New Species of Tomentella (Thelephorales, Basidiomycota) from Temperate Continental Mountain Climate of China (Xinjiang Region)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Morphological Studies
2.2. Molecular Procedures and Phylogenetic Analyses
3. Results
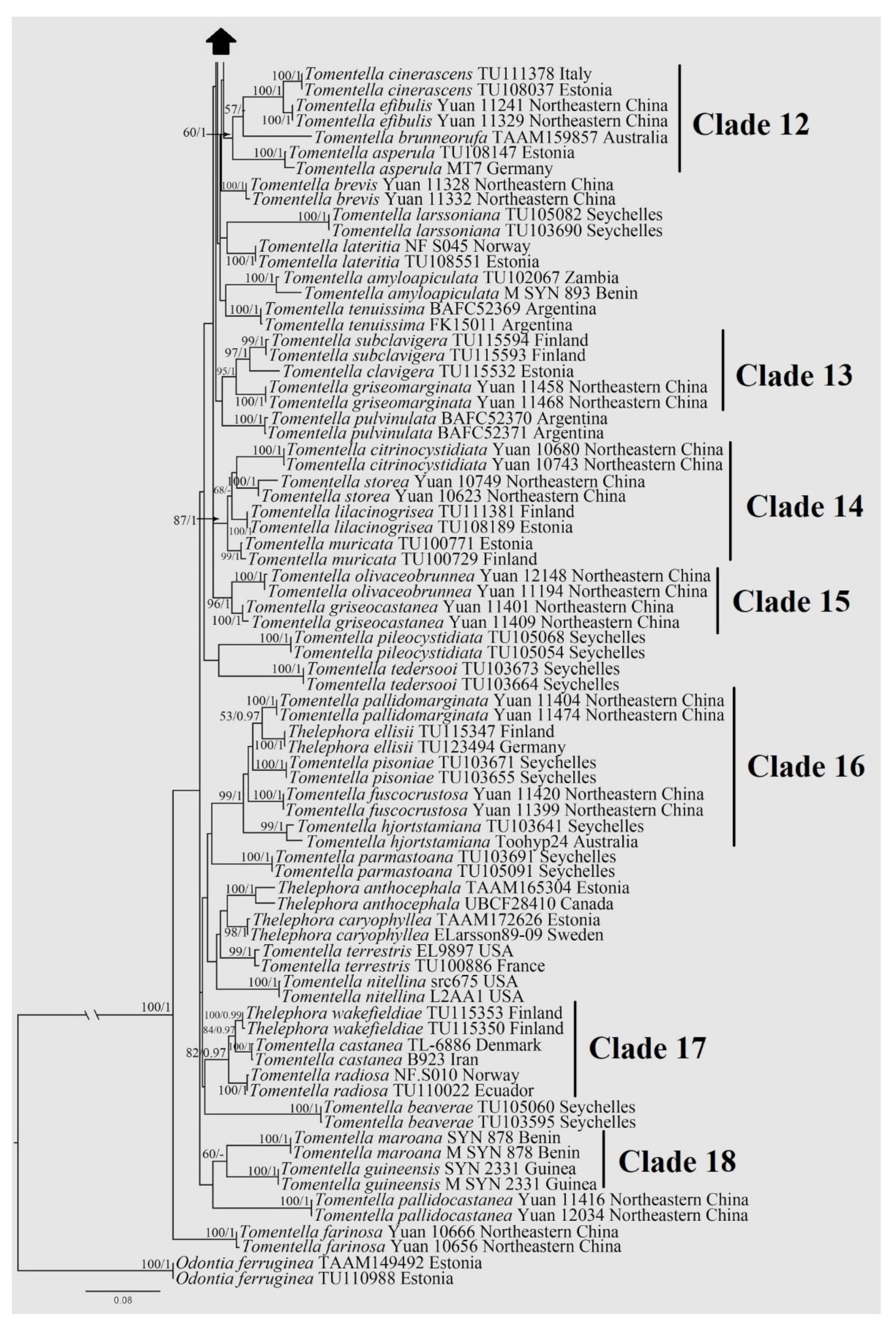
3.1. Phylogenetic Analysis
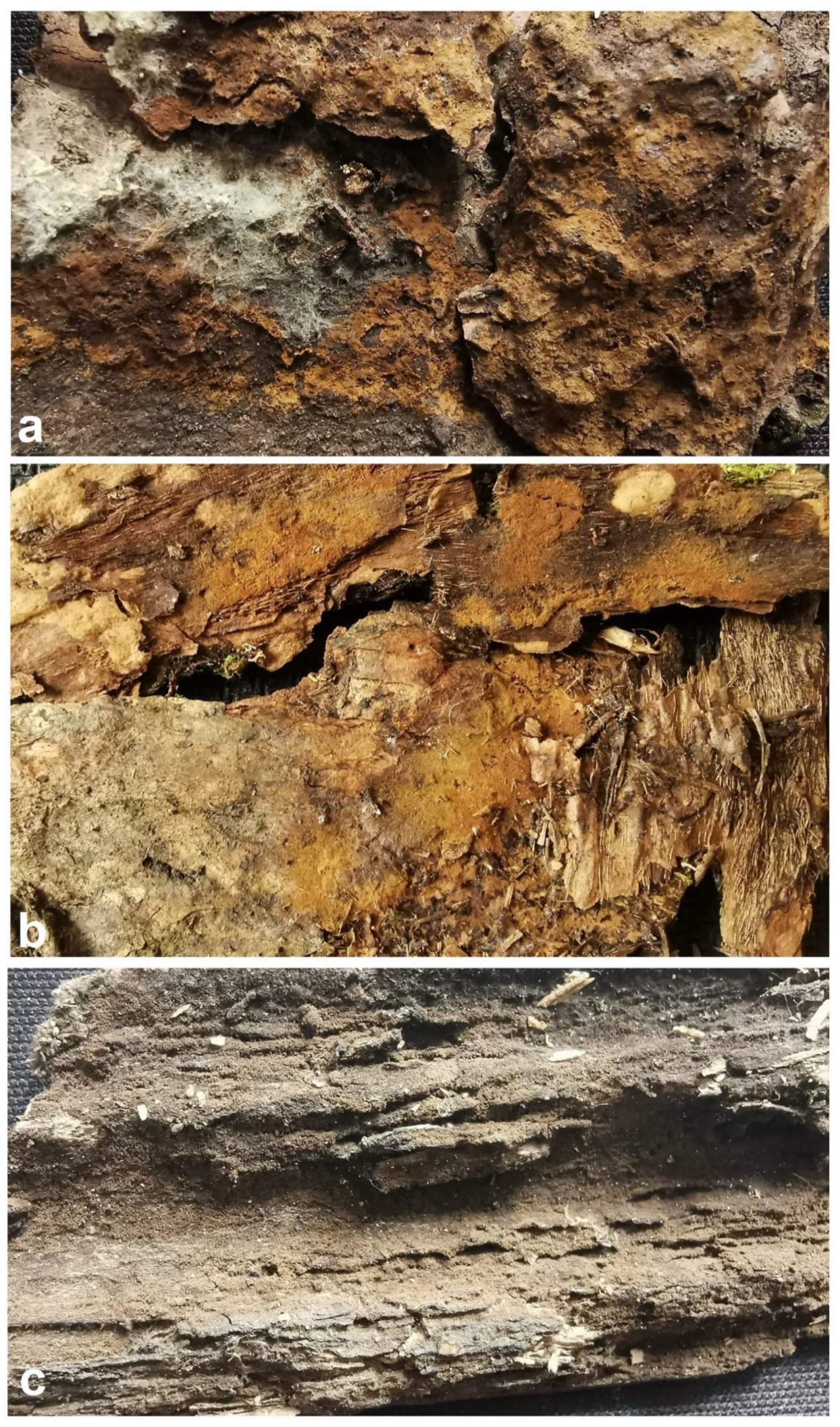
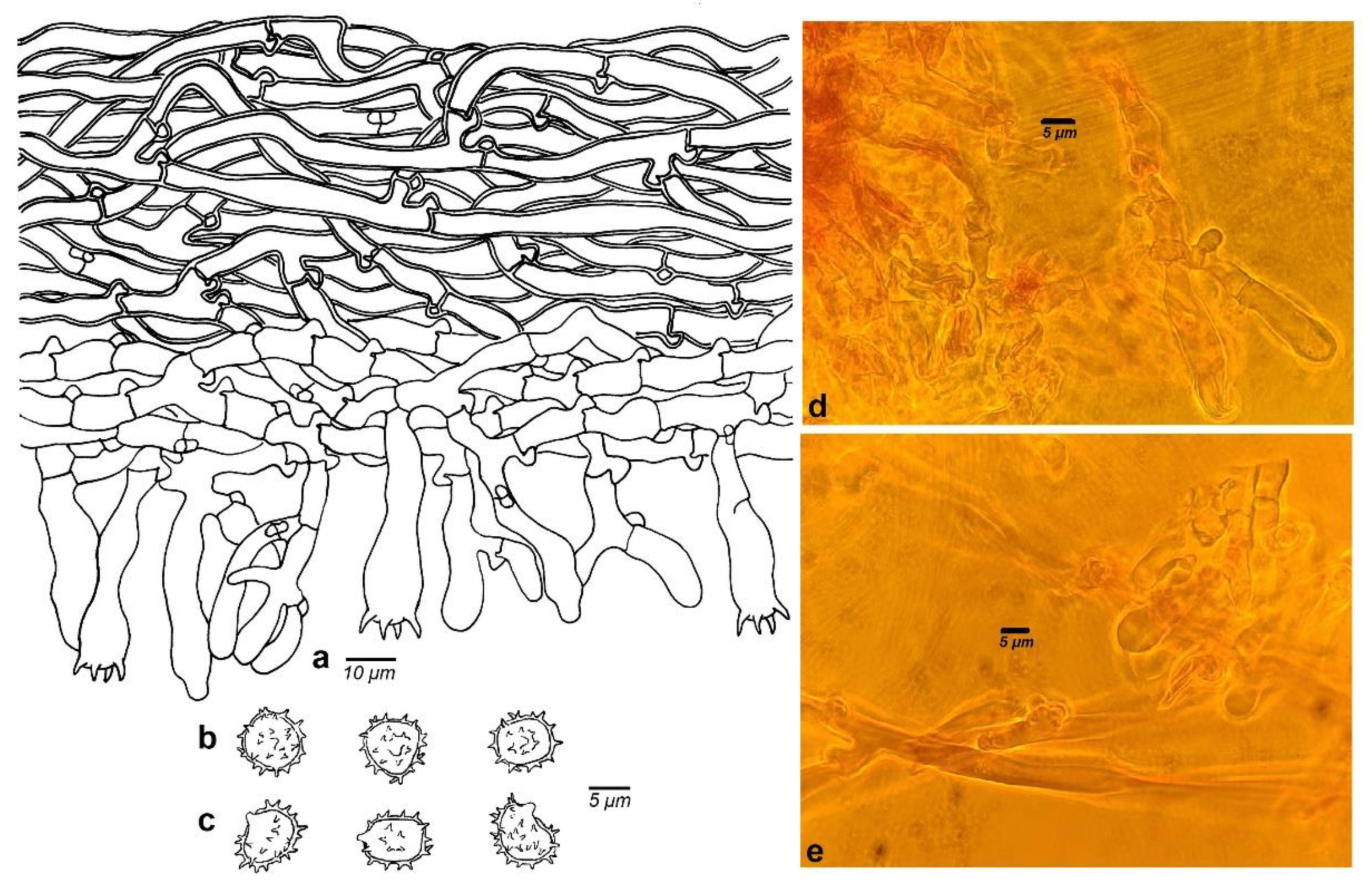
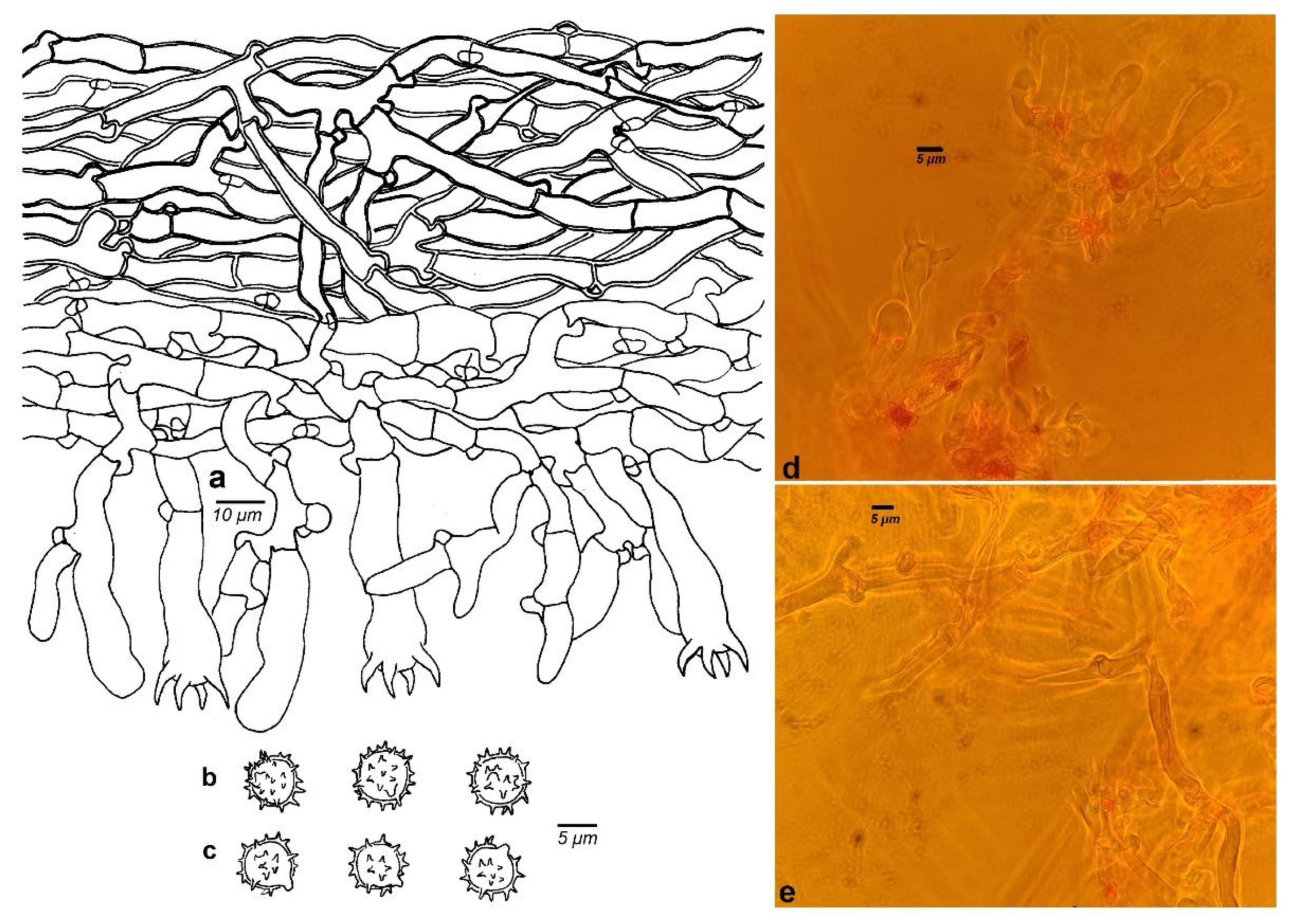
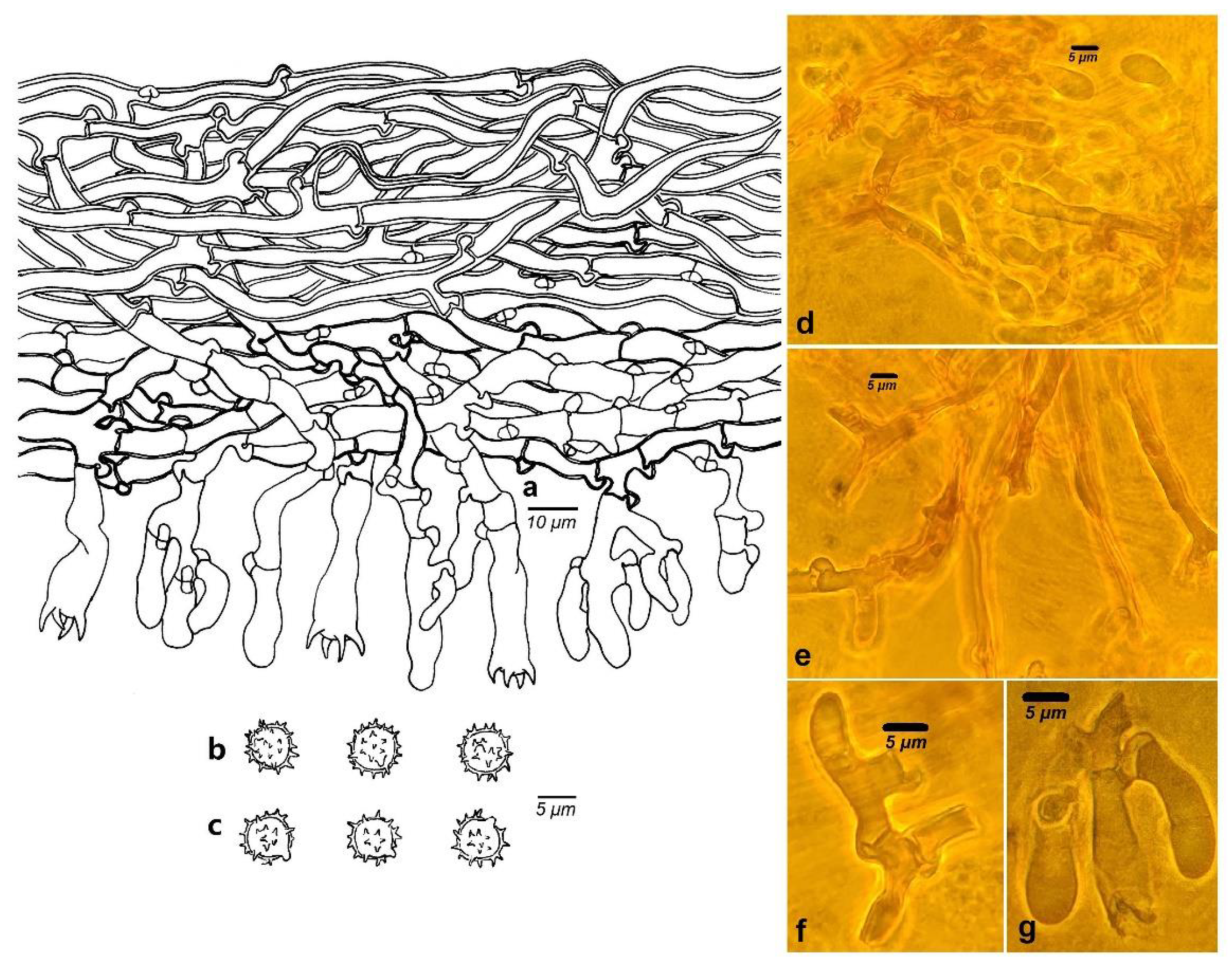
3.2. Taxonomy
4. Discussion
5. The Types of Rhizomorphs Used in the Key
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Key to Species of Tomentella from China
| 1. Basidiospores globose to subglobose in frontal view | 2 |
| 1. Basidiospores lobed in frontal view | 20 |
| 2. Rhizomorphs absent | 3 |
| 2. Rhizomorphs absent | 4 |
| 3. Length of subhymenial hyphal cell ≥ 15 μm | T. tenuirhizomorpha |
| 3. Length of subhymenial hyphal cell < 15 μm | T. brunneogrisea |
| 4. Basidiocarps separable from the substrate | 5 |
| 4. Basidiocarps adherent to the substrate | 7 |
| 5. Sterile margin byssoid | T. changbaiensis |
| 5. Sterile margin farinaceous | 6 |
| 6. Subicular hyphae without encrustation | T. atrocastanea |
| 6. Subicular hyphae with encrustation | T. longiaculeifera |
| 7. Basidiocarps arachnoid | 8 |
| 7. Basidiocarps mucedinoid, crustose, and pelliculose | 11 |
| 8. Basidia clavate | T. asiae-orientalis |
| 8. Basidia utriform | 9 |
| 9. Basidiospores L < 7 μm and W < 6 μm | T. schrenkiana |
| 9. Basidiospores L ≥ 7 μm and W ≥ 6 μm | 10 |
| 10. Echinuli of basidiospores ≤ 1 μm, sterigmata of basidia ≤ 5 μm | T. aurantispora |
| 10. Echinuli of basidiospores > 1 μm, sterigmata of basidia > 5 μm | T. kanasensis |
| 11. Basidia clavate | 12 |
| 11. Basidia utriform | 14 |
| 12. Subicular hyphae without encrustation | T. globospora |
| 12. Subicular hyphae with encrustation | 13 |
| 13. Basidiocarps pelliculose | T. duplexa |
| 13. Basidiocarps mucedinoid | T. griseomarginata |
| 14. Sterigmata of basidia ≥ 10 μm | T. conclusa |
| 14. Sterigmata of basidia < 10 μm | 15 |
| 15. Length of subhymenial hyphal cell ≥ 15 μm, not inflated | T. pertenuis |
| 15. Length of subhymenial hyphal cell < 15 μm, inflated | 16 |
| 16. Basidiocarps crustose or pelliculose | 17 |
