Abstract
Full understanding and control of pine wilt disease (PWD) is a work in progress and breeding for disease resistance constitutes an essential management strategy for reducing its impact, as evidenced by advanced breeding programs in countries such as Japan. Since Pinus radiata is one of the most commercially relevant species in northern Spain, we designed a study to assess genetic variation in susceptibility to this pathogen using 44 P. radiata half-sib families from the Galician breeding program. Three Bursaphelenchus xylophilus (pinewood nematode, PWN) inoculation experiments were performed to evaluate disease-related variables, estimate genetic parameters, and study sources of genotype by environment interaction (G × E). We also looked at differences in the constitutive chemical compounds of susceptible and non-susceptible individuals. The results showed great variation in PWN susceptibility, with survival rates for P. radiata families ranging from 0% to 90%. In addition, heritability estimates (hi2 = 0.43, hf2 = 0.72) and genetic gain (>26% selecting 50% of the families) were both moderately high for survival. Significant differences in several constitutive chemical compounds were found between susceptible and non-susceptible seedlings in the two susceptibility groups considered. These results confirm the potential of breeding to obtain P. radiata genotypes that are resistant to pine wilt disease and open possibilities for mitigating its future impact on P. radiata stands.
1. Introduction
Monterey pine, Pinus radiata D. Don, is a tree species native to very limited areas of the California coast in the United States and Baja California in Mexico [1]. However, as one of the most widely planted tree species in the world, it is cultivated in Australia, New Zealand, Chile, South Africa, and southwest Europe. In the latter region, plantations are mainly located in northern Spain, specifically in the Basque Country and Galicia, which contain 47.6% and 33.3% of the total area covered by P. radiata in Spain, respectively. In 2019, 2.2 million m3 of this species were harvested in the Basque Country and 1.8 million m3 in Galicia, representing 95.2% and 46.2% of the total conifer harvest volume in each region, respectively [2].
Tree species are under increasing threat worldwide from diseases and insect pests, many of which are non-native. One health menace to P. radiata is Bursaphelenchus xylophilus (pinewood nematode, PWN), the organism that causes pine wilt disease (PWD). Although P. radiata is only weakly affected by PWN in its native area [3], mortality in planted areas was reported in the outbreak that occurred in Sancti-Spiritus (Salamanca, Spain) [4]. In previous experiments under greenhouse conditions, this species experienced mortality rates of 40–95% following exposure to the pinewood nematode [5].
With the increasing temperatures and droughts that accompany global warming, P. radiata stands in northern Spain—where average summer temperatures reach 20 °C—will be at high risk of being affected by PWD [6]. The spread of PWN also threatens countries such as Australia and New Zealand, where this pine species constitutes a significant component of the forest industry [7]. Careful monitoring of coniferous forests and strict inspections of wood trade are ongoing in these countries to prevent the establishment of this pest.
Since the first report of B. xylophilus being introduced in Japan in 1905 [8], this pathogen has spread widely and has been reported in China [9], Korea [10], and Taiwan [11]. In Europe, PWD was first reported in Portugal [12], where the pathogen continues to cause irreparable damage to forest ecosystems and severe economic losses [13]. Spain declared the first outbreak in 2008 [14], and five other foci have been declared to date [4,15], two of which are currently eradicated.
All countries affected by PWD have dedicated significant effort to stopping it, but none have been able to fully control the disease. Management measures, such as aerial spraying of insecticides, removing dead or infected trees, and stand management, have only slowed down the spread of the disease. However, combining some of these measures with the deployment of PWN-resistant genotypes might be an important strategy for mitigating the impact of PWD [16]. Breeding for resistance to PWD has been successfully developed in Asian countries. The first PWN resistance breeding program began in Japan in 1978 with the selection of surviving trees within severely PWD-damaged stands of susceptible Pinus thunbergii Parl. and Pinus densiflora Siebold & Zucc [17,18]. More recently, the potential for breeding PWN-resistant Pinus pinaster Ait. individuals has also been confirmed [19,20] and PWN resistance has been included as a selection trait in the Galician P. pinaster breeding program [20].
Genetic variation in susceptibility to PWN has not been broadly studied for P. radiata, though some studies have shown variation in resistance to other pests, such as the pine aphid Essigella californica Essig [21], and to diseases such as dothistroma needle blight [22] or Cyclaneusma needle cast [23]. In fact, resistance to Dothistroma pini Hulbary is an important selection trait in genetic breeding programs for P. radiata in New Zealand and Australia.
One of the difficulties that forest breeders must address in the implementation of efficient breeding programs are genotype-by-environment interactions (G × E). The expression of resistance may differ among genotypes across a range of environments due to G × E, making changes in genotype ranking a primary concern for tree breeders. Consequently, the detection of G × E is relevant for estimating the expected genetic gain in any breeding program [24,25].
Although breeding forest tree species for resistance is a long-term solution, it is also a long-term process. Genomic selection using molecular markers such as single nucleotide polymorphisms (SNPs) could shorten breeding cycles [26,27] while greater command of the Pinus defences involved in resistance to B. xylophilus may prove useful for selecting resistant Pinus genotypes. Chemical compounds in pines constitute a major defence against a wide range of pests and diseases. Accordingly, different authors have demonstrated the significant role that various chemical compounds play in resistance to PWN. Phytoalexins, for instance, appear to have nematocidal action [28] while concentrations of total phenolics and condensed tannins seem to be related to PWN resistance [29,30,31]. Other studies point to the importance of nutrients in PWD development [5,31].
The main purpose of this paper was to determine whether the P. radiata breeding population in the Galician breeding program has enough genetic variation in PWN resistance to reduce disease impact through selection and breeding. Specifically, we aimed to (i) estimate genetic parameters, (ii) explore the genotype by environment interaction, and (iii) study constitutive chemical differences between susceptible and resistant P. radiata seedlings.
2. Materials and Methods
2.1. Plant Material and Experimental Design
Three experiments were performed to test half-sib families from the P. radiata genetic improvement program begun in Galicia (NW Spain) in the 1990s. Seeds for the three experiments were collected from a progeny trial located in A Coruña (Spain). They were grown in nursery beds at the Lourizán Forest Research Center (Xunta de Galicia, 42°24′35″ N 8°00′12″ W, Pontevedra, Spain) in two-litre plastic pots using a mixed substrate of peat moss and vermiculite (9:1 v/v.).
Three-year-old seedlings from 44 P. radiata open-pollinated families were evaluated; 41 in Experiment 1 of Supplementary Materials (Figure S1), 44 in Experiment 2, and 40 in Experiment 3. Each family was evaluated in at least two out of the three experiments (Table 1).

Table 1.
Main features of the P. radiata inoculation experiments.
All experiments followed a randomized complete block design with 40–44 families, ten blocks, and one-tree plots. For each experiment, we inoculated ten seedlings per family with B. xylophilus and three with distilled water as controls. The three blocks including control seedlings were randomly selected. Seedlings in Experiments 1 and 2 were inoculated in April, while those in Experiment 3 were inoculated in July.
2.2. Pinewood Nematode Culture and Inoculation Procedure
Seedlings in the three experiments were inoculated using B. xylophilus which had been isolated from the outbreak that occurred in As Neves (Pontevedra, NW Spain) in 2010 [14].
Nematodes were reared using a non-sporulating form of Botrytis cinerea Pers. fungus cultured on PDA medium at 25 °C. The day before inoculation, nematodes were extracted by a modified Baermann funnel technique and adjusted to 2000 nematodes per ml in distilled water.
For seedling inoculation, a wound was made in the previous year’s growth of the stem and a 1 cm-wide strip of gauze bandage was placed around the wound. Nematode suspension was pipetted onto the gauze bandage and the inoculation site was sealed with Parafilm® to avoid desiccation. Control seedlings were inoculated with 300 µL of distilled water while inoculated seedlings were administered a 300 µL suspension of distilled water containing 600 B. xylophilus nematodes at mixed developmental stages.
2.3. Pre-Inoculation Variables
In the three experiments, growth variables were assessed for all seedlings prior to the inoculation date. We measured height to the previous year’s growth (HPY), growth increment from the previous year’s growth to the inoculation date (IH), seedling height at the inoculation date (H), basal diameter at the inoculation date (D), and number of principal branches (NB).
