Effect of Bentonite on the Early Growth Characteristics of Teak Seedlings Planted in Sandy Soil in Northeast Thailand—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Selection of Experimental Plots and Preparation of the Land and Teak Seedlings
2.3. Experiment
2.4. Soil Analysis
2.5. Measurement of Growth of Teak Seedlings
2.6. Measurement of Photosynthetic Rate and Concentrations of Chlorophyll
2.7. Measurements of Leaf Water Potential and Concentrations of Chlorophyll
2.8. Analysis of Biomass
2.9. Analysis of Mycorrhizal Colonization and Element Concentrations in the Plant Organs
2.10. Statistical Analyses
3. Results
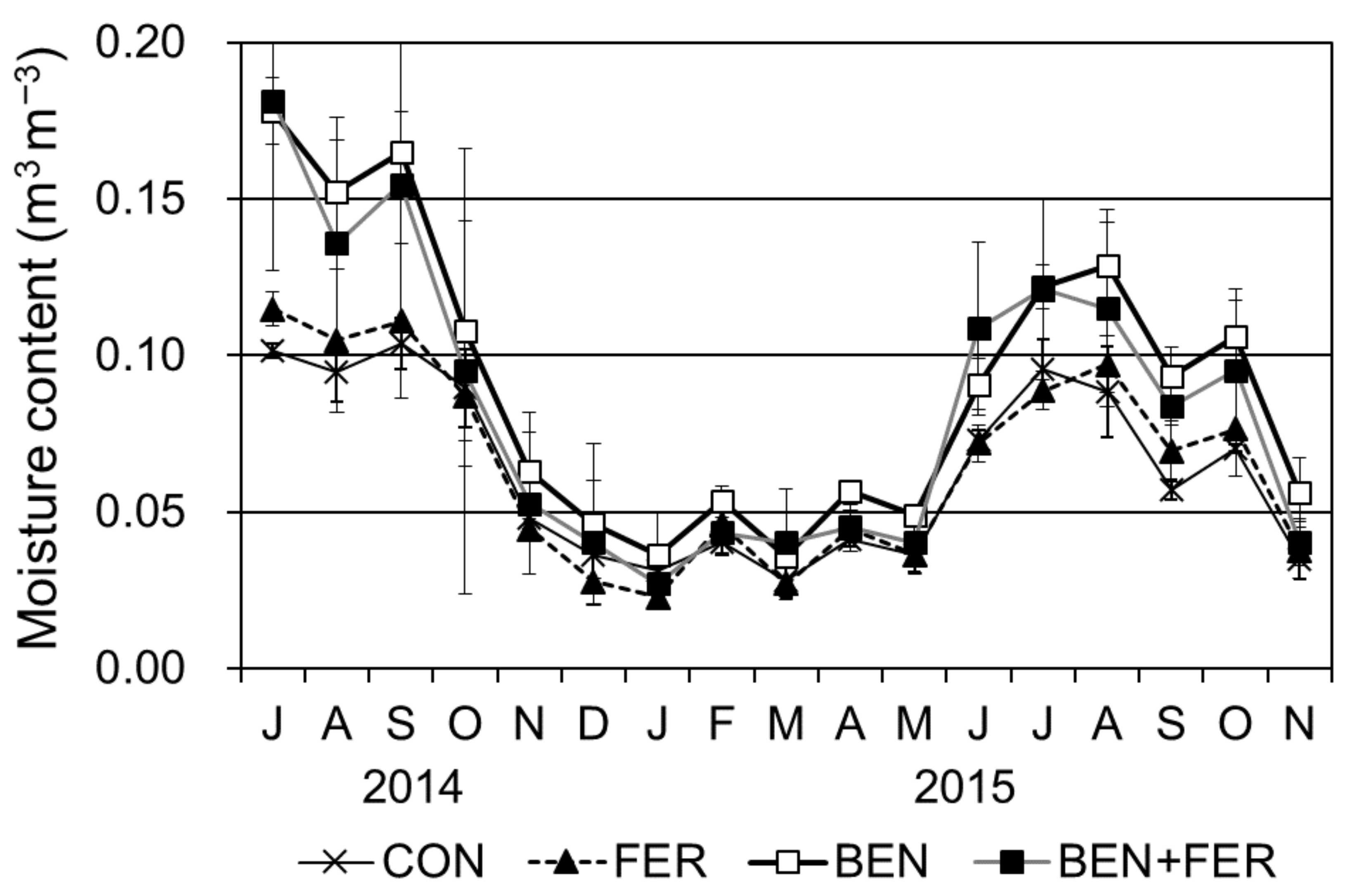
3.1. Soil Moisture Content
3.2. Soil Properties
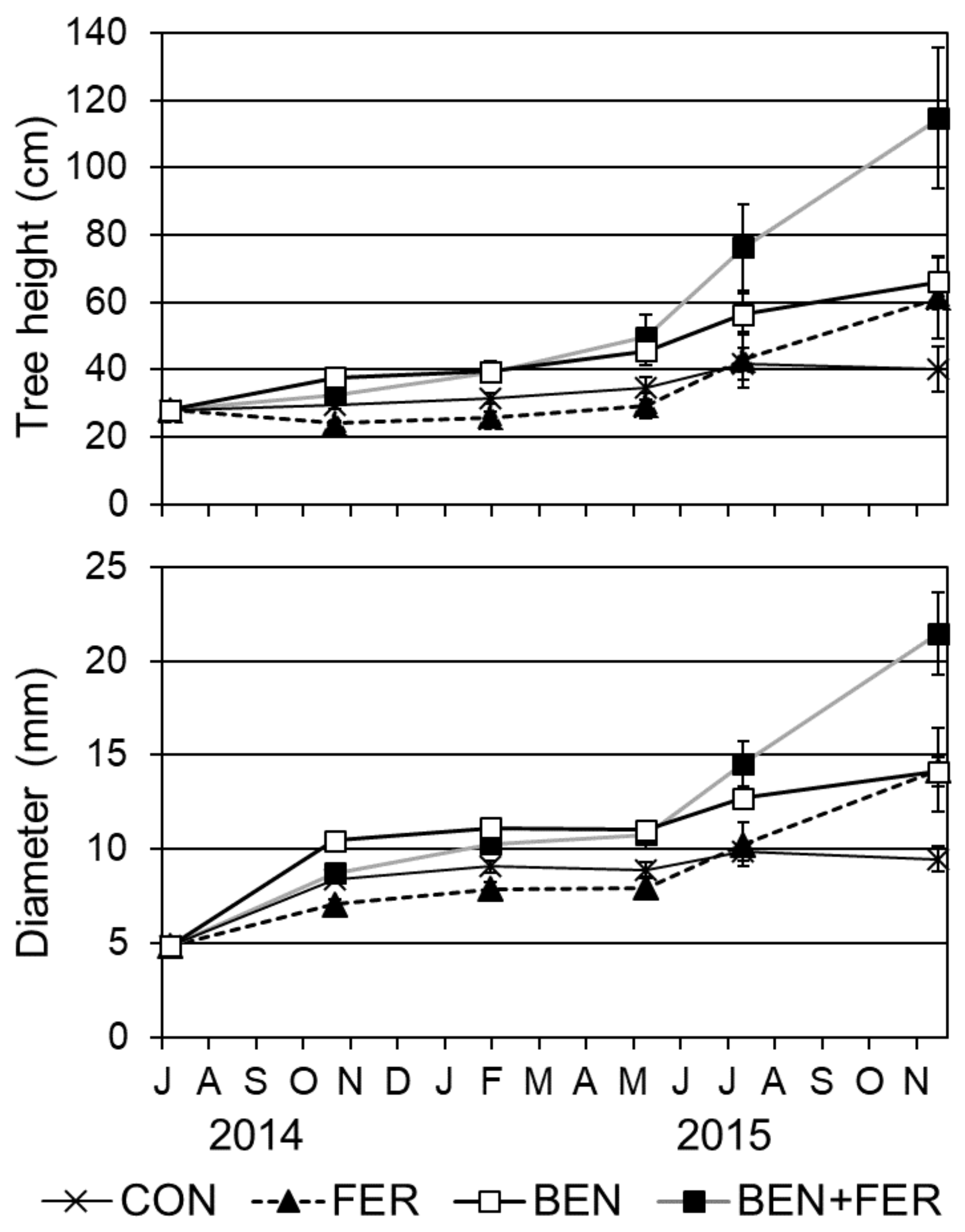
3.3. Growth of Teak Seedlings
3.4. Photosynthetic Rate
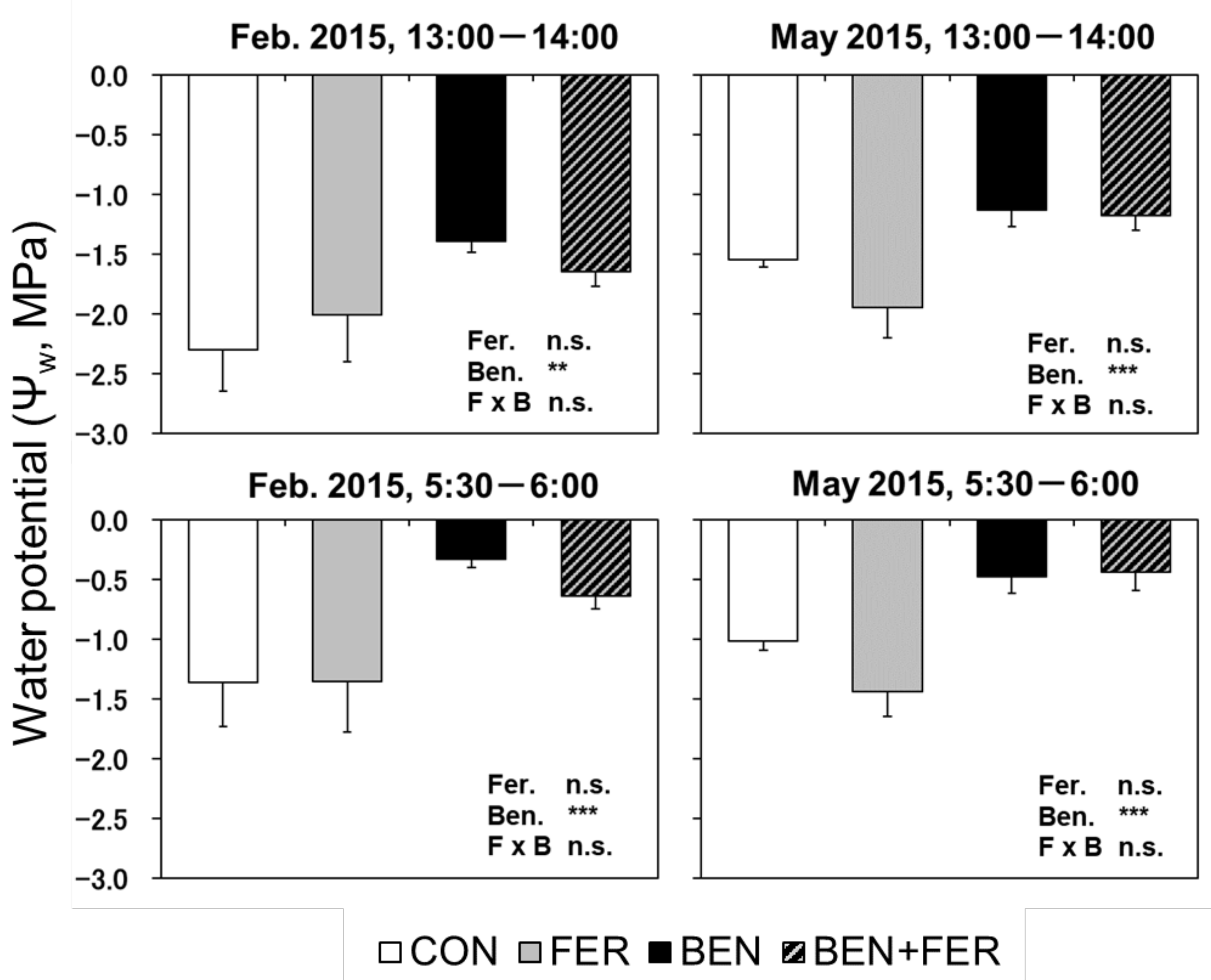
3.5. Leaf Water Potential
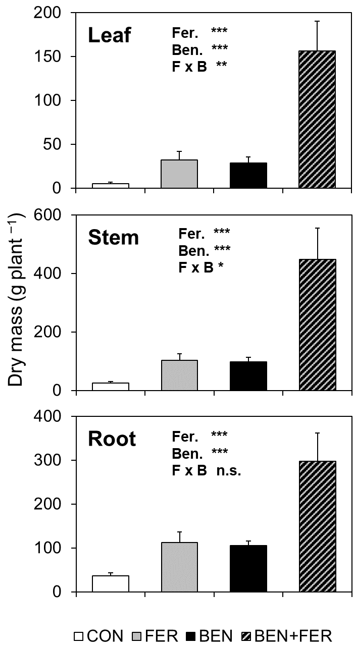
3.6. Biomass and Growth Parameters of Teak Seedlings
3.7. Nutrient Concentrations
4. Discussion
4.1. Effects of Fertilizer and Bentonite on Soil
4.2. Effects of Fertilizer and Bentonite on Teak Biomass
4.3. Effects of Fertilizer and Bentonite on Physiological Characteristics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tewari, D.N. A Monograph of Teak (Tectona grandis Linn. f.); International Book Distributors: Dehra Dun, India, 1992; p. 479. [Google Scholar]
- Kaosa-ard, A. Teak (Tectona grandis Linn. f) Its Natural Distribution and Related Factors. Nat. Hist. Bull. Siam Soc. 1989, 29, 55–74. [Google Scholar]
- Tanaka, N.; Hamazaki, T.; Vacharangkura, T. Distribution, Growth and Site Requirements of Teak. Jpn. Agric. Res. Q. 1998, 32, 65–77. [Google Scholar]
- Barroso, D.G.; Figueiredo, F.A.M.M.A.; Pereira, R.C.; Mendonça, A.V.R.; Silva, L.C. Macronutrient Deficiency Diagnosis in Teak Seedlings. Rev. Árvore 2005, 29, 671–679, (In Portuguese and English Summary). [Google Scholar] [CrossRef]
- Zhou, Z.; Liang, K.; Xu, D.; Zhang, Y.; Huang, G.; Ma, H. Effects of Calcium, Boron and Nitrogen Fertilization on the Growth of Teak (Tectona grandis) Seedlings and Chemical Property of Acidic Soil Substrate. New For. 2012, 43, 231–243. [Google Scholar] [CrossRef]
- Gopikumar, K.; Varghese, V. Sand Culture Studies of Teak (Tectona grandis) in Relation to Nutritional Deficiency Symptoms, Growth and Vigour. J. Trop. For. Sci. 2004, 16, 46–61. [Google Scholar]
- Food and Agriculture Organization. An Explanatory Note on the FAO World Soil Resources Map at 1:25,000,000 Scale; World Soil Resources Reports 66; FAO: Rome, Italy, 1993; p. 64. [Google Scholar]
- Kyuma, K. Soil Resources and Land Use in Tropical Asia. Pedosphere 2003, 13, 49–57. [Google Scholar]
- Eswaran, H.; Padmanabhan, E. Tropics and Sub-Tropics, Soil of the. In Encyclopedia of Soil Science; Lal, R., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2002; pp. 1342–1345. [Google Scholar]
