Abstract
Wood pastures are home to a variety of species, including the dung beetle. Dung beetles are an important functional group in decomposition. Specifically, in terms of livestock manure, they not only contribute to nutrient cycling but are key players in supporting human and animal health. Dung beetles, however, are declining in population, and urgent recommendations are needed to reverse this trend. Recommendations need to be based on solid evidence and specific habitats. Herein, we aimed to investigate the role of an intermediate habitat type between forests and pastures. Wood pastures are key areas for dung beetle conservation. For this reason, we compared dung beetle assemblages among forests, wood pastures, and grasslands. We complemented this with studies on the effects of dung type and season at three Hungarian locations. Pitfall traps baited with cattle, sheep, or horse dung were used in forests, wood pastures, and pasture habitats in spring, summer, and autumn. Dung beetle assemblages of wood pastures showed transient characteristics between forests and pastures regarding their abundance, species richness, Shannon diversity, assemblage composition, and indicator species. We identified a strong effect of season and a weak of dung type. Assemblage composition proved to be the most sensitive measure of differences among habitats. The conservation of dung beetles, and the decomposition services they provide, need continuous livestock grazing to provide fresh dung, as well as the maintenance of wood pastures where dung beetle assemblages typical of forests and pastures can both survive.
1. Introduction
Every year, four billion tons of livestock feces are produced in Europe [1]. Its decomposition is an essential function of ecosystems, and dung beetles (Scarabaeidae: Scarabaeinae, Aphodiinae; Geotrupidae), which are widespread across most terrestrial habitats, play an important role in it [2,3]. Therefore, the conservation of dung beetles and the sustenance of the ecosystem service they provide is an important task. Dung beetles are involved in the decomposition of feces, not only by means of consumption by both larvae and imagoes [4] but even more importantly by chopping, spreading, and burying it, thereby making it available for other decomposing organisms [5,6]. By accelerating decomposition, they contribute to the soil’s nutrient cycle and stimulate plant growth by fertilization (increasing the nutrient content of the soil) [7]. Their vertical tunnels promote the mixing of soil layers, increase aeration, and permeability [8].
They also have an important role in maintaining human and animal health by removing the feces of domestic animals from the surface, thus controlling the number of parasitic worms and dung flies [9]. In addition, they assist the secondary distribution of plant seeds present in dung [10]. All of these activities are considered as valuable ecosystem services [3], resulting in significant financial savings for agriculture [11,12].
Only sufficiently diverse assemblages and abundant populations of dung beetles are able to perform their ecological function (acknowledging that body size and functional groups are also important factors [13,14]), but they are declining worldwide. The most serious adverse effects are land use changes, habitat fragmentation [15,16], and declining wild mammal species that provide feces [17,18], all of which transform and intensify traditional animal husbandry [19]. Overgrazing or abandoning pastures leads to a decrease in both abundance and species diversity [20,21,22]. The negative effects of agricultural intensification, and increased use of pesticides and insecticides, have already been demonstrated on a number of arthropod taxa [23], including dung beetles [24]. Numerous studies have shown that veterinary medical products, widely used in animal health today, and their breakdown products can cause drastic reductions in dung beetle populations as they appear in pastures through feces [14,25,26].
Dung beetles’ lifestyles and ecological needs make them sensitive to environmental changes and they are therefore an important indicator group used in more and more studies that assess the state of the environment worldwide [27]. Farming and nature conservation practices that sustain species-rich communities able to perform their ecological functions are essential to the successful conservation of dung beetles. There are, however, still significant knowledge gaps. For example, both grassland and forest dung beetles are well-studied globally, which is important as dung beetle assemblages of forests and grasslands. These habitats are different, e.g., in terms of total biomass, species and functional compositions, and their functions in dung removal [15]. Buse and Entling [28] also found that dung removal is significantly different between forests and grasslands. These are in line with the evidence that dung beetles are highly sensitive to habitat modifications [16] and habitat structure at the local and landscape scales [29,30]. There is, however, a unique European habitat, the wood pasture, a transition between forest and grassland, where trees are scattered across pastures [31]. Wood pastures are still present in the Mediterranean and in Eastern Europe as part of farming systems [32], and present a socio-ecological framework for sustainable agriculture with high biodiversity. Such a unique habitat may provide novel information on how dung beetle assemblages form.
In Europe, including Hungary, the biggest threat to dung beetles is the decline of traditional farming systems. It has changed in many ways since the middle of the 20th century [33,34]. Grazing livestock has been significantly reduced, leaving many former pastures abandoned, while extensive animal husbandry was mostly replaced by intensive livestock farming. Although wood pastures and forest grazing were also well-established as important traditional practices in the last century [35], today only open grasslands are grazed. Nowadays, only a few wood pastures are used actively, but more and more are being restored and grazed mainly for nature conservation purposes. Forest grazing in Hungary has gradually declined and was finally completely ceased in the 1960s [35]. All these trends changed the resources for dung beetles, making them a threatened, declining group.
The objective of our study was to determine the effects of habitat type, dung type, and season on species richness, abundance, diversity, and species composition of dung beetle assemblages. Our aims were as follows:
First, we wanted to find out where (i.e., in what habitat) do dung beetles occur? Our study compared three habitat types with different levels of woodland cover: open pastures, wood pastures, and forests. Wood pastures are acknowledged as valuable habitats for several other taxa [36,37], and we were interested in whether (i) they exhibit an outstanding diversity that harbors both forest and grassland species and thereby have their own specific species pool or if (ii) they represent a transitional situation for dung beetles.
Second, we were interested in what other factors influence dung beetle assemblages. A locality effect, for example, would suggest that the generalization of any results and their applicability at other locations are limited. In addition, to analyse the effect of location, we also studied the effect of dung type and season, both of which are known to influence dung beetles [17,30] and thus may provide additional information to understand the distribution of dung beetles across habitat types. In our study, we compared the dung beetle assemblages in the feces of the three most important grazing livestock in Europe: cattle, horse, and sheep taken in different seasons (spring, summer, and autumn).
Third, we intended to provide research evidence to guide nature conservation management of wood pastures for the preservation of dung beetle assemblages and this unique habitat. Although some such results were known already from other regions or continents, management, which is context dependent, including locality, cannot directly use those results.
2. Materials and Methods
2.1. Study Area
Our study was conducted in three predominantly forested landscapes in the Northern and Transdanubian Mountains of Hungary (sampling locations are given as KMZ (Keyhole Markup language Zipped) files for Google Earth in the Supplementary Materials). The selected pastures and wood pastures were actively grazed. In the forests, there was no livestock grazing; however, wild ungulates such as red deer, roe deer, and wild boar were common. The sites were selected to meet two main criteria. First, they were to include patches of actually grazed wood pastures, as it is the rarest habitat type. Second, there had to be both actively used pastures and seminatural forests in the vicinity (within 2.5 km).
2.1.1. Cserépfalu
The site is a ca. 100 ha area of grasslands and wood pastures, surrounded by forests within the Bükk National Park to the northeast of Cserépfalu at the altitude of 250–350 m above sea level. Two-thirds of the area is wood pasture, while one-third is open pasture. On the open pastures and in the lower parts of the wood pasture, ca. 40 Hungarian grey cattle have been grazed for some years before the time of our sampling. In the year preceding the study, about 200 sheep and some goats were introduced to the wood pasture and herded across the area two or three times a week.
