Comparative Analysis of Biological Activity of Artificial and Wild Agarwood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Alcohol-Soluble Extract
2.2.2. DPPH Free Radical Clearance Capacity
2.2.3. ABTS+ Free Radical Clearance Ability
2.2.4. Total Reducing Power
2.2.5. Anti-Acetylcholinesterase Activity
2.2.6. Anti-α-Glucosidase Activity
2.2.7. Data Processing
3. Results
3.1. Antioxidation Ability
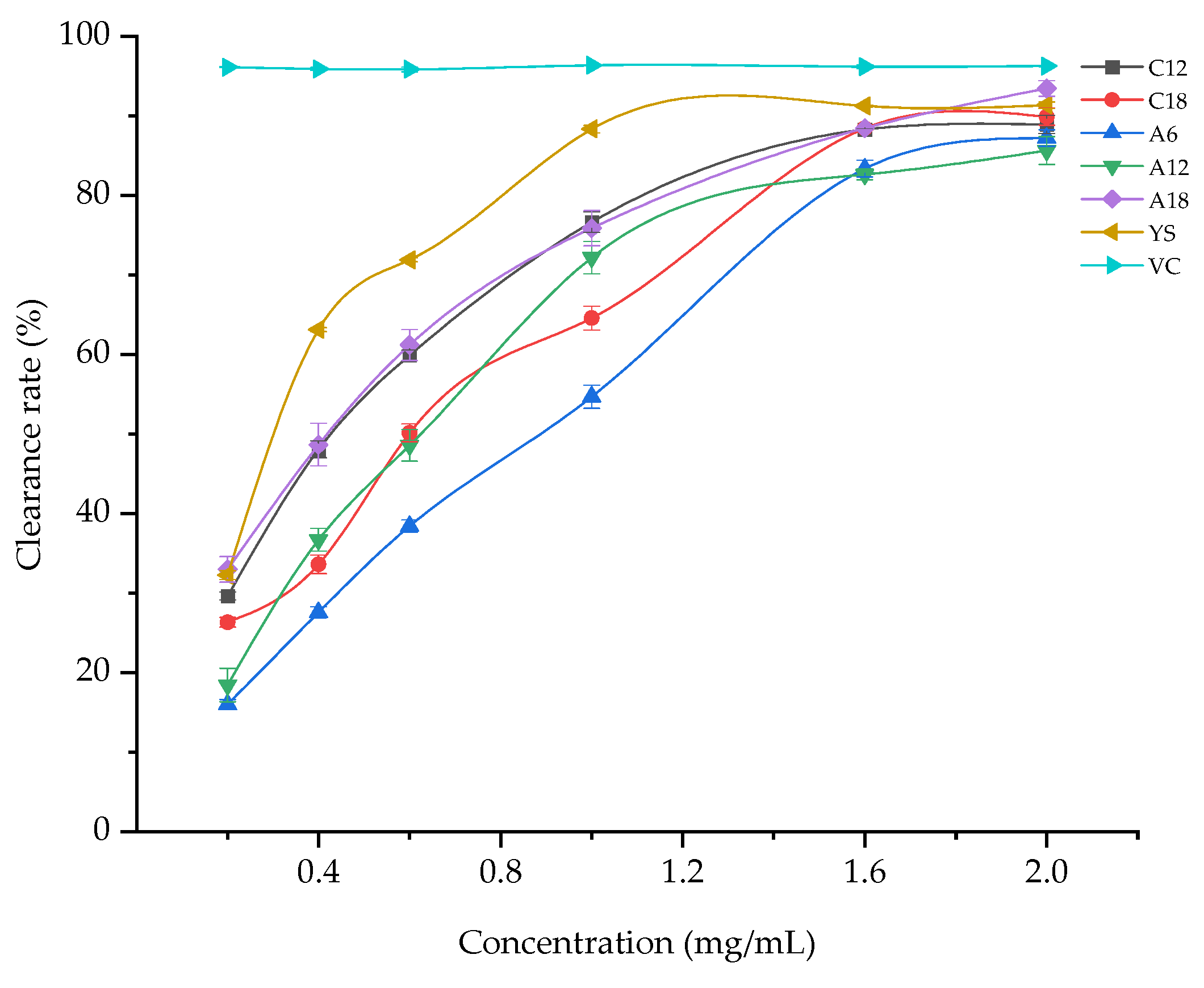
3.1.1. DPPH Free Radical Scavenging Capacity
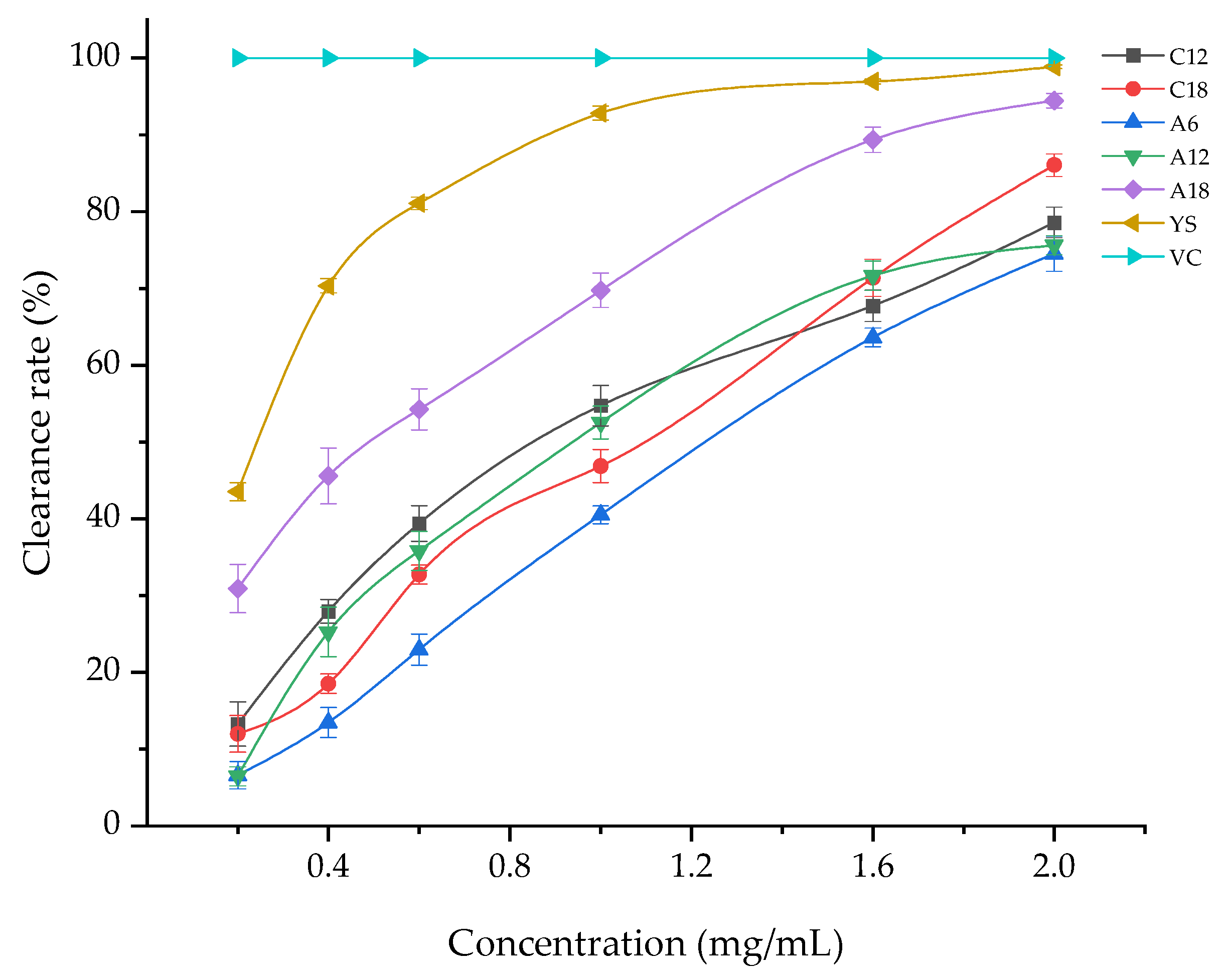
3.1.2. ABTS+ Free Radical Scavenging Capacity
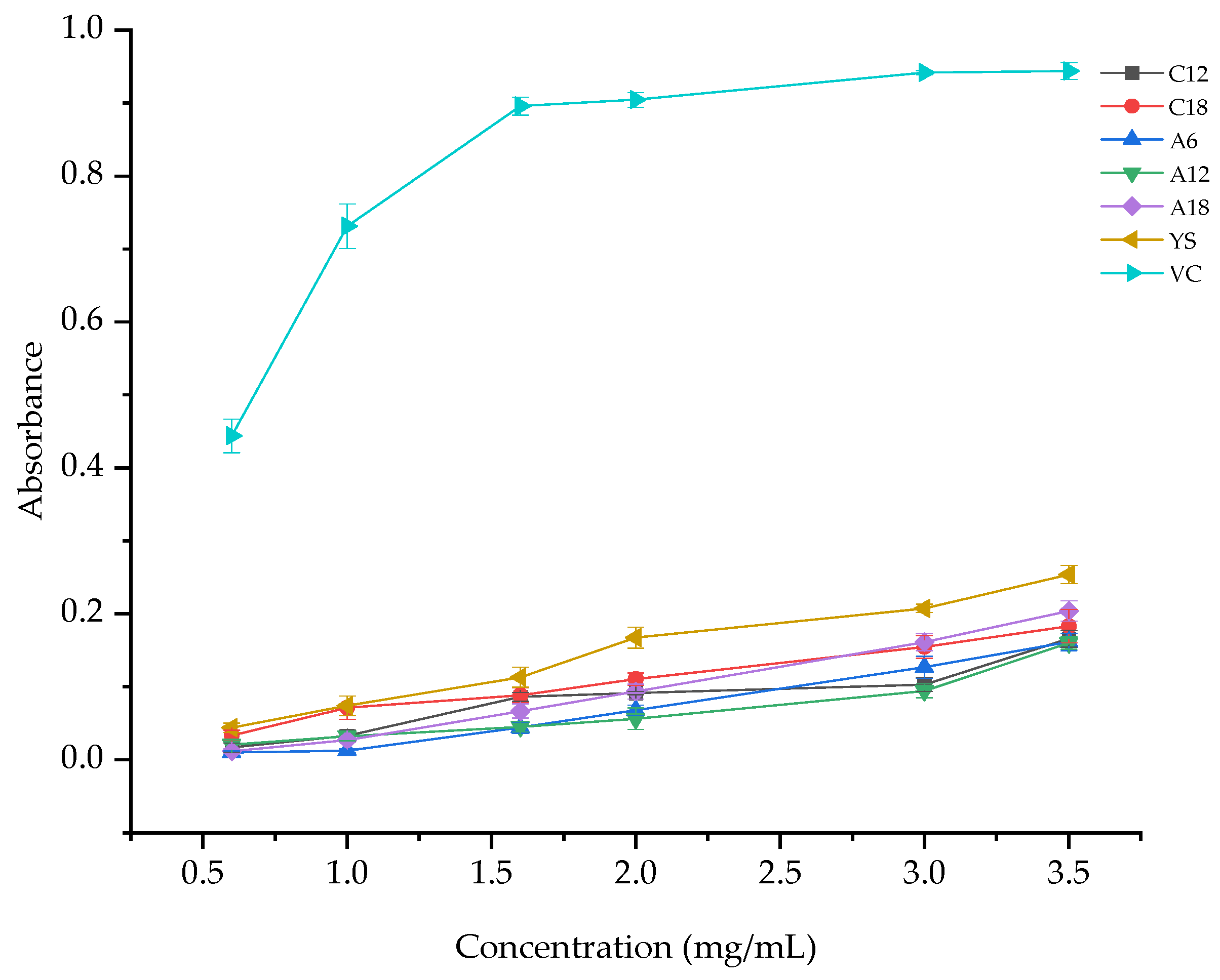
3.1.3. Total Reducing Power
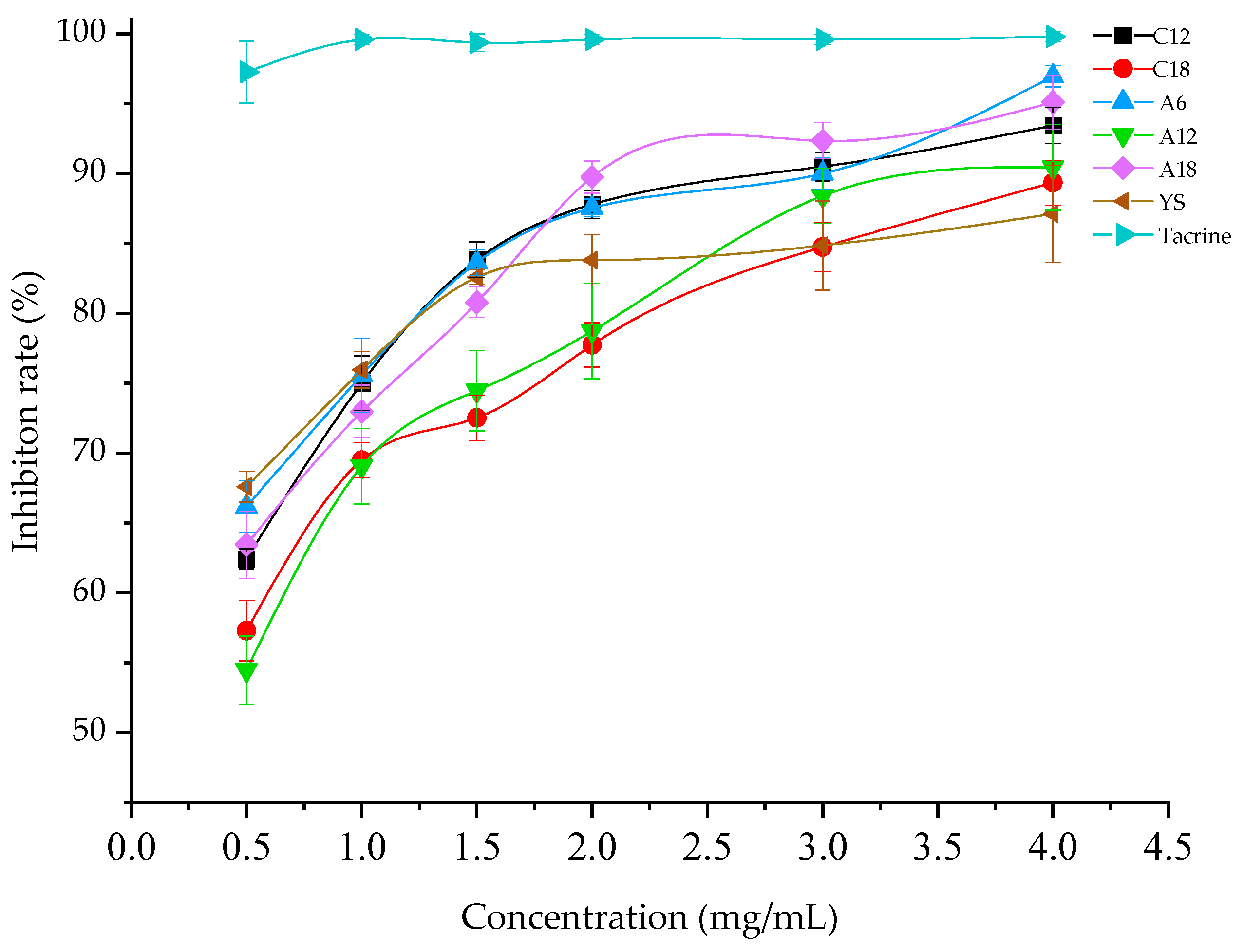
3.2. Acetylcholinesterase Activity Inhibition
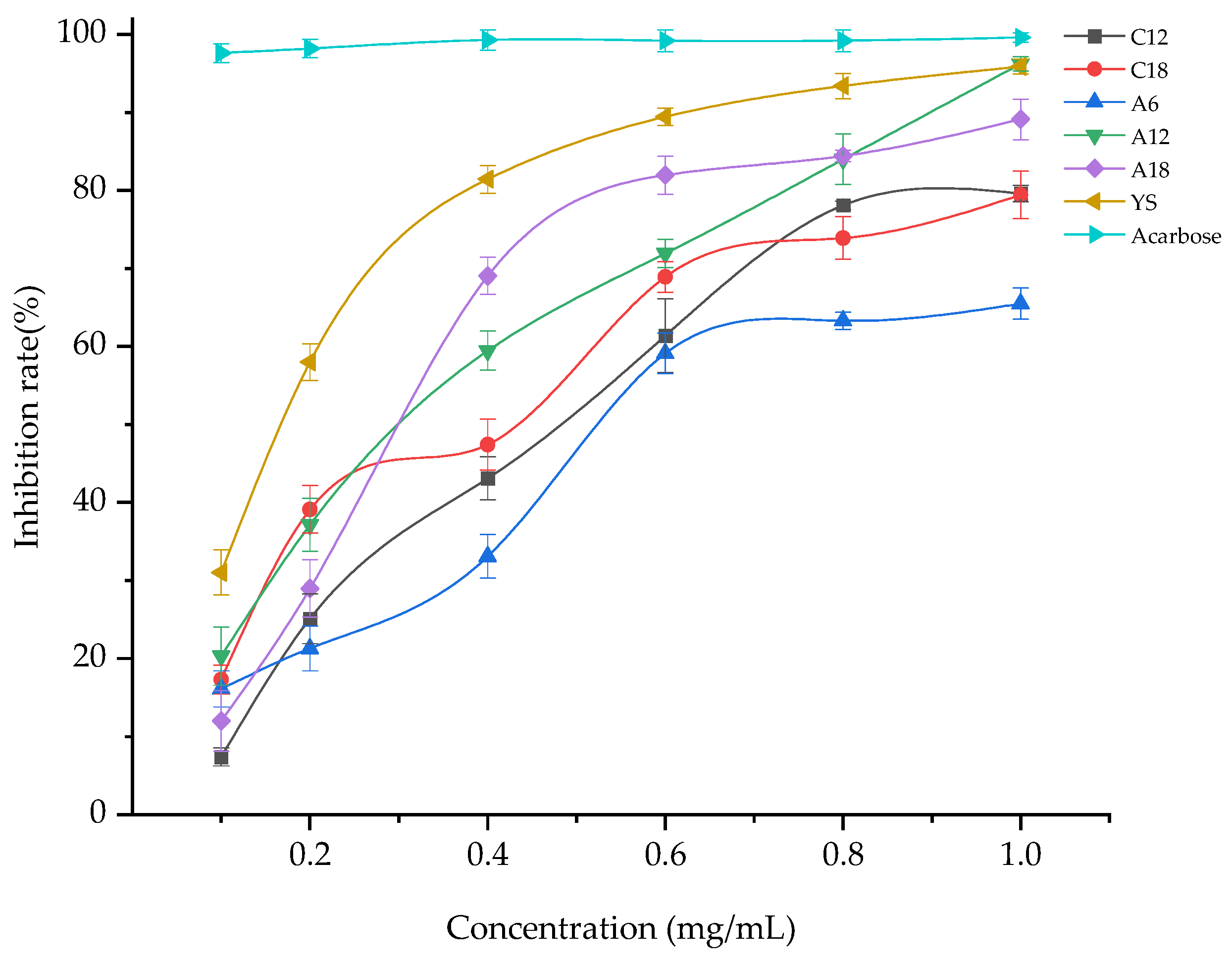