- Salifu, K.F. Site Variables Controlling Teak (Tectona grandis) Growth in the High Forest Zone of Ghana. J. Trop. For. Sci. 2001, 13, 99–108. [Google Scholar]
- Huy, B.; Tri, P.C.; Triet, T. Assessment of Enrichment Planting of Teak (Tectona grandis) in Degraded Dry Deciduous Dipterocarp Forest in the Central Highland, Vietnam. South. For. J. For. Sci. 2018, 80, 75–84. [Google Scholar] [CrossRef]
- Ghiberto, P.J.; Libardi, P.L.; Trivelin, P.C.O. Nutrient Leaching in an Ultisol Cultivated with Sugarcane. Agric. Water Manag. 2015, 148, 141–149. [Google Scholar] [CrossRef]
- Suzuki, S.; Noble, A.D.; Ruaysoongnern, S.; Chinabut, N. Improvement in Water-Holding Capacity and Structural Stability of a Sandy Soil in Northeast Thailand. Arid. Land Res. Manag. 2007, 21, 37–49. [Google Scholar] [CrossRef]
- Kayama, M.; Nimpila, S.; Hongthong, S.; Yoneda, R.; Wichiennopparat, W.; Himmapan, W.; Vacharangkura, T.; Noda, I. Effects of Bentonite, Charcoal and Corncob for Soil Improvement and Growth Characteristics of Teak Seedling Planted on Acrisols in Northeast Thailand. Forests 2016, 7, 36. [Google Scholar] [CrossRef]
- Kayama, M.; Nimpila, S.; Hongthong, S.; Himmapan, W. Growth Characteristics of Teak Seedling Planted on Different Types of Sandy Soil in Northeast Thailand. In Improvement of Utilization Techniques of Forest Resources to Promote Sustainable Forestry in Thailand; JIRCAS Working report 85; Kayama, M., Himmapan, W., Eds.; Japan International Research Center for Agricultural Sciences: Tsukuba, Japan, 2017; pp. 45–56. [Google Scholar]
- Wichiennopparat, W.; Wanpinit, M.; Nimpila, S.; Soonthornwit, N. Growth Performance of 6-Year-Old Teak Plantation under Different Soil Improvement Methods in Khon Kaen Province, Thailand. In Improvement of Utilization Techniques of Forest Resources to Promote Sustainable Forestry in Thailand; JIRCAS Working Report 85; Kayama, M., Himmapan, W., Eds.; Japan International Research Center for Agricultural Sciences: Tsukuba, Japan, 2017; pp. 57–62. [Google Scholar]
- Tangmitcharoen, S.; Nimpila, S.; Phuangjumpee, P.; Piananurak, P. Two-Year Results of a Clonal Test of Teak (Tectona grandis L.f.) in the Northeast Thailand. In Approach to Sustainable Forestry of Indigenous Tree Species in Northeast Thailand; JIRCAS Working report 74; Noda, I., Vacharangkura, T., Himmapan, W., Eds.; Japan International Research Center for Agricultural Sciences: Tsukuba, Japan, 2012; pp. 19–22. [Google Scholar]
- Kenzo, T.; Himmapan, W.; Yoneda, R.; Tedsorn, N.; Vacharangkura, T.; Hitsuma, G.; Noda, I. General Estimation Model for above- and below-Ground Biomass of Teak Plantation in Thailand. For. Ecol. Manag. 2020, 457, 117701. [Google Scholar] [CrossRef]
