Wood Pastures: A Transitional Habitat between Forests and Pastures for Dung Beetle Assemblages
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.1.1. Cserépfalu
2.1.2. Hollókő
2.1.3. Balatonakali-Dörgicse
2.2. Sampling Design and Dung Beetle Trapping
2.3. Data Analysis
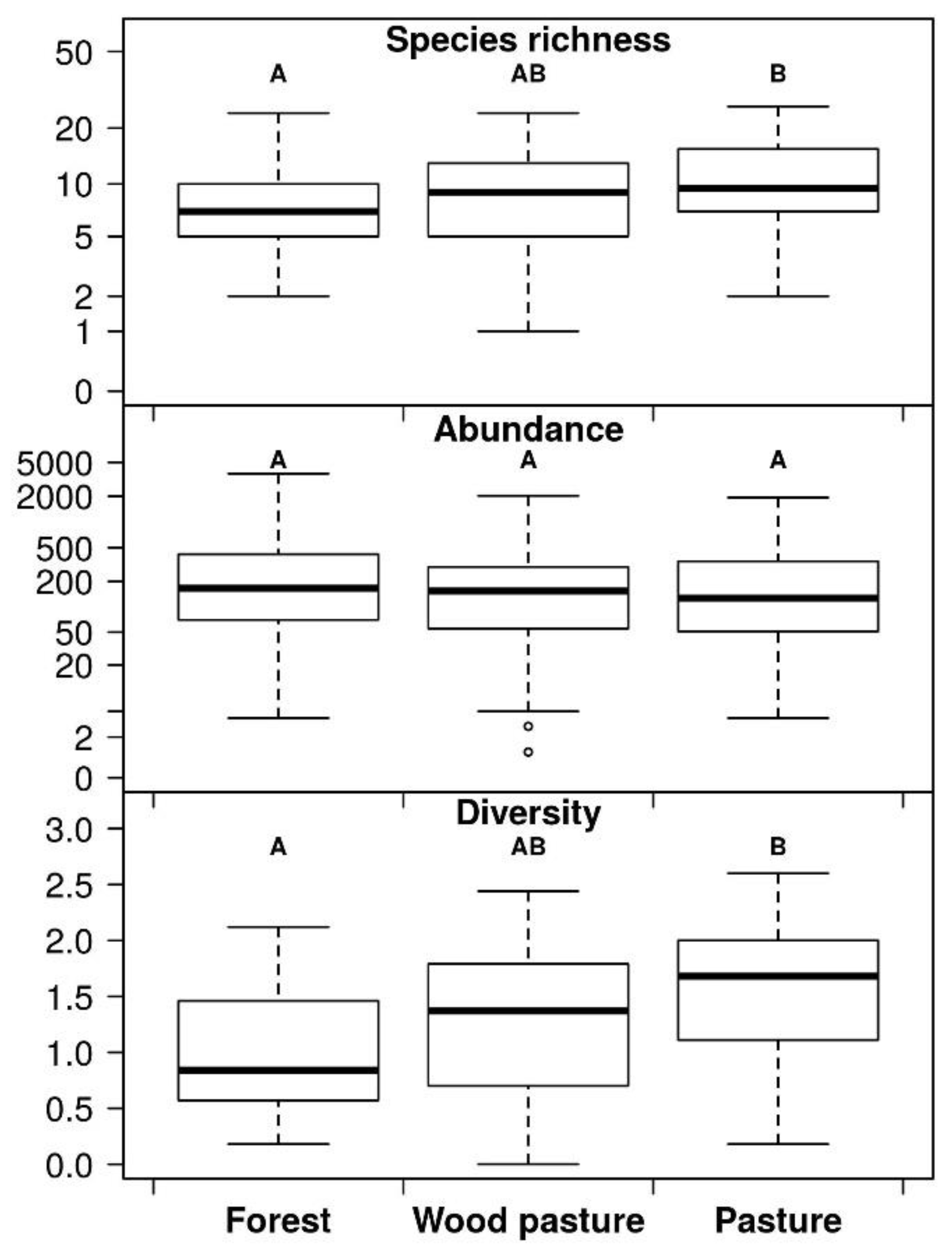
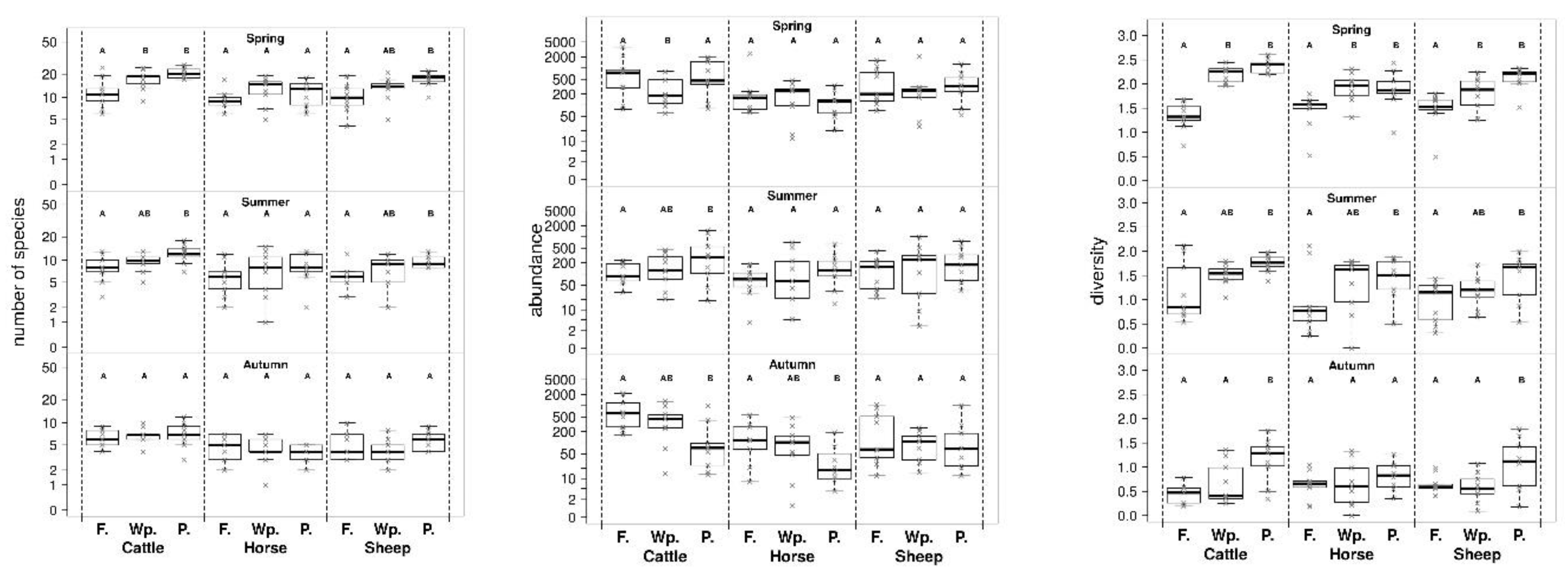
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Forest | Wood Pasture | Pasture | Total | % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbr. | B | H | C | ∑ | B | H | C | ∑ | B | H | C | ∑ | |||
| Geotrupidae | |||||||||||||||
| Anoplotrupes stercorosus (Scriba, 1791) | Ano_ste | 1 | 1671 | 117 | 1789 | 0 | 13 | 90 | 103 | 0 | 3 | 2 | 5 | 1897 | 2.43 |
| Geotrupes spiniger (Marsham, 1802) | Geo_spi | 29 | 9 | 9 | 47 | 18 | 1 | 13 | 32 | 23 | 6 | 16 | 45 | 124 | 0.16 |
| Trypocopris vernalis (Linnaeus, 1758) | Try_ver | 1534 | 156 | 914 | 2604 | 1741 | 148 | 709 | 2598 | 345 | 21 | 19 | 385 | 5587 | 7.15 |
| Scarabaeinae | |||||||||||||||
| Caccobius schreberi (Linnaeus, 1767) | Cac_sch | 0 | 0 | 0 | 0 | 442 | 10 | 0 | 452 | 685 | 231 | 7 | 923 | 1375 | 1.76 |
| Copris lunaris (Linnaeus, 1758) | Cop_lun | 11 | 1 | 15 | 27 | 14 | 1 | 49 | 64 | 33 | 12 | 74 | 119 | 210 | 0.27 |
| Euoniticellus fulvus (Goeze, 1777) | Euo_ful | 0 | 0 | 0 | 0 | 26 | 6 | 46 | 78 | 73 | 163 | 274 | 510 | 588 | 0.75 |
| Euonthophagus amyntas (Olivier, 1789) | Euo_amy | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 4 | 11 | 0 | 0 | 11 | 15 | 0.02 |
| Onthophagus coenobita (Herbst, 1783) | Ont_coe | 321 | 294 | 1159 | 1774 | 31 | 326 | 136 | 493 | 11 | 78 | 29 | 118 | 2385 | 3.05 |
| Onthophagus fracticornis (Preyssler, 1790) | Ont_fra | 15 | 20 | 1451 | 1486 | 176 | 224 | 470 | 870 | 288 | 103 | 836 | 1227 | 3583 | 4.59 |
| Onthophagus furcatus (Fabricius, 1781) | Ont_fur | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 4 | 4 | 7 | 0.01 |
| Onthophagus grossepunctatus Reitter, 1905 | Ont_gro | 226 | 0 | 157 | 383 | 2301 | 6 | 799 | 3106 | 1504 | 281 | 564 | 2349 | 5838 | 7.47 |
| Onthophagus illyricus (Scopoli, 1763) | Ont_ill | 11 | 2 | 41 | 54 | 153 | 70 | 164 | 387 | 372 | 1309 | 767 | 2448 | 2889 | 3.70 |
| Onthophagus joannae Goljan, 1953 | Ont_joa | 0 | 0 | 0 | 0 | 0 | 10 | 41 | 51 | 0 | 0 | 10 | 10 | 61 | 0.08 |
| Onthophagus lemur (Fabricius, 1781) | Ont_lem | 20 | 0 | 10 | 30 | 207 | 0 | 2 | 209 | 111 | 1 | 3 | 115 | 354 | 0.45 |
| Onthophagus medius (Kugelann, 1792) | Ont_med | 0 | 2 | 0 | 2 | 33 | 12 | 0 | 45 | 460 | 1190 | 1 | 1651 | 1698 | 2.17 |
| Onthophagus nuchicornis (Linnaeus, 1758) | Ont_nuc | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0.00 |
| Onthophagus ovatus (Linnaeus, 1767) | Ont_ova | 2 | 10 | 3 | 15 | 63 | 189 | 357 | 609 | 96 | 2614 | 617 | 3327 | 3951 | 5.06 |
| Onthophagus ruficapillus Brulle, 1832 | Ont_ruf | 1 | 0 | 2 | 3 | 403 | 6 | 15 | 424 | 1436 | 1049 | 160 | 2645 | 3072 | 3.93 |
| Onthophagus semicornis (Panzer, 1798) | Ont_sem | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 2 | 0.00 |
| Onthophagus taurus (Schreber, 1759) | Ont_tau | 1 | 1 | 2 | 4 | 49 | 17 | 9 | 75 | 179 | 1078 | 98 | 1355 | 1434 | 1.84 |
| Onthophagus vacca (Linnaeus, 1767) | Ont_vac | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 7 | 1 | 17 | 17 | 0.02 |
| Onthophagus verticicornis (Laicharting, 1781) | Ont_ver | 1003 | 101 | 5945 | 7049 | 89 | 81 | 1111 | 1281 | 95 | 448 | 475 | 1018 | 9348 | 11.96 |
| Onthophagus vitulus (Fabricius, 1777) | Ont_vit | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0.00 |
| Sisyphus schaefferi (Linnaeus, 1758) | Sis_sch | 751 | 8 | 796 | 1555 | 713 | 49 | 1943 | 2705 | 943 | 642 | 535 | 2120 | 6380 | 8.17 |
| Aphodiinae | |||||||||||||||
| Acanthobodilus immundus (Creutzer, 1799) | Aca_imm | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 2 | 0 | 27 | 0 | 27 | 30 | 0.04 |