3.3. α-Glucosidase Activity Inhibition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azren, P.D.; Azren, P.D.; Lee, S.Y.; Lee, S.Y.; Emang, D.; Emang, D.; Mohamed, R. History and perspectives of induction technology for agarwood production from cultivated Aquilaria in Asia: A review. J. For. Res. 2019, 30, 1–11. [Google Scholar] [CrossRef]
- Lee, S.Y.; Mohamed, R. The Origin and Domestication of Aquilaria, an Important Agarwood-Producing Genus; Springer: Singapore, 2016; pp. 1–20. [Google Scholar]
- Nor, A.M.; Said, A.A.; Majid, A.; Saidatul, J.; Yasmin, N. Comparison of chemical profile of selected gaharu oils from Peninsular Malaysia. Malays. J. Anal. Sci. 2008, 12, 338–340. [Google Scholar]
- López-Sampson, A.; Page, T. History of Use and Trade of Agarwood. Econ. Bot. 2018, 72, 107–129. [Google Scholar] [CrossRef]
- Nobuchi, T.; Somkid, S. Preliminary observation of Aquliaria crassna wood associated with the formation of aloeswood bult. Kyoto Univ. For. 1991, 63, 226–235. [Google Scholar]
- Faizal, A.; Esyanti, R.R.; Aulianisa, E.N.; Iriawati; Santoso, E.; Turjaman, M. Formation of agarwood from Aquilaria malaccensis in response to inoculation of local strains of Fusarium solani. Trees 2017, 31, 189–197. [Google Scholar] [CrossRef]
- Dahham, S.; Tabana, Y.; Iqbal, M.; Ahamed, M.; Ezzat, M.; Majid, A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Yang, Y.; Zhang, Z.; Wei, J.; Meng, H.; Chen, W.; Feng, J.; Gan, B.; Chen, X.; et al. Whole-tree agarwood-inducing technique: An efficient novel technique for producing high-quality agarwood in cultivated Aquilaria sinensis trees. Molecules 2013, 18, 3086–3106. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Mohamed, R. Understanding Agarwood Formation and Its Challenges; Springer: Singapore, 2016; pp. 39–56. [Google Scholar]
- Chen, H.; Yang, Y.; Xue, J.; Wei, J.; Zhang, Z.; Chen, H. Comparison of Compositions and Antimicrobial Activities of Essential Oils from Chemically Stimulated Agarwood, Wild Agarwood and Healthy Aquilaria sinensis (Lour.) Gilg Trees. Molecules 2011, 16, 4884–4896. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wang, S.; Peng, D.; Yu, Z.; Guo, P.; Wei, J. Agarwood Extract Mitigates Intestinal Injury in Fluorouracil-Induced Mice. Biol. Pharm. Bull. 2019, 42, 1112–1119. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Chen, H.; Wang, H.; Mei, W.; Dai, H. Natural products in agarwood and Aquilaria plants: Chemistry, biological activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 528–565. [Google Scholar] [CrossRef]
- Li, W.; Cai, C.; Dong, W.; Guo, Z.; Wang, H.; Mei, W.; Dai, H. 2-(2-Phenylethyl)chromone derivatives from Chinese agarwood induced by artificial holing. Fitoterapia 2014, 98, 117–123. [Google Scholar] [CrossRef]
- Kuang, T.; Chen, H.; Kong, F.; Cai, C.; Yang, L.; Mei, W.; Dai, H. Three new 2-(2-phenylethyl)chromone derivatives from artificial holing agarwood of Aquilaria sinensis. Phytochem. Lett. 2018, 26, 96–100. [Google Scholar] [CrossRef]
- Li, W.; Cai, C.H.; Guo, Z.K.; Wang, H.; Zuo, W.J.; Dong, W.H.; Mei, W.L.; Dai, H.F. Five new eudesmane-type sesquiterpenoids from Chinese agarwood induced by artificial holing. Fitoterapia 2015, 100, 44–49. [Google Scholar] [CrossRef]
- Liao, G.; Mei, W.L.; Dong, W.H.; Li, W.; Wang, P.; Kong, F.D.; Gai, C.J.; Song, X.Q.; Dai, H.F. 2-(2-Phenylethyl)chromone derivatives in artificial agarwood from Aquilaria sinensis. Fitoterapia 2016, 110, 38–43. [Google Scholar] [CrossRef]
- Wang, S.L.; Liao, H.R.; Cheng, M.J.; Shu, C.W.; Chen, C.L.; Chung, M.I.; Chen, J.J. Four New 2-(2-Phenylethyl)-4H-chromen-4-one Derivatives from the Resinous Wood of Aquilaria sinensis and Their Inhibitory Activities on Neutrophil Pro-Inflammatory Responses. Planta Med. 2018, 84, 1340–1347. [Google Scholar] [CrossRef] [Green Version]
- Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia; China Medical Science and Technology Press: Beijing, China, 2015.