| 16. Basidiocarps mucedinoid | 19 |
| 17. Basidiocarps crustose | T. griseofusca |
| 17. Basidiocarps pelliculose | 18 |
| 18. Sterigmata of basidia ≤ 5 μm, echinuli of basidiospores > 2 μm | T. megaspora |
| 18. Sterigmata of basidia > 5 μm, echinuli of basidiospores ≤ 2 μm | T. fuscopelliculosa |
| 19. Echinuli usually isolated, sterile margin farinaceous | T. segregata |
| 19. Echinuli usually grouped in 2 or more, sterile margin byssoid | T. pallidobrunnea |
| 20. Rhizomorphs present | 21 |
| 20. Rhizomorphs absent | 43 |
| 21. Cystidia present | 22 |
| 21. Cystidia absent | 25 |
| 22. Basidiocarps pelliculose | T. citrinocystidiata |
| 22. Basidiocarps arachnoid | 23 |
| 23. Basidiocarps brown or dark brown | 24 |
| 23. Basidiocarps olive yellow to olive | T. gloeocystidiata |
| 24. Echinuli of basidiospores ≤ 1.5 μm | T. cystidiata |
| 24. Echinuli of basidiospores > 1.5 μm | T. capitatocystidiata |
| 25. Rhizomorphs of type G | 26 |
| 25. Rhizomorphs of other types | 29 |
| 26. Basidiocarps pelliculose and separable from the substrate | T. incrustata |
| 26. Basidiocarps arachnoid and adherent to the substrate | 27 |
| 27. Subicular hyphae without encrustation | T. dimidiata |
| 27. Subicular hyphae with encrustation | 28 |
| 28. Rhizomorphs of type C | 29 |
| 28. Rhizomorphs of other types | 30 |
| 29. Basidiocarps mucedinoid and discontinuous | T. brevis |
| 29. Basidiocarps arachnoid and continuous | T. qingyuanensis |
| 30. Rhizomorphs of type A | 31 |
| 30. Rhizomorphs of type B | 32 |
| 31. Sterile margin of basidiocarps pale yellow | T. pallidomarginata |
| 31. Sterile margin of basidiocarps brown | T. fuscocrustosa |
| 32. Subicular hyphae with encrustation | T. fuscogranulosa |
| 32. Subicular hyphae without encrustation | 33 |
| 33. Basidiocarps discontinuous | T. interrupta |
| 33. Basidiocarps continuous | 34 |
| 34. Hymenophoral surface granulose | 35 |
| 34. Hymenophoral surface smooth | 39 |
| 35. Basidiocarps separable from the substrate | 36 |
| 35. Basidiocarps adherent to the substrate | 37 |
| 36. Basidiocarps dark brown, subicular hyphae simple-septate | T. efibulata |
| 36. Basidiocarps olive brown, subicular hyphae clamped | T. olivacea |
| 37. Hyphae of rhizomorphs simple-septate | T. efibulis |
| 37. Hyphae of rhizomorphs clamped | 38 |
