Helminths in Myomorph Rodents (Rodentia, Myomorpha) from the National Park “Smolny” and Its Surroundings (European Russia)
Abstract
:1. Introduction
2. Materials and Methods
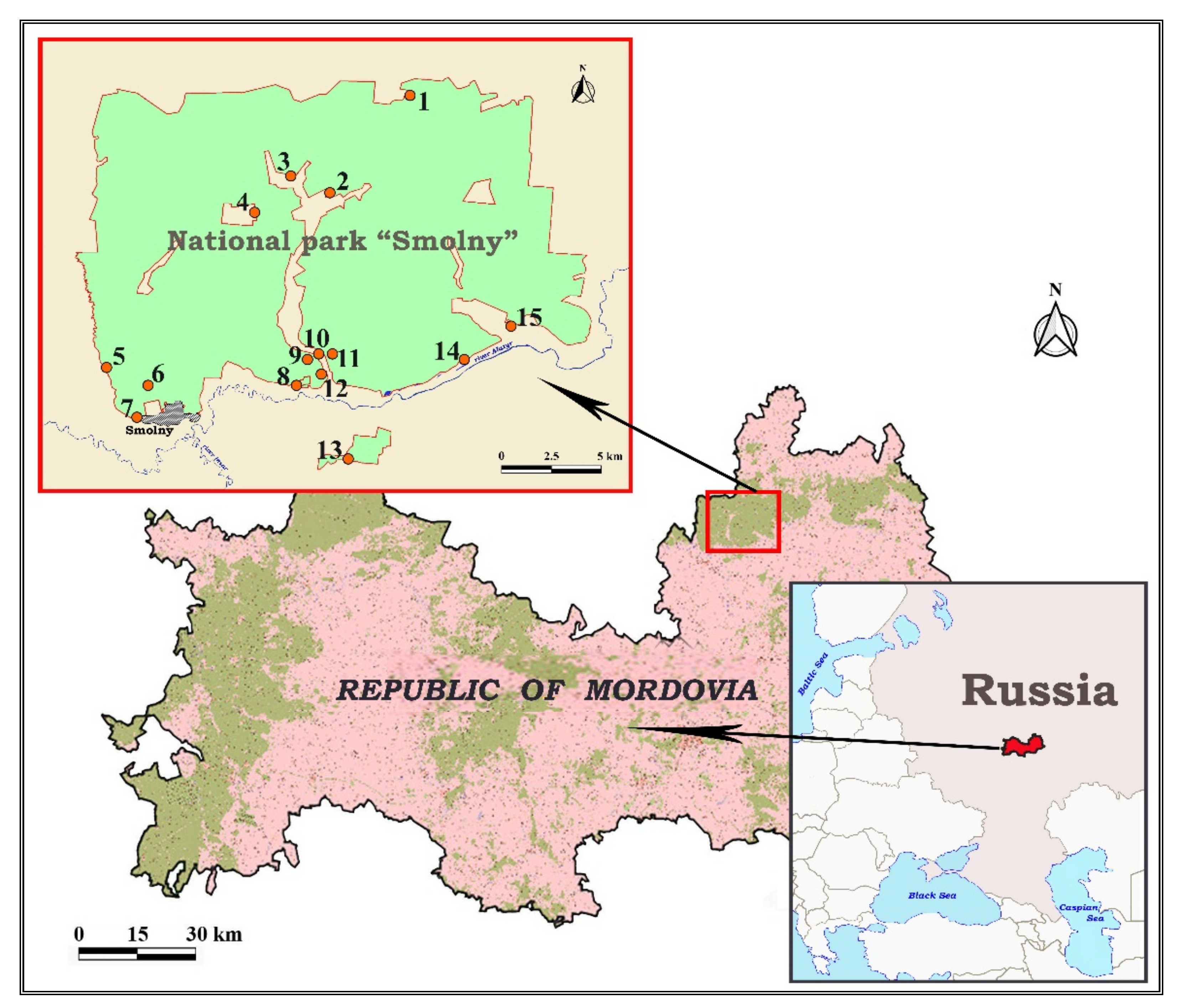
2.1. Study Area
2.2. Trapping of Rodents
2.3. Parasite Examination
2.4. Data Analysis
3. Results
3.1. Trapping Rodent Results
3.2. Helminth Fauna of Small Rodents
3.3. Comparative Analysis of the Helminth Fauna in Rodents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Price, P.W. Evolutionary Biology of Parasites; Princeton University Press: Princeton, NJ, USA, 1980; pp. 3–237. [Google Scholar]
- Poulin, R.; Morand, S. Parasite Biodiversity; Smithsonian Institution Press: Washington, DC, USA, 2004; pp. 3–216. [Google Scholar]
- Horwitz, P.; Wilcox, B. Parasites, ecosystems and sustainability: An ecological and complex systems perspective. Int. J. Parasitol. 2005, 35, 725–732. [Google Scholar] [CrossRef]
- Chikhlyaev, I.V.; Ruchin, A.B.; Fayzulin, A.I. The helminth fauna study of European common toad in the Volga basin. Nat. Environ. Pollut. Technol. 2016, 15, 1103–1109. [Google Scholar]
- Saidi, A.; Mimouni, R.; Hamadi, F.; Oubrou, W. Cross-sectional study of Eimeria spp. infection in three antelope species (Addax nasomaculatus, Gazella dorcas and Oryx dammah) maintained in the Souss-Massa National Park (Morocco). Nat. Conserv. Res. 2020, 5, 77–82. [Google Scholar] [CrossRef]
- Seryodkin, I.V.; Odoyevskaya, I.M.; Konyaev, S.V.; Spiridonov, S.E. Trichinella infection of wild carnivorans in Primorsky Krai, Russian Far East. Nat. Conserv. Res. 2020, 5, 31–40. [Google Scholar] [CrossRef]
- Chikhlyaev, I.V.; Ruchin, A.B. An overview of the helminths of moor frog Rana arvalis Nilsson, 1842 (Amphibia: Anura) in the Volga basin. Diversity 2021, 13, 61. [Google Scholar] [CrossRef]
- Preisser, W. Latitudinal gradients of parasite richness: A review and new insights from helminths of cricetid rodents. Ecography 2019, 42, 1315–1330. [Google Scholar] [CrossRef]
- Kononova, M.I.; Prisniy, Y.A. Helminthes of mouse-like rodents in the Belogorye State Nature reserve (Russia). Nat. Conserv. Res. 2020, 5, 11–18. [Google Scholar] [CrossRef]
- Poulin, R.; Presswell, B.; Jorge, F. The state of fish parasite discovery and taxonomy: A critical assessment and a look forward. Int. J. Parasitol. 2020, 50, 733–742. [Google Scholar] [CrossRef]
- Pringle, R. Upgrading protected areas to conserve wild biodiversity. Nature 2017, 546, 91–99. [Google Scholar] [CrossRef]
- Ghosh-Harihar, M.; An, R.; Athreya, R.; Borthakur, U.; Chanchani, P.; Chetry, D.; Datta, A.; Harihar, A.; Karanth, K.K.; Mariyam, D.; et al. Protected areas and biodiversity conservation in India. Biol. Conserv. 2019, 237, 114–124. [Google Scholar] [CrossRef]
- Minin, A.A.; Ananin, A.A.; Buyvolov, Y.A.; Larin, E.G.; Lebedev, P.A.; Polikarpova, N.V.; Prokosheva, I.V.; Rudenko, M.I.; Sapelnikova, I.I.; Fedotova, V.G.; et al. Recommendations to unify phenological observations in Russia. Nat. Conserv. Res. 2020, 5, 89–110. [Google Scholar] [CrossRef]
- Mohd-Azlan, J.; Lok, L.; Maiwald, M.J.; Fazlin, S.; Shen, T.D.; Kaicheen, S.S.; Dagang, P. The distribution of medium to large mammals in Samunsam Wildlife Sanctuary, Sarawak in relation to the newly constructed Pan-Borneo Highway. Nat. Conserv. Res. 2020, 5, 43–54. [Google Scholar] [CrossRef]