2.4. Wilting Symptom Assessment and Survival
We assessed wilting symptoms in all experiments twice a week from the onset of external wilting symptoms until no symptom evolution was observed. Wilting symptoms were assessed using a seven-level scale based on the percentage of discoloured needles, ranging from 1 (no external symptoms) to 7 (all needles brown and wilted) [5].
Survival and disease evolution variables were estimated from the wilting symptom assessments. Survival was determined as a binary variable: 1, dead seedling (wilting symptom Level 6 or 7), and 0, alive (Levels 1–5). For disease duration variables, Level 3 was established as the start of wilting symptoms and Level 6 as the end of wilting symptoms. Disease duration was estimated as the difference between the end and the start of wilting symptoms. All disease evolution variables were expressed in number of days.
2.5. Nematode Quantification
In the three experiments, nematodes were extracted from the stem in two control and eight inoculated seedlings per family in a subset of twelve randomly selected families. The same twelve families were used for nematode quantification in all three experiments.
Nematodes were extracted using the modified Baermann funnel technique and then quantified under a stereomicroscope (Olympus Co., Ltd., Tokyo, Japan). Stem samples were dried at 105 °C for 48 h to express nematode density as the number of nematodes per gram of dry stem weight.
2.6. Chemical Compounds Analysis
Needles were collected from all seedlings prior to B. xylophilus inoculation in each experiment. These samples were immediately frozen at −20 °C until all experiments concluded. At the end of each experiment, the 25 most and 25 least susceptible seedlings were selected and needles from these individuals were arranged into a total of 10 samples per experiment. Five samples per experiment and susceptibility group (resistant and susceptible) were composed of needles from five seedlings. The samples of the resistant individuals belonged to 30 families and the susceptible to 39. Needles from the selected seedlings were then lyophilised before blending to make the samples.
Chemical analyses were performed on the samples to determine water content and levels of lipid-soluble substances, total polyphenols, condensed tannins, macronutrients (N, P, K, Ca, and Mg), and micronutrients (Fe and Mn), as described by Menéndez-Gutiérrez et al. [5].
Soluble carbohydrates and starch concentrations were analysed as per Chow and Landhausser [32] and DuBois et al. [33], with some adjustments. Soluble carbohydrates were extracted with aqueous ethanol (EtOH:H2O) (80:20) (v/v) using an ultra turrax, followed by centrifugation. A rotatory evaporator was used for ethanol removal and soluble carbohydrates in the extract were analysed as glucose, following the Dubois method [33]. Non-structural storage carbohydrates (starch) were hydrolysed with H2SO4 5N and analysed colorimetrically as soluble carbohydrates. The results were expressed in mg glucose g−1 lyophilized tissue.
2.7. Statistical Analysis
Joint analyses of the three experiments were performed for all variables. Survival, wilting symptoms, and disease duration variables (SW, DW, EW) were analysed using the following general linear mixed model:
where Xijk is survival, wilting symptom or any of the disease duration variables of individual seedlings, µ is the overall mean, Ei is the fixed effect of the ith experiment, Fj is the random effect of the jth family, Bk(Ei) is the random effect of the kth block within the ith experiment, Ei × Fj is the random interaction between the ith experiment and the jth family, and and εijk is the residual random error.
For survival, the analysis was also performed including diameter (the trait most correlated to survival in the present work) as a covariate, to determine whether differences in susceptibility were merely due to differences in diameter or if other causes were involved.
Pre-inoculation variables (HPY, IH, H, D, NB) were analysed using the model described above but excluding the block effect.
The SAS System MIXED procedure was applied to fit the model for wilting symptoms, disease duration and pre-inoculation variables. However, binary survival data (0/1) were analysed using the SAS System GLIMMIX procedure of presuming a binary distribution and a logit link function. Accordingly, the function used to estimate predicted survival (p) was:
where ηijk is the link function g(p), µ the conditional mean, and ln [p/(1 − p)] the odds of survival.
The significance of random factors was tested by a likelihood ratio test, which was determined by the difference in two times the log-likelihood of the models, including and excluding the assessed random factor. The likelihood ratio was distributed as a one-tailed χ2 with one degree of freedom [34].
Individual (hi2) and family (hf2) heritabilities were estimated as follows from the joint analyses:
where σA2 is the additive variance, assuming true half-sib families was calculated as σA2 = 4σf2; σf2, the family variance component; σfe2, the family variance component by experiment interaction; σε2, the residual variance; N, the harmonic mean number of seedlings per plot; B, the number of blocks; and E, the number of experiments. Standard errors were calculated as in Wright [35].
For binomial variable survival, the residual variance was established as π2/3, the variance on the underlying scale for logit link function [36].
The predicted genetic gain in percentage from the across site family selection was estimated as the average best linear unbiased predictors (BLUPs) of the selected less susceptible families divided by the population mean, multiplied by 100 and the individual heritability [37].
Chemical compound analyses were carried out using the SAS MIXED procedure, including the experiment, the susceptibility group (susceptible or non-susceptible seedlings), and their interactive effect as fixed factors.
Likelihood-based analyses were performed to thoroughly examine genotype by environment interaction (G × E) causes for survival and wilting symptoms, according to Yang [38] and De la Mata and Zas [39]. Initially, a full model was fitted using an unstructured family covariance structure in the SAS PROC MIXED procedure, considering the experiment as a fixed factor and the family and block within the experiments as random factors. Then, reduced models with specific constraints to the family covariance structures were fitted and compared to the full model. The following sources of G × E were tested: homogeneity of family variance across experiments, perfect family correlation between all experiment pairs, and homogeneity of family covariance across experiment pairs. These three hypotheses were evaluated by comparing the restricted log-likelihood ratio obtained for the full model to the likelihood ratio of the reduced covariance model. Under the null hypothesis (i.e., full covariance model is not different from the reduced covariance model), the log-likelihood ratio is distributed as χ2 with degrees of freedom given by the difference in the number of covariance parameters that defined the full model and the reduced model [34].
Differences in nematode density in the stem at the end of the experiments were studied using the Kruskal–Wallis non-parametric analysis of variance. These analyses were performed separately for each experiment and then jointly. The Mann–Whitney U test was performed to compare families when significant differences were found.
Pearson correlation coefficients between all traits were calculated for individual-seedling values of the three experiments (phenotypic correlations) and for family breeding values from joint analyses (genetic correlations) using the CORR procedure in SAS. Spearman correlations were only used when nematode densities were analysed.
SAS System Software (SAS Institute Inc., Cary, NC, USA, 2014) was used to perform all statistical analyses.
3. Results
3.1. Pre-Inoculation Variables
All P. radiata seedling variables measured prior to B. xylophilus inoculation (H, IH, HPY, D, NB) showed significant differences among experiments (p < 0.05), families (p < 0.0001), and family × experiment interaction (p < 0.015) (Table 2).

Table 2.
Overall means and standard error (SD); Variance components (σ2) and likelihood ratio significance test (χ2 LRT) for random effects; F ratios and significance levels for the fixed effect, using mixed-model analysis to calculate heritability in P. radiata families inoculated with Bursaphelenchus xylophilus. Height (H), Height to the previous year’s growth (HPY), growth increment from the previous year’s growth to the inoculation date (IH), Diameter (D), Number of principal branches (NB), Wilting symptoms (W), Survival rate (S), Start (SW), End (EW) and Duration (DW) of wilting symptoms of dead seedlings.
Seedlings from the experiments inoculated in April (Experiments 1 and 2) presented significantly greater height, growth increment from the previous year’s growth to the inoculation date, height to the previous year’s growth, and basal diameter compared to seedlings inoculated in July (Experiment 3). Conversely, the number of principal branches was greater in Experiment 3 (data not shown).
Individual narrow-sense heritability for these traits was high in all cases (hi2 > 0.50), except for IH (hi2 = 0.29). Family heritability estimates were also high, with heritability ranging from 0.63 (IH) to 0.77 (HPY) (Table 2).
3.2. Disease-Related Variables
Wilting symptoms and survival differed significantly among P. radiata families, but also between experiments and among blocks. In contrast, this analysis detected no differences in the G × E interaction for both traits (Table 2). Differences among P. radiata families for both variables remained highly significant when diameter was included as covariate in the previous analyses (p < 0.01, data not shown), indicating that other genetically controlled factors might be involved in genetic susceptibility to PWN.