| Acrossus depressus (Kugelann, 1792) | Acr_dep | 1 | 4 | 20 | 25 | 0 | 1 | 1 | 2 | 0 | 0 | 3 | 3 | 30 | 0.04 |
| Acrossus luridus (Fabricius, 1775) | Acr_lur | 0 | 1 | 8 | 9 | 7 | 88 | 23 | 118 | 7 | 276 | 39 | 322 | 449 | 0.57 |
| Acrossus rufipes (Linnaeus, 1758) | Acr_ruf | 0 | 2 | 27 | 29 | 0 | 0 | 14 | 14 | 0 | 0 | 7 | 7 | 50 | 0.06 |
| Aphodius fimetarius (Linnaeus, 1758) | Aph_fim | 0 | 0 | 4 | 4 | 0 | 2 | 5 | 7 | 1 | 1 | 20 | 22 | 33 | 0.04 |
| Bodiloides ictericus (Laicharting, 1781) | Bod_ict | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 7 | 7 | 0.01 |
| Bodilopsis rufa (Moll, 1782) | Bod_ruf | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 0.00 |
| Bodilopsis sordida (Fabricius, 1775) | Bod_sor | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 3 | 0 | 3 | 5 | 0.01 |
| Bodilus lugens (Creutzer, 1799) | Bod_lug | 0 | 0 | 0 | 0 | 112 | 0 | 0 | 112 | 9 | 2 | 0 | 11 | 123 | 0.16 |
| Calamosternus granarius (Linnaeus, 1767) | Cal_gra | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 3 | 0.00 |
| Chilothorax distinctus (O. F. Muller, 1776) | Chi_dis | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 19 | 3 | 2 | 24 | 25 | 0.03 |
| Chilothorax paykulli (Bedel, 1908) | Chi_pay | 0 | 0 | 44 | 44 | 1 | 0 | 0 | 1 | 3 | 0 | 0 | 3 | 48 | 0.06 |
| Colobopterus erraticus (Linnaeus, 1758) | Col_err | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 6 | 81 | 2 | 89 | 91 | 0.12 |
| Coprimorphus scrutator (Herbst, 1789) | Cop_scr | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0.00 |
| Esymus pusillus (Herbst, 1789) | Esy_pus | 3 | 29 | 22 | 54 | 6 | 36 | 69 | 111 | 11 | 174 | 99 | 284 | 449 | 0.57 |
| Eudolus quadriguttatus (Herbst, 1783) | Euo_qua | 0 | 0 | 3 | 3 | 4 | 0 | 5 | 9 | 22 | 0 | 10 | 32 | 44 | 0.06 |
| Euorodalus paracoenosus (Balthasar and Hrubant, 1960) | Euo_par | 2 | 0 | 20 | 22 | 28 | 0 | 49 | 77 | 29 | 13 | 17 | 59 | 158 | 0.20 |
| Eupleurus subterraneus (Linnaeus, 1758) | Eup_sub | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0.00 |
| Limarus maculatus (Sturm, 1800) | Lim_mac | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.00 |
| Melinopterus consputus (Creutzer, 1799) | Mel_con | 0 | 4 | 2 | 6 | 9 | 2 | 1 | 12 | 54 | 1715 | 446 | 2215 | 2233 | 2.86 |
| Melinopterus prodromus (Brahm, 1790) | Mel_pro | 31 | 22 | 3 | 56 | 60 | 90 | 13 | 163 | 301 | 139 | 23 | 463 | 682 | 0.87 |
| Melinopterus pubescens (Sturm, 1800) | Mel_pub | 0 | 280 | 1 | 281 | 0 | 9 | 0 | 9 | 0 | 0 | 0 | 0 | 290 | 0.37 |
| Nimbus obliteratus (Panzer, 1823) | Nim_obl | 1869 | 2133 | 6801 | 10,803 | 1082 | 3681 | 849 | 5612 | 66 | 54 | 5 | 125 | 16,540 | 21.17 |
| Nobius serotinus (Panzer, 1799) | Nob_ser | 0 | 3 | 0 | 3 | 0 | 1 | 0 | 1 | 0 | 155 | 10 | 165 | 169 | 0.22 |
| Otophorus haemorrhoidalis (Linnaeus, 1758) | Oto_hae | 0 | 0 | 1 | 1 | 0 | 0 | 4 | 4 | 1 | 59 | 9 | 69 | 74 | 0.09 |
| Oxyomus sylvestris (Scopoli, 1763) | Oxy_syl | 6 | 1 | 7 | 14 | 9 | 0 | 4 | 13 | 1 | 3 | 1 | 5 | 32 | 0.04 |
| Phalacronothus biguttatus (Germar, 1824) | Pha_big | 1 | 0 | 0 | 1 | 1 | 0 | 3 | 4 | 10 | 1 | 0 | 11 | 16 | 0.02 |
| Plagiogonus arenarius (Olivier, 1789) | Pla_are | 272 | 70 | 597 | 939 | 275 | 3 | 300 | 578 | 37 | 2 | 74 | 113 | 1630 | 2.09 |
| Planolinus fasciatus (Olivier, 1789) | Pla_fas | 554 | 105 | 198 | 857 | 1 | 0 | 7 | 8 | 0 | 0 | 0 | 0 | 865 | 1.11 |
| Rhodaphodius foetens (Fabricius, 1787) | Rho_foe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0.00 |
| Sigorus porcus (Fabricius, 1792) | Sig_por | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 3 | 0 | 0 | 2 | 2 | 5 | 0.01 |
| Teuchestes fossor (Linnaeus, 1758) | Teu_fos | 0 | 1 | 1 | 2 | 0 | 1 | 0 | 1 | 0 | 8 | 0 | 8 | 11 | 0.01 |
| Volinus sticticus (Panzer, 1798) | Vol_sti | 49 | 1593 | 1293 | 2935 | 0 | 27 | 242 | 269 | 0 | 10 | 1 | 11 | 3215 | 4.11 |
| Abundance | 6715 | 6525 | 19,677 | 32,917 | 8063 | 5114 | 7547 | 20,724 | 7252 | 11,972 | 5266 | 24,490 | 78,131 | 100.00 | |