- Simonov, S.A.; Matantseva, M.V. Analysis of the current status of avifauna in Kostomuksha State Nature Reserve and Kalevala National Park (North-West Russia), taking into account influence from adjacent areas. Nat. Conserv. Res. 2020, 5, 51–65. [Google Scholar] [CrossRef]
- Maron, M.; Simmonds, J.S.; Watson, J.E.M. Bold nature retention targets are essential for the global environment agenda. Nat. Ecol. Evol. 2018, 2, 1194–1195. [Google Scholar] [CrossRef]
- Kestemont, B. The bottom-up assessment of threatened species. Nat. Conserv. Res. 2019, 4, 93–106. [Google Scholar] [CrossRef]
- Kopoteva, T.A.; Kuptsova, V.A. Effects of pyrogenic factor on wetlands of Petrovskaya Pad’ (Jewish Autonomous Region, Russia). Nat. Conserv. Res. 2019, 4, 35–44. [Google Scholar] [CrossRef]
- Lebedinskii, A.A.; Noskova, O.S.; Dmitriev, A.I. Post-fire recovery of terrestrial vertebrates in the Kerzhensky State Nature Biosphere Reserve (Central Volga Region, Russia). Nat. Conserv. Res. 2019, 4, 45–56. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Khapugin, A.A. Red data book invertebrates in a protected area of European Russia. Acta Zool. Acad. Sci. Hung. 2019, 65, 349–370. [Google Scholar] [CrossRef]
- Maxwell, S.L.; Cazalis, V.; Dudley, N.; Hoffmann, M.; Rodrigues, A.S.L.; Stolton, S.; Visconti, P.; Woodley, S.; Kingston, N.; Lewis, E.; et al. Area-based conservation in the twenty-first century. Nature 2020, 586, 217–227. [Google Scholar] [CrossRef]
- Rutovskaya, M.V.; Aleksandrov, A.N.; Podshivalina, V.N.; Soboleva, A.S.; Glushenkov, O.V. Habitat conditions of Desmana moschata (Talpidae, Eulipotyphla, Mammalia) in the buffer zone of the Prisurskiy State Nature Reserve (Russia). Nat. Conserv. Res. 2020, 5, 36–46. [Google Scholar] [CrossRef]
- Lyubimov, A.; Kryuchkov, A.; Eglit, A.; Ivanova, D.; Khumalo, N. Improvement of the strictly protected areas nets in Russian Federation. Sam. Luka Probl. Region. Glob. Ecol. 2018, 27, 17–20. [Google Scholar] [CrossRef]
- Negrobov, O.P.; Maslova, O.O.; Selivanova, O.V. Fauna of the family Dolichopodidae (Diptera) of the Astrakhan State Nature Biosphere Reserve (Russia). Nat. Conserv. Res. 2018, 3 (Suppl. 2), 91–96. [Google Scholar] [CrossRef]
- Kirillova, N.Y.; Kirillov, A.A.; Shchenkov, S.V.; Chikhlyaev, I.V. Oswaldocruzia filiformis sensu lato (Nematoda: Molineidae) from amphibians and reptiles in European Russia: Morphological and molecular data. Nat. Conserv. Res. 2020, 5 (Suppl. 2), 41–56. [Google Scholar] [CrossRef]
- Bondarenko, A.S.; Zamotajlov, A.S.; Belyi, A.I.; Khomitskiy, E.E. Fauna and ecological characteristics of ground beetles (Coleoptera, Carabidae) of the Nature Sanctuaries “Prichernomorskiy” and “Tuapsinskiy” (Russia). Nat. Conserv. Res. 2020, 5, 66–85. [Google Scholar] [CrossRef]
- Kirillova, N.Y. Helminths in Small Mammals from the Samarskaya Luka. In Fauna and Ecology; Lambert Academic Publishing: Saarbrucken, Germany, 2011; pp. 3–251. [Google Scholar]
- Kirillova, N.Y.; Kirillov, A.A. Overview of helminths in small mammals in the Zhiguli State Reserve. Nat. Conserv. Res. 2017, 2, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Romashov, B.V. Helminths of myomorph rodents of the Usmanskiy forest. Proc. Voronezh Reserve 1997, 23, 186–206. [Google Scholar]