- Wichiennopparat, W.; Wanpinit, M.; Visaratana, T.; Noda, I.; Sukchan, S.; Sasrisang, A. Soil Suitability Map for Teak Plantation in Chaiyaphum and Khon Kaen Provinces; RFD-JIRCAS Joint Research Project: Bangkok, Thailand, 2015; p. 52. (In Thai) [Google Scholar]
- Wehr, J.B.; Smith, T.E.; Menzies, N.W. Influence of Soil Characteristics on Teak (Tectona grandis L. f.) Establishment and Early Growth in Tropical Northern Australia. J. For. Res. 2017, 22, 153–159. [Google Scholar] [CrossRef]
- Rajendrudu, G.; Naidu, C.V.; Mallikarjuna, K. Effect of Water Stress on Photosynthesis and Growth in Two Teak Phenotypes. Photosynthetica 1999, 36, 627–630. [Google Scholar] [CrossRef]
- Rao, P.B.; Kaur, A.; Tewari, A. Drought Resistance in Seedlings of Five Important Tree Species in Terai Region of Uttarakhand. Trop. Ecol. 2008, 49, 43–52. [Google Scholar]
- Geleano, E.; Vasconcelos, T.S.; Novais de Oliveira, P.; Carrer, H. Physiological and Molecular Responses to Drought Stress in Teak (Tectona grandis L.f.). PLoS ONE 2019, 14, e0221571. [Google Scholar] [CrossRef]
- Bledsoe, C.S.; Zasoski, R.J. Effects of Ammonium and Nitrate on Growth and Nitrogen Uptake by Mycorrhizal Douglas-fir Seedlings. Plant Soil 1983, 71, 445–454. [Google Scholar] [CrossRef]
- Mishra, P.K.; Prasad, S.S.; Babu, B.M.; Varalakshmi, L.R. Bentonite as an Ameliorant in an Alfisol—A Laboratory Study. J. Irrig. Drain. Eng. 2001, 127, 118–122. [Google Scholar] [CrossRef]
- Noble, A.D.; Gillman, G.P.; Nath, S.; Srivastava, R.J. Changes in the Surface Charge Characteristics of Degraded Soils in the Wet Tropics Through the Addition of Beneficiated Bentonite. Soil Res. 2001, 39, 991–1001. [Google Scholar] [CrossRef]
- Brockley, R.P. Effects of Different Sources and Rates of Sulphur on the Growth and Foliar Nutrition of Nitrogen-Fertilized Lodgepole Pine. Can. J. For. Res. 2004, 34, 728–743. [Google Scholar] [CrossRef]
- Muththalib, A.; Baudet, B.A. Effect of Heavy Metal Contamination on the Plasticity of Kaolin-Bentonite Clay Mixtures and an Illite-Smectite Rich Natural Clay. E3S Web Conf. 2019, 92, 10005. [Google Scholar] [CrossRef]
- Redding, M.R.; Lewis, R.; Kearton, T.; Smith, O. Manure and Sorbent Fertilisers Increase On-Going Nutrient Availability Relative to Conventional Fertilisers. Sci. Total Environ. 2016, 569–570, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Sherriff, N.; Issa, R.; Morris, K.; Livens, F.; Heath, S.; Bryan, N. Reversibility in Radionuclide/Bentonite Bulk and Colloidal Ternary Systems. Miner. Mag. 2015, 79, 1307–1315. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.; Ni, X.; Zhou, Z.; Yu, L.; Liu, B.; Yang, Y.; Wu, Y. Performance of Matrix-Based Slow-Release Urea in Reducing Nitrogen Loss and Improving Maize Yield and Profits. Field Crops Res. 2017, 212, 73–81. [Google Scholar] [CrossRef]