| 38. Basidiocarps pelliculose | T. inconspicua |
| 38. Basidiocarps mucedinoid | T. parvispora |
| 39. Basidiocarps separable from the substrate | 40 |
| 39. Basidiocarps adherent to the substrate | 41 |
| 40. Basidiocarps mucedinoid, subicular hyphae clamped | T. flavidobadia |
| 40. Basidiocarps pelliculose, subicular hyphae clamped and simple-septate | T. separata |
| 41. Basidia utriform | T. aureomarginata |
| 41. Basidia clavate | T. brunneoflava |
| 42. Basidia clavate | 43 |
| 42. Basidia utriform | 48 |
| 43. Subicular hyphae with encrustation | 44 |
| 43. Subicular hyphae without encrustation | 45 |
| 44. Basidiocarps mat-like | T. storea |
| 44. Basidiocarps arachnoid | T. farinosa |
| 45. Sterile margin byssoid | T. pallidocastanea |
| 45. Sterile margin farinaceous | 46 |
| 46. Basidiocarps brownish grey to olive brown | T. olivaceobrunnea |
| 46. Basidiocarps grayish brown to dark blond or brown | 47 |
| 47. Length of subhymenial hyphal cell > 15 μm, not inflated | T. liaoningensis |
| 47. Length of subhymenial hyphal cell ≤ 15 μm, inflated | T. griseocastanea |
| 48. Sterile margin byssoid | T. atrobadia |
| 48. Sterile margin farinaceous | 49 |
| 49. Basidiocarps arachnoid or pelliculose | 50 |
| 49. Basidiocarps mucedinoid | 51 |
| 50. Basidiocarps arachnoid, echinuli of basidiospores ≤ 1.5 μm | T. fuscofarinosa |
| 50. Basidiocarps pelliculose, echinuli of basidiospores > 1.5 μm | T. longiechinuli |
| 51. Basidiocarps clavate, sterigmata of basidia ≤ 5 μm | T. coffeae |
| 51. Basidia utriform, sterigmata of basidia > 5 μm | T. stipitata |
References
- Persoon, C.H. Observationes Mycologicae; Gesnerus, Usterius & Wolfius: Leipzig, Germany, 1799; pp. 1–106. [Google Scholar]
- Patouillard, N.T. Les Hyménomycètes d’Europe. Anatomie Générale et Classification des Champignons Supérieurs (The Hymenomycetes of Europe. General Anatomy and Classification of the Higher Fungi); Paul Klincksieck: Paris, France, 1887; pp. 1–166. [Google Scholar]
- Kõljalg, U. Tomentella (Basidiomycota) and related genera in temperate Eurasia; Synopsis Fungorum 9.; Tõravere Trükikoda and Greif: Tartu, Estonia, 1996; pp. 1–213. [Google Scholar]
