Terhonen, E.; Blumenstein, K.; Kovalchuk, A.; Asiegbu, F.O. Forest tree microbiomes and associated fungal endophytes: Functional roles and impact on forest health. Forests 2019, 10, 42. [Google Scholar] [CrossRef]
- Partida-Martínez, L.P.; Heil, M. The microbe-free plant: Fact or artifact? Front. Plant Sci. 2011, 2. [Google Scholar] [CrossRef]
- Hacquard, S.; Schadt, C.W. Towards a holistic understanding of the beneficial interactions across the Populus microbiome. New Phytol. 2005, 205, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Ben Said, S.; Or, D. Synthetic microbial ecology: Engineering habitats for modular consortia. Front. Microbiol. 2017, 8, 1125. [Google Scholar] [CrossRef]
- Zmora, N.; Zeevi, D.; Korem, T.; Segal, E.; Elinav, E. Taking it personally: Personalized utilization of the human microbiome in health and disease. Cell Host Microbe 2016, 19, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Mosqueda, M.C.; Rocha-Granados, M.C.; Glick, B.R.; Santoyo, G. Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Lebeis, S.L. Greater than the sum of their parts: Characterizing plant microbiomes at the community-level. Curr. Opin. Plant Biol. 2015, 24, 82–86. [Google Scholar] [CrossRef]
- Vorholt, J.A.; Vogel, C.; Carlström, C.I.; Müller, D.B. Establishing causality: Opportunities of synthetic communities for plant microbiome research. Cell Host Microbe 2017, 22, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.L.; Ling, H.; Lee, Y.S.; Chang, M.W. Microbiome engineering: Current applications and its future. Biotechnol. J. 2017, 12, 1600099. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. The soil microbiome—from metagenomics to metaphenomics. Curr. Opin. Microbiol. 2018, 43, 162–168. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef] [PubMed]
- Llado, S.; Lopez-Mondejar, R.; Baldrian, P. Forest soil bacteria: Diversity, involvement in ecosystem processes, and response to global change. Microbiol. Mol. Biol. Rev. 2017, 81, e00063-16. [Google Scholar] [CrossRef]
- Lombard, N.; Prestat, E.; van Elsas, J.D.; Simonet, P. Soil-specific limitations for access and analysis of soil microbial communities by metagenomics. FEMS Microbiol. Ecol. 2011, 78, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Eloe-Fadrosh, E.A.; Ivanova, N.N.; Woyke, T.; Kyrpides, N.C. Metagenomics uncovers gaps in amplicon-based detection of microbial diversity. Nat. Microbiol. 2016, 1, 15032. [Google Scholar] [CrossRef]
- Albertsen, M.; Hugenholtz, P.; Skarshewski, A.; Nielsen, K.L.; Tyson, G.W.; Nielsen, P.H. Genome sequences of rare, uncultured bacteria obtained by differential coverage binning of multiple metagenomes. Nat. Biotechnol. 2013, 31, 533–538. [Google Scholar] [CrossRef]
- Wrighton, K.C.; Thomas, B.C.; Sharon, I.; Miller, C.S.; Castelle, C.J.; VerBerkmoes, N.C.; Wilkins, M.J.; Hettich, R.L.; Lipton, M.S.; Williams, K.H.; et al. Fermentation, hydrogen, and sulfur metabolism in multiple uncultivated bacterial phyla. Science 2012, 337, 1661–1665. [Google Scholar] [CrossRef]
- Alteio, L.V.; Schulz, F.; Seshadri, R.; Varghese, N.; Rodriguez-Reillo, W.; Ryan, E.; Goudeau, D.; Eichorst, S.A.; Malmstrom, R.R.; Bowers, R.M.; et al. Complementary metagenomic approaches improve reconstruction of microbial diversity in a forest soil. Msystems 2020, 5, e00768-19. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.M.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M.; et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef]
- Yuan, J.; Zhao, J.; Wen, T.; Zhao, M.; Li, R.; Goossens, P.; Huang, Q.; Bai, Y.; Vivanco, J.M.; Kowalchuk, G.A.; et al. Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome 2018, 6, 156. [Google Scholar] [CrossRef]
- Garcia-Lemos, A.M.; Großkinsky, D.K.; Stokholm, M.S.; Lund, O.S.; Nicolaisen, M.H.; Roitsch, T.G.; Veierskov, B.; Nybroe, O. Root-associated microbial communities of Abies nordmanniana: Insights into interactions of microbial communities with antioxidative enzymes and plant growth. Front. Microbiol. 2019, 10, 1937. [Google Scholar] [CrossRef] [PubMed]