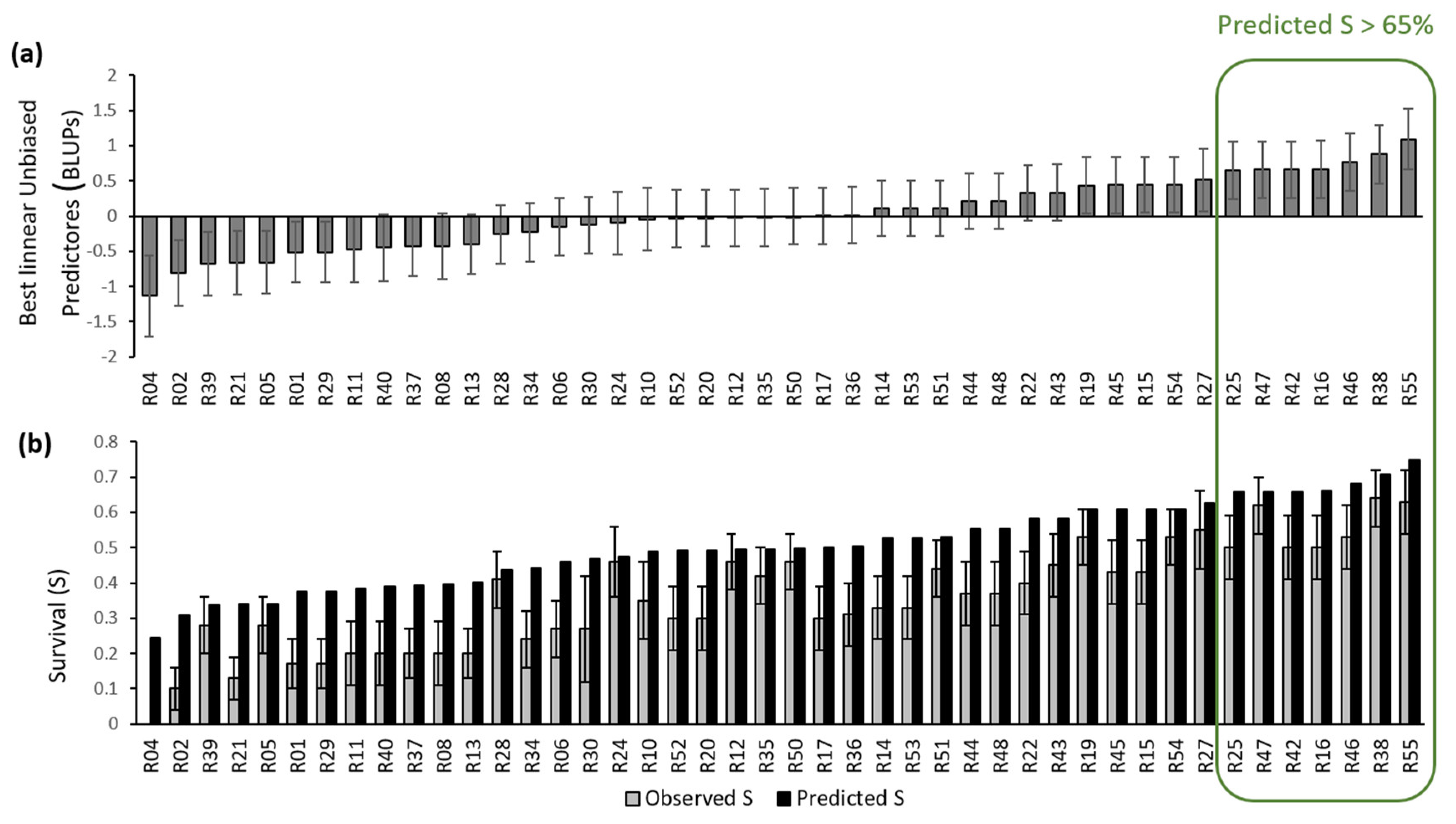
In Experiment 2, 50% of the seedlings survived B. xylophilus, compared to 20% in Experiment 1 and 26% in Experiment 3. In those two experiments, the mean diurnal and nocturnal temperatures were higher than in Experiment 2 (Table 1). Despite the low P. radiata survival rates, great variation in survival rates was observed among families. The observed family survival rates ranged from 0% to 90 % in Experiment 2 and from 0% and 70% in the other two experiments. One group of families stood out as having reasonably high predicted survival rate (over 65%, a range oscillating from 24% to 75%) and fewer wilting symptoms in all the experiments (Figure 1).
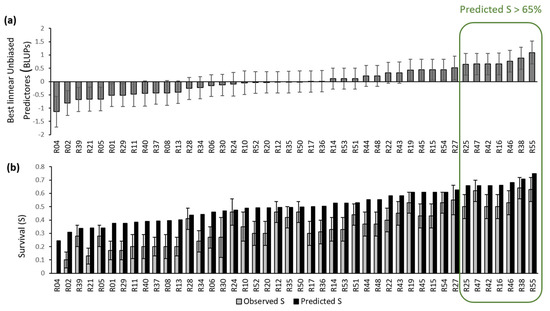
Figure 1.
(a) BLUPs survival ranking for the 44 Pinus radiata families (b) Predicted and observed survival 83 days after inoculation. Error bars are standard errors.
Significant differences among families were also observed for the end and duration of wilting symptoms, though not for the start of symptoms. The G × E interaction was only significant for the disease duration variable SW (Table 2).
On average, wilting symptoms started 33 days after inoculation (DAI) and ended 55 DAI, so the average duration of the disease was 22 days. However, all these variables differed significantly among experiments (Table 2).
Both individual and family heritability estimates were especially high for survival and all pre-inoculation variables (H, HPY, D, NB) except height increment, which showed lower values (Table 2). Wilting symptoms and disease duration variable (SW, EW, DW) values were moderately high for both individual and family heritabilities (Table 2).
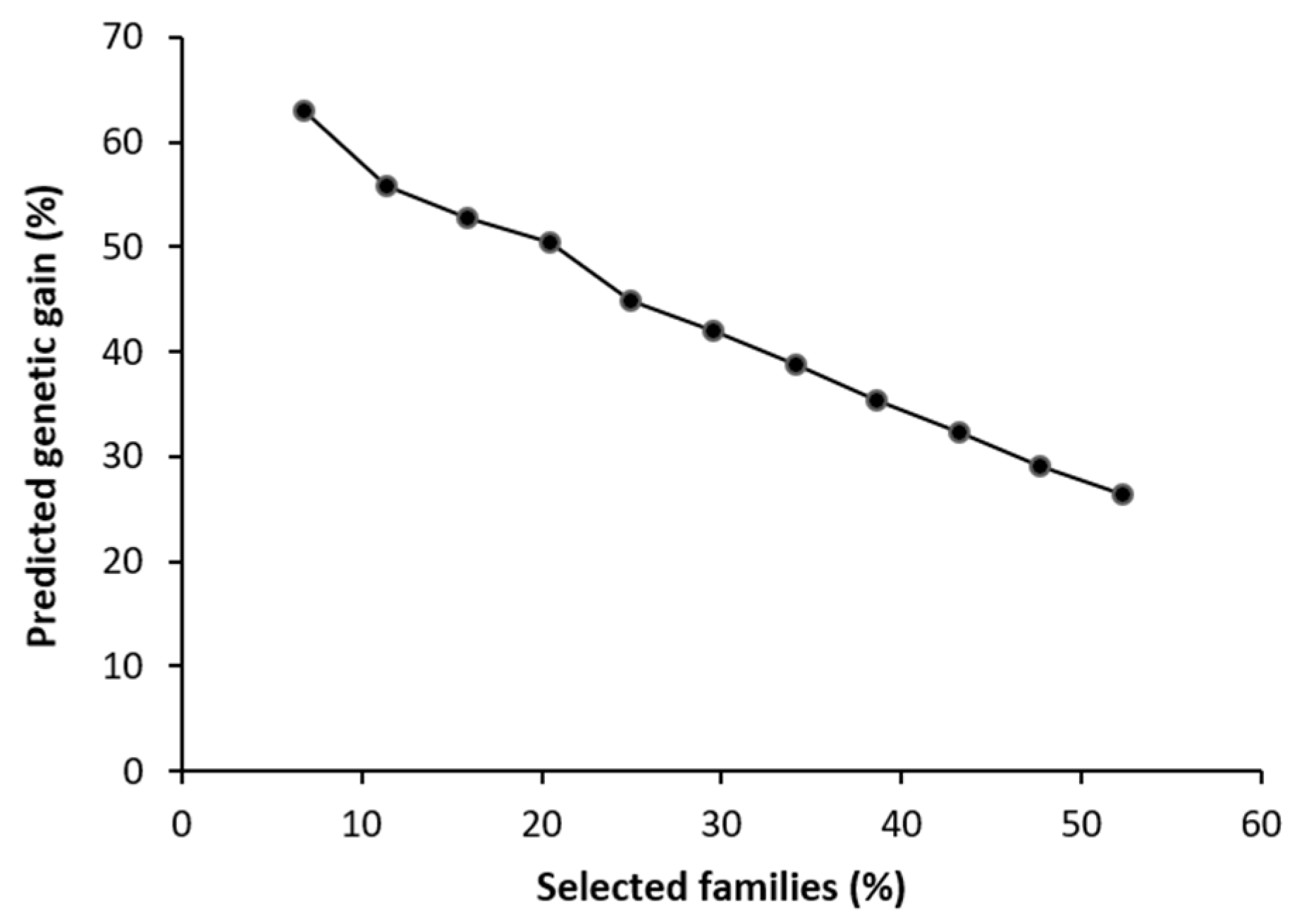
The value for predicted genetic gain for survival was high. By selecting the 50% of the families with the highest survival, we obtained a genetic gain of up to 26.4%. As the number of families selected decreased, this value grew to almost 52.7% for the seven families that were finally selected (Figure 2).