- Ivanov, V.M.; Kalmykov, A.P.; Fedorovich, V.V.; Semenova, N.N.; Parshina, O.Y. Structural changes in helminthofauna of rodents resulted from introduction and settling of animals in the Volga delta. Arid Ecosyst. 2011, 17, 76–82. [Google Scholar]
- Romashova, N.B. Recent fauna and ecology of helminths of myomorph rodents of the Usmanskiy forest. Proc. Voronezh Reserve 2012, 42, 184–194. [Google Scholar]
- Vlasov, E.F.; Malysheva, N.S.; Krivopalov, A.V. Helminth fauna of myomorh rodents (Rodentia, Myomorha) in the Central Chernozem State Nature Reserve. Rus. Parasitol. J. 2015, 4, 24–33. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Kirillov, A.A.; Chikhlyaev, I.V.; Kirillova, N.Y. Parasitic Worms of Land Vertebrates of the Mordovia Nature Reserve. In Flora and Fauna of Reserves; Committee of RAS for the Conservation of Biological Diversity: Moscow, Russia, 2016; Volume 124, pp. 3–72. [Google Scholar]
- Kirillov, A.A.; Kirillova, N.Y.; Krasnobaev, Y.P.; Vekhnik, V.P. Parasitic worms of small mammals of Zhiguli State Reserve. In Flora and Fauna of Reserves; Committee of RAS for the Conservation of Biological Diversity: Moscow, Russia, 2017; Volume 128, pp. 3–77. [Google Scholar]
- Meerburg, B.G.; Singleton, G.R.; Kijlstra, A. Rodent-borne diseases and their risks for public health. Crit. Rev. Microbiol. 2009, 35, 221–270. [Google Scholar] [CrossRef]
- Bordes, F.; Blasdell, K.; Morand, S. Transmission ecology of rodent-borne diseases: New frontiers. Integr. Zool. 2015, 10, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Krucken, J.; Blumke, J.; Maaz, D.; Demeler, J.; Ramunke, S.; Antolova, D.; Schaper, R.; von Samson-Himmelstjerna, G. Small rodents as paratenic or intermediate hosts of carnivore parasites in Berlin, Germany. PLoS ONE 2017, 12, e0172829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levykh, A.Y.; Panin, V.V. Species composition and community structure of small mammals in Parapolsky Dol (Koryak State Nature Reserve, Kamchatka). Nat. Conserv. Res. 2019, 4, 1–12. [Google Scholar] [CrossRef]
- Ahissa, L.; Akpatou, B.K.; Bohoussou, H.K.; Kadjo, B.; Kone, I. Species composition and community structure of terrestrial small mammals in Tanoé-Ehy Swamp Forest (South-East Ivory Coast): Implication for conservation. Nat. Conserv. Res. 2020, 5, 53–63. [Google Scholar] [CrossRef]
- Kirillova, N.Y.; Kirillov, A.A.; Ruchin, A.B. First record of helminths of the European pine vole, Microtus subterraneus (Rodentia, Cricetidae) in Russia with overview on the rodent’s range. Rus. J. Theriol. 2021. 20, 19–24. [CrossRef]
- Grishutkin, G.F.; Lapshin, A.S.; Spiridonov, S.N.; Artaev, O.N.; Ruchin, A.B.; Kuznetsov, V.A.; Andreychev, A.V. Vertebrate animals of National Park “Smolny”. In Flora and Fauna of Reserves; Issue 124; Committee of RAS for the Reserve of Biological Diversity: Moscow, Russia, 2013; pp. 3–56. [Google Scholar]
- Kirillova, N.Y.; Krystufek, B.; Kirillov, A.A.; Ruchin, A.B.; Grishutkin, G.F. The first record of Microtus subterraneus (de Selys-Longchamps, 1836) (Rodentia, Cricetidae) for Mordovia, Russia. Acta Biol. Sibir. 2019, 5, 145–149. [Google Scholar] [CrossRef]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. OJEU 2010, L276, 33–79.