- Raghu, H.B.; Ashwin, R.; Ravi, J.E.; Bagyaraj, D.J. Microbial Consortium Improved Growth and Performance of Teak (Tectona grandis L.f.) in Nursery and Field Trials. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 20, 903–909. [Google Scholar] [CrossRef]
- Himmapan, W.; Noda, I.; Furuya, N. The Study on the Administration of Private Forest Plantation Cooperative of Thailand: A Case Study of Nomgbua Lamphu Private Forest Plantation Cooperative Limited. J. For. Manag. 2010, 4, 1–12. [Google Scholar]
- Van Reeuwijk, L.P. Procedures for Soil Analysis, 6th ed.; International Soil Reference and Information Centre: Wagningen, The Netherlands, 2002; p. 100. [Google Scholar]
- Klute, A. Methods of Soil Analysis, Part 1. Physical and Mineralogical Methods, 2nd ed.; Soil Science Society of America Inc.: Madison, WI, USA, 1986; p. 1188. [Google Scholar]
- Muto, K.; Sakurai, K.; Ito, A. Simplified Analysis of Cation-Exchange-Capacity (CEC). Tohoku Agric. Res. 1984, 35, 271–272. (In Japanese) [Google Scholar]
- Weatherburn, M.W. Phenol-Hypochlorite Reaction for Determination of Ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnson, C.T.; Sumner, M.E. Methods of Soil Analysis, Part 3. Chemical Methods; Soil Science Society of America Inc.: Madison, WI, USA, 1996; p. 1390. [Google Scholar]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998; p. 1220. [Google Scholar]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A. A Reappraisal of the Use of DMSO for the Extraction and Determination of Chlorophylls a and b in Lichens and Higher Plants. Environ. Exp. Bot. 1992, 32, 85–100. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology, 4th ed.; Springer: Berlin, Germany, 2003; p. 513. [Google Scholar]
- Tondjo, K.; Brancheriau, L.; Sabatier, S.A.; Kokutse, A.D.; Akossou, A.; Kokou, K.; Fourcaud, T. Non-Destructive Measurement of Leaf Area and Dry Biomass in Tectona grandis. Trees 2015, 29, 1625–1631. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An Evaluation of Techniques for Measuring Vesicular Arbuscular Mycorrhizal Infection in Roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Goto, S. Digestion Method. In Manual of Plant Nutrition; Editorial Committee of Methods for Experiments in Plant Nutrition, Ed.; Hakuyusha: Tokyo, Japan, 1990; pp. 125–128. (In Japanese) [Google Scholar]
- Radulov, I.; Berbecea, A.; Sala, F.; Crista, F.; Lato, A. Mineral Fertilization Influence on Soil pH, Cationic Exchange Capacity and Nutrient Content. Res. J. Agric. Sci. 2011, 43, 160–165. [Google Scholar]
- Bhatt, M.K.; Labanya, R.; Joshi, H.C. Influence of Long-Term Chemical Fertilizer and Organic Manures on Soil Fertility—A Review. Univers. J. Agric. Res. 2019, 7, 177–188. [Google Scholar] [CrossRef]
- Soda, W.; Noble, A.D.; Suzuki, S.; Simmons, R.; Sindhusen, L.; Bhuthorndharaj, S. Co-composting of Acid Waste Bentonites and Their Effects on Soil Properties and Crop Biomass. J. Environ. Qual. 2006, 35, 2293–2301. [Google Scholar] [CrossRef] [PubMed]