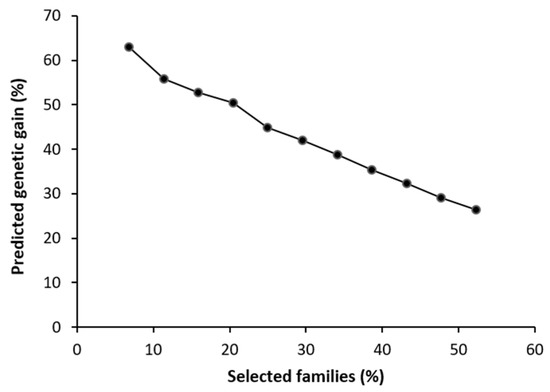
Figure 2.
Predicted genetic gain in survival of Bursaphelenchus xylophilus in relation to the number of Pinus radiata families selected.
3.3. Genotype by Environment Interaction
The G × E interaction was not significant for wilting symptoms or survival in the conventional mixed model analyses (Table 2). However, more specific G × E analyses involving various sources of interaction showed a significant G × E effect (Table 3) that was not explained by any of the sources studied. In fact, one of the key implications of G × E in a breeding program, the family ranking changes, was not significant (Table 3). This interaction did not seem relevant in the end, since the ratio family variance component to G × E variance for these traits did not exceed the interaction relevance threshold proposed by Shelbourne [40] (σf2/σfe2 < 2). The values for survival and wilting symptoms were 0.39 and 0.29, respectively (data not shown).

Table 3.
Likelihood ratio tests from analyses of different sources of genotype by environment interaction (G × E) across the three experiments. Chi-squared values (χ2) and significance of the sources of G × E are estimated by comparing the likelihood ratio of the full and reduced models. Degrees of freedom (df) are the difference between the number of (co)variance parameters estimated in the full and reduced models.
3.4. Nematodes
The number of nematodes sampled from inoculated stems differed significantly among the three experiments (Kruskal–Wallis χ2 > 10.12, p < 0.0064; data not shown). The median number of nematodes was higher in Experiment 1 than in the other experiments, but we only found significant differences among families in Experiment 3 (Kruskal–Wallis χ2 > 23.87, p < 0.0324; data not shown) when data were analysed separately for each experiment.
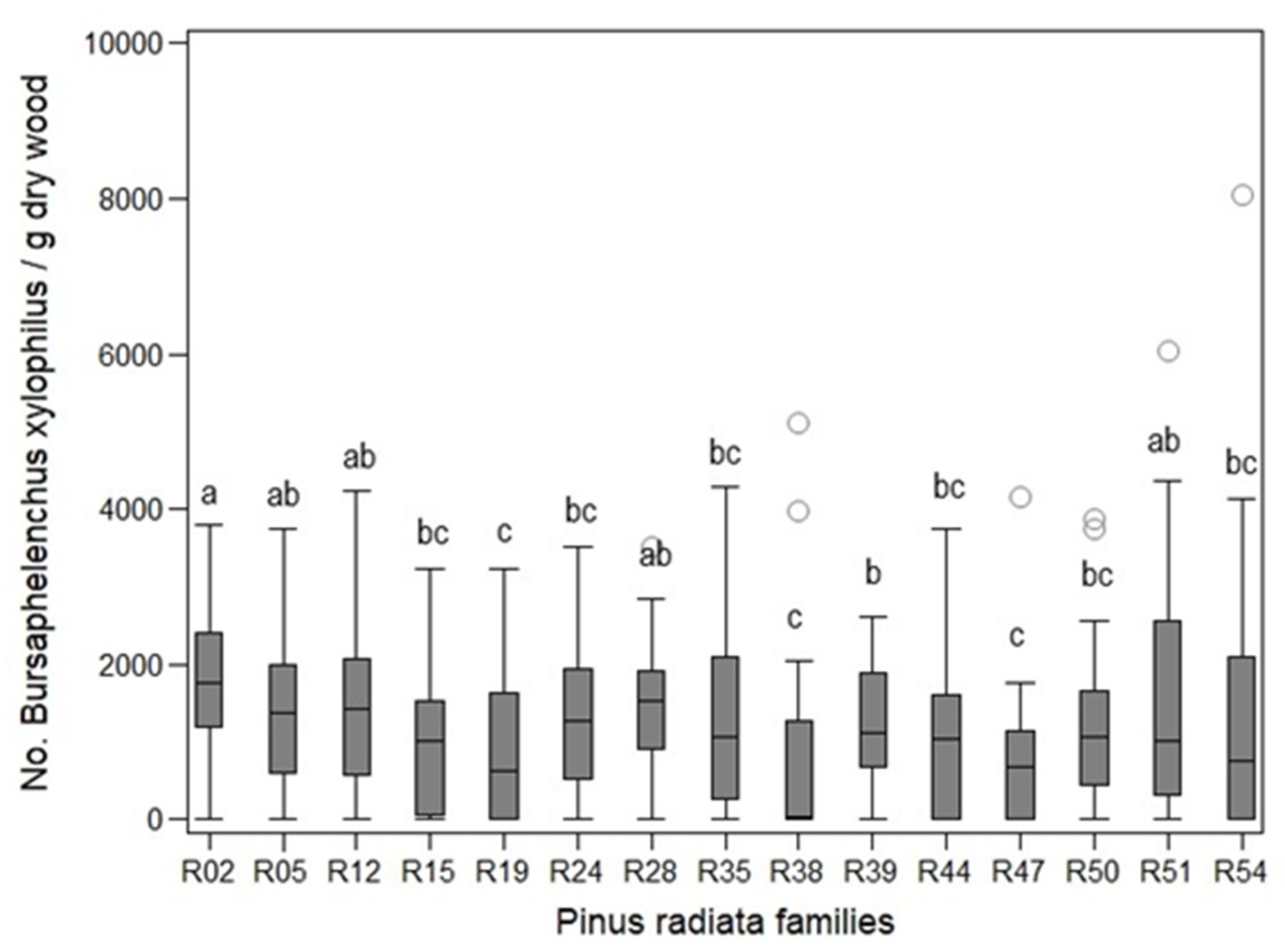
In the joint analyses, the number of nematodes differed significantly among families (Kruskal–Wallis χ2 > 34.01, p < 0.0021; Figure 3, data not shown).
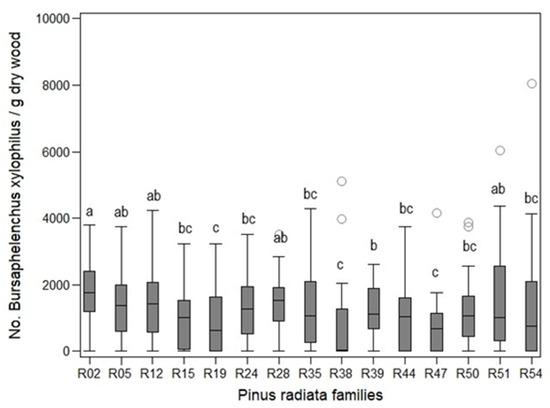
Figure 3.
