The Contribution of Roots, Mycorrhizal Hyphae, and Soil Free-Living Microbes to Soil Respiration and Its Temperature Sensitivity in a Larch Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Chamber Experimental Design
- Type-A = total soil respiration (Rsoil).
- Type-A − Type-B = root respiration (Rroot)
- Type-B − Type-C = mycorrhizal respiration (Rmyc)
- Type-C = soil organic matter (SOM) respiration (Rsom).
2.3. Respiration Manual Measurement and Calculations
3. Results
3.1. Soil Environmental Factors and Respiration
3.2. Response of Respiration to Soil Temperature and Moisture
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bond-Lamberty, B.; Thomson, A. A global database of soil respiration data. Biogeosciences 2010, 7, 1915–1926. [Google Scholar] [CrossRef] [Green Version]
- Bond-Lamberty, B.; Thomson, A. Temperature-associated increases in the global soil respiration record. Nature 2010, 464, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Epron, D.; Dantec, V.L.; Dufrene, E.; Granier, A. Seasonal dynamics of soil carbon dioxide efflux and simulated rhizosphere respiration in a beech forest. Tree Physiol. 2001, 21, 145–152. [Google Scholar] [CrossRef]
- Savage, K.; Davidson, E.A.; Tang, J. Diel patterns of autotrophic and heterotrophic respiration among phenological stages. Glob. Chang. Biol. 2013, 19, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Janssens, I.A.; Pilegaard, K. Large seasonal changes in Q10 of soil respiration in a beech forest. Glob. Chang. Biol. 2003, 9, 911–918. [Google Scholar]
- Davidson, E.A.; Belk, E.; Boone, R.D. Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob. Chang. Biol. 1998, 4, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Boone, R.D.; Nadelhoffer, K.J.; Canary, J.D.; Kaye, J.P. Roots exert a strong influence on the temperature sensitivity of soil respiration. Nature 1998, 396, 570–572. [Google Scholar]
- Zhou, T.; Shi, P.J.; Hui, D.F.; Luo, Y.Q. Global pattern of temperature sensitivity of soil heterotrophic respiration Q10 and its implications for carbon-climate feedback. J. Geophys. Res. Biogeosci. 2009, 114, 9. [Google Scholar] [CrossRef] [Green Version]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.K.; Hodge, A.; Fitter, A.H.; Atkin, O.K. Mycorrhizal respiration: Implications for global scaling relationships. Trends Plant Sci. 2008, 13, 583–588. [Google Scholar] [CrossRef]