- Egloffstein, T.A. Natural Bentonite—Influence of the Ion Exchange and Partial Desiccation on Permeability and Self-Healing Capacity of Bentonites Used in GCLs. Geotext. Geomembr. 2001, 19, 427–444. [Google Scholar] [CrossRef]
- El-Sergany, M.; Shanableh, A. Phosphorus Removal Using Al-Modified Bentonite Clay—Effect of Particle Size. In Proceedings of the 2012 Asia Pacific Conference on Environmental Science and Technology, Kuala Lumpur, Malaysia, 1–2 February 2012; pp. 323–329. [Google Scholar]
- Dong, D.; Wang, C.; Zwieten, L.V.; Wang, H.; Jiang, P.; Zhou, M.; Wu, W. An Effective Biochar-Based Slow-Release Fertilizer for Reducing Nitrogen Loss in Paddy Fields. J. Soil Sediment 2020, 20, 3027–3040. [Google Scholar] [CrossRef]
- Croker, J.; Poss, R.; Hartmann, C.; Bhuthorndharaj, S. Effects of Recycled Bentonite Addition on Soil Properties, Plant Growth and Nutrient Uptake in A tropical Sandy Soil. Plant Soil 2004, 267, 155–163. [Google Scholar] [CrossRef]
- Zech, W.; Drechsel, P. Relationships between Growth, Mineral Nutrition and Site Factors of Teak (Tectona grandis) Plantations in the Rainforest Zone of Liberia. For. Ecol. Manag. 1991, 41, 221–235. [Google Scholar] [CrossRef]
- Bücking, H.; Kafle, A. Role of Arbuscular Mycorrhizal Fungi in the Nitrogen Uptake of Plants: Current Knowledge and Research Gaps. Agronomy 2015, 5, 587–612. [Google Scholar] [CrossRef]
- Behling, M.; Neves, J.C.L.; de Barros, N.F.; Kishimoto, C.B.; Smit, L. Nutrient Use Efficiency for the Formation of Fine and Medium Roots in Teak Stands. Rev. Árvore 2014, 38, 837–846, (In Portuguese and English Summary). [Google Scholar] [CrossRef][Green Version]
- Chanan, M.; Hardiwinoto, S.; Abus, C.; Purwanto, R.H.; Purwanta, S. The Identification of Macro Nutrient Status of Superior Teak Plantation (Tectona grandis Lin.F) by Means of DRIS Norms (Diagnosis and Recommendation Integrated System) in Indonesia. For. Sci. Technol. 2019, 15, 7–12. [Google Scholar] [CrossRef]
- Óskarsson, H.; Sigurgeirsson, A.; Raulund-Rasmussen, K. Survival, Growth, and Nutrition of Tree Seedlings Fertilized at Planting on Andisol Soils in Iceland: Six-Year Results. For. Ecol. Manag. 2006, 229, 88–97. [Google Scholar] [CrossRef]
- Wolf, B. The Fertile Triangle: The Interrelationship of Air, Water, and Nutrients in Maximizing Soil Productivity, 1st ed.; Food Products Press: Binghamton, NY, USA, 1999; p. 484. [Google Scholar]
- Evans, J.R. Photosynthesis and Nitrogen Relationships in Leaves of C3 Plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Raaimakers, D.; Boot, R.G.A.; Dijkstra, P.; Pot, S.; Pons, T. Photosynthetic Rates in Relation to Leaf Phosphorus Content in Pioneer Versus Climax Tropical Rainforest Trees. Oecologia 1995, 102, 120–125. [Google Scholar] [CrossRef]