Number of Bursaphelenchus xylophilus per dry gram of wood extracted from stem of Pinus radiata families. Box-whisker plot, Line (median), Box (25%–75% quartiles of values), Whisker (min-max span of values), Circles (outliers).
At the end of the assays, the number of nematodes recovered from the stems of seedlings at wilting levels 1–4 ranged from 0 to 69.50 B. xylophilus per dry gram of wood. In seedlings at wilting levels 6 and 7, the number ranged from 40.73 to 8059.16 B. xylophilus per dry gram of wood.
We did not recover any nematodes from the control seedlings.
3.5. Correlations
Survival and wilting symptoms presented a markedly strong negative correlation at phenotypic and genetic levels. Hence, they had relationships with the same traits but opposite signs (Table 4). At the phenotypic level, all pre-inoculation traits except IH were correlated positively with wilting symptoms and negatively with survival, especially NB and D. Similarly, nematode density was also strongly correlated with both traits. Disease duration variables (SW, EW, DW) had a negative relationship with wilting (Table 4).

Table 4.
Pearson correlation matrix of phenotypic values of the three experiments (below the diagonal) and genotypic values from joint analyses of the three experiments (above the diagonal) coefficients between pairs of variables. Height (H), Height to the previous year’s growth (HPY), Growth increment from the previous year’s growth to the inoculation date (IH), Diameter (D), Number of principal branches (NB), Wilting symptoms (W), Survival rate (S), Start (SW), End (EW) and Duration (DW) of wilting symptoms in dead seedlings, Number of B. xylophilus in the stem per gram of dry weight (ND).
The same relationships were observed at the genetic level, except for all height traits, which were not significantly correlated with survival or wilting symptoms (Table 4).
3.6. Chemical Compounds
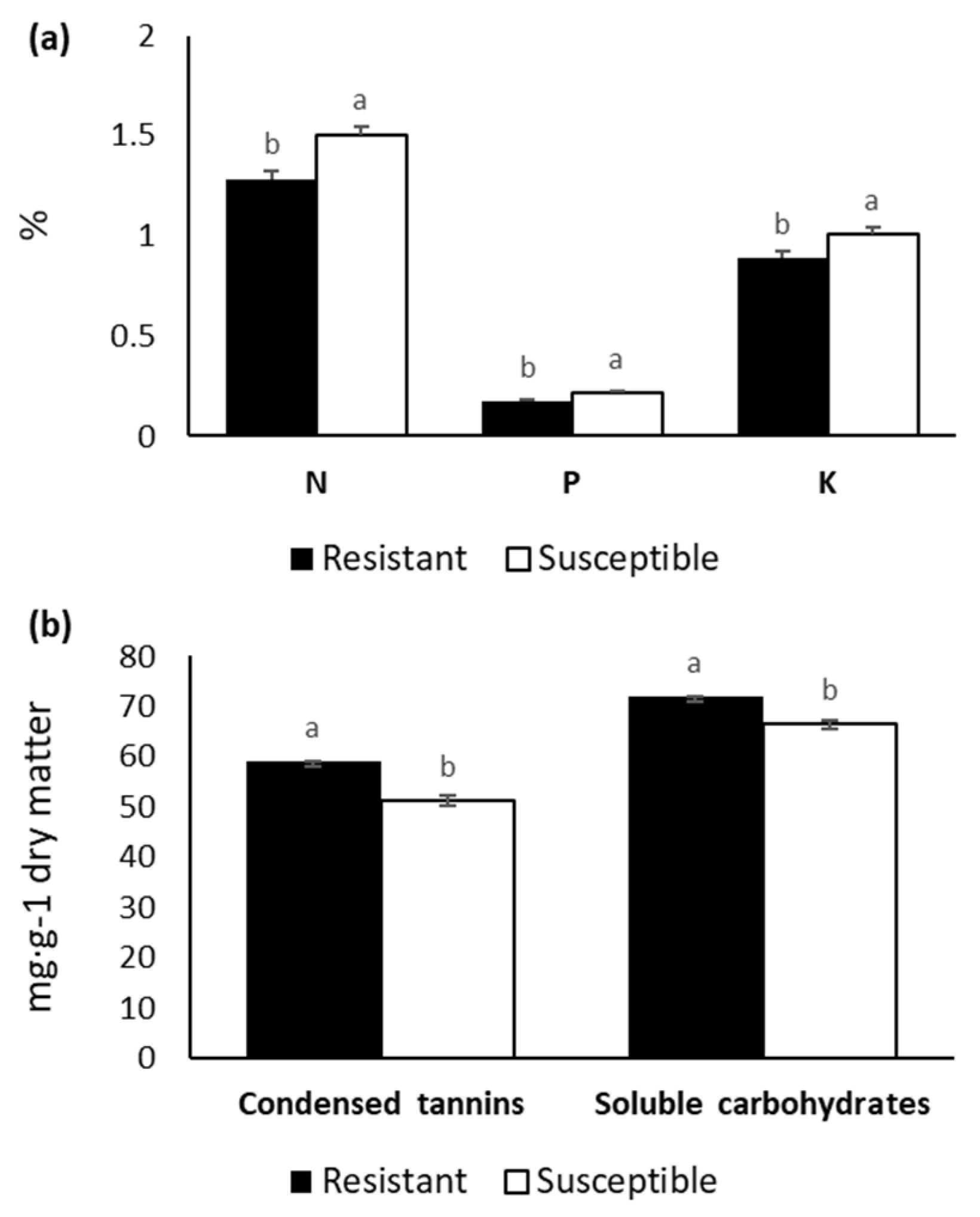
The constitutive macronutrients N, P, and K and the micronutrient Mn, along with condensed tannins and soluble carbohydrates, showed significant differences among susceptibility groups. In addition, we observed a significant interaction effect between susceptibility group and experiment for Mn and lipid-soluble substances (Table 5). The group comprised of resistant individuals had the highest concentration of condensed tannins and soluble carbohydrates but was lower in N, P, and K contents (Figure 4). We only found significant differences for lipid-soluble substances between the resistant and susceptible groups in Experiment 3, where concentrations of this chemical were much greater in the resistant group. Mn concentrations were also significantly higher for the resistant individuals in Experiment 2 (data not shown).

Table 5.
Results of mixed-model analysis of the constitutive chemical compounds in needles. F ratios (degrees of freedom are shown as a subscript, F factor, error) and associated p values are shown. WC: Water content, N: Nitrogen, P: Phosphorus, K: Potassium, Ca: Calcium, Mg: Magnesium, Fe: Iron, Mn: Manganese, LS: Lipid-soluble substances, POL: Total polyphenols, TAN: Condensed tannins, CAR: Soluble carbohydrates STA: Starch. Significant p-values (p ≤ 0.05) are shown in bold.
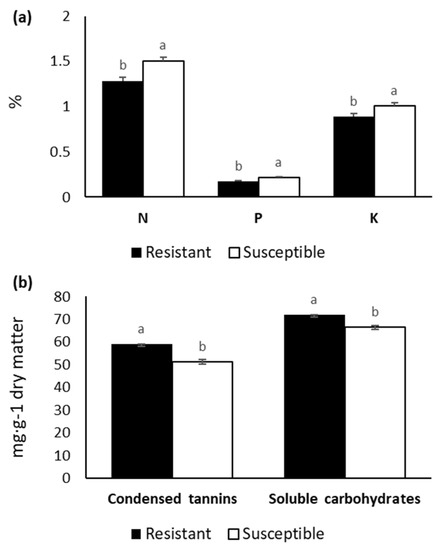
Figure 4.
Constitutive concentration of the macronutrients N, P, K (a); condensed tannins and soluble carbohydrates (b) in Pinus radiata needles from resistant and susceptible groups.
4. Discussion
Our results revealed significant genetic variation with a broad range of susceptibility to pinewood nematode among P. radiata half-sib families from the Galician breeding program. The moderately high heritability estimates and genetic gain obtained from these experiments confirm the potential of this species for breeding PWD-resistant trees to mitigate future damage to P. radiata plantations.