- Yang, B.; Wang, J.; Zhao, M.; Liu, Y.; Wang, W.; Jiang, Y. Identification of polysaccharides from pericarp tissues of litchi (Litchi chinensis Sonn.) fruit in relation to their antioxidant activities. Carbohydr. Res. 2006, 341, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Pan, L.; Han, Z.; Xie, Y.; Hu, H.; Liu, X.; Wu, L.; Yang, L.; Wang, Z. Antioxidative 2-(2-phenylethyl)chromones in Chinese eaglewood from Aquilaria sinensis. J. Asian Nat. Prod. Res. 2020, 22, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Berker, K.I.; Demirata, B.; Apak, R. Determination of Total Antioxidant Capacity of Lipophilic and Hydrophilic Antioxidants In the Same Solution by Using Ferric–Ferricyanide Assay. Food Anal. Methods 2012, 5, 1150–1158. [Google Scholar] [CrossRef]
- Wang, H.N.; Dong, W.H.; Huang, S.Z.; Li, W.; Kong, F.D.; Wang, H.; Wang, J.; Mei, W.L.; Dai, H.F. Three new sesquiterpenoids from agarwood of Aquilaria crassna. Fitoterapia 2016, 114, 7–11. [Google Scholar] [CrossRef]
- Ting, L.I.; Zhang, X.D.; Song, Y.W. A microplate-based screening method for alpha-glucosidase inhibitors. Chin. J. Clin. Pharmacol. Ther. 2005, 10, 1128–1134. [Google Scholar]
- Poljšak, B.; Dahmane, R. Free Radicals and Extrinsic Skin Aging. Dermatol. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Li, W.; Luo, S.; Zhao, X.; Ma, C.; Liu, S. GC-MS Study of the Chemical Components of Different Aquilaria sinensis (Lour.) Gilgorgans and Agarwood from Different Asian Countries. Molecules 2018, 23, 2168. [Google Scholar] [CrossRef] [Green Version]
- Amini, M.; Zayeri, F.; Moghaddam, S.S. Years Lived with Disability due to Alzheimer’s Disease and Other Dementias in Asian and North African Countries: A Trend Analysis. J. Epidemiol. Glob. Health 2019, 9, 29–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celik, I.E.; Acar, B.; Cay, S. An unusual cardiovascular adverse effect of donepezil. Intern. Med. J. 2015, 45, 877–878. [Google Scholar] [CrossRef]
- Neely, G.A.; Sabir, S.; Kohli, A.; Neostigmine. StatPearls. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470596/?report=printable (accessed on 5 March 2021).
- Hernandez-Fernandez, F.; Pardal-Fernandez, J.M.; Garcia-Martinez, E.; Segura, T. Respiratory myoclonus, a side effect of galantamine. Farm. Hosp. 2011, 35, 97–99. [Google Scholar] [CrossRef]
- Liao, G.; Mei, W.; Kong, F.; Li, W.; Yuan, J.; Dai, H. 5,6,7,8-Tetrahydro-2-(2-phenylethyl)chromones from artificial agarwood of Aquilaria sinensis and their inhibitory activity against acetylcholinesterase. Phytochemistry 2017, 139, 98–108. [Google Scholar] [CrossRef]
- Papatheodorou, K.; Banach, M.; Bekiari, E.; Rizzo, M.; Edmonds, M. Complications of Diabetes 2017. J. Diabetes Res. 2018, 2018, 1–4. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, D.; Zhou, A.; Zhang, Y.; Du, Z.; Zhang, K. α-Glucosidase Inhibition and Antihyperglycemic Activity of Phenolics from the Flowers of Edgeworthia gardneri. J. Agric. Food Chem. 2015, 63, 8162–8169. [Google Scholar] [CrossRef]
