Modelling Current and Future Potential Habitats for Plantations of Eucalyptus grandis Hill ex Maiden and E. dunnii Maiden in Uruguay
Abstract
1. Introduction
2. Materials and Methods
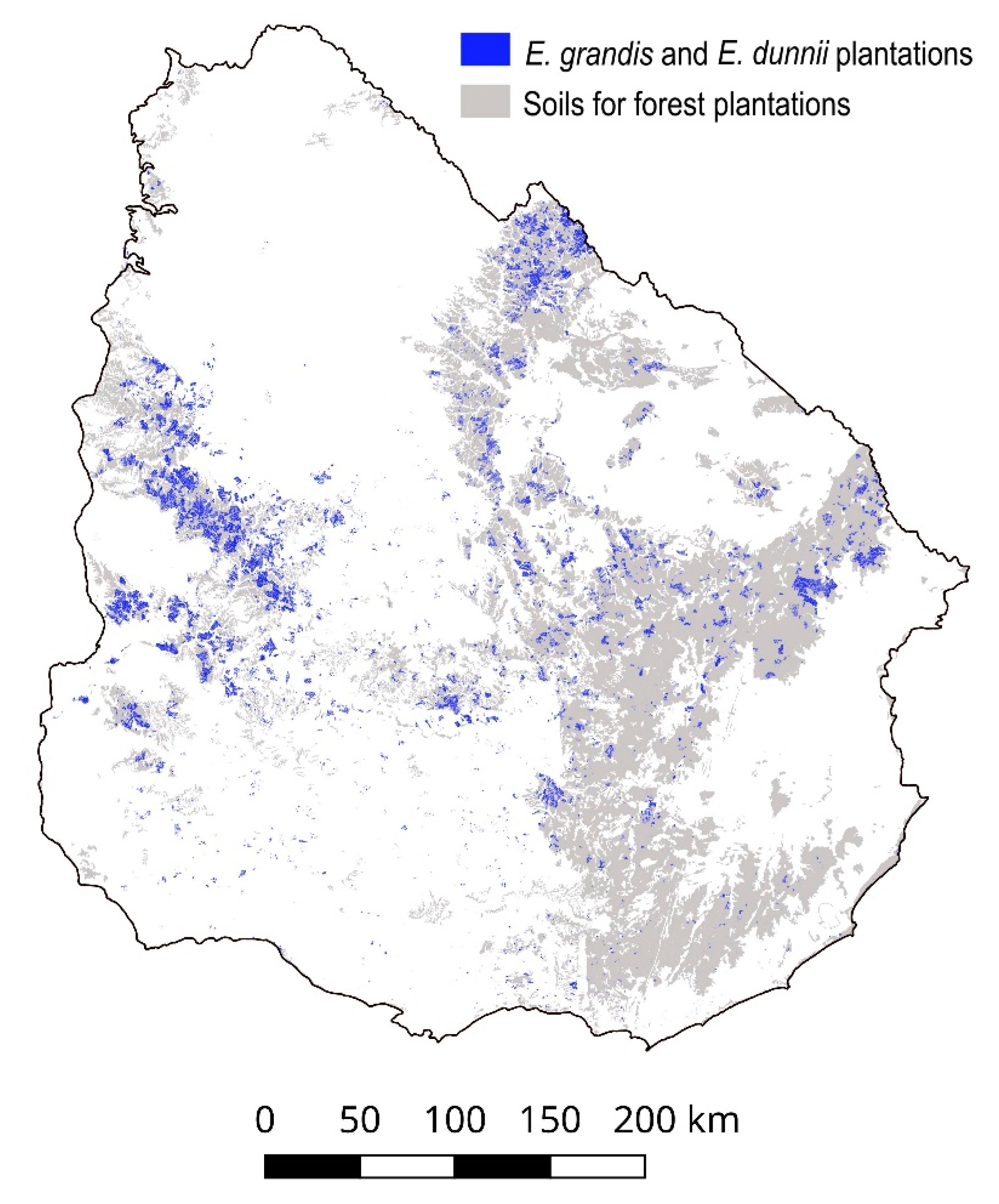