2.1.2. Hollókő
The site is at 250–300 m above sea level within the Hollókő Landscape Protection Area, mostly to the west of the village of Hollókő. In Hollókő, as part of a habitat reconstruction program completed in 2013, nearly 20 hectares of afforested land were cleared and the former wooded pasture was restored. The relatively small wood pasture on the western slope of the Várhegy is completely surrounded by forests. Pastures and hay meadows are situated in larger blocks around the village. On the large western pastures, a herd of ca. 100 head of cattle has been grazed. These animals were herded through the wood pastures for a few times a year for short periods. One paddock on the grassland bordering the southeastern part of the village has been used to keep about 30 sheep and a couple of horses.
2.1.3. Balatonakali-Dörgicse
The site is between the villages of Balatonakali and Dörgicse, 145–240 m above sea level. The ca. 200 ha wood pasture is part of the Natura 2000 network. The western part of the area was cleared a few years ago and sparse wooded vegetation was left behind. The open pasture with a sheep paddock stretches more or less continuously along the southern border of the village of Dörgicse. The southwestern and northwestern parts of the area are covered by continuous forests. In the sheep paddock near Dörgicse, about 1000 head of sheep are kept. They are grazed in smaller flocks mostly on the open pasture and the nearest parts of the wood pasture.
2.2. Sampling Design and Dung Beetle Trapping
The dung beetle assemblages have been sampled three times in 2016, in spring (May), summer (July), and autumn (October). Sampling was carried out simultaneously in all three sites. In total, 81 pitfall trap units baited with either cattle, horse, or sheep dung (three of each for every habitat in all three locations) were installed. The pitfall traps baited with different dungs were placed as if they were vertices of a triangle with sides slightly more than 10 m. The location of the sampling plots was selected to meet two basic criteria: they should be at least 100 m away from each other [38] and the edge of the habitat type should be at least 50 m away. As far as the terrain allowed, traps were installed in locations sheltered by stumps, smaller trees, or shrubs to avoid damage caused by trampling.
The 1 L containers (diameter: 11 cm, height: 15 cm) were dug into the soil up to their rim. About 3 dl of diluted (50%) propylene-glycol was used as a preservative in each container. The mouth was covered by a hexagonal chicken wire (with a mesh diameter of 25 mm) that was fixed to the soil by U-shaped wires. The dung bait was wrapped in mosquito net and fixed to the wire mesh covering the mouth by bailing wire.
The dung used as bait was always collected freshly for each sampling period from animals grazing on the specific sites. The same batch of dung was used in each trap of a particular site. Livestock were not treated with anthelmintics prior to dung collection. The collected dung was portioned into 400 (cattle, horse) and 100 g (sheep) packs within laboratory conditions. Packs were sterilised by freezing at −20 ℃ for at least 72 h. The pitfall traps were emptied after one week. In one case, the container was dug up by animals; this sample was excluded from the analysis. Dung beetles were identified at the species level in the laboratory. Nomenclature follows the Catalogue of Palaearctic Coleoptera [39].
2.3. Data Analysis
We separately tested the effect of four explanatory variables (habitat, season, dung, and locality) on abundance and species richness by generalised linear mixed models (GLMMs; [40]) and on Shannon diversity using linear mixed models (LMMs), where the response variables were the number of species (with Poisson distribution), the abundance (with negative binomial distribution), or the calculated Shannon diversity (with Gaussian distribution) of dung beetles. Replicates (sampling plots with three pitfall traps) were included as random factors. We compared the models fitted with explanatory and random factors to null models (including the random factor only) by ANOVA [41].
Our main aim was to reveal the differences in dung beetle assemblages of forest, wood pasture, and pasture habitat types. Thus, first, we compared species richness, abundances, and Shannon diversity among habitat types by a pairwise comparison with Tukey corrections, based on models described above. Second, we created subsets of season and dung type (thereby creating 9 datasets both for abundances and for number of species) and separately analysed the effect of habitat type. We applied GLMMs and LMMs [40], where the response variables were the number of species, the abundance, or the diversity of dung beetles, while the explanatory variable was only the habitat type (forest, pasture, and wood pasture). Replicates within the locality were included as random factors, and we repeated these analyses for all 9 subsets (3 seasons × 3 dung types) of our dataset. We applied pairwise comparisons with Tukey corrections between habitat types based on model results.
We explored the differences between the composition of dung beetle assemblages with nonmetric multidimensional scaling (NMDS) using a Bray–Curtis similarity measure [42]. We applied a Permutational Multivariate Analysis of Variance (PERMANOVA) [42] to analyse the effect of habitat, season, locality, and dung type as explanatory variables on assemblage composition. We evaluated the association of dung beetle species to habitat, dung type, season, and locality separately, by indicator species analysis [42]. The indicator values of the species were tested via the Monte-Carlo simulation using 10,000 permutations. The accepted significance level was p < 0.05.
The statistical analyses were carried out using R 3.4.4 statistical environment [43]. We used “lme4” 1.1-17 and “MASS” 7.3-51.4 packages for models [44,45], “lsmeans” 2.27-62 and “multcomp” 1.4-10 packages for pairwise comparison [46,47], “vegan” 2.5-2. package for nonmetric multidimensional scaling [48], and “labdsv” ver. 1.8-0 for indicator species analysis [49].
3. Results
Altogether, we recorded 57 species and 78,131 individuals across the three locations (Cserépfalu: 48 species, 32,490 individuals, Hollókő: 46 species, 23,611 individuals, and Balatonakali: 44 species, 22,030 individuals). Three species of the family Geotrupidae, 33 species of the subfamily Aphodiinae, and 21 species of the subfamily Scarabaeinae were recorded (Appendix Table A1).
The most numerous was the Scarabaeinae subfamily (43,209 individuals, 55.3%), then the Aphodiinae subfamily (27,314 individuals, 35.0%), and finally the Geotrupidae family (7608 individuals, 9.7%). The most dominant species was Nimbus obliteratus, with 16,540 individuals collected, which was 21% of all individuals. In total, 18 species accounted for nearly 95% of the total abundance, while 39 species accounted for less than 1% (Appendix Table A1).
Our models did not reveal a significant effect of locality on species richness (p = 0.116), abundance (p = 0.383), or diversity (p = 0.145), while habitat had an effect on diversity (p = 0.001), but no effect on species number (p = 0.070) nor abundance (p = 0.302). Both dung type (p(sp) = 0.024, p(abu) = 0.001) and season (p(sp) < 0.001, p(abu) = 0.022, p(div) < 0.001) had a significant effect. However, we did not find an effect of dung type on diversity (p = 0.473).
Considering habitat types, 32,917 individuals of 41 species were recorded from forests, 20,724 individuals of 47 species from wood pastures, and 24,490 individuals of 53 species from grasslands. Species richness and Shannon diversity were lower in forests than in pastures. In the case of wood pastures, these values were between the two distinct habitats. There was no difference in abundances between the three habitats (Figure 1).
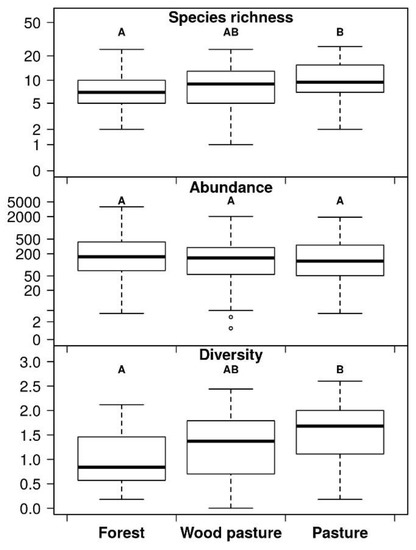
Figure 1.
Summarised dung beetle species richness, abundance, and Shannon diversity per habitat types. Box plots show medians (thick line), lower, and upper quartiles (boxes), whiskers include the range of distribution without outliers. Different letters above the boxes show the significant differences between habitats.