- Sukito, A.; Darmawan, S.; Turjaman, M. Anti-oxidant and anti-diabetes activities of agarwood extracts from Gyrinops versteegii (Gilg.) Domke and their cytotoxicity. IOP Conf. Ser. Earth Environ. Sci. 2020, 415, 012001. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.L.; Dong, W.H.; Li, W.; Wang, P.; Cao, X.; Yuan, J.Z.; Chen, H.Q.; Mei, W.L.; Dai, H.F. Sesquiterpenoids and 2-(2-phenylethyl)chromones respectively acting as α-glucosidase and tyrosinase inhibitors from agarwood of an Aquilaria plant. J. Enzyme Inhib. Med. Chem. 2019, 34, 853–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, C.; Yuan, J.; Zhu, M.; Yang, L.; Wei, Y.; Wang, H.; Long, W.; Mei, W.; Dai, H. 2-(2-Phenylethyl)chromone derivatives: Promising α-glucosidase inhibitors in agarwood from Aquilaria filaria. Phytochemistry 2021, 181, 112578. [Google Scholar] [CrossRef] [PubMed]

| Sample No. | Species Name | Inoculation Time | Number of Samples |
|---|---|---|---|
| C12 | A. crassna | 12 months | N = 3 |
| C18 | A. crassna | 18 months | N = 3 |
| A6 | A. sinensis | 6 months | N = 3 |
| A12 | A. sinensis | 12 months | N = 3 |
| A18 | A. sinensis | 18 months | N = 3 |
| Samples | Alcohol-Soluble Extract Solution Concentration (mg/mL) | DPPH Free Radical Scavenging Capacity (%) |
|---|---|---|
| C12 | 2 | 88.92 ± 1.15 d |
| C18 | 2 | 89.88 ± 1.07 c |
| A6 | 2 | 87.20 ± 1.03 d,e |
| A12 | 2 | 85.62 ± 1.76 e |
| A18 | 2 | 93.45 ± 0.98 b |
| YS | 2 | 91.34 ± 0.41 c |
| VC | 2 | 96.27 ± 0.09 a |
| Samples | Alcohol-Soluble Extract Solution Concentration (mg/mL) | ABTS+ Free Radical Scavenging Capacity (%) |
|---|---|---|
| C12 | 2 | 78.59 ± 2 d |
| C18 | 2 | 86.06 ± 1.47 c |
| A6 | 2 | 74.53 ± 2.31 e |
| A12 | 2 | 75.62 ± 1.25 d,e |
| A18 | 2 | 94.44 ± 0.94 b |
| YS | 2 | 98.87 ± 0.25 a |
| VC | 2 | 100 a |
| Samples | Alcohol-Soluble Extract Solution Concentration (mg/mL) | Total Reducing Power |
|---|---|---|
| C12 | 3.5 | 0.1657 ± 0.0115 d |
| C18 | 3.5 | 0.1830 ± 0.0230 c,d |
| A6 | 3.5 | 0.1613 ± 0.0121 d |
| A12 | 3.5 | 0.1600 ± 0.0085 d |
| A18 | 3.5 | 0.2037 ± 0.0140 c |
| YS | 3.5 | 0.2537 ± 0.0127 b |
| VC | 3.5 | 0.9437 ± 0.0116 a |
| Samples | Alcohol-Soluble Extract Solution Concentration (mg/mL) | Acetylcholinesterase Activity Inhibition (%) |
|---|---|---|
| C12 | 4 | 93.44 ± 1.28 b,c |
| C18 | 4 | 89.34 ± 1.61 d |
| A6 | 4 | 96.95 ± 0.76 a,b |
| A12 | 4 | 90.44 ± 3.06 c,d |
| A18 | 4 | 95.09 ± 1.95 b |
| YS | 4 | 87.11 ± 3.48 d |
| Tacrine | 4 | 99.79 ± 0.37 a |
| Samples | Alcohol-Soluble Extract Solution Concentration (mg/mL) | α-Glucosidase Activity Inhibition (%) |
|---|---|---|
| C12 | 1 | 79.60 ± 1.07 d |
| C18 | 1 | 79.42 ± 3.05 d |
| A6 | 1 | 65.50 ± 2.00 e |
| A12 | 1 | 96.24 ± 0.89 b |
| A18 | 1 | 89.12 ± 2.62 c |
| YS | 1 | 95.87 ± 0.94 b |
| Acarbose | 1 | 99.60 ± 0.60 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Qiao, M.; Fu, Y.; Wei, P.; Li, Y.; Liu, Z. Comparative Analysis of Biological Activity of Artificial and Wild Agarwood. Forests 2021, 12, 1532. https://doi.org/10.3390/f12111532
Ma S, Qiao M, Fu Y, Wei P, Li Y, Liu Z. Comparative Analysis of Biological Activity of Artificial and Wild Agarwood. Forests. 2021; 12(11):1532. https://doi.org/10.3390/f12111532
Chicago/Turabian StyleMa, Sheng, Mengji Qiao, Yunlin Fu, Penglian Wei, Yingjian Li, and Zhigao Liu. 2021. "Comparative Analysis of Biological Activity of Artificial and Wild Agarwood" Forests 12, no. 11: 1532. https://doi.org/10.3390/f12111532
APA StyleMa, S., Qiao, M., Fu, Y., Wei, P., Li, Y., & Liu, Z. (2021). Comparative Analysis of Biological Activity of Artificial and Wild Agarwood. Forests, 12(11), 1532. https://doi.org/10.3390/f12111532