- Enríquez, S.; Duarte, C.M.; Sand-Jensen, K.; Nielsen, S.L. Broad-Scale Comparison of Photosynthetic Rate across Phototrophic Organisms. Oecologia 1996, 108, 197–206. [Google Scholar] [CrossRef]
- Gratani, L.; Pesoli, P.; Crescente, M.F. Relationship between Photosynthetic Activity and Chlorophyll Content in an Isolated Quercus ilex L. Tree during the Year. Photosynthetica 1998, 35, 445–451. [Google Scholar] [CrossRef]
- Nemec, S. Effects of Soil Amendments and Tillage on Vesicular-Arbuscular Mycorrhizal Fungus Colonization of Citrus. HortTechnology 1998, 8, 51–54. [Google Scholar] [CrossRef]
- Hepper, C.M.; O’Shea, J. Vesicular-Arbuscular Mycorrhizal Infection in Lettuce (Lactuca sativa) in Relation to Calcium Supply. Plant Soil 1984, 82, 61–68. [Google Scholar] [CrossRef]
- Juice, S.M.; Fahey, T.J.; Siccama, T.G.; Driscoll, C.T.; Denny, E.G.; Eagar, C.; Cleavitt, N.L.; Minocha, R.; Richardson, A.D. Response of Sugar Maple to Calcium Addition to Northern Hardwood Forest. Ecology 2006, 87, 1267–1280. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N. Chapter 2 Arbuscular Mycorrhizal Fungi and Tolerance of Drought Stress in Plants. In Arbuscular mycorrhiza and Stress Taolerance of Plantsanual of Plant Nutrition; Wu, Q.S., Ed.; Springer: Singapore, 2017; pp. 25–41. [Google Scholar]
- Saleth, R.M.; Inocencio, A.; Noble, A.; Ruaysoongnern, S. Economic Gains of Improving Soil Fertility and Water Holding Capacity with Clay Application; IWMI Research Report 130; International Water Management Institute: Colombo, Sri Lanka, 2009; p. 30. [Google Scholar]
- Fernandez-Moya, J.; Murillo, R.; Portuguez, E.; Fallas, J.L.; Rios, V.; Kottman, F.; Verjans, J.M.; Mata, R.; Alvarado, A. Nutrient Concentration Age Dynamics of Teak (Tectona grandis L.f.) Plantations in Central America. For. Syst. 2013, 22, 123–133. [Google Scholar] [CrossRef]
- Nakaji, T.; Fukami, M.; Dokiya, Y.; Izuta, T. Effects of High Nitrogen Load on Growth, Photosynthesis and Nutrient Status of Cryptomeria japonica and Pinus densiflora Seedlings. Trees 2001, 15, 453–461. [Google Scholar] [CrossRef]

| Parameter | Treatment | Statistical Analysis | ||
|---|---|---|---|---|
| Sand (%) | CON FER BEN BEN + FER | 80.2 ± 0.5 79.8 ± 1.1 80.2 ± 0.5 78.9 ± 0.8 | Fer. Ben. F × B | n.s. n.s. n.s. |
| Silt (%) | CON FER BEN BEN + FER | 14.1 ± 0.7 15.4 ± 1.1 14.6 ± 0.4 14.5 ± 0.3 | Fer. Ben. F × B | n.s. n.s. n.s. |
| Clay (%) | CON FER BEN BEN + FER | 5.7 ± 0.9 4.8 ± 0.7 5.2 ± 0.4 6.6 ± 1.0 | Fer. Ben. F × B | n.s. n.s. n.s. |
| Parameter | Treatment | Jul. 2014 | Nov. 2015 | Statistical Analysis | |||
|---|---|---|---|---|---|---|---|
| pH | CON FER BEN BEN + FER | 4.53 ± 0.08 4.62 ± 0.05 4.67 ± 0.04 4.36 ± 0.06 | 4.68 ± 0.03 5.09 ± 0.08 5.03 ± 0.05 5.08 ± 0.05 | Fer. Ben. Per. | n.s. n.s. *** | F × B F × P B × P F × B × P | *** *** ** n.s. |