- Ivashkin, V.M.; Kontrimavichus, V.L.; Nasarova, N.S. Methods of the Collection and Studies of Helminths of Land Mammals; Nauka: Moscow, Russia, 1971; pp. 3–123. [Google Scholar]
- Anikanova, V.S.; Bugmyrin, S.V.; Ieshko, E.P. Methods of the Collection and Studies of Helminths of Small Mammals; Karelian Scientific Center of RAS: Petrozavodsk, Russia, 2007; pp. 3–145. [Google Scholar]
- Ryzhikov, K.M.; Gvozdev, E.V.; Tokobaev, M.M.; Shaldybin, L.C.; Matsaberidze, G.V.; Merkusheva, I.V.; Nadtochiy, E.V.; Khokhlova, I.G.; Sharpilo, L.D. Keys to the helminths of rodents in the USSR fauna. In Cestodes and Trematodes; Nauka: Moscow, Russia, 1978; pp. 3–232. [Google Scholar]
- Ryzhikov, K.M.; Gvozdev, E.V.; Tokobaev, M.M.; Shaldybin, L.C.; Matsaberidze, G.V.; Merkusheva, I.V.; Nadtochiy, E.V.; Khokhlova, I.G.; Sharpilo, L.D. Keys to the helminths of rodents in the USSR fauna. In Nematodes and Acanthocephalans; Nauka: Moscow, Russia, 1979; pp. 3–272. [Google Scholar]
- Genov, T. Helminths of Insectivores and Rodents in Bulgaria; Bulgarian Academy of Sciences: Sofia, Bulgaria, 1984; pp. 3–348. [Google Scholar]
- Makarikov, A.A.; Tkach, V.V. Two new species of Hymenolepis (Cestoda: Hymenolepididae) from Spalacidae and Muridae (Rodentia) from eastern Palearctic. Acta Parasitol. 2013, 58, 37–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haukisalmi, V. A taxonomic revision of the genus Anoplocephaloides Baer, 1923 sensu Rausch (1976), with the description of four new genera (Cestoda: Anoplocephalidae). Zootaxa 2009, 2057, 1–31. [Google Scholar]
- Haukisalmi, V.; Hardman, L.M.; Henttonen, H. Taxonomic review of cestodes oft the genus Catenotaenia Janicki, 1904 in Eurasia and molecular phylogeny of the Catenotaeniidae (Cyclophyllidea). Zootaxa 2010, 2489, 1–33. [Google Scholar] [CrossRef]
- Dawes, B. The Trematode; Cambridge University Press: Cambridge, UK, 1968; pp. 3–660. [Google Scholar]
- Sharpilo, V.P.; Iskova, N.P. Fauna of Ukraine. Trematodes. Plagiorchiata; Issue 3; Naukova Dumka: Kiev, Ukraine, 1989; Volume 34, pp. 3–280. [Google Scholar]
- Kirillov, A.A.; Kirillova, N.Y.; Chikhlyaev, I.V. Trematodes of Land Vertebrates of Middle Volga Region; Cassandra: Togliatti, Russia, 2012; pp. 3–329. [Google Scholar]
- Feliu, C.; Spakulova, M.; Casanova, J.C.; Renaud, F.; Morand, S.; Hugot, J.P.; Santalla, F.; Durand, P. Genetic and morphological heterogeneity in small rodent whipworms in southwestern Europe: Characterization of Trichuris muris and description of Trichuris arvicolae n. sp. (Nematoda: Trichuridae). J. Parasitol. 2000, 86, 442–449. [Google Scholar] [CrossRef]
- Fauna Europaea. Available online: https://fauna-eu.org/ (accessed on 12 September 2021).
- Global Cestode Database. Available online: http://tapewormdb.uconn.edu (accessed on 12 September 2021).
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004; pp. 3–256. [Google Scholar]
- Bakanov, A.I. Quantitated Estimation of Dominance in Ecological Communities; The Manuscript Was Deposited in All-Union Institute of Scientific and Technical Information (VINITI, Russia) 08.12.1987, No. 8593–B87; IBIW RAS Publishing: Borok, Russia, 1987; pp. 3–64. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Version 2.16. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Lavikainen, A.; Iwaki, T.; Nakao, M.; Konyaev, S.V. Genetic diversity of the cryptic Hydatigera taeniaformis complex. In New Knowledge for Parasites. Parasitological Studies in Siberia and the Far East, Proceedings of the V Interregional Conference, Novosibirsk, Russia, 14–16 September 2015; Yurlova, N.I., Konyaev, S.V., Eds.; Garamond: Novosibirsk, Russia, 2015; pp. 65–66. [Google Scholar]
- Lavikainen, A.; Iwaki, T.; Haukisalmi, V.; Konyaev, S.V.; Casiraghi, M.; Dokuchaev, N.E.; Galimberti, A.; Haljian, A.; Henttonen, H.; Ichikawa-Seki, M.; et al. Reappraisal of Hydatigera taeniaeformis (Batsch, 1786) (Cestoda: Taeniidae) sensu lato with description of Hydatigera kamiyai n. sp. Int. J. Parasitol. 2016, 46, 361–374. [Google Scholar] [CrossRef] [Green Version]