2.1. Study Area
2.2. Sources of Data and Environmental and Edaphic Variables
2.3. Variable Selection
2.4. Statistical Models
2.5. Selection and Validation of the Model
2.6. Comparison of Current and Future Distributions
3. Results
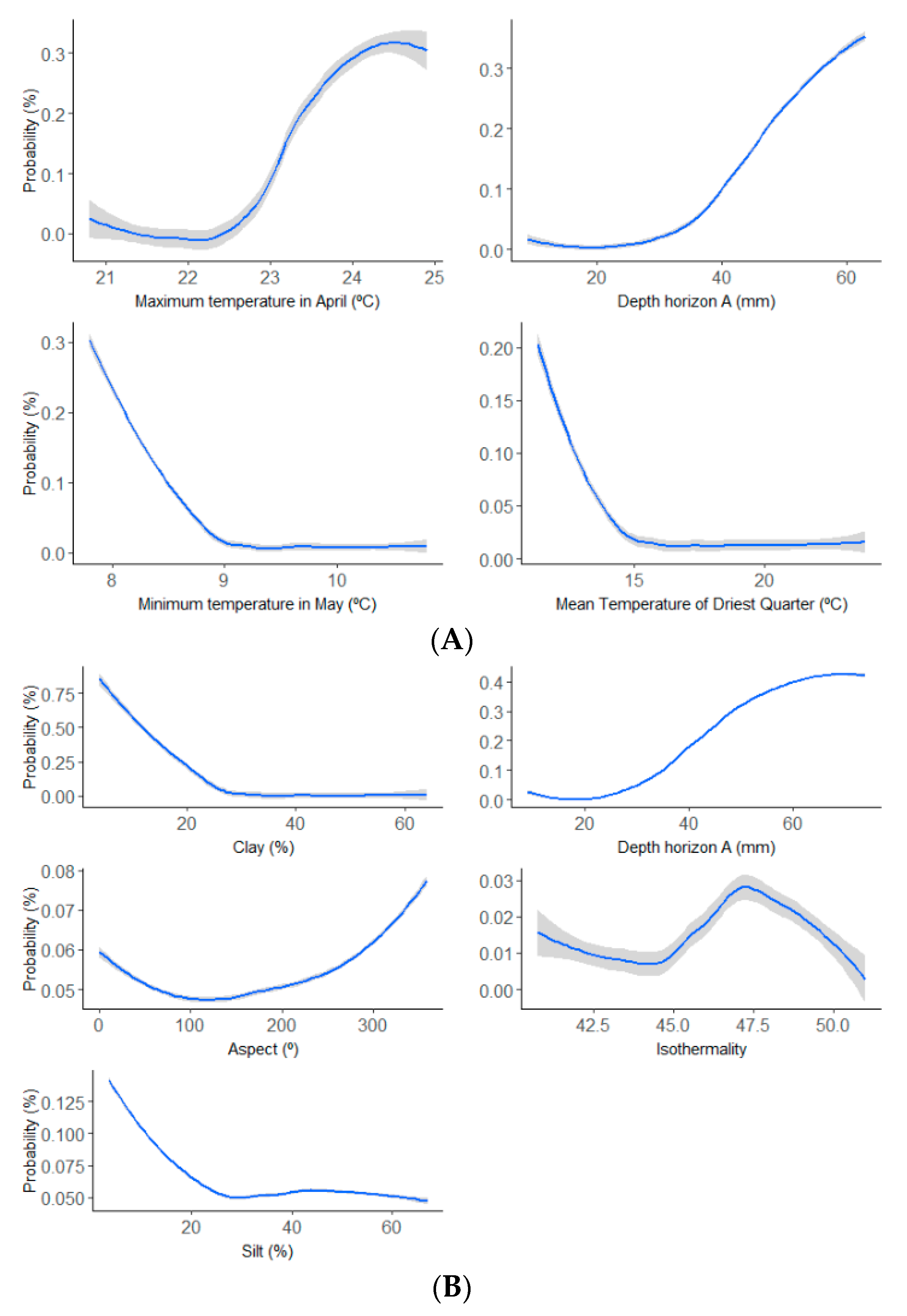
3.1. Selection of Variables
3.2. Model Selection and Validation
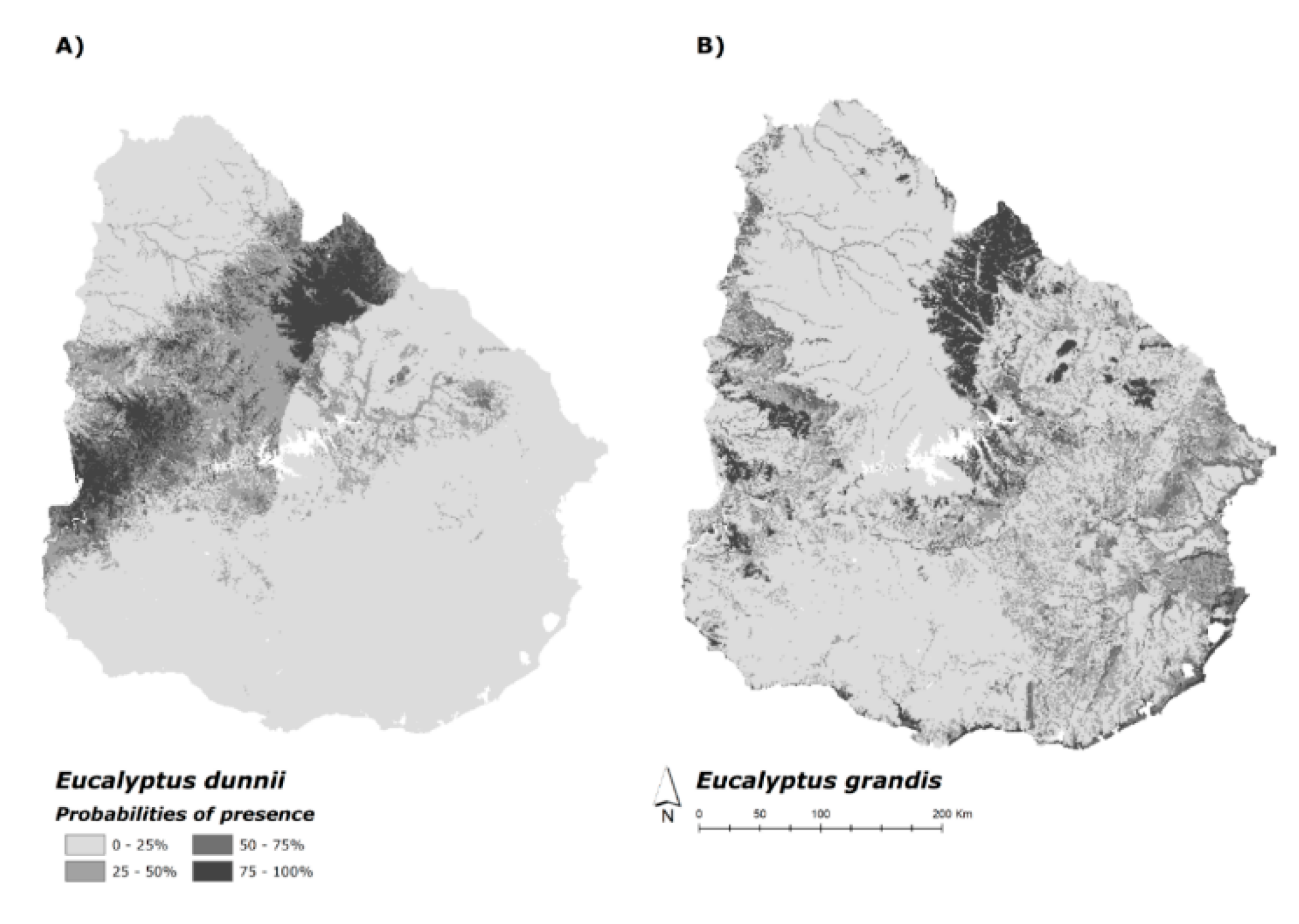
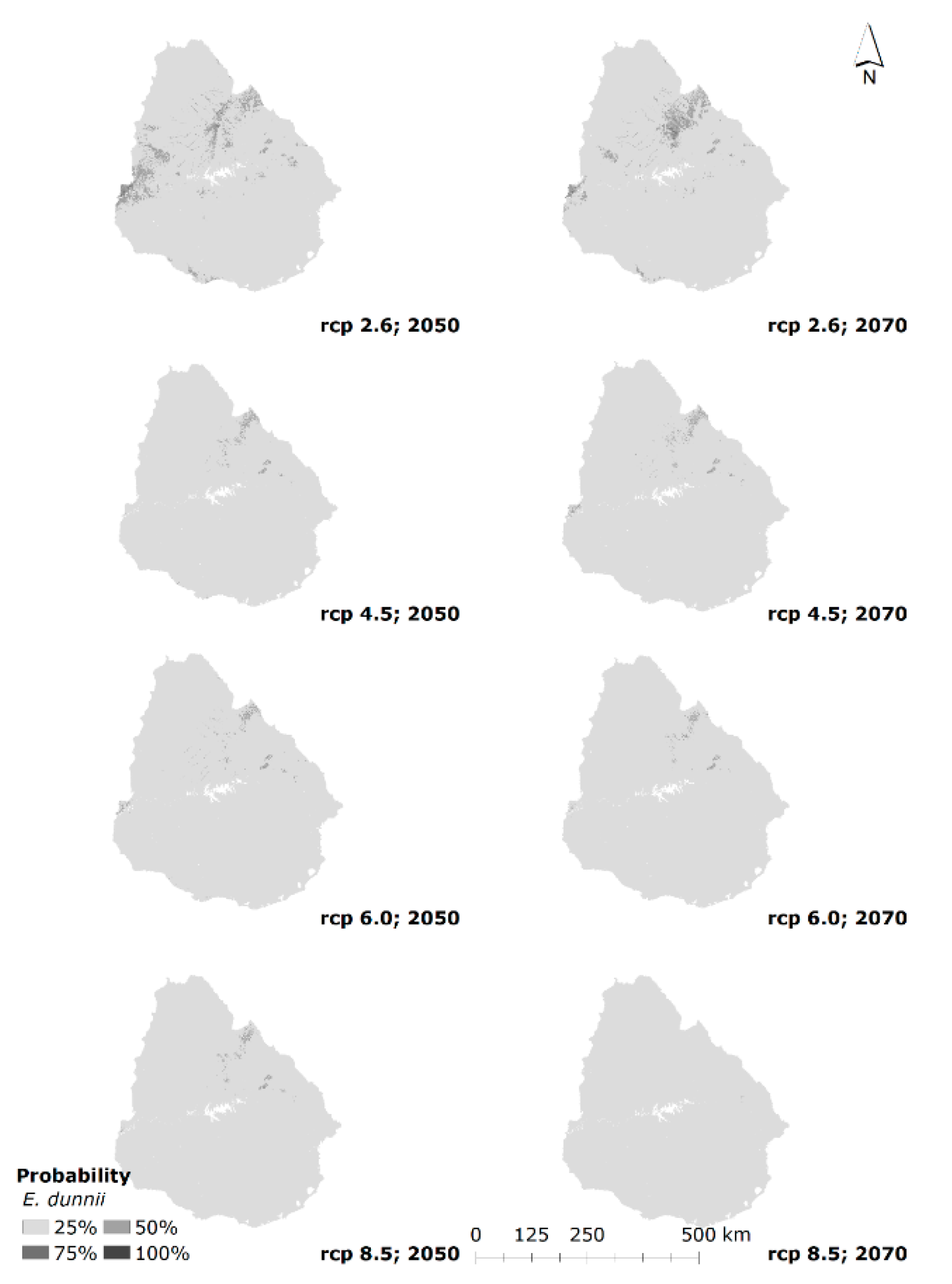
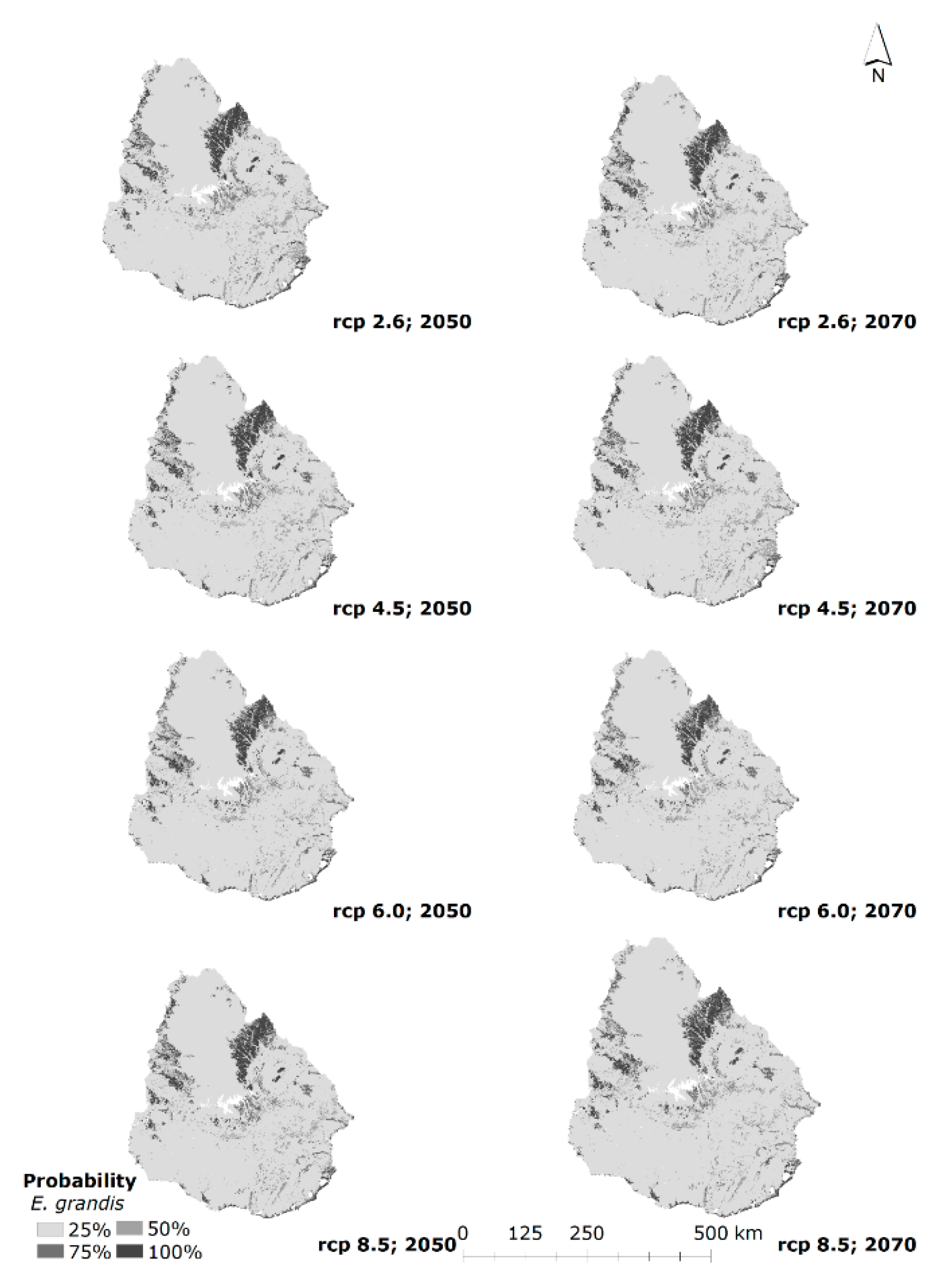
3.3. Current and Future Habitat Projection
4. Discussion
4.1. Variable Selection and Model Precision
4.2. Current Potential Habitat and Future Projection
4.3. Management Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- MGAP-DGF. Bosques Plantados de Eucaliptos Registrados. 2018. Available online: http://www.mgap.gub.uy/unidad-organizativa/direccion-general-forestal/informacion-tecnica/estadisticas-y-mercados/recurso-forestal (accessed on 1 February 2018).