| Species number | 25 | 28 | 35 | 41 | 34 | 32 | 34 | 47 | 35 | 41 | 42 | 53 | 57 | ||
| Contrast | Adjusted p-Value | |||||
|---|---|---|---|---|---|---|
| Abundance | Number of Species | Diversity | ||||
| Cattle | Spring | Forest | Wood pasture | 0.0037 ** | 0.0124 * | <0.0001 *** |
| Forest | Pasture | 0.9996 | 0.0001 *** | <0.0001 *** | ||
| Wood pasture | Pasture | 0.0053 ** | 0.3134 | 0.2063 | ||
| Summer | Forest | Wood pasture | 0.7217 | 0.6062 | 0.1177 | |
| Forest | Pasture | 0.0083 ** | 0.0123 * | 0.0055 ** | ||
| Wood pasture | Pasture | 0.0701 | 0.1337 | 0.3470 | ||
| Autumn | Forest | Wood pasture | 0.4623 | 0.8890 | 0.4605 | |
| Forest | Pasture | 0.0067 ** | 0.5829 | 0.0024 ** | ||
| Wood pasture | Pasture | 0.1523 | 0.8565 | 0.0380 * | ||
| Horse | Spring | Forest | Wood pasture | 0.2544 | 0.0764 | 0.0106 * |
| Forest | Pasture | 0.0995 | 0.1767 | 0.0214 * | ||
| Wood pasture | Pasture | 0.8438 | 0.9165 | 0.9486 | ||
| Summer | Forest | Wood pasture | 0.8803 | 0.4303 | 0.1982 | |
| Forest | Pasture | 0.2188 | 0.1953 | 0.0443 * | ||
| Wood pasture | Pasture | 0.5154 | 0.8739 | 0.7153 | ||
| Autumn | Forest | Wood pasture | 0.8827 | 0.9741 | 0.9830 | |
| Forest | Pasture | 0.0344 * | 0.6473 | 0.5260 | ||
| Wood pasture | Pasture | 0.1058 | 0.7753 | 0.6300 | ||
| Sheep | Spring | Forest | Wood pasture | 0.8016 | 0.1234 | 0.0473 * |
| Forest | Pasture | 0.9111 | 0.0004 *** | 0.0001 *** | ||
| Wood pasture | Pasture | 0.9834 | 0.1358 | 0.0537 | ||
| Summer | Forest | Wood pasture | 0.4881 | 0.4314 | 0.3512 | |
| Forest | Pasture | 0.4728 | 0.0420 * | 0.0166 * | ||
| Wood pasture | Pasture | 0.9993 | 0.4548 | 0.2640 | ||
| Autumn | Forest | Wood pasture | 0.0843 | 0.5931 | 0.9162 | |
| Forest | Pasture | 0.4491 | 0.7658 | 0.0278 * | ||
| Wood pasture | Pasture | 0.6574 | 0.2209 | 0.0114 * | ||
References
- Berendes, D.M.; Yang, P.J.; Lai, A.; Hu, D.; Brown, J. Estimation of global recoverable human and animal faecal biomass. Nat. Sustain. 2018, 1, 679–685. [Google Scholar] [CrossRef]
- Hanski, I.; Cambefort, Y. Dung Beetle Ecology; Princeton University Press: Princeton, NJ, USA, 1991; ISBN 978-0-691-08739-9. [Google Scholar]
- Nichols, E.; Spector, S.; Louzada, J.; Larsen, T.; Amezquita, S.; Favila, M. Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol. Conserv. 2008, 141, 1461–1474. [Google Scholar] [CrossRef]
- Holter, P. Herbivore dung as food for dung beetles: Elementary coprology for entomologists. Ecol. Entomol. 2016, 41, 367–377. [Google Scholar] [CrossRef]
- Tixier, T.; Bloor, J.M.G.; Lumaret, J.-P. Species-specific effects of dung beetle abundance on dung removal and leaf litter decomposition. Acta Oecol. 2015, 69, 31–34. [Google Scholar] [CrossRef]
- Slade, E.M.; Roslin, T.; Santalahti, M.; Bell, T. Disentangling the ‘brown world’ faecal-detritus interaction web: Dung beetle effects on soil microbial properties. Oikos 2016, 125, 629–635. [Google Scholar] [CrossRef]
- Bang, H.S.; Lee, J.-H.; Kwon, O.S.; Na, Y.E.; Jang, Y.S.; Kim, W.H. Effects of paracoprid dung beetles (Coleoptera: Scarabaeidae) on the growth of pasture herbage and on the underlying soil. Appl. Soil Ecol. 2005, 29, 165–171. [Google Scholar] [CrossRef]
- Brown, J.; Scholtz, C.H.; Janeau, J.-L.; Grellier, S.; Podwojewski, P. Dung beetles (Coleoptera: Scarabaeidae) can improve soil hydrological properties. Appl. Soil Ecol. 2010, 46, 9–16. [Google Scholar] [CrossRef]
- Ridsdill-Smith, T.J.; Edwards, P.B. Biological Control: Ecosystem Functions Provided by Dung Beetles. In Ecology and Evolution of Dung Beetles; Simmons, L.E., Ridsdill-Smith, T.J., Eds.; Wiley-Blackwell: Chichester, UK, 2011; pp. 245–266. ISBN 978-1-4443-4200-0. [Google Scholar]
- D’Hondt, B.; Bossuyt, B.; Hoffmann, M.; Bonte, D. Dung beetles as secondary seed dispersers in a temperate grassland. Basic Appl. Ecol. 2008, 9, 542–549. [Google Scholar] [CrossRef]