- Franklin, O.; Näsholm, T.; Högberg, P.; Högberg, M.N. Forests trapped in nitrogen limitation—An ecological market perspective on ectomycorrhizal symbiosis. New Phytol. 2014, 203, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Ficken, C.D.; Warren, J.M. The carbon economy of drought: Comparing respiration responses of roots, mycorrhizal fungi, and free-living microbes to an extreme dry-rewet cycle. Plant Soil 2019, 435, 407–422. [Google Scholar] [CrossRef]
- Talbot, J.M.; Allison, S.D.; Treseder, K.K. Decomposers in disguise: Mycorrhizal fungi as regulators of soil C dynamics in ecosystems under global change. Funct. Ecol. 2008, 22, 955–963. [Google Scholar] [CrossRef] [Green Version]
- Wallander, H.; Ekblad, A.; Godbold, D.L.; Johnson, D.; Bahr, A.; Baldrian, P.; Björk, R.G.; Kieliszewska-Rokicka, B.; Kjøller, R.; Kraigher, H.; et al. Evaluation of methods to estimate production, biomass and turnover of ectomycorrhizal mycelium in forests soils—A review. Soil Biol. Biochem. 2013, 57, 1034–1042. [Google Scholar] [CrossRef]
- Heinemeyer, A.; Ineson, P.; Ostle, N.; Fitter, A.H. Respiration of the external mycelium in the arbuscular mycorrhizal symbiosis shows strong dependence on recent photosynthates and acclimation to temperature. New Phytol. 2006, 171, 159–170. [Google Scholar] [CrossRef]
- Moyano, F.E.; Kutsch, W.L.; Schulze, E.D. Response of mycorrhizal, rhizosphere and soil basal respiration to temperature and photosynthesis in a barley field. Soil Biol. Biochem. 2007, 39, 843–853. [Google Scholar] [CrossRef]
- Heinemeyer, A.; Hartley, I.P.; Evans, S.P.; De la Fuente, J.A.C.; Ineson, P. Forest soil CO2 flux: Uncovering the contribution and environmental responses of ectomycorrhizas. Glob. Chang. Biol. 2007, 13, 1786–1797. [Google Scholar] [CrossRef]
- Heinemeyer, A.; Wilkinson, M.; Vargas, R.; Subke, J.A.; Casella, E.; Morison, J.I.L.; Ineson, P. Exploring the “overflow tap” theory: Linking forest soil CO2 fluxes and individual mycorrhizosphere components to photosynthesis. Biogeosciences 2012, 9, 79–95. [Google Scholar] [CrossRef] [Green Version]
- Mamet, S.D.; Brown, C.D.; Trant, A.J.; Laroque, C.P. Shifting global Larix distributions: Northern expansion and southern retraction as species distributions: Northern expansion and southern retraction as species respond to changing climate. J. Biogeogr. 2019, 46, 30–44. [Google Scholar] [CrossRef] [Green Version]
- Ozeki, M.; Kuribayashi, M. Tree growth of old man-made larch stands in lizuna Heights, Ngano Prefecture, central Japan. Bull. Nagano Environ. Conserv. Res. Inst. 2019, 15, 45–49, (In Japanese with English summary). [Google Scholar]
- Lloyd, J.; Taylor, J.A. On the temperature dependence of soil respiration. Funct. Ecol. 1994, 8, 315–323. [Google Scholar] [CrossRef]