Resistance breeding has proven successful in controlling diverse pests and pathogens that affect forest tree species [41,42,43]. In PWD resistance breeding, numerous resistant P. densiflora and P. thunbergii clones have been identified in Japan since 1972, with respective survival rates that are 40% and 16% higher than the non-selected population [44]. These data justify the need to continue advancing this research line as part of the strategy to control the disease. Resistance breeding programs are also underway for other susceptible species, though they are in earlier stages than the Japanese program. A PWD resistance breeding program for P. massoniana was initiated in China in 2001 with the selection of 1201 resistant P. massoniana individuals [45]. On the Iberian Peninsula, significant genetic variation in susceptibility to PWD was found among half-sib families of P. pinaster, prompting both Portugal and Spain (Galicia in particular) to start improvement programs [19,20]. Six families from the Galician P. pinaster breeding program (NW Spain) have been registered as parents of families resistant to PWD in the National Catalogue of Base Materials [46] and are currently ready to be commercialized.
Australia and New Zealand, where P. radiata has a great economic importance, have prioritized breeding for resistance to diverse foliar diseases, especially Dothisthoma needle blight and Cyclaneusma needle cast, which have been studied and included as selection criteria for several decades [47]. The variation in resistance to these diseases has a strong additive genetic basis [22,23].
The results of this study also justify starting a PWD resistance breeding line for P. radiata as part of the Galician breeding program for this species. As in the previous examples, significant genetic variation in susceptibility to PWD was observed, along with reasonably high heritability estimates, indicating that resistance is inherited and mainly due to additive genetic effects. The individual narrow-sense heritability estimate for survival was similar to the maximum heritability estimates reported for open-pollinated P. thunbergii families [18] and P. pinaster families, which were selected from Portuguese areas severely affected by PWD and studied under controlled conditions [19]. However, the heritability values obtained for P. pinaster families from the Galician breeding population (NW Spain) for mortality were considerably lower [20].
In this study, the isolate used for screening was obtained from a dead Maritime pine from the outbreak that occurred in As Neves (Galicia, NW Spain). Many authors have emphasized the importance of using the most virulent isolate for selecting the most resistant genotypes in breeding programs [48]. Accordingly, we chose the isolate for this assay based on a previous study of isolates of diverse origins to determine the most virulent isolate for P. radiata [49].
Many factors seem to affect variation in PWN susceptibility, including the physicochemical characteristics of the host and environmental factors, such as temperature and drought. In this study, we observed a significant genetic and phenotypic relationship between growth traits and diseased-related variables, in which vigorously growing families tended to have higher mortality and develop more wilting symptoms. This relationship was also reported for P. pinaster families from the Galician breeding population: trees from the most susceptible families also had greater height growth [20]. The same has been reported for other pests [50,51] and points to a growth–defence trade-off, though other studies found no relation or a negative correlation between growth and mortality [52,53].
Temperature is also known to be widely associated to PWD development, as illustrated by the differential survival rate obtained across experiments. We observed that the highest survival rate occurred in the experiment subjected to the lowest day and night temperatures. Differences in temperature were even greater when we only considered temperature during the three first weeks after inoculation. This finding is in accordance with a previous study that described the great relevance of early cumulative temperatures in PWD development, especially in resistant P. thunbergii families [54]. Another work demonstrated the heavy influence of high nocturnal temperature on tree mortality after PWN inoculation and the likely contribution of other factors to PWD development [53].
While our results suggested a low level of G × E, we have to take into consideration that we studied G × E under greenhouse conditions where the sources of variation are less pronounced. In a study by Matsunaga et al. [55] of six P. thunbergii families at three sites with dissimilar climates, only a low proportion of the total variance was explained by site x family interaction and no changes were observed in the resistance family ranking. Similarly, Suontama [23] did not find a significant G × E interaction that affected P. radiata resistance to Cyclaneusma needle cast.
Genetic variation in secondary metabolites has been reported for conifers [56]. For this reason, we focused on certain constitutive chemicals in P. radiata that may also influence disease development in resistant families. Given the rapid multiplication and spread of the nematode throughout the tree, we hypothesized that the defence system could not inhibit PNW infection unless defence metabolites such as terpenoids or tannin-like substances were already present in the tree or produced in the early stage of the disease [57]. Condensed tannins are phenolic compounds with great defensive capacity. Indeed, these rather potent antibiotics act to protect against insect herbivores [58,59]. In the present work, we observed higher levels of constitutive condensed tannins in resistant individuals. Our findings concur with those of Ohyama et al. [60], who reported a higher proanthocyanidin (condensed tannin) content in pine-wilt resistant P. thunbergii and P. densiflora clones than in susceptible pines. Resistant individuals also had larger amounts of soluble carbohydrates. These substances are involved in various stress responses and are related to important changes in the balance of reactive oxygen species (ROS) [61], which are known to be released in response to PWN infection [62]. However, this relationship requires more thorough study since soluble sugar can also contribute to ROS scavenging [61].
Plant nutrients can also play an important role in disease resistance by enhancing disease resistant mechanisms. We found higher constitutive levels of Mn in resistant individuals in two of the experiments. This compound plays an important role in the biosynthesis of some defensive substances, such as lignin and phenol. It is also involved in photosynthesis and many other tree functions [63]. By the same principles, the highly PWN-susceptible species P. sylvestris was found to have about ten times less Mn than other, less susceptible pine species [5]. Conversely, we observed higher levels of N, P, and K in the susceptible group than in the resistant one. The effect of N and P on disease resistance seems inconsistent and sometimes depends on the plant pathogen [63]. For instance, one study indicated higher levels of constitutive N in the phloem of P. sylvestris, which is highly susceptible to PWN, than in other less susceptible species [31], while another study reported much lower amounts of N in the xylem of P. sylvestris than in other species [5]. Though we found lower levels of K in the resistant individuals, fertilization with K seems to reduce disease incidence in some crops by promoting the synthesis of various compounds involved in disease resistance [63].
Neither field-based assessments nor field selection of PWN-resistant candidate trees in affected areas were possible in Spain due to EU quarantine organism restrictions, since the disease is currently found in isolated foci and has not spread over the entire territory. Given the impossibility of validating our findings in adult trees in the field, the results must be considered cautiously. However, the use of two- or three-year-old seedlings for testing resistance against pinewood nematode is broadly accepted. In Japan, the evaluation in seedlings has been carried out for many years, obtaining resistant candidates which are being planted. Additionally, Maehara et al. [64] used P. thunbergii adult trees, obtaining similar results to those of Kanzaki et al. [65] who used three-year-old seedlings.
Experiences with managing pine wilt disease in affected countries have shown that conventional disease control measures are insufficient and must be complemented with strategies such as breeding. This work confirms that breeding PWN-resistant trees through selection is possible for P. radiata. Our results confirm the existence of genetic variation among P. radiata families, with high heritability estimates and a moderately high genetic gain for survival. These findings imply that resistance to PWD can be included as a new selection factor in the P. radiata breeding program and survival seems to be the best trait to assess in operational breeding. Furthermore, this work provides evidence indicating the importance of the constitutive chemistry of P. radiata seedlings in host resistance to PWN.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/f12111474/s1, Figure S1: Pinus radiata experiment one month after inoculation with Bursaphelenchus xylophilus.