- Haukisalmi, V.; Hardman, L.M.; Henttonen, H.; Laakkonen, J.; Niemimaa, J.; Hardman, M.; Gubanyi, A. Molecular systematics and morphometrics of Anoplocephaloides dentata (Cestoda, Anoplocephalidae) and related species in voles and lemmings. Zool. Scr. 2009, 38, 199–220. [Google Scholar] [CrossRef]
- Haukisalmi, V.; Wickstrom, L.M.; Henttonen, H.; Hantula, J.; Gubanyi, A. Molecular and morphological evidence for multiple species within Paranoplocephala omphalodes (Cestoda, Anoplocephalidae) in Microtus voles (Arvicolinae). Zool. Scr. 2004, 33, 277–290. [Google Scholar] [CrossRef]
- Vlasenko, P.; Abramov, S.; Bugmyrin, S.; Dupal, T.; Fomenko, N.; Gromov, A.; Zakharov, E.; Ilyashenko, V.; Kabdolov, Z.; Tikunov, A.; et al. Geographical distribution and hosts of the cestode Paranoplocephala omphalodes (Hermann, 1783) Lühe, 1910 in Russia and adjacent territories. Parasitol. Res. 2019, 118, 3543–3548. [Google Scholar] [CrossRef] [PubMed]
- Haukisalmi, V.; Hardman, L.M.; Foronda, P.; Feliu, C.; Laakkonen, J.; Niemimaa, J.; Lehtonen, J.T.; Henttonen, H. Systematic relationships of hymenolepidid cestodes of rodents and shrews inferred from sequences of 28S ribosomal RNA. Zool. Scr. 2010, 39, 631–641. [Google Scholar] [CrossRef]
- Montgomery, S.S.J.; Montgomery, W.I.; Dunn, T.S. Biochemical, physiological and morphological variation in unarmed hymenolepids (Eucestoda: Cyclophyllidea). Zool. J. Linn. Soc. 1987, 91, 293–324. [Google Scholar] [CrossRef]
- Kostadinova, A.; Gibson, D.I.; Biserkov, V.; Ivanova, R. A quantitative approach to the evaluation of the morphological variability of two echinostomes, Echinostoma miyagawai lshii, 1932 and, E. revolutum (Frolich, 1802), from Europe. Syst. Parasitol. 2000, 45, 1–15. [Google Scholar] [CrossRef]
- Kostadinova, A.; Gibson, D.I.; Biserkov, V.; Chipev, N. Re-validation of Echinostoma miyagawai Ishii, 1932 (Digenea: Echinostomatidae) on the basis of the experimental completion of its life-cycle. Syst. Parasitol. 2000, 45, 81–108. [Google Scholar] [CrossRef]
- Faltynkova, A.; Georgieva, S.; Soldanova, M.; Kostadinova, A. A re-assessment of species diversity within the ‘revolutum’ group of Echinostoma Rudolphi, 1809 (Digenea: Echinostomatidae) in Europe. Syst. Parasitol. 2015, 90, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Shaykenov, B. Helminths in Rodents from Kazakhstan; Nauka: Alma-Ata, Kazakhstan, 1981; pp. 3–172. [Google Scholar]
- Cutillas, C.; Oliveros, R.; Rojas, M.; Guevarra, D.C. Determination of Trichuris muris from murid hosts and T. arvicolae (Nematoda) from arvicolid rodents by amplification and sequestration of the ITS1-5.8SITS2 segment of the ribosomal DNA. Parasitol. Res. 2002, 88, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Callejon, R.; de Rojas, M.; Feliu, C.; Balao, F.; Marrugal, A.; Henttonen, H.; Guevara, D.; Cutillas, C. Phylogeography of Trichuris populations isolated from different Cricetidae rodents. Parasitology 2012, 139, 1795–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quentin, J.C. Morphogenese larvarie du spiruride Mastophorus muris (Gmelin, 1790). Ann. Parasitol. Hum. Comp. 1970, 45, 839–855. [Google Scholar] [CrossRef]
- Kozlov, D.P. Keys to Helminths of Carnivores of the USSR; Nauka: Moscow, Russia, 1977; pp. 3–275. [Google Scholar]
- Akbaev, M.S.; Vodyanov, A.A.; Kosminkov, N.E. Parasitology and Invasive Diseases of Animals; Kolos: Moscow, Russia, 1998; pp. 3–743. [Google Scholar]
- Kirillova, N.Y.; Kirillov, A.A. Ecological analysis of helminth fauna of mouse rodents of the Samarskaya Luka. Proc. Samara Sci. Center RAS 2005, 4, 261–275. [Google Scholar]
- Roberts, M.; Rodrigo, A.; McArdle, B.; Charleston, W.A.G. The effect of habitat on the helminth parasites of an island population of the Polynesian rat (Rattus exularis). J. Zool. 1992, 227, 109–125. [Google Scholar] [CrossRef]