| CEC (cmol kg−1) | CON FER BEN BEN + FER | 1.30 ± 0.10 1.61 ± 0.14 1.75 ± 0.08 2.20 ± 0.13 | 1.42 ± 0.06 1.44 ± 0.13 1.78 ± 0.13 2.14 ± 0.25 | Fer. Ben. Per. | ** *** n.s. | F × B F × P B × P F × B × P | n.s. n.s. n.s. n.s. |
| OM (g kg−1) | CON FER BEN BEN + FER | 7.00 ± 2.32 6.76 ± 1.61 9.60 ± 1.90 9.84 ± 2.03 | 1.54 ± 0.23 2.16 ± 0.23 1.32 ± 0.12 2.53 ± 0.28 | Fer. Ben. Per. | n.s. n.s. *** | F × B F × P B × P F × B × P | n.s. n.s. n.s. n.s. |
| N (mg kg−1) | CON FER BEN BEN + FER | 96 ± 16 346 ± 72 104 ± 16 644 ± 41 | 367 ± 17 411 ± 11 278 ± 32 556 ± 41 | Fer. Ben. Per. | *** *** *** | F × B F × P B × P F × B × P | *** *** * n.s. |
| P (mg kg−1) | CON FER BEN BEN + FER | 9 ± 2 36 ± 8 10 ± 2 80 ± 14 | 4 ± 0 44 ± 12 4 ± 0 91 ± 15 | Fer. Ben. Per. | *** *** n.s. | F × B F × P B × P F × B × P | *** n.s. n.s. n.s. |
| Ca (mg kg−1) | CON FER BEN BEN + FER | 53 ± 8 78 ± 7 70 ± 4 99 ± 17 | 33 ± 5 73 ± 5 154 ± 19 171 ± 24 | Fer. Ben. Per. | ** *** *** | F × B F × P B × P F × B × P | n.s. n.s. *** n.s. |
| Mg (mg kg−1) | CON FER BEN BEN + FER | 22.9 ± 2.9 26.9 ± 2.6 30.1 ± 1.5 29.0 ± 2.4 | 7.9 ± 2.1 28.8 ± 4.6 26.3 ± 5.0 41.0 ± 8.5 | Fer. Ben. Per. | ** ** n.s. | F × B F × P B × P F × B × P | n.s. ** n.s. n.s. |
| K (mg kg−1) | CON FER BEN BEN + FER | 23 ± 3 239 ± 43 25 ± 3 240 ± 38 | 16 ± 2 40 ± 5 34 ± 9 109 ± 10 | Fer. Ben. Per. | *** n.s. *** | F × B F × P B × P F × B × P | n.s. *** n.s. n.s. |
| Na (mg kg−1) | CON FER BEN BEN + FER | 2.4 ± 0.6 11.5 ± 2.0 12.2 ± 2.1 22.9 ± 2.6 | 11.1 ± 1.1 11.5 ± 1.4 10.1 ± 1.0 09.4 ± 2.6 | Fer. Ben. Per. | *** *** n.s. | F × B F × P B × P F × B × P | n.s. *** *** n.s. |
| Parameter | Jul. 2014 | Oct. 2014 | Feb. 2015 | May 2015 | Jul. 2015 | Nov. 2015 | |
|---|---|---|---|---|---|---|---|
| Tree height | Fer. Ben. F × B | n.s. n.s. n.s. | *** *** n.s. | * *** n.s. | n.s. *** n.s. | n.s. *** n.s. | *** *** n.s. |
| Diameter | Fer. Ben. F × B | n.s. n.s. n.s. | *** *** n.s. | n.s. *** n.s. | n.s. *** n.s. | n.s. *** n.s. | *** *** n.s. |
| Oct. 2014 | Feb. 2015 | May 2015 | Jul. 2015 | Oct. 2015 | |||
| Psat | Fer. Ben. F × B | n.s. *** n.s. | *** *** n.s. | n.s. *** n.s. | * n.s. n.s. | * *** n.s. | |
| gs | Fer. Ben. F × B | n.s. *** n.s. | * ** n.s. | n.s. * n.s. | n.s. n.s. n.s. | n.s. * n.s. | |
| Chl.a + b | Fer. Ben. F × B | n.s. *** n.s. | * ** n.s. | n.s. n.s. n.s. | n.s. * n.s. | n.s. ** n.s. | |
| Parameter | Treatment | Statistical Analysis | ||
|---|---|---|---|---|
| Survival rate (%) | CON FER BEN BEN + FER | 55.0 ± 13.2 20.0 ± 7.6 66.7 ± 7.3 44.3 ± 3.3 | Fer. Ben. F × B | * n.s. n.s. |
| RGR (g g−1 yr) | CON FER BEN BEN + FER | 3.21 ± 0.22 4.54 ± 0.47 4.96 ± 0.15 6.42 ± 0.36 | Fer. Ben. F × B | *** *** n.s. |
| Leaf area (cm2) | CON FER BEN BEN + FER | 92 ± 34 528 ± 106 454 ± 84 1214 ± 149 | Fer. Ben. F × B | *** *** n.s. |