Author Contributions
Conceptualization, M.M.-G., R.D. and M.A.; methodology, M.M.-G., R.D. and M.A.; formal analysis, M.M.-G., R.D. and M.A.; investigation, M.M.-G., R.D. and M.A.; resources, R.D. and M.A.; data curation, M.M.-G.; writing—original draft preparation, M.M.-G.; writing—review and editing, M.M.-G., M.A. and R.D.; supervision, M.M.-G. and R.D.; project administration, R.D.; funding acquisition, R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Institute for Agricultural and Food Research and Technology (RTA 2014-042-C2 and RTA2017-012-C2), and co-funded by the European Regional Development Fund (Plan de Mejora e Innovación Forestal de Galicia 2010–2020) and INDITEX.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank Ricardo Ferradás, Maribel Juncal, and Francis Ignacio for their technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ledig, F.T.; Vargas-Hernández Jesús, J.; Johnsen, H.K. The conservation of forest genetic resources: Case histories from Canada, Mexico, and the United States. J. For. 1998, 96, 32–41. [Google Scholar]
- MITECO. Anuario de Estadística Forestal 2019; Ministerio fpara la Transición Ecológica y el Reto Demográfico: Madrid, Spain, 2020. [Google Scholar]
- Mota, M.M.; Futai, K.; Vieira, P. Pine wilt disease and the pinewood nematode, Bursaphelenchus xylophilus. In Integrated Management of Fruit Crops Nematodes; Ciancio, A., Mukerji, K.G., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 253–274. ISBN 978-1-4020-9858-1. [Google Scholar]
- Zamora, P.; Rodríguez, V.; Renedo, F.; Sanz, A.V.; Domínguez, J.C.; Pérez-Escolar, G.; Miranda, J.; Álvarez, B.; González-Casas, A.; Mayor, E.; et al. First report of Bursaphelenchus xylophilus causing pine wilt disease on Pinus radiata in Spain. Plant Dis. 2015, 99, 1449. [Google Scholar] [CrossRef]
- Menéndez-Gutiérrez, M.; Alonso, M.; Jiménez, E.; Toval, G.; Mansilla, P.; Abelleira, A.; Abelleira-Sanmartín, A.; Díaz, R. Interspecific variation of constitutive chemical compounds in Pinus spp. xylem and susceptibility to pinewood nematode (Bursaphelenchus xylophilus). Eur. J. Plant Pathol. 2018, 150, 939–953. [Google Scholar] [CrossRef]
- De la Fuente, B.; Saura, S. Long-term projections of the natural expansion of the pine wood nematode in the Iberian Peninsula. Forests 2021, 12, 849. [Google Scholar] [CrossRef]
- Lawson, S.A.; Sathyapala, S. The risk of pine wilt disease to Australia and New Zealand. In Pine Wilt Disease: A Worldwide Threat to Forest Ecosystems; Mota, M.M., Vieira, P., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 41–58. [Google Scholar]
- Yano, M. Investigations on the cause of mortality in Nagasaki prefecture. Sanron-Koho 1913, 4, 1–14. [Google Scholar]
- Cheng, H.; Lin, M.; Li, W.; Fang, Z. The ocurrence of a pine wilting disease caused by a nematode found in Nanjing. For. Pest Dis. 1983, 4, 1–5. [Google Scholar]
- Yi, C.K.; Byun, B.H.; Park, J.D.; Yang, S.I.; Chang, K.H. First finding of the pine wood nematode, Bursaphelenchus xylophilus (Steiner et Buhrer) Nickle and its insect vector in Korea. Res. Rep. For. Res. Inst. (Seoul) 1989, 38, 141–149. [Google Scholar]
- Tzean, S.; Jan, S. The ocurrence of pinewood nematode, Bursaphelenchus xylophilus in Taiwan. In Proceedings of the 6th ROC Symposium of Electron Microscopy, 1985; pp. 38–39. [Google Scholar]
- Mota, M.; Braasch, H.; Bravo, M. First Report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1999, 1, 727–734. [Google Scholar] [CrossRef]
- Soliman, T.; Mourits, M.C.M.; van der Werf, W.; Hengeveld, G.M.; Robinet, C.; Lansink, A.G.J.M.O. Framework for modelling economic impacts of invasive species, applied to pine wood nematode in europe. PLoS ONE 2012, 7, e45505. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.; Cobacho Arcos, S.; Escuer, M.; Santiago Merino, R.; Esparrago, G.; Abelleira, A.; Navas, A. Incidence of the pinewood nematode Bursaphelenchus xylophilus Steiner & Buhrer, 1934 (Nickle, 1970) in Spain. Nematology 2011, 13, 755–757. [Google Scholar] [CrossRef]
- Abelleira, A.; Picoaga, A.; Mansilla, J.P.; Aguin, O. Detection of Bursaphelenchus xylophilus, Causal Agent of Pine Wilt Disease on Pinus pinaster in Northwestern Spain. Plant Dis. 2011, 95, 776. [Google Scholar] [CrossRef]
- Matsunaga, K.; Ohira, M.; Hirao, T.; Takahashi, M.; Fukatsu, E.; Kurita, M.; Kuramoto, N.; Togashi, K. The current state of breeding for Japanese pine resistance against pine wilt disease and planned gain realization by seed orchard establishment. In Proceedings of the IUFRO Seed Orchard Conference 2017, Balsta, Sweden, 4–6 September 2017; p. 79. [Google Scholar]
- Kurinobu, S. Current status of resistance breeding of Japanese pine species to pine wilt disease. For. Sci. Tech. 2008, 4, 51–57. [Google Scholar] [CrossRef]
- Toda, T.; Kurinobu, S. Genetic improvement in pine wilt disease resistance in Pinus thunbergii: The effectiveness of pre-screening with an artificial inoculation at the nursery. J. For. Res. 2001, 6, 197–201. [Google Scholar] [CrossRef]
- Carrasquinho, I.; Lisboa, A.; Inácio, M.L.; Gonçalves, E. Genetic variation in susceptibility to pine wilt disease of maritime pine (Pinus pinaster Aiton) half-sib families. Ann. For. Sci. 2018, 75, 85. [Google Scholar] [CrossRef] [Green Version]
- Menéndez-Gutiérrez, M.; Alonso, M.; Toval, G.; Díaz, R. Testing of selected Pinus pinaster half-sib families for tolerance to pinewood nematode (Bursaphelenchus xylophilus). Forestry 2018, 91, 38–48. [Google Scholar] [CrossRef]
- Sasse, J.; Elms, S.; Kube, P. Genetic resistance in Pinus radiata to defoliation by the pine aphid Essigella californica. Aust. For. 2009, 72, 25–31. [Google Scholar] [CrossRef]
- Ivković, M.; Baltunis, B.; Gapare, W.; Sasse, J.; Dutkowski, G.; Elms, S.; Wu, H. Breeding against Dothistroma needle blight of Radiata pine in Australia. Can. J. For. Res. 2010, 40, 1653–1660. [Google Scholar] [CrossRef]
- Suontama, M.; Li, Y.; Low, C.B.; Dungey, H.S. Genetic improvement of resistance to Cyclaneusma needle cast in Pinus radiata. Can. J. For. Res. 2019, 49, 128–133. [Google Scholar] [CrossRef]
- Burdon, R.D.; Li, Y. Genotype-Environment interaction involving site differences in expression of genetic variation along with genotypic rank changes: Simulations of economic significance. Tree Genet. Genomes 2019, 15, 2. [Google Scholar] [CrossRef]
- Li, Y.; Suontama, M.; Burdon, R.D.; Dungey, H.S. Genotype by environment interactions in forest tree breeding: Review of methodology and perspectives on research and application. Tree Genet. Genomes 2017, 13, 60. [Google Scholar] [CrossRef] [Green Version]
- Cortés, A.J.; Restrepo-Montoya, M.; Bedoya-Canas, L.E. Modern strategies to assess and breed forest tree adaptation to changing climate. Front. Plant Sci. 2020, 11, 1606. [Google Scholar] [CrossRef] [PubMed]
- Sniezko, R.A.; Koch, J. Breeding trees resistant to insects and diseases: Putting theory into application. Biol. Invasions 2017, 19, 3377–3400. [Google Scholar] [CrossRef]
- Hanawa, F.; Yamada, T.; Nakashima, T. Phytoalexins from Pinus strobus bark infected with pinewood nematode, Bursaphelenchus xylophilus. Phytochemistry 2001, 57, 223–228. [Google Scholar] [CrossRef]
- Pimentel, C.S.; Gonçalves, E.V.; Firmino, P.N.; Calvão, T.; Fonseca, L.; Abrantes, I.; Correia, O.; Máguas, C. differences in constitutive and inducible defences in pine species determining susceptibility to pinewood nematode. Plant Pathol. 2017, 66, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Simões, R.; Pimentel, C.; Ferreira-Dias, S.; Miranda, I.; Pereira, H. Phytochemical characterization of phloem in maritime pine and stone pine in three sites in Portugal. Heliyon 2021, 7, e06718. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, C.S.; Firmino, P.N.; Calvão, T.; Ayres, M.P.; Miranda, I.; Pereira, H. Pinewood nematode population growth in relation to pine phloem chemical composition. Plant Pathol. 2017, 66, 856–864. [Google Scholar] [CrossRef]