- Losey, J.E.; Vaughan, M. The Economic Value of Ecological Services Provided by Insects. BioScience 2006, 56, 311–323. [Google Scholar] [CrossRef]
- Beynon, S.A.; Wainwright, W.; Christie, M. The application of an ecosystem services framework to estimate the economic value of dung beetles to the U.K. cattle industry. Ecol. Entomol. 2015, 40, 124–135. [Google Scholar] [CrossRef]
- Tonelli, M.; Verdú, J.R.; Morelli, F.; Zunino, M. Dung beetles: Functional identity, not functional diversity, accounts for ecological process disruption caused by the use of veterinary medical products. J. Insect Conserv. 2020, 24, 643–654. [Google Scholar] [CrossRef]
- Verdu, J.R.; Lobo, J.M.; Sánchez-Piñero, F.; Gallego, B.; Numa, C.; Lumaret, J.-P.; Cortez, V.; Ortiz, A.J.; Tonelli, M.; García-Teba, J.P.; et al. Ivermectin residues disrupt dung beetle diversity, soil properties and ecosystem functioning: An interdisciplinary field study. Sci. Total Environ. 2018, 618, 219–228. [Google Scholar] [CrossRef]
- Frank, K.; Hülsmann, M.; Assmann, T.; Schmitt, T.; Blüthgen, N. Land use affects dung beetle communities and their ecosystem service in forests and grasslands. Agric. Ecosyst. Environ. 2017, 243, 114–122. [Google Scholar] [CrossRef]
- Nichols, E.; Larsen, T.; Spector, S.; Davis, A.L.; Escobar, F.; Favila, M.; Vulinec, K. Global dung beetle response to tropical forest modification and fragmentation: A quantitative literature review and meta-analysis. Biol. Conserv. 2007, 137, 1–19. [Google Scholar] [CrossRef]
- Nichols, E.; Gardner, T.A.; Peres, C.A.; Spector, S. The Scarabaeinae Research Network Co-declining mammals and dung beetles: An impending ecological cascade. Oikos 2009, 118, 481–487. [Google Scholar] [CrossRef]
- Bogoni, J.A.; Da Silva, P.G.; Peres, C.A. Co-declining mammal–dung beetle faunas throughout the Atlantic Forest biome of South America. Ecography 2019, 42, 1803–1818. [Google Scholar] [CrossRef]
- Hutton, S.A.; Giller, P.S. The effects of the intensification of agriculture on northern temperate dung beetle communities. J. Appl. Ecol. 2003, 40, 994–1007. [Google Scholar] [CrossRef]
- Negro, M.; Rolando, A.; Palestrini, C. The Impact of Overgrazing on Dung Beetle Diversity in the Italian Maritime Alps. Environ. Entomol. 2011, 40, 1081–1092. [Google Scholar] [CrossRef]
- Tonelli, M.; Verdu, J.R.; Zunino, M. Effects of the progressive abandonment of grazing on dung beetle biodiversity: Body size matters. Biodivers. Conserv. 2018, 27, 189–204. [Google Scholar] [CrossRef]
- Tonelli, M.; Verdú, J.R.; Zunino, M. Grazing abandonment and dung beetle assemblage composition: Reproductive behaviour has something to say. Ecol. Indic. 2019, 96, 361–367. [Google Scholar] [CrossRef]
- Seibold, S.; Gossner, M.M.; Simons, N.K.; Blüthgen, N.; Müller, J.; Ambarlı, D.; Ammer, C.; Bauhus, J.; Fischer, M.; Habel, J.C.; et al. Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 2019, 574, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Geiger, F.; van der Lubbe, S.C.T.M.; Brunsting, A.M.H.; de Snoo, G.R. Insect abundance in cow dung pats of different farming systems. Entomol. Ber. 2010, 70, 106–110. [Google Scholar]
- Lumaret, J.-P.; Errouissi, F. Use of anthelmintics in herbivores and evaluation of risks for the non target fauna of pastures. Vet. Res. 2002, 33, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Pecenka, J.R.; Lundgren, J.G. Effects of herd management and the use of ivermectin on dung arthropod communities in grasslands. Basic Appl. Ecol. 2019, 40, 19–29. [Google Scholar] [CrossRef]
- Spector, S. Scarabaeine Dung Beetles (coleoptera: Scarabaeidae: Scarabaeinae): An Invertebrate Focal Taxon for Biodiversity Research and Conservation. Coleopt. Bull. 2006, 60, 71–83. [Google Scholar] [CrossRef]
- Buse, J.; Entling, M.H. Stronger dung removal in forests compared with grassland is driven by trait composition and biomass of dung beetles. Ecol. Entomol. 2020, 45, 223–231. [Google Scholar] [CrossRef]