- Larsen, M.J. A Contribution to the Taxonomy of the Genus Tomentella; Mycologia Memoir 4.; New York Botanical Garden in Collaboration with The Mycological Society of America: New York, NY, USA, 1974; pp. 1–145. [Google Scholar]
- Cunningham, G.H. The Thelephoraceae of Australia and New Zealand, 1st ed.; R.E. Owen: New Zealand, UK, 1963; pp. 1–359. [Google Scholar]
- Welden, A.L. West Indian Species of dark-spored Thelephoraceae. Sydowia 1968, XXII, 269–273. [Google Scholar]
- Wakefield, E.M. Tomentelloideae in the British Isles. Transactions of the British Mycol. Soc. 1969, 53, 161–206. [Google Scholar] [CrossRef]
- Yorou, N.S.; Gardt, S.; Guissou, M.L.; Diabaté, M.; Agerer, R. Three new Tomentella species from West Africa identified by anatomical and molecular data. Mycol. Prog. 2012, 11, 449–462. [Google Scholar] [CrossRef]
- Kuhar, F.; Barroetaveña, C.; Rajchenberg, M. New species of Tomentella (Thelephorales) from the Patagonian Andes forests. Mycologia 2016, 108, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Mu, Y.H.; Yuan, H.S. Two new species of Tomentella (Thelephorales, Basidiomycota) from Lesser Xingan Mts., northeastern China. Phytotaxa 2018, 369, 080–092. [Google Scholar] [CrossRef]
- Yuan, H.S.; Lu, X.; Dai, Y.C.; Hyde, K.D.; Kan, Y.H.; Kušan, I.; He, S.H.; Liu, N.G.; Sarma, V.V.; Zhao, C.L.; et al. Fungal diversity notes 1277–1386: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2020, 1, 1–260. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, F.; Dai, Y.C.; Qin, W.M.; Yuan, H.S. Odontia aculeata and O. sparsa, two new species of tomentelloid fungi (Thelephorales, Basidiomycota) from the secondary forests of northeast China. Phytotaxa 2018, 372, 183–192. [Google Scholar] [CrossRef]
- Gorjón, S.P.; Greslebin, A.G. Type studies of the species of Odontia described by GH Cunningham. N. Z. J. Bot. 2012, 50, 289–301. [Google Scholar] [CrossRef] [Green Version]
- Danielson, R.M.; Visser, S.; Parkinson, D. Microbial activity and mycorrhizal potential of four overburden types used in the reclamation of extracted oil sands. Can. J. Soil Sci. 1983, 63, 363–375. [Google Scholar] [CrossRef] [Green Version]
- Tedersoo, L.; Harend, H.; Buegger, F.; Pritsch, K.; Saar, I.; Kõljalg, U. Stable isotope analysis, field observations and synthesis experiments suggest that Odontia is a non-mycorrhizal sister genus of Tomentella and Thelephora. Fungal Ecol. 2014, 11, 80–90. [Google Scholar] [CrossRef]