- Chow, P.S.; Landhausser, S.M. A Method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Fry, J. Estimation of genetic variances and covariances by restricted maximum likelihood using PROC MIXED. In Genetic Analysis of Complex Traits Using SAS; SAS Institute Inc.: Cary, NC, USA, 2004; pp. 11–34. [Google Scholar]
- Wright, J.W. Introduction to Forest Genetics, 1st ed.; Academic Press: Cambridge, MA, USA, 1976; ISBN 9780323148887. [Google Scholar]
- Gilmour, A.R.; Anderson, R.D.; Rae, A.L. The analysis of binomial data by a generalized linear mixed model. Biometrika 1985, 72, 593–599. [Google Scholar] [CrossRef]
- Nantongo, J.S.; Potts, B.M.; Fitzgerald, H.; Newman, J.; Elms, S.; Aurik, D.; Dungey, H.; O’Reilly-Wapstra, J.M. Quantitative genetic variation in bark stripping of Pinus radiata. Forests 2020, 11, 1356. [Google Scholar] [CrossRef]
- Yang, R. Likelihood-based analysis of genotype–environment interactions. Crop Sci. 2002, 42, 1434–1440. [Google Scholar] [CrossRef]
- De la Mata, R.; Zas, R. Transferring Atlantic maritime pine improved material to a region with marked mediterranean influence in inland NW Spain: A likelihood-based approach on spatially adjusted field data. Eur. J. For. Res. 2010, 129, 645–658. [Google Scholar] [CrossRef] [Green Version]
- Shelbourne, C.J.A. Genotype-Environment interaction: Its study and its implications in forest tree improvement. In Proceedings of the IUFRO Genetics and SABRAO Joint Symposium, Tokyo, Japan, 1972; pp. 1–28. [Google Scholar]
- Alfaro, R.I.; King, J.N.; VanAkker, L. Delivering sitka spruce with resistance against white pine weevil in British Columbia, Canada. For. Chron. 2013, 89, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Carson, M.; Carson, S.; Te Rinni, C. Successful varietal forestry with radiata pine in New Zealand. N. Z. J. For. Sci. 2015, 60, 8–11. [Google Scholar]
- Sniezko, R.; Smith, J.; Liu, J.-J.; Hamelin, R. Genetic resistance to fusiform rust in southern pines and white pine blister rust in white pines—A Contrasting tale of two rust pathosystems—Current status and future prospects. Forests 2014, 5, 2050–2083. [Google Scholar] [CrossRef]
- Toda, T. Studies on breeding for resistance to pine wilt disease in Pinus densiflora and P. thunbergia. Bull. For. Tree Breed. Inst. 2004, 20, 83–217. [Google Scholar]
- Xu, L.-Y.; Zhang, J.; Gao, J.-B.; Chen, X.-L.; Jiang, C.-W.; Hao, Y.-P. Study on the disease resistance of candidate clones in Pinus massonina to Bursaphelenchus xylophilus. China For. Sci. Techonol. 2012, 26, 27–30. [Google Scholar]
- Resolución de 3 de Junio de 2020, de La Dirección General de BiodiversidadBosques y Desertificación, por la que se Publican las Incorporaciones al Catálogo Nacional de Materiales de Base, para la Producción de Materiales Forestales de Reproducción de la Categoría “Cualificado” de la Especie Pinus Pinaster Ait, Situados en el Territorio de Galicia; Ministerio para la Transición Ecológica y el Reto Demográfico: Madrid, Spain, 2020; Available online: https://www.boe.es/diario_boe/txt.php?lang=en&id=BOE-A-2020-6076 (accessed on 8 February 2021).
- Burdon, R.D.; Carson, M.J.; Shelbourne, C.J.A. Achievements in forest tree genetic improvement in Australia and New Zealand 10: Pinus radiata in New Zealand. Aust. For. 2008, 71, 263–279. [Google Scholar] [CrossRef]
- Akiba, M.; Ishihara, M.; Sahashi, N.; Nakamura, K.; Ohira, M.; Toda, T. Virulence of Bursaphelenchus xylophilus isolated from naturally infested pine forests to five resistant families of Pinus thunbergii. Plant Dis. 2012, 96, 249–252. [Google Scholar] [CrossRef] [Green Version]
- Menéndez-Gutiérrez, M.; Villar, L.; Díaz, R. Virulence of seven pathogenic Bursaphelenchus xylophilus isolates in Pinus pinaster and Pinus radiata seedlings and its relation with multiplication. For. Pathol. 2021, 51, e12677. [Google Scholar] [CrossRef]
- Verrez, A.; Quiring, D.; Le Cocq, T.L.; Adams, G.; Park, Y.S. Genetically based resistance to the white pine weevil in jack pine and eastern white pine. For. Chron. 2010, 86, 775–779. [Google Scholar] [CrossRef] [Green Version]
- Kleinhentz, M.; Raffinz, A.; Jactel, H. Genetic parameters and gain expected from direct selection for resistance to Dioryctria sylvestrella Ratz. (Lepidoptera:Pyralidae) in Pinus pinaster Ait., using a full diallel mating design. For. Genet. 1998, 5, 147–154. [Google Scholar]
- Yamanobe, T. Relationships between morphological traits and resistance to pine wood nematode in two Japanese pines. Eur. J. Plant Pathol. 2009, 124, 543–552. [Google Scholar] [CrossRef]
- Menéndez-Gutiérrez, M.; Alonso, M.; Toval, G.; Díaz, R. Variation in pinewood nematode susceptibility among Pinus pinaster Ait. provenances from the iberian peninsula and france. Ann. For. Sci. 2017, 74, 76. [Google Scholar] [CrossRef] [Green Version]
- Iki, T.; Matsunaga, K.; Hirao, T.; Ohira, M.; Yamanobe, T.; Iwaizumi, M.G.; Miura, M.; Isoda, K.; Kurita, M.; Takahashi, M.; et al. Effects of temperature factors on resistance against pine wood nematodes in Pinus thunbergii, based on multiple location sites nematode inoculation tests. Forests 2020, 11, 922. [Google Scholar] [CrossRef]
- Matsunaga, K.; Iki, T.; Hirao, T.; Ohira, M.; Yamanobe, T.; Iwaizumi, M.G.; Miura, M.; Isoda, K.; Kurita, M.; Takahashi, M.; et al. Do seedlings derived from pinewood nematode-resistant Pinus thunbergii Parl. clones selected in southwestern region perform well in northern regions in Japan? Inferences from nursery inoculation tests. Forests 2020, 11, 955. [Google Scholar] [CrossRef]
- Ott, D.S.; Yanchuk, A.D.; Huber, D.P.W.; Wallin, K.F. Genetic variation of lodgepole pine, Pinus contorta var. latifolia, chemical and physical defenses that affect mountain pine beetle, Dendroctonus ponderosae, attack and tree mortality. J. Chem. Ecol. 2011, 37, 1002–1012. [Google Scholar] [CrossRef]
- Kuroda, K. Physiological incidences related to symptom development and wilting mechanism. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: Tokyo, Japan, 2008; pp. 204–222. ISBN 978-4-431-75655-2. [Google Scholar]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A.; Amendola, V. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In Phytochemistry: Advances in Research; Trivandrum: Kerala, India, 2006; Volume 661, ISBN 8130800349. [Google Scholar]
- Ayres, M.P.; Clausen, T.P.; MacLean, S.F.; Redman, A.M.; Relchardt, P.B. Diversity of structure and antiherbivore activity in condensed tannins. Ecology 1997, 78, 1696–1712. [Google Scholar] [CrossRef]
- Ohyama, N.; Shiraishi, S.; Takagi, T. Characteristics in the graftings of the resistant pine against wood nematodes. For. Tree Breed. 1986, 140, 17–21. [Google Scholar]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef]
- Fukuda, K. Physiological process of the symptom development and resistance mechanism in pine wilt disease. J. For. Res. 1997, 2, 171–181. [Google Scholar] [CrossRef]
- Dordas, C. Role of nutrients in controlling plant diseases in sustainable agriculture. A Review. Agron. Sus. Dev. 2008, 28, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Maehara, N.; Aikawa, T.; Kanzaki, N. Inoculation of several Bursaphelenchus xylophilus group nematodes into adult trees of Pinus thunbergii and their survival in the trees. For. Pathol. 2011, 41, 477–481. [Google Scholar] [CrossRef]
- Kanzaki, N.; Aikawa, T.; Maehara, N.; Ichihara, Y. An inoculation experiment of Japanese Bursaphelenchus nematodes on Japanese black and red pine, Pinus thunbergii and P. densiflora. J. For. Res. 2011, 16, 325–330. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).