- Hrynkiewicz, K.; Szymanska, S.; Piernik, A.; Thiem, D. Ectomycorrhizal community structure of Salix and Betula spp. at a saline site in central Poland in relation to the seasons and soil parameters. Water Air Soil Pollut. 2015, 226, 99. [Google Scholar] [CrossRef]
- Khabou, W.; Hajji, B.; Mohamed Zouari, M.; Rigane, H.; Ben Abdallah, F. Arbuscular mycorrhizal fungi improve growth and mineral uptake of olive tree under gypsum substrate. Ecol. Eng. 2014, 73, 290–296. [Google Scholar] [CrossRef]
- Lau, J.A.; Lennon, J.T.; Heath, K.D. Trees harness the power of microbes to survive climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 11009–11011. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular mycorrhizal fungi as natural biofertilizers: Let’s benefit from past successes. Front. Microbiol. 2015, 6, 1559. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Y.; Zeng, R.S.; Xu, J.F.; Li, J.; Shen, X.; Yihdego, W.J. Interplant communication of tomato plants through underground common mycorrhizal networks. PLoS ONE 2010, 5, e13324. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, G.; Sbodio, A.; Tech, J.J.; Suslow, T.V.; Coaker, G.L.; Leveau, J.H.J. Leaf microbiota in an agroecosystem: Spatiotemporal variation in bacterial community composition on field-grown lettuce. ISME J. 2012, 6, 1812–1822. [Google Scholar] [CrossRef]
- Stamets, P. Mycelium Running: How Mushrooms Can Help Save the World; Random House Digital, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Delmotte, N.; Knief, C.; Chaffron, S.; Innerebner, G.; Roschitzki, B.; Schlapbach, R.; von Mering, C.; Vorholt, J.A. Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc. Natl. Acad. Sci. USA 2009, 106, 16428–16433. [Google Scholar] [CrossRef] [PubMed]
- Finkel, O.M.; Burch, A.Y.; Lindow, S.E.; Post, A.F.; Belkin, S. Geographical location determines the population structure in phyllosphere microbial communities of a salt-excreting desert tree. Appl. Environ. Microbiol. 2011, 77, 7647–7655. [Google Scholar] [CrossRef]
- Peñuelas, J.; Terradas, J. The foliar microbiome. Trends Plant Sci. 2014, 19, 278–280. [Google Scholar] [CrossRef]
- Galippe, V. Note sur la présence de micro-organismes dans les tissus végétaux (deuxième note). C R Seances Soc. Biol. Fil. 1887, 39, 557–560. [Google Scholar]
- Porras-Alfaro, A.; Bayman, P. Hidden fungi, emergent properties: Endophytes and microbiomes. Ann. Rev. Phytopathol. 2009, 49, 291–315. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Christian, N.; Sullivan, C.; Visser, N.D.; Clay, K. Plant host and geographic location drive endophyte community composition in the face of perturbation. Microb. Ecol. 2016, 72, 621–632. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Wicaksono, W.A.; Jones, E.E.; Casonato, S.; Monk, J.; Ridgway, H.J. Biological control of Pseudomonas syringae pv. actinidiae (Psa), the causal agent of bacterial canker of kiwifruit, using endophytic bacteria recovered from a medicinal plant. Biol. Control 2018, 116, 103–112. [Google Scholar] [CrossRef]
- Yaish, W.M.; Al-Harrasi, I.; Alansari, A.S.; Al-Yahyai, R.; Glick, B.R. The use of high throughput DNA sequence analysis to assess the endophytic microbiome of date palm roots grown under different levels of salt stress. Int. Microbiol. 2017, 19, 143–155. [Google Scholar]
- Yuan, Z.; Druzhinina, I.; Labbé, J.; Redman, R.; Qin, Y.; Rodriguez, R.; Zhang, C.; Tuskan, G.A.; Lin, F. Specialized microbiome of a halophyte and its role in helping non-host plants to withstand salinity. Sci. Rep. 2016, 6, 32467. [Google Scholar] [CrossRef]
- Rojas-Solís, D.; Hernández-Pacheco, C.E.; Santoyo, G. Evaluation of Bacillus and Pseudomonas to colonize the rhizosphere and their effect on growth promotion in tomato (Physalis ixocarpa Brot. ex Horm.). Rev. Chapingo Ser. Hortic. 2016, 22, 45–57. [Google Scholar] [CrossRef]