- Numa, C.; Verdú, J.R.; Sánchez, A.; Galante, E. Effect of landscape structure on the spatial distribution of Mediterranean dung beetle diversity. Divers. Distrib. 2009, 15, 489–501. [Google Scholar] [CrossRef]
- Roslin, T.; Viljanen, H. Dung Beetle Populations: Structure and Consequences. In Ecology and Evolution of Dung Beetles; Simmons, L.E., Ridsdill-Smith, T.J., Eds.; Wiley-Blackwell: Chichester, UK, 2011; pp. 220–244. ISBN 978-1-4443-4200-0. [Google Scholar]
- Hartel, T.; Plieninger, T. European Wood-Pastures in Transition: A Social-Ecological Approach; Routledge: Abingdon, UK, 2014; ISBN 978-1-135-13911-7. [Google Scholar]
- Plieninger, T.; Hartel, T.; Martín-López, B.; Beaufoy, G.; Bergmeier, E.; Kirby, K.; Montero, M.J.; Moreno, G.; Oteros-Rozas, E.; Van Uytvanck, J. Wood-pastures of Europe: Geographic coverage, social–ecological values, conservation management, and policy implications. Biol. Conserv. 2015, 190, 70–79. [Google Scholar] [CrossRef]
- Stoate, C.; Báldi, A.; Beja, P.; Boatman, N.; Herzon, I.; Van Doorn, A.; De Snoo, G.; Rakosy, L.; Ramwell, C. Ecological impacts of early 21st century agricultural change in Europe—A review. J. Environ. Manag. 2009, 91, 22–46. [Google Scholar] [CrossRef]
- Mihók, B.; Biró, M.; Molnár, Z.; Kovacs, E.; Bölöni, J.; Erős, T.; Standovár, T.; Török, P.; Csorba, G.; Margóczi, K.; et al. Biodiversity on the waves of history: Conservation in a changing social and institutional environment in Hungary, a post-soviet EU member state. Biol. Conserv. 2017, 211, 67–75. [Google Scholar] [CrossRef]
- Varga, A.; Molnár, Z.; Biró, M.; Demeter, L.; Gellény, K.; Miókovics, E.; Molnár, Á.; Molnár, K.; Ujházy, N.; Ulicsni, V.; et al. Changing year-round habitat use of extensively grazing cattle, sheep and pigs in East-Central Europe between 1940 and 2014: Consequences for conservation and policy. Agric. Ecosyst. Environ. 2016, 234, 142–153. [Google Scholar] [CrossRef]
- Hartel, T.; Hanspach, J.; Abson, D.; Máthé, O.; Moga, C.I.; Fischer, J. Bird communities in traditional wood-pastures with changing management in Eastern Europe. Basic Appl. Ecol. 2014, 15, 385–395. [Google Scholar] [CrossRef]
- Gallé, R.; Urák, I.; Nikolett, G.-S.; Hartel, T. Sparse trees and shrubs confers a high biodiversity to pastures: Case study on spiders from Transylvania. PLoS ONE 2017, 12, e0183465. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.G.; Hernández, M.I.M. Spatial Patterns of Movement of Dung Beetle Species in a Tropical Forest Suggest a New Trap Spacing for Dung Beetle Biodiversity Studies. PLoS ONE 2015, 10, e0126112. [Google Scholar] [CrossRef] [PubMed]
- Löbl, I.; Löbl, D. Scarabaeoidea–Scirtoidea–Dascilloidea–Buprestoidea–Byrrhoidea: Revised and Updated Edition; Catalogue of Palaearctic Coleoptera; Brill: Leiden, The Netherlands, 2016; ISBN 978-90-04-30914-2. [Google Scholar]
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Statistics for Biology and Health; Springer: New York, NY, USA, 2009; ISBN 978-0-387-87457-9. [Google Scholar]
- Chambers, J.M.; Hastie, T. Statistical Models in S; Wadsworth & Brooks/Cole Advanced Books & Software: Monterey, CA, USA, 1992; ISBN 978-0-534-16764-6. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R, 2nd ed.; Use R!; Springer International Publishing: New York, NY, USA, 2018; ISBN 978-3-319-71403-5. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2018. Available online: https://www.r-project.org (accessed on 20 November 2020).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Statistics and Computing; Springer: New York, NY, USA, 2002; ISBN 978-0-387-95457-8. [Google Scholar]
- Lenth, R.V. Least-Squares Means: The R Package lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biometr. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 20 November 2020).