- Arneberg, P. Host population density and body mass as determinants of species richness in parasite communities: Comparative analyses of directly transmitted nematodes of mammals. Ecography 2002, 25, 88–94. [Google Scholar] [CrossRef]
- Anikanova, V.S.; Ieshko, E.P.; Bugmyrin, S.V.; Borodina, K.A. Peculiarities of biotopic distribution of cestodes from the common shrew Sorex araneus in Southern Karelia. Parasitologiia 2003, 37, 479–487. [Google Scholar]
- Kirillova, N.Y.; Kirillov, A.A. Influence of the population density of the host (mouse rodents) on its helminth fauna. Proc. Samara Sci. Center RAS 2006, 8, 548–555. [Google Scholar]
- Torres, J.; Miquel, J.; Casanova, J.C.; Ribas, A.; Feliu, C.; Morand, S. Endoparasite species richness of Iberian carnivores: Influences of host density and range distribution. Biodivers. Conserv. 2006, 15, 4619–4632. [Google Scholar] [CrossRef]
- Kirillova, N.Y.; Kirillov, A.A. Island isolation influence on parasite fauna of murine rodents. Proc. Samara Sci. Center RAS 2009, 11, 119–126. [Google Scholar]
- Rosalino, L.M.; Santos, M.J.; Fernandes, C.; Santos-Reis, M. Biogeographical region and host trophic level determine carnivore endoparasite richness in the Iberian Peninsula. Parasitology 2011, 138, 758–765. [Google Scholar] [CrossRef]
- Froeschke, G.; Matthee, S. Landscape characteristics influence helminth infestations in a peri-domestic rodent—Implications for possible zoonotic disease. Parasites Vectors 2014, 7, 393. [Google Scholar] [CrossRef] [Green Version]
- Parshina, O.Y.; Kalmykov, A.P.; Ivanov, V.M.; Semenova, N.N. Helminth fauna in rodents of family Muridae Illiger, 1811 in biogeocoenoses of Astrakhan region. In Biota and Soil Diversity of Nothern and Central Asia, Proceedings of II International Conference, Volume 2, Ulan-Ude, Russia, 20–25 June 2011; Buryatian Scientific Center of Siberian Branch of RAS: Ulan-Ude, Russia, 2011; pp. 87–90. [Google Scholar]
- Bugmyrin, S.V.; Korosov, A.V.; Bespyatova, L.A.; Ieshko, E.P. Helminth fauna of the bank vole Myodes glareolus (Schreber, 1780) in the Kizhi archipelago. Parasitologiia 2015, 49, 61–71. [Google Scholar]
- Kirillova, N.Y.; Kirillov, A.A.; Ruchin, A.B.; Trukhachev, M.V. Helminth fauna of Microtus cf. arvalis (Rodentia, Cricetidae) in Russia and adjacent countries. Biodiversitas 2020, 21, 1961–1979. [Google Scholar] [CrossRef]

| Rodent Species | 1 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2018 | |||||||||||||||
| Clethrionomys glareolus | 0 2 | – 3 | 71 | – | – | – | – | 4 | – | – | – | – | – | 36 | – |
| Microtus agrestis | 0 | – | 3 | – | – | – | – | 0 | – | – | – | – | – | 0 | – |
| Arvicola amphibius | 0 | – | 7 | – | – | – | – | 0 | – | – | – | – | – | 0 | – |
| Apodemus agrarius | 0 | – | 27 | – | – | – | – | 0 | – | – | – | – | – | 23 | – |
| Sylvaemus flavicollis | 3 | – | 8 | – | – | – | – | 0 | – | – | – | – | – | 8 | – |
| Sylvaemus uralensis | 0 | – | 10 | – | – | – | – | 0 | – | – | – | – | – | 15 | – |
| 2019 | |||||||||||||||
| Clethrionomys glareolus | – | – | 69 | – | – | – | – | – | 10 | 14 | 5 | – | – | 64 | 5 |
| Microtus arvalis | – | – | 7 | – | – | – | – | – | 2 | 0 | 5 | – | – | 1 | 9 |
| Microtus agrestis | – | – | 5 | – | – | – | – | – | 0 | 0 | 0 | – | – | 2 | 2 |
| Microtus subterraneus | – | – | 8 | – | – | – | – | – | 1 | 0 | 0 | – | – | 0 | 0 |
| Apodemus agrarius | – | – | 40 | – | – | – | – | – | 15 | 8 | 1 | – | – | 37 | 49 |
| Sylvaemus flavicollis | – | – | 35 | – | – | – | – | – | 4 | 3 | 5 | – | – | 18 | 3 |
| Sylvaemus uralensis | – | – | 38 | – | – | – | – | – | 15 | 15 | 10 | – | – | 36 | 16 |
| Mus musculus | – | – | 0 | – | – | – | – | – | 0 | 0 | 0 | – | – | 1 | 0 |
| Micromys minutus | – | – | 1 | – | – | – | – | – | 0 | 0 | 0 | – | – | 0 | 0 |
| 2020 | |||||||||||||||
| Clethrionomys glareolus | – | 14 | 16 | 0 | 30 | 15 | 16 | – | 0 | 0 | 0 | 0 | 21 | 18 | 12 |