| AM colonization (%) | CON FER BEN BEN + FER | 90.7 ± 3.6 84.2 ± 3.5 94.8 ± 1.5 93.4 ± 1.5 | Fer. Ben. F × B | n.s. * n.s. |
| Element | Treatment | Concentration (mg g−1) | Statistical Analysis | |||
|---|---|---|---|---|---|---|
| Leaf | Root | Leaf | Root | |||
| N | CON FER BEN BEN + FER | 18.9 ± 1.0 24.2 ± 1.0 21.5 ± 0.8 25.7 ± 1.4 | 7.6 ± 0.3 9.9 ± 0.6 8.8 ± 0.3 9.1 ± 0.4 | Fer. Ben. F × B | *** n.s. n.s. | ** n.s. * |
| P | CON FER BEN BEN + FER | 0.76 ± 0.06 1.54 ± 0.17 0.90 ± 0.06 1.64 ± 0.13 | 0.33 ± 0.03 0.67 ± 0.09 0.33 ± 0.03 0.56 ± 0.05 | Fer. Ben. F × B | *** n.s. n.s. | *** n.s. n.s. |
| K | CON FER BEN BEN + FER | 5.20 ± 0.53 4.87 ± 0.62 6.73 ± 0.41 5.88 ± 0.84 | 3.99 ± 0.24 5.31 ± 0.30 4.17 ± 0.26 6.53 ± 0.37 | Fer. Ben. F × B | n.s. n.s. n.s. | *** * n.s. |
| Ca | CON FER BEN BEN + FER | 3.88 ± 0.75 9.25 ± 1.35 6.01 ± 0.97 9.00 ± 0.89 | 3.16 ± 0.25 3.18 ± 0.51 3.10 ± 0.27 2.91 ± 0.35 | Fer. Ben. F × B | *** n.s. n.s. | n.s. n.s. n.s. |
| Mg | CON FER BEN BEN + FER | 5.05 ± 0.36 6.31 ± 0.46 4.46 ± 0.29 4.58 ± 0.39 | 1.02 ± 0.06 1.25 ± 0.09 0.92 ± 0.06 1.22 ± 0.10 | Fer. Ben. F × B | n.s. ** n.s. | n.s. *** n.s. |
| Elements | Leaves | Roots | |||
|---|---|---|---|---|---|
| r | F | r | F | ||
| Elements in soils—Elements in leaves or roots | |||||
| N P K Ca Mg | 0.427 0.506 –0.041 0.073 –0.204 | 7.58 ** 11.1 ** 0.06 0.18 1.48 | 0.208 0.272 0.469 –0.171 0.076 | 1.54 2.72 9.61 *** 1.02 0.20 | |
| Elements in leaves or roots–RGR | |||||
| N P K Ca Mg | 0.140 0.493 0.129 0.222 –0.250 | 0.92 14.7 *** 0.78 2.40 3.05 | –0.014 <0.001 0.520 –0.369 –0.034 | <0.01 <0.01 17.1 *** 7.23 ** 0.05 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayama, M.; Nimpila, S.; Hongthong, S.; Yoneda, R.; Himmapan, W.; Noda, I. Effect of Bentonite on the Early Growth Characteristics of Teak Seedlings Planted in Sandy Soil in Northeast Thailand—A Pilot Study. Forests 2021, 12, 26. https://doi.org/10.3390/f12010026
Kayama M, Nimpila S, Hongthong S, Yoneda R, Himmapan W, Noda I. Effect of Bentonite on the Early Growth Characteristics of Teak Seedlings Planted in Sandy Soil in Northeast Thailand—A Pilot Study. Forests. 2021; 12(1):26. https://doi.org/10.3390/f12010026
Chicago/Turabian StyleKayama, Masazumi, Suchat Nimpila, Sutjaporn Hongthong, Reiji Yoneda, Woraphun Himmapan, and Iwao Noda. 2021. "Effect of Bentonite on the Early Growth Characteristics of Teak Seedlings Planted in Sandy Soil in Northeast Thailand—A Pilot Study" Forests 12, no. 1: 26. https://doi.org/10.3390/f12010026
APA StyleKayama, M., Nimpila, S., Hongthong, S., Yoneda, R., Himmapan, W., & Noda, I. (2021). Effect of Bentonite on the Early Growth Characteristics of Teak Seedlings Planted in Sandy Soil in Northeast Thailand—A Pilot Study. Forests, 12(1), 26. https://doi.org/10.3390/f12010026