- Liang, N.; Hirano, T.; Zheng, Z.M.; Tang, J.; Fujimura, Y. Soil CO₂ efflux of a larch forest in northern Japan. Biogeosciences 2010, 7, 3447–3457. [Google Scholar] [CrossRef] [Green Version]
- Teramoto, M.; Liang, N.; Takahashi, Y.; Zeng, J.; Saigusa, N.; Ide, R.; Zhao, X. Enhanced understory carbon flux components and robustness of net CO2 exchange after thinning in a larch forest in central Japan. Agric. For. Meteorol. 2019, 274, 106–117. [Google Scholar] [CrossRef]
- Harmon, M.E.; Bond-Lamberty, B.; Tang, J.W.; Vargas, R. Heterotrophic respiration in disturbed forests: A review with examples from North America. J. Geophys. Res. Biogeosci. 2011, 116, 1–17. [Google Scholar] [CrossRef]
- Kominami, Y.; Jomura, M.; Ataka, M.; Tamai, K.; Miyama, T.; Dannoura, M.; Makita, N.; Yoshimura, K. Heterotrophic respiration causes seasonal hysteresis in soil respiration in a warm-temperate forest. J. For. Res. 2012, 17, 296–304. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Wang, C.; Gower, S.T. A global relationship between the heterotrophic and autotrophic components of soil respiration? Glob. Chang. Biol. 2004, 10, 1756–1766. [Google Scholar] [CrossRef]
- Vargas, R.; Baldocchi, D.D.; Allen, M.F.; Bahn, M.; Black, T.A.; Collins, S.L.; Yuste, J.C.; Hirano, T.; Jassal, R.S.; Pumpanen, J.; et al. Looking deeper into the soil: Biophysical controls and seasonal lags of soil CO2 production and efflux. Ecol. Appl. 2010, 20, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Makita, N.; Kosugi, Y.; Sakabe, A.; Kanazawa, A.; Ohkubo, S.; Tani, M. Seasonal and diurnal patterns of soil respiration in an evergreen coniferous forest: Evidence from six years of observation with automatic chambers. PLoS ONE 2018, 13, e0192622. [Google Scholar]
- Newman, G.S.; Arthur, M.A.; Muller, R.N. Above- and belowground net primary production in a temperate mixed deciduous forest. Ecosystems 2006, 9, 317–329. [Google Scholar] [CrossRef]
- Moyano, F.E.; Kutsch, L.W.; Rebmann, C. Soil respiration fluxes in relation to photosynthetic activity in broad-leaf and needle-leaf forest stands. Agric. For. Meteorol. 2008, 148, 135–143. [Google Scholar] [CrossRef]
- Ekblad, A.; Wallander, H.; Godbold, D.L.; Cruz, C.; Johnson, D.; Baldrian, P.; Bjork, R.G.; Epron, D.; Kieliszewska-Rokicka, B.; Kjoller, R.; et al. The production and turnover of extramatrical mycelium of ectomycorrhizal fungi in forest soils: Role in carbon cycling. Plant Soil 2013, 366, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Hamdi, S.; Moyano, F.; Sall, S.; Bernoux, M.; Chevallier, T. Synthesis analysis of the temperature sensitivity of soil respiration from laboratory studies in relation to incubation methods and soil conditions. Soil Biol. Biochem. 2013, 58, 115–126. [Google Scholar] [CrossRef]