- Timm, C.M.; Pelletier, D.A.; Jawdy, S.S.; Gunter, L.E.; Henning, J.A.; Engle, N.; Lu, T.Y. Two poplar-associated bacterial isolates induce additive favorable responses in a constructed plant-microbiome system. Front. Plant Sci. 2016, 7, 497. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Smith, F.A.; Smith, S.E. What is the significance of the arbuscular mycorrhizal colonization of many economically important crop plants? Plant Soil 2011, 348, 63. [Google Scholar] [CrossRef]
- Yuan, Z.-L.; Zhang, C.; Lin, F.-C. Role of diverse non-systemic fungal endophytes in plant performance and response to stress: Progress and approaches. J. Plant Growth Regul. 2010, 29, 116–126. [Google Scholar] [CrossRef]
- Oelmüller, R.; Sherameti, I.; Tripathi, S.; Varma, A. Piriformospora indica, a cultivable root endophyte with multiple biotechnological applications. Symbiosis 2009, 49, 1–17. [Google Scholar] [CrossRef]
- Narayan, O.P.; Verma, N.; Singh, A.K.; Oelmüller, R.; Kumar, M.; Prasad, D.; Kapoor, R.; Dua, M.; Johri, A.K. Antioxidant enzymes in chickpea colonized by Piriformospora indica participate in defense against the pathogen Botrytis cinerea. Sci. Rep. 2017, 7, 13553. [Google Scholar] [CrossRef] [PubMed]
- Likar, M.; Regvar, M. Isolates of dark septate endophytes reduce metal uptake and improve physiology of Salix caprea L. Plant Soil 2013, 370, 593–604. [Google Scholar] [CrossRef]
- Zachow, C.; Berg, C.; Muller, H.; Monk, J.; Berg, G. Endemic plants harbour specific Trichoderma communities with an exceptional potential for biocontrol of phytopathogens. J. Biotechnol. 2016, 235, 162–170. [Google Scholar] [CrossRef]
- Bae, H.; Roberts, D.P.; Lim, H.-S.; Strem, M.D.; Park, S.-C.; Ryu, C.-M.; Rachel Melnick, R.; Bailey, B.R. Endophytic-Trichoderma isolates from tropical environments delay disease onset and induce resistance against Phytophthora capsici in hot pepper using multiple mechanisms. Mol. Plant Micr. Interact. 2011, 24, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Romeralo, C.; Witzell, J.; Romeralo-Tapia, R.; Botella, L.; Javier Diez, J. Antagonistic activity of fungal endophyte filtrates against Gremmeniella abietina infections on Aleppo pine seedlings. Eur. J. Plant Pathol. 2015, 143, 691–704. [Google Scholar] [CrossRef]
- Ben Mefteh, F.; Daoud, A.; Chenari Bouket, A.; Thissera, B.; Kadri, Y.; Cherif-Silini, H.; Eshelli, M.; Alenezi, F.N.; Vallat, A.; Oszako, T.; et al. Date palm trees root-derived endophytes as fungal cell factories for diverse bioactive metabolites. Int. J. Mol. Sci. 2018, 19, 1986. [Google Scholar] [CrossRef]
- Halecker, S.; Wennrich, J.-P.; Rodrigo, S.; Andrée, N.; Rabsch, L.; Baschien, C.; Steinert, M.; Stadler, M.; Surup, F.; Schulz, B. Fungal endophytes for biocontrol of ash dieback: The antagonistic potential of Hypoxylon rubiginosum. Fungal Ecol. 2020, 45, 100918. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Dash, C.K.; Akutse, K.S.; Keppanan, R.; Afolabi, O.G.; Hussain, M.; Qasim, M.; Wang, L. Prospects of endophytic fungal entomopathogens as biocontrol and plant growth promoting agents: An insight on how artificial inoculation methods affect endophytic colonization of host plants. Microbiol. Res. 2018, 217, 34–50. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, Y.; Dennis, P.G.; Paungfoo-Lonhienne, C.; Weber, L.; Brackin, R.; Ragan, M.A.; Schmidt, S.; Hugenholgz, P. Evolutionary conservation of a core root microbiome across plant phyla along a tropical soil chronosequence. Nat. Commun. 2017, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Oter, R.; Nakano, R.T.; Dombrowski, N.; Ma, K.-W.; McHardy, A.C.; Schulze-Lefert, P. Modular traits of the Rhizobiales root microbiota and their evolutionary relationship with symbiotic rhizobia. Cell Host Microbe 2018, 24, 155–167. [Google Scholar] [CrossRef]
- Doty, S.L.; Oakley, B.; Xin, G.; Kang, J.W.; Singleton, G.; Khan, Z.; Vajzovic, A.; Staley, J.T. Diazotrophic endophytes of native black cottonwood and willow. Symbiosis 2009, 47, 23–33. [Google Scholar] [CrossRef]
- Santi, C.; Bogusz, D.; Franche, C. Biological nitrogen fixation in non-legume plants. Ann. Bot. 2013, 111, 743–767. [Google Scholar] [CrossRef]