- Brazeiro, A.; Panario, D.; Soutullo, A.; Gutierrez, O.; Segura, A.; Mai, P. Clasificación y Delimitación de las Eco-regiones de Uruguay; Informe Técnico; Convenio MGAP/PPR—Facultad de Ciencias/Vida Silvestre/Sociedad Zoológica del Uruguay/CIEDUR: Montevideo, Uruguay, 2012; 40p. [Google Scholar]
- Prior, L.; Bowman, D. Big eucalypts grow more slowly in a warm climate: Evidence of an interaction between tree size and temperature. Glob. Chang. Biol. 2014, 20, 2793–2799. [Google Scholar] [CrossRef] [PubMed]
- Booth, T.; Broadhurst, L.; Pinkard, E.; Prober, S.; Dillon, S.; Bush, D.; Pinyopusarerk, K.; Doran, J.; Ivkovich, M.; Young, A. Native forests and climate change: Lessons from eucalypts. For. Ecol. Manag. 2015, 347, 18–29. [Google Scholar] [CrossRef]
- Hughes, L.; Cawsey, E.; Westoby, M. Climatic range sizes of Eucalyptus species in relation to future climate change. Glob. Ecol. Biogeogr. 1996, 5, 23–29. [Google Scholar] [CrossRef]
- Drake, J.; Aspinwall, M.; Pfautsch, S.; Rymer, P.; Reich, P.; Smith, R.; Crous, K.; Tissue, D.; Ghannoum, O.; Tjoelker, M. The capacity to cope with climate warming declines from temperate to tropical latitudes in two widely distributed Eucalyptus species. Glob. Chang. Biol. 2015, 21, 459–472. [Google Scholar] [CrossRef]
- Buckeridge, J. Some biological consequences of environmental change: A study using barnacles (Cirripedia: Balanomorpha) and gum trees (Angiospermae: Myrtaceae). Integr. Zool. 2010, 5, 122–131. [Google Scholar] [CrossRef]
- Booth, T. Eucalypt plantations and climate change. For. Ecol. Manag. 2013, 301, 28–34. [Google Scholar] [CrossRef]
- Giménez, A.; Castaño, J.; Baethgen, W.; Lanfranco, B. Cambio climático en Uruguay, posibles impactos y medidas de adaptación en el sector agropecuario. Serie Técnica 2009, 178, 1–23. [Google Scholar]
- Garcia, L.; Ferraz, S.; Alvares, C.; Ferraz, B.; Higa, R. Modelagem da aptidão climática do Eucalyptus grandis frente aos cenários de mudanças climáticas no Brasil Modeling suitable climate for Eucalyptus grandis under future climates scenarios in Brazil. Sci. For. 2014, 42, 503–511. [Google Scholar]
- Pereira, V.; Blain, G.; Maria, A.; Avila, H.; Célia, R.; Pires, D.; Pinto, H. Impacts of climate change on drought: Changes to drier conditions at the beginning of the crop growing season in southern Brazil. Agrometeoroly 2018, 77, 201–211. [Google Scholar] [CrossRef]
- IPCC. Global Warming of 1.5 Oc. 2019. Available online: https://report.ipcc.ch/sr15/pdf/sr15_spm_final.pdf (accessed on 25 January 2019).
- Rogers, H.; Bingham, G.; Cure, J.; Smith, J.; Surano, K. Responses of Selected Plant Species to Elevated Carbon Dioxide in the Field. J. Environ. Qual. 1983, 12, 569–574. [Google Scholar] [CrossRef]
- Fearnside, P. Plantation forestry in Brazil: The potential impacts of climatic change. Biomass Bioenergy 1999, 16, 91–102. [Google Scholar] [CrossRef]
- Karnosky, D.; Pregitzer, K.; Zak, D.; Kubiske, M.; Hendrey, G.; Weinstein, D.; Nosal, M.; Percy, J. Scaling ozone responses of forest trees to the ecosystem level in a changing climate. Plant Cell Environ. 2005, 28, 965–981. [Google Scholar] [CrossRef]
- Apgaua, D.; Tng, D.; Forbes, S.; Ishida, Y.; Vogado, N.; Cernusak, L.; Laurance, S. Elevated temperature and CO2 cause differential growth stimulation and drought survival responses in eucalypt species from contrasting habitats. Tree Physiol. 2019, 39, 1806–1820. [Google Scholar] [CrossRef] [PubMed]
- Lama Gutiérrez, G. Atlas del Eucalipto. Monografias INIA 15; Instituto Nacional de Investigaciones Agrarias (INIA)/Instituto Nacional para la Conservación de la Naturaleza (ICONA): Sevilla, Spain, 1976. [Google Scholar]
- Hamann, A.; Wang, T. Potencial effects of climate change on ecosystem and tree species distribution in British Columbia. Ecology 2006, 87, 2773–2886. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Breiner, F.; Guisan, A.; Bergamini, A.; Nobis, M. Overcoming limitations of modelling rare species by using ensembles of small models. Methods Ecol. Evol. 2015, 6, 1210–1218. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.; Guisan, A.; Mcmahon, S.; Normand, S.; Thuiller, W.; Rafael, W.; Zimmermann, N.; Elith, J. What do we gain from simplicity versus complexity in species distribution models? Ecography 2014, 37, 1267–1281. [Google Scholar] [CrossRef]
- Thuiller, W.; Georges, D.; Engler, R. Biomod2: Ensemble Platform for Species Distribution Modeling. R Package Version 3.3.1. Available online: https://cran.r-project.org/web/packages/biomod2/biomod2.pdf (accessed on 30 May 2020).