- Raich, J.W.; Schlesinger, W.H. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus 1992, 44, 81–99. [Google Scholar] [CrossRef] [Green Version]
- Liang, N.; Nakadai, T.; Hirano, T.; Qu, L.; Koike, T.; Fujinuma, Y.; Inoue, G. In situ comparison of four approaches to estimating soil CO2 efflux in a northern larch (Larix kaempferi Sarg.) forest. Agr. For. Meteorol. 2004, 123, 97–117. [Google Scholar] [CrossRef]
- Neumann, J.; Matzner, E. Contribution of newly grown extrametrical ectomycorrhizal mycelium and fine roots to soil respiration in a young Norway spruce site. Plant Soil 2014, 378, 73–82. [Google Scholar] [CrossRef]
- Reichstein, M.; Janssens, I.A. Semi-empirical modeling of the response of soil respiration to environmental factors in laboratory and field conditions. In Soil Carbon Dynamics: An Integrated Methodology; Kutsch, W.L., Michael, B., Heinemeyer, A., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 207–220. [Google Scholar]
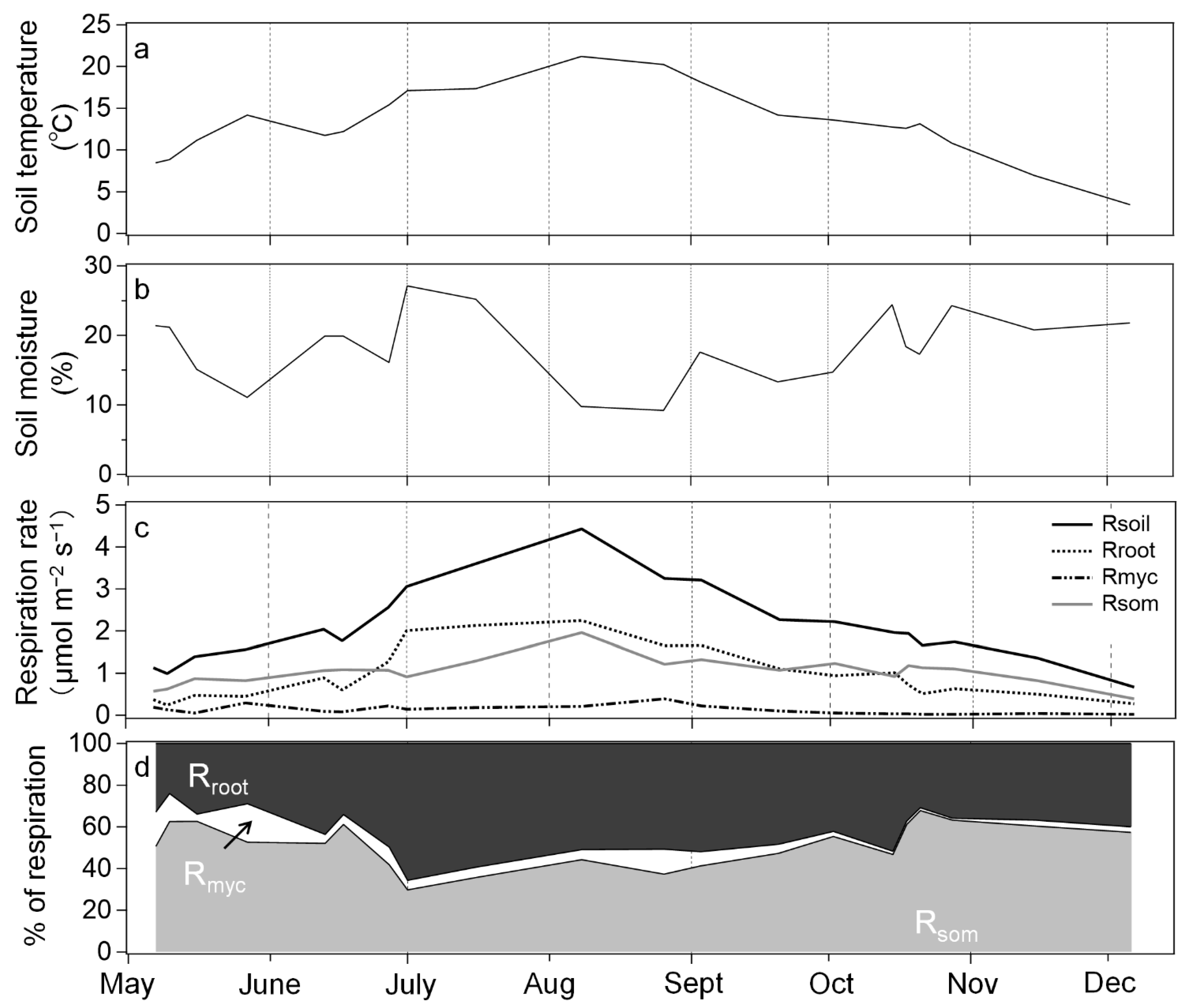

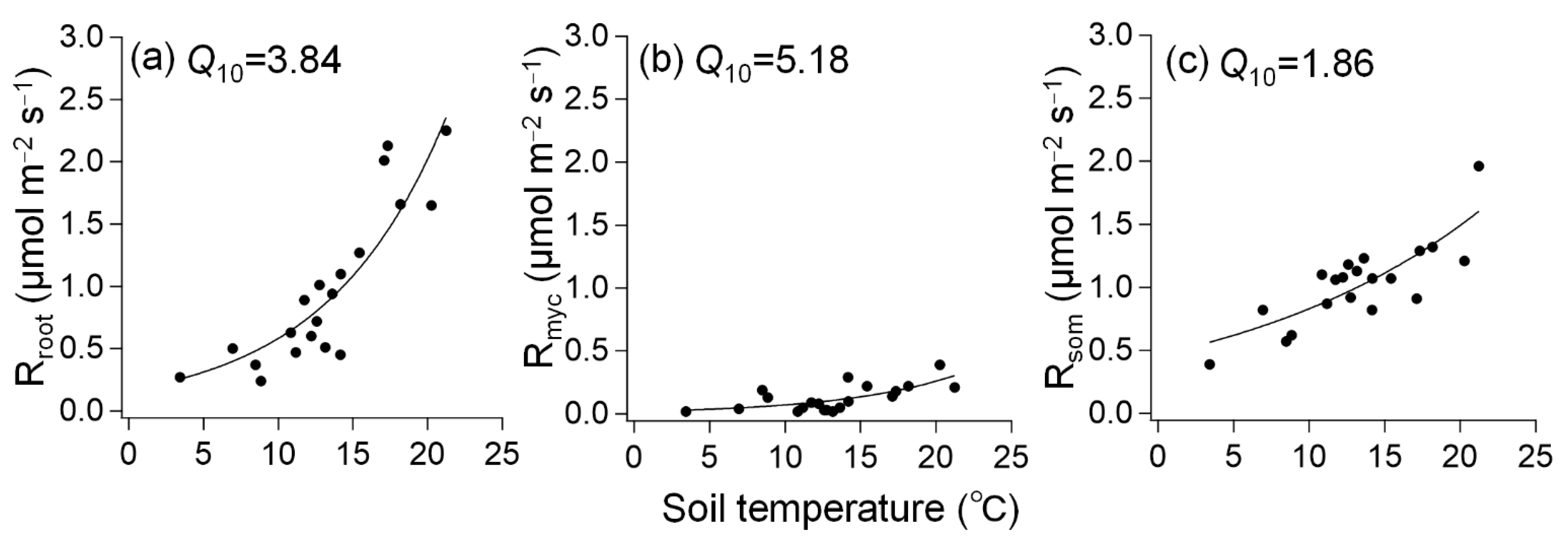
| Rsoil | Rroot | Rmyc | Rsom | |
|---|---|---|---|---|
| α | 0.5210 | 0.1346 | 0.0086 | 0.4349 |
| β | 0.0998 | 0.1345 | 0.1644 | 0.0618 |
| R2 | 0.89 | 0.78 | 0.55 | 0.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makita, N.; Fujimoto, R.; Tamura, A. The Contribution of Roots, Mycorrhizal Hyphae, and Soil Free-Living Microbes to Soil Respiration and Its Temperature Sensitivity in a Larch Forest. Forests 2021, 12, 1410. https://doi.org/10.3390/f12101410
Makita N, Fujimoto R, Tamura A. The Contribution of Roots, Mycorrhizal Hyphae, and Soil Free-Living Microbes to Soil Respiration and Its Temperature Sensitivity in a Larch Forest. Forests. 2021; 12(10):1410. https://doi.org/10.3390/f12101410
Chicago/Turabian StyleMakita, Naoki, Roma Fujimoto, and Azusa Tamura. 2021. "The Contribution of Roots, Mycorrhizal Hyphae, and Soil Free-Living Microbes to Soil Respiration and Its Temperature Sensitivity in a Larch Forest" Forests 12, no. 10: 1410. https://doi.org/10.3390/f12101410
APA StyleMakita, N., Fujimoto, R., & Tamura, A. (2021). The Contribution of Roots, Mycorrhizal Hyphae, and Soil Free-Living Microbes to Soil Respiration and Its Temperature Sensitivity in a Larch Forest. Forests, 12(10), 1410. https://doi.org/10.3390/f12101410