| Microtus arvalis | – | 0 | 2 | 0 | 0 | 0 | 0 | – | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Microtus subterraneus | – | 0 | 6 | 0 | 0 | 0 | 0 | – | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Arvicola amphibius | – | 0 | 1 | 1 | 0 | 0 | 0 | – | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Apodemus agrarius | – | 1 | 5 | 0 | 0 | 0 | 0 | – | 1 | 0 | 0 | 0 | 3 | 1 | 2 |
| Sylvaemus flavicollis | – | 17 | 15 | 0 | 26 | 27 | 16 | – | 4 | 6 | 5 | 10 | 18 | 19 | 13 |
| Sylvaemus uralensis | – | 18 | 18 | 0 | 29 | 20 | 14 | – | 5 | 3 | 10 | 0 | 3 | 22 | 12 |
| Sicista betulina | – | 0 | 4 | 0 | 0 | 0 | 0 | – | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Parasite | Distribution | Host | P, % | IR | MA | Localities |
|---|---|---|---|---|---|---|
| Family Anoplocephalidae Anoplocephaloides dentata (Galli-Valerio, 1905) | Holarctic | Clethrionomys glareolus | 0.7 | 1 2 | 0.01 | 14 |
| Microtus arvalis | 7.7 | 2 | 0.2 | 3, 14 | ||
| Microtus subterraneus | 6.7 | 2 | 0.1 | 3 | ||
| Apodemus agrarius | 1.9 | 1–4 | 0.04 | 3 | ||
| Paranoplocephala omphalodes (Hermann, 1783) | Palaearctic | Clethrionomys glareolus | 14.1 | 1–6 | 0.2 | 3, 6, 7, 9, 10, 13, 14 |
| Microtus arvalis | 53.9 | 1–11 | 1.1 | 3, 9, 11, 15 | ||
| Microtus agrestis | in 1 (11) 1 | 1 | 0.1 | 3 | ||
| Family Catenotaeniidae Catenotaenia henttoneni Haukisalmi et Tenora, 1993 | Europe | Clethrionomys glareolus | 16.2 | 1–4 | 0.2 | 3, 5, 7, 10, 11, 14, 15 |
| Catenotaenia sp. 1 | Europe | Microtus arvalis | 3.9 | 1 | 0.04 | 9 |
| Microtus agrestis | in 1 (11) | 1 | 0.1 | 14 | ||
| Catenotaenia sp. 2 | Europe | Sicista betulina | in 3 (4) | 1–2 | 1.3 | 3 |
| Spasskijela lobata (Baer, 1925) | Palaearctic | Sylvaemus flavicollis | 4.9 | 1–14 | 0.02 | 3, 5, 7, 12, 13, 14 |
| Sylvaemus uralensis | 4.2 | 1–12 | 0.02 | 3, 5, 7, 14 | ||
| Apodemus agrarius | 0.5 | 1 | 0.005 | 15 | ||
| Family Dilepididae Dilepis undula (Schrank, 1788), larva | Palaearctic | Clethrionomys glareolus | 0.2 | 1 | 0.002 | 3 |
| Family Hymenolepididae Hymenolepis apodemi Makarikov et Tkach, 2013 | Sylvaemus flavicollis | 0.8 | 1–2 | 0.01 | 3 | |
| Cosmopolitan | Sylvaemus uralensis | 0.3 | 1 | 0.003 | 15 | |
| Apodemus agrarius | 0.9 | 1 | 0.01 | 14, 15 | ||
| Family Taeniidae Taenia martis Zeder, 1803, larva | Europe | Clethrionomys glareolus | 0.5 | 1 | 0.005 | 3, 14 |
| Versteria mustelae (Gmelin, 1790), larva | Clethrionomys glareolus | 1.9 | 1–2 | 0.03 | 3, 9, 11, 15 | |
| Holarctic | Microtus arvalis | 3.9 | 1 | 0.04 | 3 | |
| Apodemus agrarius | 0.5 | 2 | 0.01 | 3 | ||
| Hydatigera taeniaeformis (Batsch, 1786) s. l., larva | Cosmopolitan | Clethrionomys glareolus | 1.0 | 1 | 0.01 | 3 |
| Microtus subterraneus | 20.0 | 1–2 | 0.3 | 3, 9 | ||
| Sylvaemus flavicollis | 1.1 | 1 | 0.01 | 3, 7, 14 | ||
| Sylvaemus uralensis | 1.3 | 1 | 0.01 | 3, 10, 14 | ||
| Apodemus agrarius | 0.9 | 1 | 0.01 | 10, 14 | ||
| Family Brachylaimidae Brachylaima aequans (Looss 1899) | Palaearctic | Apodemus agrarius | 0.9 | 1–2 | 0.01 | 3 |
| Family Plagiorchiidae Plagiorchis elegans (Rudolphi, 1802) | Sylvaemus flavicollis | 3.4 | 1–6 | 0.12 | 6, 14, 15 | |
| Holarctic | Sylvaemus uralensis | 7.8 | 1–51 | 0.4 | 3, 11, 14, 15 | |
| Apodemus agrarius | 14.4 | 1–27 | 0.8 | 14, 15 | ||
| Plagiorchis maculosus (Rudolphi, 1802) | Cosmopolitan | Clethrionomys glareolus | 0.2 | 3 | 0.01 | 14 |
| Family Dicrocoeliidae Brachylecithum rodentini Agapova, 1955 | Palaearctic | Clethrionomys glareolus | 1.7 | 2–15 | 0.1 | 3, 8 |
| Corrigia vitta (Dujardin, 1845) | Palaearctic | Sylvaemus uralensis | 1.3 | 1–2 | 0.02 | 14 |
| Family Echinostomatidae Echinostoma miyagawai Ishii, 1932 | Palaearctic | Apodemus agrarius | 3.7 | 1–2 | 0.06 | 14, 15 |
| Family Trichuridae Trichuris arvicolae Feliu, Spakulova, Casanova, Renaud, Morand, Hugot, Santalla et Durand, 2000 | Palaearctic | Clethrionomys glareolus | 1.0 | 1–2 | 0.07 | 3 |
| Microtus arvalis | 3.9 | 1 | 0.04 | 14 | ||
| Microtus agrestis | in 1 (11) | 1 | 0.1 | 15 | ||