- Roberts, D.W. Labdsv: Ordination and Multivariate Analysis for Ecology. 2019. Available online: https://CRAN.R-project.org/package=labdsv (accessed on 20 November 2020).
- Buse, J.; Šlachta, M.; Sladecek, F.X.J.; Carpaneto, G.M. Summary of the morphological and ecological traits of Central European dung beetles. Entomol. Sci. 2018, 21, 315–323. [Google Scholar] [CrossRef]
- Ríos-Díaz, C.L.; Moreno, C.E.; Ortega-Martínez, I.J.; Zuria, I.; Escobar, F.; Castellanos, I. Sheep herding in small grasslands promotes dung beetle diversity in a mountain forest landscape. J. Insect Conserv. 2020. [Google Scholar] [CrossRef]
- Jugovic, J.; Koren, T.; Koprivnikar, N. Competition and Seasonal Co-Existence of Coprophagous Scarabaeoidea (Coleoptera) in Differently Managed Habitat Patches of Sub-Mediterranean Grasslands in Slovenia. Pol. J. Ecol. 2019, 67, 247–263. [Google Scholar] [CrossRef]
- Numa, C.; Verdú, J.R.; Rueda, C.; Galante, E. Comparing Dung Beetle Species Assemblages Between Protected Areas and Adjacent Pasturelands in a Mediterranean Savanna Landscape. Rangel. Ecol. Manag. 2012, 65, 137–143. [Google Scholar] [CrossRef]
- Tocco, C.; Negro, M.; Rolando, A.; Palestrini, C. Does natural reforestation represent a potential threat to dung beetle diversity in the Alps? J. Insect Conserv. 2013, 17, 207–217. [Google Scholar] [CrossRef]
- Barton, P.S.; Manning, A.D.; Gibb, H.; Lindenmayer, D.B.; Cunningham, S.A. Conserving ground-dwelling beetles in an endangered woodland community: Multi-scale habitat effects on assemblage diversity. Biol. Conserv. 2009, 142, 1701–1709. [Google Scholar] [CrossRef]
- Lövei, G.L.; Magura, T.; Tóthmérész, B.; Ködöböcz, V. The influence of matrix and edges on species richness patterns of ground beetles (Coleoptera: Carabidae) in habitat islands. Glob. Ecol. Biogeogr. 2006, 15, 283–289. [Google Scholar] [CrossRef]
- Magura, T. Carabids and forest edge: Spatial pattern and edge effect. For. Ecol. Manag. 2002, 157, 23–37. [Google Scholar] [CrossRef]
- Gallé, R.; Kanizsai, O.; Ács, V.; Molnár, B. Functioning of Ecotones—Spiders and Ants of Edges between Native and Non-Native Forest Plantations. Pol. J. Ecol. 2014, 62, 815–820. [Google Scholar] [CrossRef]
- Bogyó, D.; Magura, T.; Nagy, D.D.; Tóthmérész, B. Distribution of millipedes (Myriapoda, Diplopoda) along a forest interior–forest edge–grassland habitat complex. ZooKeys 2015, 510, 181–195. [Google Scholar] [CrossRef]
- Piccini, I.; Palestrini, C.; Rolando, A.; Roslin, T. Local management actions override farming systems in determining dung beetle species richness, abundance and biomass and associated ecosystem services. Basic Appl. Ecol. 2019, 41, 13–21. [Google Scholar] [CrossRef]
- Bradford, M.A.; Berg, B.; Maynard, D.S.; Wieder, W.R.; Wood, S.A. Understanding the dominant controls on litter decomposition. J. Ecol. 2016, 104, 229–238. [Google Scholar] [CrossRef]
- Von Hoermann, C.; Weithmann, S.; Deißler, M.; Ayasse, M.; Steiger, S. Forest habitat parameters influence abundance and diversity of cadaver-visiting dung beetles in Central Europe. R. Soc. Open Sci. 2020, 7, 191722. [Google Scholar] [CrossRef]
- Perrin, W.; Moretti, M.; Vergnes, A.; Borcard, D.; Jay-Robert, P. Response of dung beetle assemblages to grazing intensity in two distinct bioclimatic contexts. Agric. Ecosyst. Environ. 2020, 289, 106740. [Google Scholar] [CrossRef]