Pairwise comparisons of species richness, abundances, and Shannon diversities between habitat types revealed several significant differences between forests and pastures, while wood pastures exhibited an intermediate position (e.g., species richness for sheep dung during spring and summer), except for one case (spring abundance on cattle dung) where wood pasture dung beetle number was lower than in both habitats (Figure 2, Appendix Table A2).

Figure 2.
Habitat effect on dung beetle species richness, abundance, and diversity separately for dung type (columns) and seasons (rows). Box plots show medians (thick line), lower, and upper quartiles (boxes), whiskers include the range of distribution without outliers. Different letters above the boxes show the significant differences between habitats within a given season and dung type, applying generalised linear mixed models (GLMMs), linear mixed models (LMMs), and their pairwise comparisons. F.: forest, Wp.: wood pasture, and P.: pasture.
For composition of dung beetle assemblages, the seasonal distinction was the most noticeable effect according to the NMDS analysis (Figure 3A, PERMANOVA test: R2 = 0.237, p = 0.001). There was no overlap between forest and grassland dung beetle samples, but wood pastures had an intermediate position between the two other habitat types (Figure 3B, PERMANOVA test: R2 = 0.109, p = 0.001). Furthermore, assemblage composition showed significant differences among the three localities in Hungary (Figure 3C, PERMANOVA test: R2 = 0.081, p = 0.001), but the effect of locality was smaller compared to the effect of season and habitat. Dung type had no significant effect on the composition of dung beetle assemblages (Figure 3D, PERMANOVA test: R2 = 0.017, p = 0.322).
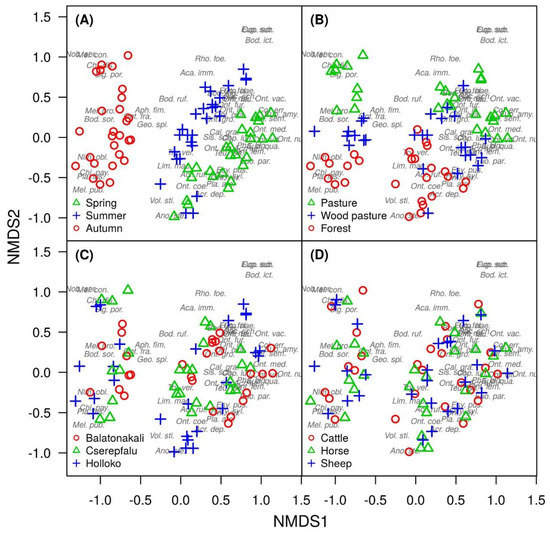
Figure 3.
Assembly composition of dung beetles according to (A) season, (B) habitat, (C) locality, and (D) dung type, applying nonmetric multidimensional scaling (NMDS).
The analysis of indicator species regarding habitat, dung type, and season showed that 12 species were linked to pastures, 6 to forests, and only one to wood pastures (Table 1). Only cattle feces had three indicator species, while the other two dung types had none. Spring had 20 indicator species, while summer and autumn had less than half of that (Table 1).

Table 1.
Dung beetle species with significant indicator values for habitat, dung type, and season.
4. Discussion
Our main question concerned whether wood pastures have distinct dung beetle assemblages compared to forests and pastures. Using various types of analyses to process data on around 80,000 individuals, we found that assemblages in wood pastures showed an intermediate or transient position between the assemblages of forests and pastures, which were distinct. In Central Europe, the majority of dung beetle species inhabit open or semiopen grasslands, and there are fewer woodland species [50] (Scarabaeinae: mostly grassland species, Geotrupidae: mostly forest species, Aphodiinae: both forest and grassland species [15]). Our study showed similar patterns. Dung beetle assemblages on wood pastures showed an intermediate position between forest and pasture assemblages in composition, species richness, and Shannon diversity. In general, this position was present in all but one comparison when broken down according to seasons and dung types. This transient position is also supported by the distribution of the indicator species, wherein we found only one dung beetle (Onthophagus joannae) species specific for the wood pastures from the total of 57 dung beetle species and total of 19 habitat indicator species. This pattern in assemblage characteristics probably had an effect on the decomposition function of dung beetles, as Buse and Entling [28] showed.
The role of habitat in structuring dung beetle assemblages is well-known. Several studies found differences in various parameters between forest and pasture assemblages. Ríaz-Díaz et al. [51] found significant differences in species composition between forest and grassland habitats. Jugovic et al. [52] found significant differences in species composition, species richness, and abundance among grazed and overgrown sites. Numa et al. [53] found significant differences for species composition, species dominance, and abundance between sites with wild versus livestock herbivory. All of these studies showed a strong effect of habitat on the structure of dung beetle assemblages. In our case, we also found significant differences between forests and pastures in species richness and Shannon diversity, but not for wood pastures and forests or wood pastures and pastures. This pattern is remarkable, as based on the literature. We may expect three different assemblages in the three different habitats. Similar studies, where forest, pasture, and a transitional habitat (usually shrubland) were involved, found significant differences between habitats, indicating that neither was in a transitional position [29,54]. It seems that this is the general pattern, as this was found for other taxa as well. For example, spider [37] and ground-dwelling beetle assemblages [55] of open pasture habitats and scattered trees (and other habitats) were statistically distinct. Lövei et al. [56] categorised carabids to the forest edge and matrix species as these habitats had distinct assemblages. The transitional position of wood pastures between forests and pastures is interesting, if compared to forest, forest edge, and grassland patterns. Such studies showed that all three habitats had different assemblages for carabids [57], ants and spiders [58], and millipedes [59]. Therefore, the habitat effect in the forest, wood pasture, and pasture system seems to be rather different from forest, edge, and pasture (or grassland) systems, highlighting the uniqueness of the former system.
Our second question was on the potential effects of locality, dung type, and season on assemblages. We found that dung beetle assemblages are similar across three distant locations in Hungary for all studied measures, namely for species number, abundance, Shannon diversity, and composition. The similarity of the three localities is probably linked to the overwhelming role of locally relevant factors in shaping dung beetle assemblages (and decomposition in general); thus, differences between habitat or management types are expected to be larger than differences between locations [60,61]. This similarity of dung beetle assemblages across locations indicates that our results are relevant to other locations (at least in the Pannonian region), which supports the wider applicability of our findings. The habitat effect and lack of locality effect on dung beetle assemblages are probably linked to vegetation characteristics. Several studies showed that vegetation structure (e.g., plant diversity [62]), fine-scale heterogeneity in grazing intensity [63], or vegetation cover [64] highly influenced dung beetle assemblages. Our results on habitat and locality can be explained on the assumption that differences between forests or pastures or wood pastures are smaller across locations than among the three habitat types within a given location.
The majority of Palearctic dung beetle species are considered to be generalists, feeding on various types of dung available. This suggests that a dung type effect on assemblage structure is likely weak. Indeed, we could not clearly identify distinct dung beetle assemblages across the three dung types, which is supported also by a low number of indicator species associated with each dung type. However, we found a significant effect of dung type on abundance and species number, indicating that even generalist species can show different preferences if they have a choice of several dung types [65].
Season seems to be a profound factor in shaping dung beetle assemblages. We already know from several studies that dung beetle species appear in a determined phenological order during the year [52,66,67]. We found a clear distinction in assemblage composition in spring, summer, and autumn for all assemblage measures. Indicator species are also in line with former results [68]. In autumn, the wood pasture samples were more distinct from forests and pastures, while both the spring and the summer samples showed a greater similarity among dung beetle assemblages in the three habitats, suggesting that the seasonality influenced the effect of habitats on dung beetles.
Dung beetle assemblage composition was different in this study for habitats, seasons, and dung types. It seems that composition is a sensitive measure of differences in dung beetle assemblages across a range of conditions [51,69].