| Trichuris muris (Schrank, 1788) | Palaearctic | Sylvaemus uralensis | 0.3 | 1 | 0.003 | 9 |
| Family Heligmosomidae Heligmosomoides glareoli (Baylis, 1928) | Palaearctic | Clethrionomys glareolus | 1.4 | 1–3 | 0.02 | 3, 5, 14 |
| Heligmosomoides laevis (Dujardin, 1845) | Palaearctic | Arvicola amphibius | in 1 (9) | 7 | 0.8 | 3 |
| Microtus arvalis | 7.7 | 2–3 | 0.2 | 3 | ||
| Microtus agrestis | in 3 (11) | 2–4 | 0.7 | 3, 14, 15 | ||
| Microtus subterraneus | 20.0 | 1–2 | 0.3 | 3 | ||
| Heligmosomoides polygyrus (Dujardin, 1845) | Palaearctic | Sylvaemus flavicollis | 48.7 | 1–83 | 0.5 | 2, 3, 5, 6, 7, 10, 11, 12, 13, 14, 15 |
| Sylvaemus uralensis | 29.4 | 1–71 | 2.4 | 2, 3, 5, 6, 7, 9, 10, 11, 13, 14 | ||
| Apodemus agrarius | 2.8 | 1–2 | 0.04 | 14, 15 | ||
| Heligmosomum mixtum Schulz, 1954 | Palaearctic | Clethrionomys glareolus | 59.8 | 1–27 | 2.7 | 2, 3, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 |
| Family Spirocercidae Mastophorus muris (Gmelin, 1790) | Cosmopolitan | Sicista betulina | in 1 (4) | 5 | 1.3 | 3 |
| Clethrionomysglareolus | 2.9 | 1–13 | 0.09 | 3, 7 | ||
| Sylvaemus uralensis | 0.3 | 1 | 0.003 | 3 | ||
| Apodemus agrarius | 0.5 | 1 | 0.005 | 15 | ||
| Family Heterakidae Heterakis spumosa Schneider, 1866 | Cosmopolitan | Apodemus agrarius | 15.3 | 1–16 | 0.5 | 3, 9, 10, 14, 15 |
| Family Oxyuridae Syphacia agraria Sharpilo, 1973 | Palaearctic | Apodemus agrarius | 28.7 | 1–62 | 4.4 | 3, 9, 10, 11, 14, 15 |
| Syphacia nigeriana Baylis, 1928 | Holarctic | Microtus arvalis | 11.5 | 11–31 | 2.2 | 14, 15 |
| Microtus agrestis | in 1 (11) | 57 | 5.2 | 15 | ||
| Syphacia obvelata (Rudolphi, 1802) | Cosmopolitan | Sylvaemus flavicollis | 12.9 | 1–190 | 5.2 | 1, 3, 5, 6, 10, 12, 13, 14, 15 |
| Sylvaemus uralensis | 21.7 | 2–340 | 8.2 | 3, 5, 6, 7, 9, 10, 11, 14, 15 | ||
| Syphacia petrusewiczi Bernard, 1966 | Holarctic | Clethrionomysglareolus | 13.6 | 3–600 | 14.9 | 3, 5, 7, 11, 13, 14, 15 |
| Syphacia stroma (Linstow, 1884) | Palaearctic | Sylvaemus flavicollis | 51.3 | 1–450 | 21.1 | 1, 2, 3, 5, 11, 13, 14 |
| Sylvaemus uralensis | 9.7 | 1–110 | 1.3 | 3, 5, 6, 14 |
| Title 1 | Ms | Cg | Marv | Magr | Aam | Sf | Su | Sb | Aagr |
|---|---|---|---|---|---|---|---|---|---|
| M. subterraneus | 1 | 0.24 | 0.40 | 0.29 | 0.50 | 0.20 | 0.15 | 0.00 | 0.25 |
| C. glareolus | 0.24 | 1 | 0.38 | 0.22 | 0.00 | 0.10 | 0.17 | 0.13 | 0.30 |
| M. arvalis | 0.40 | 0.38 | 1 | 0.73 | 0.25 | 0.00 | 0.00 | 0.00 | 0.20 |
| M. agrestis | 0.29 | 0.22 | 0.73 | 1 | 0.40 | 0.00 | 0.00 | 0.00 | 0.00 |
| A. amphibius | 0.50 | 0.00 | 0.25 | 0.40 | 1 | 0.00 | 0.00 | 0.00 | 0.00 |
| S. flavicollis | 0.20 | 0.10 | 0.00 | 0.00 | 0.00 | 1 | 0.82 | 0.00 | 0.50 |
| S. uralensis | 0.15 | 0.17 | 0.00 | 0.00 | 0.00 | 0.82 | 1 | 0.17 | 0.52 |
| S. betulina | 0.00 | 0.13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.17 | 1 | 0.13 |
| A. agrarius | 0.25 | 0.30 | 0.20 | 0.00 | 0.00 | 0.50 | 0.52 | 0.13 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirillova, N.; Ruchin, A.; Kirillov, A. Helminths in Myomorph Rodents (Rodentia, Myomorpha) from the National Park “Smolny” and Its Surroundings (European Russia). Forests 2021, 12, 1510. https://doi.org/10.3390/f12111510
Kirillova N, Ruchin A, Kirillov A. Helminths in Myomorph Rodents (Rodentia, Myomorpha) from the National Park “Smolny” and Its Surroundings (European Russia). Forests. 2021; 12(11):1510. https://doi.org/10.3390/f12111510
Chicago/Turabian StyleKirillova, Nadezhda, Alexander Ruchin, and Alexander Kirillov. 2021. "Helminths in Myomorph Rodents (Rodentia, Myomorpha) from the National Park “Smolny” and Its Surroundings (European Russia)" Forests 12, no. 11: 1510. https://doi.org/10.3390/f12111510
APA StyleKirillova, N., Ruchin, A., & Kirillov, A. (2021). Helminths in Myomorph Rodents (Rodentia, Myomorpha) from the National Park “Smolny” and Its Surroundings (European Russia). Forests, 12(11), 1510. https://doi.org/10.3390/f12111510