- Errouissi, F.; Jay-Robert, P. Consequences of habitat change in euromediterranean landscapes on the composition and diversity of dung beetle assemblages (Coleoptera, Scarabaeoidea). J. Insect Conserv. 2019, 23, 15–28. [Google Scholar] [CrossRef]
- Dormont, L.; Rapior, S.; McKey, D.B.; Lumaret, J.-P. Influence of dung volatiles on the process of resource selection by coprophagous beetles. Chemoecology 2007, 17, 23–30. [Google Scholar] [CrossRef]
- Wassmer, T. Seasonality of Coprophagous Beetles in the Kaiserstuhl Area near Freiburg (Sw Germany) Including the Winter Months. Acta Oecol. Int. J. Ecol. 1994, 15, 607–631. [Google Scholar]
- Galante, E.; Mena, J.; Lumbreras, C. Dung Beetles (Coleoptera: Scarabaeidae, Geotrupidae) Attracted to Fresh Cattle Dung in Wooded and Open Pasture. Environ. Entomol. 1995, 24, 1063–1068. [Google Scholar] [CrossRef]
- Agoglitta, R.; Moreno, C.E.; Zunino, M.; Bonsignori, G.; Dellacasa, M. Cumulative annual dung beetle diversity in Mediterranean seasonal environments. Ecol. Res. 2012, 27, 387–395. [Google Scholar] [CrossRef]
- Senyuz, Y.; Lobo, J.M.; Dindar, K. Altitudinal gradient in species richness and composition of dung beetles (Coleoptera: Scarabaeidae) in an eastern Euro-Mediterranean locality: Functional, seasonal and habitat influences. Eur. J. Entomol. 2019, 116, 309–319. [Google Scholar] [CrossRef]
- Piccini, I.; Caprio, E.; Palestrini, C.; Rolando, A. Ecosystem functioning in relation to species identity, density, and biomass in two tunneller dung beetles. Ecol. Entomol. 2020, 45, 311–320. [Google Scholar] [CrossRef]
- Birkett, A.J.; Blackburn, G.A.; Menéndez, R. Linking species thermal tolerance to elevational range shifts in upland dung beetles. Ecography 2018, 41, 1510–1519. [Google Scholar] [CrossRef]
- Storck-Tonon, D.; Da Silva, R.J.; Sawaris, L.; Vaz-De-Mello, F.Z.; Da Silva, D.J.; Peres, C.A. Habitat patch size and isolation drive the near-complete collapse of Amazonian dung beetle assemblages in a 30-year-old forest archipelago. Biodivers. Conserv. 2020, 29, 2419–2438. [Google Scholar] [CrossRef]
- Jay-Robert, P.; Niogret, J.; Errouissi, F.; Labarussias, M.; Paoletti, É.; Luis, M.V.; Lumaret, J.-P. Relative efficiency of extensive grazing vs. wild ungulates management for dung beetle conservation in a heterogeneous landscape from Southern Europe (Scarabaeinae, Aphodiinae, Geotrupinae). Biol. Conserv. 2008, 141, 2879–2887. [Google Scholar] [CrossRef]
- Buse, J.; Šlachta, M.; Sladecek, F.X.; Pung, M.; Wagner, T.; Entling, M.H. Relative importance of pasture size and grazing continuity for the long-term conservation of European dung beetles. Biol. Conserv. 2015, 187, 112–119. [Google Scholar] [CrossRef]
- Forgie, S.A.; Paynter, Q.; Zhao, Z.; Flowers, C.; Fowler, S. Newly released non-native dung beetle species provide enhanced ecosystem services in New Zealand pastures. Ecol. Entomol. 2018, 43, 431–439. [Google Scholar] [CrossRef]
- Vojta, J.; Drhovská, L. Are abandoned wooded pastures suitable refugia for forest species? J. Veg. Sci. 2012, 23, 880–891. [Google Scholar] [CrossRef]
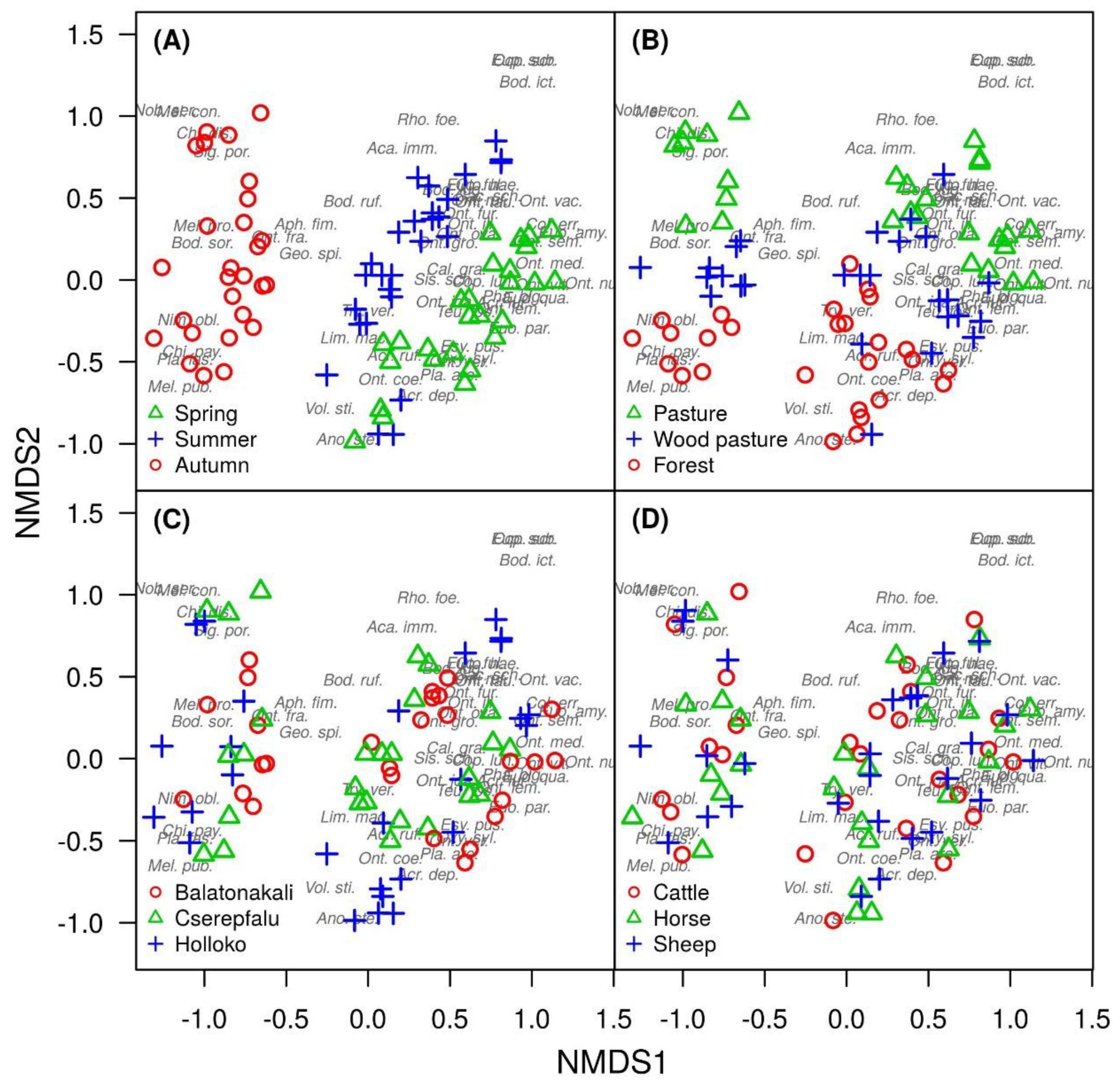
| Species | Indicator Value | p-Value | Habitat |
| Anoplotrupes stercorosus | 0.664 | 0.000 | Forest |
| Melinopterus pubescens | 0.251 | 0.011 | Forest |
| Onthophagus coenobita | 0.606 | 0.002 | Forest |
| Plagiogonus arenarius | 0.341 | 0.028 | Forest |
| Planolinus fasciatus | 0.404 | 0.000 | Forest |
| Volinus sticticus | 0.778 | 0.000 | Forest |
| Caccobius schreberi | 0.398 | 0.002 | Pasture |
| Chilothorax distinctus | 0.249 | 0.003 | Pasture |