The third aim of this study was to provide information for nature conservation management. Dung beetle assemblages of Hungary and the Pannonian biogeographic region are still understudied. During this one-year sampling in three locations, nearly half of the species of Hungarian dung beetle fauna were recorded; this seems to be outstanding for the results of the mosaic-like structural complexity of the studied habitats. The conservation of dung beetles should focus on maintaining compositional diversity, as different species have different roles, and the loss of a species cannot be compensated by the presence of others [70]. Species-specific thermal tolerance is very important [71], as is poor flight capacity limiting dispersal that potentially leads to local extinctions if habitats are further degraded (e.g., Storck-Tonon et al. [72]). The provisioning of fresh dung throughout the year—irrespective of what livestock species produces it—is of major importance for all habitats. The manure of wild ungulates is not sufficient to ensure the conservation of the regional dung beetle species pool [73]. From a practical point of view, this means that if we want to maintain species-rich communities and the associated ecosystem services, we can do so by increasing the availability of the right amount and quality of manure throughout the year. Extensive animal husbandry (i.e., grazing livestock from spring to late autumn) is one way to enhance the habitat for dung beetles. It is imperative for the conservation of diverse assemblages that grazing take place in the long term. Grazing continuity has a positive effect on species numbers both for generalists and specialists [74]. The use of veterinary medical products needs to be reconsidered to avoid harm to dung beetles [14]. Long-term conservation needs to consider climate change effects and connectivity of suitable dung beetle habitats to provide dispersal possibilities. Active conservation planning is needed, as only sufficiently stable and abundant populations as well as diverse assemblages of dung beetles are able to perform their ecological functions that benefit people [15,75].
5. Conclusions
We concluded that wood pastures are key habitats for dung beetle conservation, as they harbor dung beetle assemblages that show a transition between forest and grassland assemblages. Wood pastures should not be viewed as a refuge for all forest species [76], but as a transitional habitat where some forest and pasture species can be conserved into one habitat. Therefore, wood pastures must be maintained and their characteristically large trees need to be conserved and replaced if needed. Proper management by grazing livestock is also essential not only for dung beetles but for various ecologically important taxa. The conservation of such ecosystems should be a priority. Throughout Europe, there are already several completed or ongoing projects aimed at the restoration and conservation management of wood pastures. Such actions should be funded more widely for the benefits they provide both in terms of their unique biodiversity and local livelihoods not only in Europe, but worldwide.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4907/12/1/25/s1, Sampling locations (KMZ file).
Author Contributions
Conceptualization, L.S.; methodology, L.S.; field and lab work, L.S. and R.Á.; data curation, L.S., R.Á., and V.S.; formal analysis, V.S.; writing—original draft preparation, L.S., V.S., G.B., R.Á. and A.B.; writing—review and editing, L.S., V.S., G.B., R.Á. and A.B.; supervision, A.B.; funding acquisition, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Doctoral School of Environmental Sciences of the Szent István University, the “Sustainable use of ecosystem services—research for mitigating the negative effect of climate change, land use change and biological invasion” project (GINOP-2.3.2-15-2016-00019), and partly by the EU H2020 project SUPER-G (https://www.super-g.eu, grant agreement N. 774124). V.S. was funded by the National Research, Development and Innovation Office (FK 123813).
Acknowledgments
We would like to thank the two anonymous reviewers for their valuable comments. We would also like to thank Krisztián Harmos (Bükk National Park) for field assistance, as well as Brigitta Palotás and Anikó Zölei for their help in preparing the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
List of dung beetle species, and their abundances according to habitats at the three regions in Hungary (B—Balatonakali, H—Hollókő, C—Cserépfalu).
Table A1.
List of dung beetle species, and their abundances according to habitats at the three regions in Hungary (B—Balatonakali, H—Hollókő, C—Cserépfalu).
| Forest | Wood Pasture | Pasture | Total | % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbr. | B | H | C | ∑ | B | H | C | ∑ | B | H | C | ∑ | |||
| Geotrupidae | |||||||||||||||
| Anoplotrupes stercorosus (Scriba, 1791) | Ano_ste | 1 | 1671 | 117 | 1789 | 0 | 13 | 90 | 103 | 0 | 3 | 2 | 5 | 1897 | 2.43 |
| Geotrupes spiniger (Marsham, 1802) | Geo_spi | 29 | 9 | 9 | 47 | 18 | 1 | 13 | 32 | 23 | 6 | 16 | 45 | 124 | 0.16 |
| Trypocopris vernalis (Linnaeus, 1758) | Try_ver | 1534 | 156 | 914 | 2604 | 1741 | 148 | 709 | 2598 | 345 | 21 | 19 | 385 | 5587 | 7.15 |
| Scarabaeinae | |||||||||||||||
| Caccobius schreberi (Linnaeus, 1767) | Cac_sch | 0 | 0 | 0 | 0 | 442 | 10 | 0 | 452 | 685 | 231 | 7 | 923 | 1375 | 1.76 |
| Copris lunaris (Linnaeus, 1758) | Cop_lun | 11 | 1 | 15 | 27 | 14 | 1 | 49 | 64 | 33 | 12 | 74 | 119 | 210 | 0.27 |
| Euoniticellus fulvus (Goeze, 1777) | Euo_ful | 0 | 0 | 0 | 0 | 26 | 6 | 46 | 78 | 73 | 163 | 274 | 510 | 588 | 0.75 |
| Euonthophagus amyntas (Olivier, 1789) | Euo_amy | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 4 | 11 | 0 | 0 | 11 | 15 | 0.02 |
| Onthophagus coenobita (Herbst, 1783) | Ont_coe | 321 | 294 | 1159 | 1774 | 31 | 326 | 136 | 493 | 11 | 78 | 29 | 118 | 2385 | 3.05 |
| Onthophagus fracticornis (Preyssler, 1790) | Ont_fra | 15 | 20 | 1451 | 1486 | 176 | 224 | 470 | 870 | 288 | 103 | 836 | 1227 | 3583 | 4.59 |