- Nisa, H.; Kamili, A.N.; Nawchoo, I.A.; Shafi, S.; Shameem, N.; Bandh, S.A. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microb. Pathogenesis 2015, 82, 50–59. [Google Scholar] [CrossRef]
- Brader, G.; Compant, G.; Mitter, B.; Trognitz, F.; Sessitsch, A. Metabolic potential of endophytic bacteria. Curr. Opin. Biotech. 2014, 27, 30–37. [Google Scholar] [CrossRef]
- Bordiec, S.; Paquis, S.; Lacroix, H.; Dhondt, S.; Ait Barka, E.; Kauffmann, S.; Jeandet, P.; Mazeyrat-Gourbeyre, F.; Clément, C.; Baillieul, F.; et al. Comparative analysis of defense responses induced by the endophytic plant growth-promoting rhizobacterium Burkholderia phytofirmans strain PsJN and the non-host bacterium Pseudomonas syringae pv. pisi in grapevine cell suspensions. J. Exp. Bot. 2011, 62, 595–603. [Google Scholar] [CrossRef]
- Denance, N.; Sanchez-Vallet, A.; Goffner, D.; Molina, A. Disease resistance or growth: The role of plant hormones in balancing immune responses and fitness costs. Front. Plant Sci. 2013, 4, 155. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, B.G.; Licastro, D.; Mendonça-Previato, L.; Cámara, M.; Venturi, V. Plant-influenced gene expression in the rice endophyte Burkholderia kururiensis M130. Mol. Plant Micr. Interact 2015, 28, 10–21. [Google Scholar] [CrossRef]
- Mitter, B.N.; Pfaffenbichler, R.; Flavell, S.; Compant, L.; Antonielli, A.; Petric, A.; Sessitsch, A. A new approach to modify plant microbiomes and traits by introducing beneficial bacteria at flowering into progeny seeds. Front. Microbiol. 2017, 8, 11. [Google Scholar] [CrossRef]
- Vainio, E.J.; Muller, M.M.; Korhonen, K.; Piri, T.; Hantula, J. Viruses accumulate in aging infection centers of a fungal forest pathogen. ISME J. 2015, 9, 497–507. [Google Scholar] [CrossRef]
- Žifčáková, L.; Větrovský, T.; Howe, A.; Baldrian, P. Microbial activity in forest soil reflects the changes in ecosystem properties between summer and winter. Environ. Microbiol. 2016, 18, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Hungate, B.A.; Mau, R.L.; Schwartz, E.; Caporaso, J.G.; Dijkstra, P.; van Gestel, N.; Koch, B.J.; Liu, C.M.; McHugh, T.A.; Marks, J.C.; et al. Quantitative microbial ecology through stable isotope probing. Appl. Environ. Microb. 2015, 81, 7570–7581. [Google Scholar] [CrossRef]
- Ihrmark, K.; Bodeker, I.T.M.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandström-Durling, M.; Clemmensen, K.E.; et al. New primers to amplify the fungal ITS2 region–evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 2016, 24, 3315–3335. [Google Scholar] [CrossRef]
- Pandey, A.; Yarzábal, L.A. Bioprospecting cold-adapted plant growth promoting microorganisms from mountain environments. Appl. Microbiol. Biotechnol. 2019, 103, 643–657. [Google Scholar] [CrossRef]
- Rao, M.V.; Rice, R.A.; Fleischer, R.C.; Muletz-Wolz, C.R. Soil fungal communities differ between shaded and sun-intensive coffee plantations in El Salvador. PLoS ONE 2020, 15, e0231875. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-H.; Jeon, Y.-D.; Lee, O.-M.; Kim, J.-D.; Lee, N.-R.; Park, G.-T.; Son, H.-J. Characterization of a multifunctional feather-degrading Bacillus subtilis isolated from forest soil. Biodegradation 2010, 21, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Haruna, A.O.; Majid, N.M.A.; Jalloh, M.B. Potential PGPR properties of cellulolytic, nitrogen-fixing, phosphate-solubilizing bacteria in rehabilitated tropical forest soil. Microorganisms 2020, 8, 442. [Google Scholar] [CrossRef]
- Puri, A.; Padda, K.P.; Chanway, C.P. Sustaining the growth of Pinaceae trees under nutrient-limited edaphic conditions via plant-beneficial bacteria. PLoS ONE 2020, 15, e0238055. [Google Scholar] [CrossRef] [PubMed]
- Hortal, S.; Pera, J.; Parladé, J. Field persistence of the edible ectomycorrhizal fungus Lactarius deliciosus: Effect of inoculation strain, initial colonization level, and site characteristics. Mycorrhiza 2009, 19, 167–177. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).