| Colobopterus erraticus | 0.290 | 0.001 | Pasture |
| Euoniticellus fulvus | 0.642 | 0.000 | Pasture |
| Melinopterus consputus | 0.294 | 0.022 | Pasture |
| Onthophagus illyricus | 0.628 | 0.000 | Pasture |
| Onthophagus medius | 0.252 | 0.021 | Pasture |
| Onthophagus ovatus | 0.655 | 0.000 | Pasture |
| Onthophagus ruficapillus | 0.606 | 0.000 | Pasture |
| Onthophagus taurus | 0.665 | 0.000 | Pasture |
| Onthophagus vacca | 0.259 | 0.001 | Pasture |
| Otophorus haemorrhoidalis | 0.311 | 0.002 | Pasture |
| Onthophagus joannae | 0.248 | 0.003 | Wood pasture |
| Species | Indicator Value | p-Value | Dung Type |
| Aphodius fimetarius | 0.287 | 0.002 | Cattle |
| Otophorus haemorrhoidalis | 0.238 | 0.025 | Cattle |
| Teuchestes fossor | 0.185 | 0.009 | Cattle |
| Species | Indicator Value | p-Value | Season |
| Acrossus depressus | 0.333 | 0.000 | Spring |
| Acrossus luridus | 0.630 | 0.000 | Spring |
| Colobopterus erraticus | 0.264 | 0.001 | Spring |
| Copris lunaris | 0.763 | 0.000 | Spring |
| Esymus pusillus | 0.922 | 0.000 | Spring |
| Eudolus quadriguttatus | 0.444 | 0.000 | Spring |
| Euoniticellus fulvus | 0.411 | 0.002 | Spring |
| Euorodalus paracoenosus | 0.593 | 0.000 | Spring |
| Onthophagus coenobita | 0.941 | 0.000 | Spring |
| Onthophagus illyricus | 0.607 | 0.001 | Spring |
| Onthophagus joannae | 0.204 | 0.014 | Spring |
| Onthophagus lemur | 0.573 | 0.000 | Spring |
| Onthophagus medius | 0.481 | 0.000 | Spring |
| Onthophagus vacca | 0.163 | 0.043 | Spring |
| Onthophagus verticicornis | 0.960 | 0.000 | Spring |
| Oxyomus sylvestris | 0.454 | 0.000 | Spring |
| Phalacronothus biguttatus | 0.208 | 0.006 | Spring |
| Plagiogonus arenarius | 0.782 | 0.000 | Spring |
| Sisyphus schaefferi | 0.672 | 0.000 | Spring |
| Volinus sticticus | 0.604 | 0.000 | Spring |
| Acanthobodilus immundus | 0.200 | 0.033 | Summer |
| Bodilus lugens | 0.259 | 0.001 | Summer |
| Caccobius schreberi | 0.327 | 0.017 | Summer |
| Geotrupes spiniger | 0.633 | 0.000 | Summer |
| Onthophagus grossepunctatus | 0.471 | 0.012 | Summer |
| Onthophagus taurus | 0.444 | 0.036 | Summer |
| Trypocopris vernalis | 0.701 | 0.000 | Summer |
| Chilothorax distinctus | 0.249 | 0.004 | Autumn |
| Chilothorax paykulli | 0.222 | 0.002 | Autumn |
| Melinopterus consputus | 0.593 | 0.000 | Autumn |
| Melinopterus prodromus | 0.911 | 0.000 | Autumn |
| Nimbus obliteratus | 0.926 | 0.000 | Autumn |
| Nobius serotinus | 0.222 | 0.004 | Autumn |
| Planolinus fasciatus | 0.443 | 0.000 | Autumn |
| Sigorus porcus | 0.148 | 0.029 | Autumn |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somay, L.; Szigeti, V.; Boros, G.; Ádám, R.; Báldi, A. Wood Pastures: A Transitional Habitat between Forests and Pastures for Dung Beetle Assemblages. Forests 2021, 12, 25. https://doi.org/10.3390/f12010025
Somay L, Szigeti V, Boros G, Ádám R, Báldi A. Wood Pastures: A Transitional Habitat between Forests and Pastures for Dung Beetle Assemblages. Forests. 2021; 12(1):25. https://doi.org/10.3390/f12010025
Chicago/Turabian StyleSomay, László, Viktor Szigeti, Gergely Boros, Réka Ádám, and András Báldi. 2021. "Wood Pastures: A Transitional Habitat between Forests and Pastures for Dung Beetle Assemblages" Forests 12, no. 1: 25. https://doi.org/10.3390/f12010025
APA StyleSomay, L., Szigeti, V., Boros, G., Ádám, R., & Báldi, A. (2021). Wood Pastures: A Transitional Habitat between Forests and Pastures for Dung Beetle Assemblages. Forests, 12(1), 25. https://doi.org/10.3390/f12010025