| Onthophagus furcatus (Fabricius, 1781) | Ont_fur | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 4 | 4 | 7 | 0.01 |
| Onthophagus grossepunctatus Reitter, 1905 | Ont_gro | 226 | 0 | 157 | 383 | 2301 | 6 | 799 | 3106 | 1504 | 281 | 564 | 2349 | 5838 | 7.47 |
| Onthophagus illyricus (Scopoli, 1763) | Ont_ill | 11 | 2 | 41 | 54 | 153 | 70 | 164 | 387 | 372 | 1309 | 767 | 2448 | 2889 | 3.70 |
| Onthophagus joannae Goljan, 1953 | Ont_joa | 0 | 0 | 0 | 0 | 0 | 10 | 41 | 51 | 0 | 0 | 10 | 10 | 61 | 0.08 |
| Onthophagus lemur (Fabricius, 1781) | Ont_lem | 20 | 0 | 10 | 30 | 207 | 0 | 2 | 209 | 111 | 1 | 3 | 115 | 354 | 0.45 |
| Onthophagus medius (Kugelann, 1792) | Ont_med | 0 | 2 | 0 | 2 | 33 | 12 | 0 | 45 | 460 | 1190 | 1 | 1651 | 1698 | 2.17 |
| Onthophagus nuchicornis (Linnaeus, 1758) | Ont_nuc | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0.00 |
| Onthophagus ovatus (Linnaeus, 1767) | Ont_ova | 2 | 10 | 3 | 15 | 63 | 189 | 357 | 609 | 96 | 2614 | 617 | 3327 | 3951 | 5.06 |
| Onthophagus ruficapillus Brulle, 1832 | Ont_ruf | 1 | 0 | 2 | 3 | 403 | 6 | 15 | 424 | 1436 | 1049 | 160 | 2645 | 3072 | 3.93 |
| Onthophagus semicornis (Panzer, 1798) | Ont_sem | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 2 | 0.00 |
| Onthophagus taurus (Schreber, 1759) | Ont_tau | 1 | 1 | 2 | 4 | 49 | 17 | 9 | 75 | 179 | 1078 | 98 | 1355 | 1434 | 1.84 |
| Onthophagus vacca (Linnaeus, 1767) | Ont_vac | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 7 | 1 | 17 | 17 | 0.02 |
| Onthophagus verticicornis (Laicharting, 1781) | Ont_ver | 1003 | 101 | 5945 | 7049 | 89 | 81 | 1111 | 1281 | 95 | 448 | 475 | 1018 | 9348 | 11.96 |
| Onthophagus vitulus (Fabricius, 1777) | Ont_vit | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0.00 |
| Sisyphus schaefferi (Linnaeus, 1758) | Sis_sch | 751 | 8 | 796 | 1555 | 713 | 49 | 1943 | 2705 | 943 | 642 | 535 | 2120 | 6380 | 8.17 |
| Aphodiinae | |||||||||||||||
| Acanthobodilus immundus (Creutzer, 1799) | Aca_imm | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 2 | 0 | 27 | 0 | 27 | 30 | 0.04 |
| Acrossus depressus (Kugelann, 1792) | Acr_dep | 1 | 4 | 20 | 25 | 0 | 1 | 1 | 2 | 0 | 0 | 3 | 3 | 30 | 0.04 |
| Acrossus luridus (Fabricius, 1775) | Acr_lur | 0 | 1 | 8 | 9 | 7 | 88 | 23 | 118 | 7 | 276 | 39 | 322 | 449 | 0.57 |
| Acrossus rufipes (Linnaeus, 1758) | Acr_ruf | 0 | 2 | 27 | 29 | 0 | 0 | 14 | 14 | 0 | 0 | 7 | 7 | 50 | 0.06 |
| Aphodius fimetarius (Linnaeus, 1758) | Aph_fim | 0 | 0 | 4 | 4 | 0 | 2 | 5 | 7 | 1 | 1 | 20 | 22 | 33 | 0.04 |
| Bodiloides ictericus (Laicharting, 1781) | Bod_ict | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 7 | 7 | 0.01 |
| Bodilopsis rufa (Moll, 1782) | Bod_ruf | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 0.00 |
| Bodilopsis sordida (Fabricius, 1775) | Bod_sor | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 3 | 0 | 3 | 5 | 0.01 |
| Bodilus lugens (Creutzer, 1799) | Bod_lug | 0 | 0 | 0 | 0 | 112 | 0 | 0 | 112 | 9 | 2 | 0 | 11 | 123 | 0.16 |
| Calamosternus granarius (Linnaeus, 1767) | Cal_gra | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 3 | 0.00 |
| Chilothorax distinctus (O. F. Muller, 1776) | Chi_dis | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 19 | 3 | 2 | 24 | 25 | 0.03 |
| Chilothorax paykulli (Bedel, 1908) | Chi_pay | 0 | 0 | 44 | 44 | 1 | 0 | 0 | 1 | 3 | 0 | 0 | 3 | 48 | 0.06 |
| Colobopterus erraticus (Linnaeus, 1758) | Col_err | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 6 | 81 | 2 | 89 | 91 | 0.12 |
| Coprimorphus scrutator (Herbst, 1789) | Cop_scr | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0.00 |
| Esymus pusillus (Herbst, 1789) | Esy_pus | 3 | 29 | 22 | 54 | 6 | 36 | 69 | 111 | 11 | 174 | 99 | 284 | 449 | 0.57 |
| Eudolus quadriguttatus (Herbst, 1783) | Euo_qua | 0 | 0 | 3 | 3 | 4 | 0 | 5 | 9 | 22 | 0 | 10 | 32 | 44 | 0.06 |
| Euorodalus paracoenosus (Balthasar and Hrubant, 1960) | Euo_par | 2 | 0 | 20 | 22 | 28 | 0 | 49 | 77 | 29 | 13 | 17 | 59 | 158 | 0.20 |
| Eupleurus subterraneus (Linnaeus, 1758) | Eup_sub | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0.00 |
| Limarus maculatus (Sturm, 1800) | Lim_mac | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.00 |
| Melinopterus consputus (Creutzer, 1799) | Mel_con | 0 | 4 | 2 | 6 | 9 | 2 | 1 | 12 | 54 | 1715 | 446 | 2215 | 2233 | 2.86 |
| Melinopterus prodromus (Brahm, 1790) | Mel_pro | 31 | 22 | 3 | 56 | 60 | 90 | 13 | 163 | 301 | 139 | 23 | 463 | 682 | 0.87 |
| Melinopterus pubescens (Sturm, 1800) | Mel_pub | 0 | 280 | 1 | 281 | 0 | 9 | 0 | 9 | 0 | 0 | 0 | 0 | 290 | 0.37 |
| Nimbus obliteratus (Panzer, 1823) | Nim_obl | 1869 | 2133 | 6801 | 10,803 | 1082 | 3681 | 849 | 5612 | 66 | 54 | 5 | 125 | 16,540 | 21.17 |
| Nobius serotinus (Panzer, 1799) | Nob_ser | 0 | 3 | 0 | 3 | 0 | 1 | 0 | 1 | 0 | 155 | 10 | 165 | 169 | 0.22 |
| Otophorus haemorrhoidalis (Linnaeus, 1758) | Oto_hae | 0 | 0 | 1 | 1 | 0 | 0 | 4 | 4 | 1 | 59 | 9 | 69 | 74 | 0.09 |
| Oxyomus sylvestris (Scopoli, 1763) | Oxy_syl | 6 | 1 | 7 | 14 | 9 | 0 | 4 | 13 | 1 | 3 | 1 | 5 | 32 | 0.04 |
| Phalacronothus biguttatus (Germar, 1824) | Pha_big | 1 | 0 | 0 | 1 | 1 | 0 | 3 | 4 | 10 | 1 | 0 | 11 | 16 | 0.02 |
| Plagiogonus arenarius (Olivier, 1789) | Pla_are | 272 | 70 | 597 | 939 | 275 | 3 | 300 | 578 | 37 | 2 | 74 | 113 | 1630 | 2.09 |
| Planolinus fasciatus (Olivier, 1789) | Pla_fas | 554 | 105 | 198 | 857 | 1 | 0 | 7 | 8 | 0 | 0 | 0 | 0 | 865 | 1.11 |
| Rhodaphodius foetens (Fabricius, 1787) | Rho_foe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0.00 |
| Sigorus porcus (Fabricius, 1792) | Sig_por | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 3 | 0 | 0 | 2 | 2 | 5 | 0.01 |
| Teuchestes fossor (Linnaeus, 1758) | Teu_fos | 0 | 1 | 1 | 2 | 0 | 1 | 0 | 1 | 0 | 8 | 0 | 8 | 11 | 0.01 |
| Volinus sticticus (Panzer, 1798) | Vol_sti | 49 | 1593 | 1293 | 2935 | 0 | 27 | 242 | 269 | 0 | 10 | 1 | 11 | 3215 | 4.11 |
| Abundance | 6715 | 6525 | 19,677 | 32,917 | 8063 | 5114 | 7547 | 20,724 | 7252 | 11,972 | 5266 | 24,490 | 78,131 | 100.00 | |
| Species number | 25 | 28 | 35 | 41 | 34 | 32 | 34 | 47 | 35 | 41 | 42 | 53 | 57 | ||

Table A2.