- Marx, D.H.; Bryan, W.C.; Davey, C.B. Influence of temperature on aseptic synthesis of ectomycorrhizae by Thelephora terrestris and Pisolithus tinctorius on loblolly pine. For. Sci. 1970, 16, 424–431. [Google Scholar] [CrossRef]
- Erland, S.; Taylor, A.F.S. Resupinate Ectomycorrhizal Fungal Genera. In Ectomycorrhizal Fungi Key Genera in Profile, 1st ed.; Cairney, J.W.G., Chambers, S.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 325–363. [Google Scholar] [CrossRef]
- Alvarez-Manjarrez, J.; Villegas-Ríos, M.; Garibay-Orijel, R.; Contreras-Pacheco, M.; Kõljalg, U. Tomentella brunneoincrustata, the first described species of the Pisonieae-associated Neotropical Tomentella clade, and phylogenetic analysis of the genus in Mexico. Mycol. Prog. 2016, 15, 1–11. [Google Scholar] [CrossRef]
- Corner, E.J.H. A Monograph of Thelephora (Basidiomycetes). In Beihefte zur Nova Hedwigia; Cramer: Berlin, Germany, 1968; Volume 27, pp. 1–110. [Google Scholar]
- Tedersoo, L.; May, T.W.; Smith, M.E. Ectomycorrhizal lifestyle in fungi: Global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 2010, 20, 217–263. [Google Scholar] [CrossRef]
- Põlme, S. Ticodendron incognitum and Neea pittieri associated ectomycorrhizal fungi in Neotropical mountain forest. Asian J. Mycol. 2018, 1, 137–145. [Google Scholar] [CrossRef]
- Read, D.J.; Perez-Moreno, J. Mycorrhizas and nutrient cycling in ecosystems—A journey towards relevance? New Phytol. 2003, 157, 475–492. [Google Scholar] [CrossRef]
- Tedersoo, L.; Gates, G.; Dunk, C.W.; Lebel, T.; May, T.W.; Kõljalg, U.; Jairus, T. Establishment of ectomycorrhizal fungal community on isolated Nothofagus cunninghamii seedlings regenerating on dead wood in Australian wet temperate forests: Does fruit-body type matter? Mycorrhiza 2009, 19, 403–416. [Google Scholar] [CrossRef]
- Selosse, M.A.; Bouchard, D.; Martin, F.; Tacon, F.L. Effect of Laccaria bicolor strains inoculated on Douglas-fir (Pseudotsuga menziesii) several years after nursery inoculation. Can. J. For. Res. 2000, 30, 360–371. [Google Scholar] [CrossRef]
- Menkis, A.; Vasiliauskas, R.; Taylor, A.F.S.; Stenlid, J.; Finlay, R. Afforestation of abandoned farmland with conifer seedlings inoculated with three ectomycorrhizal fungi—impact on plant performance and ectomycorrhizal community. Mycorrhiza 2007, 17, 337–348. [Google Scholar] [CrossRef]