Significance values for the comparisons in Figure 2, on dung beetle abundance and species number according to dung type, season and habitat (* p ≤ 0.05,** p ≤ 0.01,*** p ≤ 0.001).
Table A2.
Significance values for the comparisons in Figure 2, on dung beetle abundance and species number according to dung type, season and habitat (* p ≤ 0.05,** p ≤ 0.01,*** p ≤ 0.001).
| Contrast | Adjusted p-Value | |||||
|---|---|---|---|---|---|---|
| Abundance | Number of Species | Diversity | ||||
| Cattle | Spring | Forest | Wood pasture | 0.0037 ** | 0.0124 * | <0.0001 *** |
| Forest | Pasture | 0.9996 | 0.0001 *** | <0.0001 *** | ||
| Wood pasture | Pasture | 0.0053 ** | 0.3134 | 0.2063 | ||
| Summer | Forest | Wood pasture | 0.7217 | 0.6062 | 0.1177 | |
| Forest | Pasture | 0.0083 ** | 0.0123 * | 0.0055 ** | ||
| Wood pasture | Pasture | 0.0701 | 0.1337 | 0.3470 | ||
| Autumn | Forest | Wood pasture | 0.4623 | 0.8890 | 0.4605 | |
| Forest | Pasture | 0.0067 ** | 0.5829 | 0.0024 ** | ||
| Wood pasture | Pasture | 0.1523 | 0.8565 | 0.0380 * | ||
| Horse | Spring | Forest | Wood pasture | 0.2544 | 0.0764 | 0.0106 * |
| Forest | Pasture | 0.0995 | 0.1767 | 0.0214 * | ||
| Wood pasture | Pasture | 0.8438 | 0.9165 | 0.9486 | ||
| Summer | Forest | Wood pasture | 0.8803 | 0.4303 | 0.1982 | |
| Forest | Pasture | 0.2188 | 0.1953 | 0.0443 * | ||
| Wood pasture | Pasture | 0.5154 | 0.8739 | 0.7153 | ||
| Autumn | Forest | Wood pasture | 0.8827 | 0.9741 | 0.9830 | |
| Forest | Pasture | 0.0344 * | 0.6473 | 0.5260 | ||
| Wood pasture | Pasture | 0.1058 | 0.7753 | 0.6300 | ||
| Sheep | Spring | Forest | Wood pasture | 0.8016 | 0.1234 | 0.0473 * |
| Forest | Pasture | 0.9111 | 0.0004 *** | 0.0001 *** | ||
| Wood pasture | Pasture | 0.9834 | 0.1358 | 0.0537 | ||
| Summer | Forest | Wood pasture | 0.4881 | 0.4314 | 0.3512 | |
| Forest | Pasture | 0.4728 | 0.0420 * | 0.0166 * | ||
| Wood pasture | Pasture | 0.9993 | 0.4548 | 0.2640 | ||
| Autumn | Forest | Wood pasture | 0.0843 | 0.5931 | 0.9162 | |
| Forest | Pasture | 0.4491 | 0.7658 | 0.0278 * | ||
| Wood pasture | Pasture | 0.6574 | 0.2209 | 0.0114 * | ||
References
- Berendes, D.M.; Yang, P.J.; Lai, A.; Hu, D.; Brown, J. Estimation of global recoverable human and animal faecal biomass. Nat. Sustain. 2018, 1, 679–685. [Google Scholar] [CrossRef]
- Hanski, I.; Cambefort, Y. Dung Beetle Ecology; Princeton University Press: Princeton, NJ, USA, 1991; ISBN 978-0-691-08739-9. [Google Scholar]
- Nichols, E.; Spector, S.; Louzada, J.; Larsen, T.; Amezquita, S.; Favila, M. Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol. Conserv. 2008, 141, 1461–1474. [Google Scholar] [CrossRef]
- Holter, P. Herbivore dung as food for dung beetles: Elementary coprology for entomologists. Ecol. Entomol. 2016, 41, 367–377. [Google Scholar] [CrossRef]
- Tixier, T.; Bloor, J.M.G.; Lumaret, J.-P. Species-specific effects of dung beetle abundance on dung removal and leaf litter decomposition. Acta Oecol. 2015, 69, 31–34. [Google Scholar] [CrossRef]
- Slade, E.M.; Roslin, T.; Santalahti, M.; Bell, T. Disentangling the ‘brown world’ faecal-detritus interaction web: Dung beetle effects on soil microbial properties. Oikos 2016, 125, 629–635. [Google Scholar] [CrossRef]
- Bang, H.S.; Lee, J.-H.; Kwon, O.S.; Na, Y.E.; Jang, Y.S.; Kim, W.H. Effects of paracoprid dung beetles (Coleoptera: Scarabaeidae) on the growth of pasture herbage and on the underlying soil. Appl. Soil Ecol. 2005, 29, 165–171. [Google Scholar] [CrossRef]
- Brown, J.; Scholtz, C.H.; Janeau, J.-L.; Grellier, S.; Podwojewski, P. Dung beetles (Coleoptera: Scarabaeidae) can improve soil hydrological properties. Appl. Soil Ecol. 2010, 46, 9–16. [Google Scholar] [CrossRef]
- Ridsdill-Smith, T.J.; Edwards, P.B. Biological Control: Ecosystem Functions Provided by Dung Beetles. In Ecology and Evolution of Dung Beetles; Simmons, L.E., Ridsdill-Smith, T.J., Eds.; Wiley-Blackwell: Chichester, UK, 2011; pp. 245–266. ISBN 978-1-4443-4200-0. [Google Scholar]
- D’Hondt, B.; Bossuyt, B.; Hoffmann, M.; Bonte, D. Dung beetles as secondary seed dispersers in a temperate grassland. Basic Appl. Ecol. 2008, 9, 542–549. [Google Scholar] [CrossRef]
- Losey, J.E.; Vaughan, M. The Economic Value of Ecological Services Provided by Insects. BioScience 2006, 56, 311–323. [Google Scholar] [CrossRef]
- Beynon, S.A.; Wainwright, W.; Christie, M. The application of an ecosystem services framework to estimate the economic value of dung beetles to the U.K. cattle industry. Ecol. Entomol. 2015, 40, 124–135. [Google Scholar] [CrossRef]
- Tonelli, M.; Verdú, J.R.; Morelli, F.; Zunino, M. Dung beetles: Functional identity, not functional diversity, accounts for ecological process disruption caused by the use of veterinary medical products. J. Insect Conserv. 2020, 24, 643–654. [Google Scholar] [CrossRef]
- Verdu, J.R.; Lobo, J.M.; Sánchez-Piñero, F.; Gallego, B.; Numa, C.; Lumaret, J.-P.; Cortez, V.; Ortiz, A.J.; Tonelli, M.; García-Teba, J.P.; et al. Ivermectin residues disrupt dung beetle diversity, soil properties and ecosystem functioning: An interdisciplinary field study. Sci. Total Environ. 2018, 618, 219–228. [Google Scholar] [CrossRef]
- Frank, K.; Hülsmann, M.; Assmann, T.; Schmitt, T.; Blüthgen, N. Land use affects dung beetle communities and their ecosystem service in forests and grasslands. Agric. Ecosyst. Environ. 2017, 243, 114–122. [Google Scholar] [CrossRef]
- Nichols, E.; Larsen, T.; Spector, S.; Davis, A.L.; Escobar, F.; Favila, M.; Vulinec, K. Global dung beetle response to tropical forest modification and fragmentation: A quantitative literature review and meta-analysis. Biol. Conserv. 2007, 137, 1–19. [Google Scholar] [CrossRef]
- Nichols, E.; Gardner, T.A.; Peres, C.A.; Spector, S. The Scarabaeinae Research Network Co-declining mammals and dung beetles: An impending ecological cascade. Oikos 2009, 118, 481–487. [Google Scholar] [CrossRef]
- Bogoni, J.A.; Da Silva, P.G.; Peres, C.A. Co-declining mammal–dung beetle faunas throughout the Atlantic Forest biome of South America. Ecography 2019, 42, 1803–1818. [Google Scholar] [CrossRef]
- Hutton, S.A.; Giller, P.S. The effects of the intensification of agriculture on northern temperate dung beetle communities. J. Appl. Ecol. 2003, 40, 994–1007. [Google Scholar] [CrossRef]
- Negro, M.; Rolando, A.; Palestrini, C. The Impact of Overgrazing on Dung Beetle Diversity in the Italian Maritime Alps. Environ. Entomol. 2011, 40, 1081–1092. [Google Scholar] [CrossRef]
- Tonelli, M.; Verdu, J.R.; Zunino, M. Effects of the progressive abandonment of grazing on dung beetle biodiversity: Body size matters. Biodivers. Conserv. 2018, 27, 189–204. [Google Scholar] [CrossRef]
- Tonelli, M.; Verdú, J.R.; Zunino, M. Grazing abandonment and dung beetle assemblage composition: Reproductive behaviour has something to say. Ecol. Indic. 2019, 96, 361–367. [Google Scholar] [CrossRef]
- Seibold, S.; Gossner, M.M.; Simons, N.K.; Blüthgen, N.; Müller, J.; Ambarlı, D.; Ammer, C.; Bauhus, J.; Fischer, M.; Habel, J.C.; et al. Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 2019, 574, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Geiger, F.; van der Lubbe, S.C.T.M.; Brunsting, A.M.H.; de Snoo, G.R. Insect abundance in cow dung pats of different farming systems. Entomol. Ber. 2010, 70, 106–110. [Google Scholar]
- Lumaret, J.-P.; Errouissi, F. Use of anthelmintics in herbivores and evaluation of risks for the non target fauna of pastures. Vet. Res. 2002, 33, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Pecenka, J.R.; Lundgren, J.G. Effects of herd management and the use of ivermectin on dung arthropod communities in grasslands. Basic Appl. Ecol. 2019, 40, 19–29. [Google Scholar] [CrossRef]
- Spector, S. Scarabaeine Dung Beetles (coleoptera: Scarabaeidae: Scarabaeinae): An Invertebrate Focal Taxon for Biodiversity Research and Conservation. Coleopt. Bull. 2006, 60, 71–83. [Google Scholar] [CrossRef]
- Buse, J.; Entling, M.H. Stronger dung removal in forests compared with grassland is driven by trait composition and biomass of dung beetles. Ecol. Entomol. 2020, 45, 223–231. [Google Scholar] [CrossRef]
- Numa, C.; Verdú, J.R.; Sánchez, A.; Galante, E. Effect of landscape structure on the spatial distribution of Mediterranean dung beetle diversity. Divers. Distrib. 2009, 15, 489–501. [Google Scholar] [CrossRef]
- Roslin, T.; Viljanen, H. Dung Beetle Populations: Structure and Consequences. In Ecology and Evolution of Dung Beetles; Simmons, L.E., Ridsdill-Smith, T.J., Eds.; Wiley-Blackwell: Chichester, UK, 2011; pp. 220–244. ISBN 978-1-4443-4200-0. [Google Scholar]
- Hartel, T.; Plieninger, T. European Wood-Pastures in Transition: A Social-Ecological Approach; Routledge: Abingdon, UK, 2014; ISBN 978-1-135-13911-7. [Google Scholar]
- Plieninger, T.; Hartel, T.; Martín-López, B.; Beaufoy, G.; Bergmeier, E.; Kirby, K.; Montero, M.J.; Moreno, G.; Oteros-Rozas, E.; Van Uytvanck, J. Wood-pastures of Europe: Geographic coverage, social–ecological values, conservation management, and policy implications. Biol. Conserv. 2015, 190, 70–79. [Google Scholar] [CrossRef]
- Stoate, C.; Báldi, A.; Beja, P.; Boatman, N.; Herzon, I.; Van Doorn, A.; De Snoo, G.; Rakosy, L.; Ramwell, C. Ecological impacts of early 21st century agricultural change in Europe—A review. J. Environ. Manag. 2009, 91, 22–46. [Google Scholar] [CrossRef]
- Mihók, B.; Biró, M.; Molnár, Z.; Kovacs, E.; Bölöni, J.; Erős, T.; Standovár, T.; Török, P.; Csorba, G.; Margóczi, K.; et al. Biodiversity on the waves of history: Conservation in a changing social and institutional environment in Hungary, a post-soviet EU member state. Biol. Conserv. 2017, 211, 67–75. [Google Scholar] [CrossRef]
- Varga, A.; Molnár, Z.; Biró, M.; Demeter, L.; Gellény, K.; Miókovics, E.; Molnár, Á.; Molnár, K.; Ujházy, N.; Ulicsni, V.; et al. Changing year-round habitat use of extensively grazing cattle, sheep and pigs in East-Central Europe between 1940 and 2014: Consequences for conservation and policy. Agric. Ecosyst. Environ. 2016, 234, 142–153. [Google Scholar] [CrossRef]
- Hartel, T.; Hanspach, J.; Abson, D.; Máthé, O.; Moga, C.I.; Fischer, J. Bird communities in traditional wood-pastures with changing management in Eastern Europe. Basic Appl. Ecol. 2014, 15, 385–395. [Google Scholar] [CrossRef]
- Gallé, R.; Urák, I.; Nikolett, G.-S.; Hartel, T. Sparse trees and shrubs confers a high biodiversity to pastures: Case study on spiders from Transylvania. PLoS ONE 2017, 12, e0183465. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.G.; Hernández, M.I.M. Spatial Patterns of Movement of Dung Beetle Species in a Tropical Forest Suggest a New Trap Spacing for Dung Beetle Biodiversity Studies. PLoS ONE 2015, 10, e0126112. [Google Scholar] [CrossRef] [PubMed]
- Löbl, I.; Löbl, D. Scarabaeoidea–Scirtoidea–Dascilloidea–Buprestoidea–Byrrhoidea: Revised and Updated Edition; Catalogue of Palaearctic Coleoptera; Brill: Leiden, The Netherlands, 2016; ISBN 978-90-04-30914-2. [Google Scholar]
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Statistics for Biology and Health; Springer: New York, NY, USA, 2009; ISBN 978-0-387-87457-9. [Google Scholar]
- Chambers, J.M.; Hastie, T. Statistical Models in S; Wadsworth & Brooks/Cole Advanced Books & Software: Monterey, CA, USA, 1992; ISBN 978-0-534-16764-6. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R, 2nd ed.; Use R!; Springer International Publishing: New York, NY, USA, 2018; ISBN 978-3-319-71403-5. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2018. Available online: https://www.r-project.org (accessed on 20 November 2020).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Statistics and Computing; Springer: New York, NY, USA, 2002; ISBN 978-0-387-95457-8. [Google Scholar]
- Lenth, R.V. Least-Squares Means: The R Package lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biometr. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 20 November 2020).
- Roberts, D.W. Labdsv: Ordination and Multivariate Analysis for Ecology. 2019. Available online: https://CRAN.R-project.org/package=labdsv (accessed on 20 November 2020).