- Simard, S.W.; Molina, R.; Smith, J.E.; Perry, D.A.; Jones, M.D. Shared compatibility of ectomycorrhizae on Pseudotsuga menziesii and Betula papyrifera seedlings grown in mixture in soils from southern British Columbia. Can. J. For. Res. 1997, 27, 331–342. [Google Scholar] [CrossRef]
- Kõljalg, U.; Dahlberg, A.; Taylor, A.F.S.; Larsson, E.; Hallenberg, N.; Stenlid, J.; Larsson, K.H.; Fransson, P.M.; Kårén, O.; Jonsson, L. Diversity and abundance of resupinate thelephoroid fungi as ectomycorrhizal symbionts in Swedish boreal forests. Mol. Ecol. 2000, 9, 1985–1996. [Google Scholar] [CrossRef]
- Ishida, T.A.; Nara, K.; Ma, S.; Takano, T.; Liu, S.K. Ectomycorrhizal fungal community in alkaline-saline soil in northeastern China. Mycorrhiza 2009, 19, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Nara, K.; Zong, K.; Wang, J.; Xue, S.; Peng, K.; Shen, Z.; Lian, C. Ectomycorrhizal fungal communities associated with masson pine (Pinus massoniana) and white oak (Quercus fabri) in a manganese mining region in Hunan Province, China. Fungal Ecol. 2014, 9, 1–10. [Google Scholar] [CrossRef]
- Han, Q.; Huang, J.; Long, D.; Wang, X.; Liu, J. Diversity and community structure of ectomycorrhizal fungi associated with Larix chinensis across the alpine treeline ecotone of Taibai Mountain. Mycorrhiza 2017, 27, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.W.; Gao, C.; Chen, L.; Buscot, F.; Goldmann, K.; Purahong, W.; Ji, N.N.; Wang, Y.L.; Lü, P.P.; Li, X.C.; et al. Host phylogeny is a major determinant of Fagaceae-associated ectomycorrhizal fungal community assembly at a regional scale. Front. Microbiol. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Wang, Q.; He, X.H.; Guo, L.D. Ectomycorrhizal fungus communities of Quercus liaotungensis Koidz of different ages in a northern China temperate forest. Mycorrhiza 2012, 22, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Taniguchi, T.; Xu, M.; Du, S.; Liu, G.B.; Yamanaka, N. Ectomycorrhizal fungal communities of Quercus liaotungensis along different successional stands on the Loess Plateau, China. J. For. Res. 2014, 19, 395–403. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, Y.; Shi, N.N.; Zheng, Y.; Chen, L.; Wubet, T.; Bruelheide, H.; Both, S.; Buscot, F.; Ding, Q.; et al. Community assembly of ectomycorrhizal fungi along a subtropical secondary forest succession. New Phytol. 2015, 205, 771–785. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, W.; Li, H.; Liu, D.; Guo, Z.; Liu, H. Change of forest ecosystem service function and its value in Kanas Nature Reserve. J. Landsc. Res. 2019, 11, 80–83. [Google Scholar] [CrossRef]
- Liu, X.; Pan, C. Effects of recovery time after fire and fire severity on stand structure and soil of larch forest in the Kanas National Nature Reserve, Northwest China. J. Arid. Land 2019, 11, 811–823. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zhu, J.; Zhang, Y.; Wang, Q.; Li, T.T. Comprehensive evaluation of tourism resources in Xinjiang Tianshan Grand Canyon National Forest Park. For. Eng. 2015, 15, 30–34. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Ni, J.; Kong, Z.; Wang, L.; Li, Y.; Chen, L. The correlation between airborne Betula pollen content and meteorological factors in the Tianshan Mountains, Xinjiang, China. Chin. Sci. Bull. 2019, 64, 1909–1921. [Google Scholar] [CrossRef] [Green Version]
- Kornerup, A.; Wanscher, J. Methuen Handbook of Colour Fletcher, 3rd ed.; Eyre Methuen: Norwich, UK, 1981; pp. 1–252. [Google Scholar]
- Taylor, D.L.; McCormick, M.K. Internal transcribed spacer primers and sequences for improved characterization of basidiomycetous orchid mycorrhizas. New Phytol. 2008, 177, 1020–1033. [Google Scholar] [CrossRef] [PubMed]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.N.; Rouse, D.I.; German, T.L. PCR primers that allow intergeneric differentiation of ascomycetes and their application to Verticillium spp. Appl. Environ. Microbiol. 1994, 60, 4324–4331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, R.H.; Tedersoo, L.; Abarenkov, K.; Ryberg, M.; Kristiansson, E.; Hartmann, M.; Schoch, C.L.; Nylander, J.A.A.; Bergsten, J.; Porter, T.M.; et al. Five simple guidelines for establishing basic authenticity and reliability of newly generated fungal ITS sequences. Mycokeys 2012, 4, 37–63. [Google Scholar] [CrossRef] [Green Version]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Ostell, J.; Pruitt, K.D.; Sayers, E.W. GenBank. Nucleic Acids Res. 2018, 46, D41–D47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, R.H.; Larsson, K.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.L.; Chen, C.J.; Arab, D.A.; Du, Z.G.; He, Y.H.; Ho, S.Y.W. EasyCodeML: A visual tool for analysis of selection using CodeML. Ecol. Evol. 2019, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Silvestro, D.; Michalak, I. raxmlGUI: A graphical front-end for RAxML. Org. Divers. Evol. 2012, 12, 335–337. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Mark, P.V.D.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Steffen, K.; Yuan, H.S. Morphological and molecular identification of three new species of Tomentella from Finland. Mycologia 2018, 110, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Suvi, T.; Tedersoo, L.; Abarenkov, K.; Beaver, K.; Gerlach, J.; Kõljalg, U. Mycorrhizal symbionts of Pisonia grandis and P. sechellarum in Seychelles: Identification of mycorrhizal fungi and description of new Tomentella species. Mycologia 2010, 102, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; He, H.J.; Fan, C.Y.; Tian, L.Z.; Zhang, C.Y.; Zhao, X.H. Height-diameter models of main tree species in broadleaf-conifer mixed forest in Jiaohe, Jilin, China. For. Res. 2018, 31, 11–18. [Google Scholar] [CrossRef]
- Agerer, R. Colour Atlas of Ectomycorrhizae; 1st–9th Delivery; Einhorn: Schwäbisch Gmünd, Germany, 1987–1995; pp. 25i–28i. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Yuan, H.-S. New Species of Tomentella (Thelephorales, Basidiomycota) from Temperate Continental Mountain Climate of China (Xinjiang Region). Forests 2021, 12, 1531. https://doi.org/10.3390/f12111531
Lu X, Yuan H-S. New Species of Tomentella (Thelephorales, Basidiomycota) from Temperate Continental Mountain Climate of China (Xinjiang Region). Forests. 2021; 12(11):1531. https://doi.org/10.3390/f12111531
Chicago/Turabian StyleLu, Xu, and Hai-Sheng Yuan. 2021. "New Species of Tomentella (Thelephorales, Basidiomycota) from Temperate Continental Mountain Climate of China (Xinjiang Region)" Forests 12, no. 11: 1531. https://doi.org/10.3390/f12111531
APA StyleLu, X., & Yuan, H.-S. (2021). New Species of Tomentella (Thelephorales, Basidiomycota) from Temperate Continental Mountain Climate of China (Xinjiang Region). Forests, 12(11), 1531. https://doi.org/10.3390/f12111531