- Buse, J.; Šlachta, M.; Sladecek, F.X.J.; Carpaneto, G.M. Summary of the morphological and ecological traits of Central European dung beetles. Entomol. Sci. 2018, 21, 315–323. [Google Scholar] [CrossRef]
- Ríos-Díaz, C.L.; Moreno, C.E.; Ortega-Martínez, I.J.; Zuria, I.; Escobar, F.; Castellanos, I. Sheep herding in small grasslands promotes dung beetle diversity in a mountain forest landscape. J. Insect Conserv. 2020. [Google Scholar] [CrossRef]
- Jugovic, J.; Koren, T.; Koprivnikar, N. Competition and Seasonal Co-Existence of Coprophagous Scarabaeoidea (Coleoptera) in Differently Managed Habitat Patches of Sub-Mediterranean Grasslands in Slovenia. Pol. J. Ecol. 2019, 67, 247–263. [Google Scholar] [CrossRef]
- Numa, C.; Verdú, J.R.; Rueda, C.; Galante, E. Comparing Dung Beetle Species Assemblages Between Protected Areas and Adjacent Pasturelands in a Mediterranean Savanna Landscape. Rangel. Ecol. Manag. 2012, 65, 137–143. [Google Scholar] [CrossRef]
- Tocco, C.; Negro, M.; Rolando, A.; Palestrini, C. Does natural reforestation represent a potential threat to dung beetle diversity in the Alps? J. Insect Conserv. 2013, 17, 207–217. [Google Scholar] [CrossRef]
- Barton, P.S.; Manning, A.D.; Gibb, H.; Lindenmayer, D.B.; Cunningham, S.A. Conserving ground-dwelling beetles in an endangered woodland community: Multi-scale habitat effects on assemblage diversity. Biol. Conserv. 2009, 142, 1701–1709. [Google Scholar] [CrossRef]
- Lövei, G.L.; Magura, T.; Tóthmérész, B.; Ködöböcz, V. The influence of matrix and edges on species richness patterns of ground beetles (Coleoptera: Carabidae) in habitat islands. Glob. Ecol. Biogeogr. 2006, 15, 283–289. [Google Scholar] [CrossRef]
- Magura, T. Carabids and forest edge: Spatial pattern and edge effect. For. Ecol. Manag. 2002, 157, 23–37. [Google Scholar] [CrossRef]
- Gallé, R.; Kanizsai, O.; Ács, V.; Molnár, B. Functioning of Ecotones—Spiders and Ants of Edges between Native and Non-Native Forest Plantations. Pol. J. Ecol. 2014, 62, 815–820. [Google Scholar] [CrossRef]
- Bogyó, D.; Magura, T.; Nagy, D.D.; Tóthmérész, B. Distribution of millipedes (Myriapoda, Diplopoda) along a forest interior–forest edge–grassland habitat complex. ZooKeys 2015, 510, 181–195. [Google Scholar] [CrossRef]
- Piccini, I.; Palestrini, C.; Rolando, A.; Roslin, T. Local management actions override farming systems in determining dung beetle species richness, abundance and biomass and associated ecosystem services. Basic Appl. Ecol. 2019, 41, 13–21. [Google Scholar] [CrossRef]
- Bradford, M.A.; Berg, B.; Maynard, D.S.; Wieder, W.R.; Wood, S.A. Understanding the dominant controls on litter decomposition. J. Ecol. 2016, 104, 229–238. [Google Scholar] [CrossRef]
- Von Hoermann, C.; Weithmann, S.; Deißler, M.; Ayasse, M.; Steiger, S. Forest habitat parameters influence abundance and diversity of cadaver-visiting dung beetles in Central Europe. R. Soc. Open Sci. 2020, 7, 191722. [Google Scholar] [CrossRef]
- Perrin, W.; Moretti, M.; Vergnes, A.; Borcard, D.; Jay-Robert, P. Response of dung beetle assemblages to grazing intensity in two distinct bioclimatic contexts. Agric. Ecosyst. Environ. 2020, 289, 106740. [Google Scholar] [CrossRef]
- Errouissi, F.; Jay-Robert, P. Consequences of habitat change in euromediterranean landscapes on the composition and diversity of dung beetle assemblages (Coleoptera, Scarabaeoidea). J. Insect Conserv. 2019, 23, 15–28. [Google Scholar] [CrossRef]
- Dormont, L.; Rapior, S.; McKey, D.B.; Lumaret, J.-P. Influence of dung volatiles on the process of resource selection by coprophagous beetles. Chemoecology 2007, 17, 23–30. [Google Scholar] [CrossRef]
- Wassmer, T. Seasonality of Coprophagous Beetles in the Kaiserstuhl Area near Freiburg (Sw Germany) Including the Winter Months. Acta Oecol. Int. J. Ecol. 1994, 15, 607–631. [Google Scholar]
- Galante, E.; Mena, J.; Lumbreras, C. Dung Beetles (Coleoptera: Scarabaeidae, Geotrupidae) Attracted to Fresh Cattle Dung in Wooded and Open Pasture. Environ. Entomol. 1995, 24, 1063–1068. [Google Scholar] [CrossRef]
- Agoglitta, R.; Moreno, C.E.; Zunino, M.; Bonsignori, G.; Dellacasa, M. Cumulative annual dung beetle diversity in Mediterranean seasonal environments. Ecol. Res. 2012, 27, 387–395. [Google Scholar] [CrossRef]
- Senyuz, Y.; Lobo, J.M.; Dindar, K. Altitudinal gradient in species richness and composition of dung beetles (Coleoptera: Scarabaeidae) in an eastern Euro-Mediterranean locality: Functional, seasonal and habitat influences. Eur. J. Entomol. 2019, 116, 309–319. [Google Scholar] [CrossRef]
- Piccini, I.; Caprio, E.; Palestrini, C.; Rolando, A. Ecosystem functioning in relation to species identity, density, and biomass in two tunneller dung beetles. Ecol. Entomol. 2020, 45, 311–320. [Google Scholar] [CrossRef]
- Birkett, A.J.; Blackburn, G.A.; Menéndez, R. Linking species thermal tolerance to elevational range shifts in upland dung beetles. Ecography 2018, 41, 1510–1519. [Google Scholar] [CrossRef]
- Storck-Tonon, D.; Da Silva, R.J.; Sawaris, L.; Vaz-De-Mello, F.Z.; Da Silva, D.J.; Peres, C.A. Habitat patch size and isolation drive the near-complete collapse of Amazonian dung beetle assemblages in a 30-year-old forest archipelago. Biodivers. Conserv. 2020, 29, 2419–2438. [Google Scholar] [CrossRef]
- Jay-Robert, P.; Niogret, J.; Errouissi, F.; Labarussias, M.; Paoletti, É.; Luis, M.V.; Lumaret, J.-P. Relative efficiency of extensive grazing vs. wild ungulates management for dung beetle conservation in a heterogeneous landscape from Southern Europe (Scarabaeinae, Aphodiinae, Geotrupinae). Biol. Conserv. 2008, 141, 2879–2887. [Google Scholar] [CrossRef]
- Buse, J.; Šlachta, M.; Sladecek, F.X.; Pung, M.; Wagner, T.; Entling, M.H. Relative importance of pasture size and grazing continuity for the long-term conservation of European dung beetles. Biol. Conserv. 2015, 187, 112–119. [Google Scholar] [CrossRef]
- Forgie, S.A.; Paynter, Q.; Zhao, Z.; Flowers, C.; Fowler, S. Newly released non-native dung beetle species provide enhanced ecosystem services in New Zealand pastures. Ecol. Entomol. 2018, 43, 431–439. [Google Scholar] [CrossRef]
- Vojta, J.; Drhovská, L. Are abandoned wooded pastures suitable refugia for forest species? J. Veg. Sci. 2012, 23, 880–891. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).