- Becerra-López, J.; Romero-Méndez, U.; Ramírez-Bautista, A.; Becerra-López, S. Review of techniques for modeling species distribution. Rev. Biológico Agropecu. Tuxpan. 2016, 5, 1514–1525. [Google Scholar]
- Watling, J.; Brandt, L.; Bucklin, D.; Fujisaki, I.; Mazzotti, F.; Roma, S.; Speroterra, C. Performance metrics and variance partitioning reveal sources of uncertainty in species distribution model. Ecol. Model. 2015, 309, 48–59. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Peterson, A.; Soberón, J.; Overton, J.; Aragón, P.; Lobo, J. Use of niche models in invasive species risk assessments. Biol. Invasions 2011, 13, 2785–2797. [Google Scholar] [CrossRef]
- Califra, H.; Durán, A. 10 Años de Investigación en Producción Forestal. Productividad y Preservación de los Recursos Suelo y Agua; Departamento de Suelos y Aguas, Facultad de Agronomía, UdelaR: Montevideo, Uruguay, 2010. [Google Scholar]
- Dirección General Forestal-MGAP. Inventario Forestal Nacional Manual de Campo; Ministerio de Ganadería Agircultura y Pesca: Montevideo, Uruguay, 2014.
- Duque-Lazo, J.; Van Gils, H.; Groen, T.; Navarro-Cerrillo, R.M. Transferability of species distribution models: The case of Phytophthora cinnamomi in Southwest Spain and Southwest Australia. Ecol. Model. 2016, 320, 62–70. [Google Scholar] [CrossRef]
- Shabani, F.; Kumar, L.; Ahmadi, M. A comparison of absolute performance of different correlative and mechanistic species distribution models in an independent area. Ecol. Evol. 2016, 6, 5973–5986. [Google Scholar] [CrossRef] [PubMed]
- Lyam, P.; Duque-Lazo, J.; Durka, W.; Hauenschild, F.; Schnitzler, J.; Michalak, I.; Ogundipe, O.; Muellner-Riehl, A. Genetic diversity and distribution of Senegalia senegal (L.) Britton under climate change scenarios in West Africa. PLoS ONE 2018, 13, e0194726. [Google Scholar] [CrossRef] [PubMed]
- Silvério, E.; Duque-Lazo, J.; Navarro-Cerrillo, R.M.; Pereña, F.; Palacios-Rodriguez, G. Resilience or Vulnerability of the Rear-Edge Distributions of Pinus halepensis and Pinus pinaster Plantations versus that of Natural Populations, under Climate-Change Scenarios. Forest Sci. 2019, 1–13. [Google Scholar] [CrossRef]
- Hijmans, R.; Cameron, S.; Parra, J.; Jones, P.; Jarvis, A. WORLDCLIM–A set of global climate layers (climate grids). Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Vadillo, F. Modelamiento EspacialAaplicado al Desarrollo del Ecoturismo y la Conservación de la Avifauna en la Vertiente Occidental de Perú, Pontificia Universidad Católica del Perú; Facultad de Letras y Ciencias Humanas, Pontificia Universidad Católica del Perú (PUCP): Lima, Perú, 2017. [Google Scholar]
- Lovino, M.; Müller, O.; Berbery, H.; Müller, G. Evaluation of CMIP5 retrospective simulations of temperature and precipitation in northeastern Argentina. Int. J. Climatol. 2018, 38, 1158–1175. [Google Scholar] [CrossRef]
- Molfino, J. Características Grupos CONEAT (MGAP), INIA-GRASS. 2012. Available online: http://sig.inia.org.uy/sigras/#InformacionGeográfi (accessed on 10 May 2018).
- Calle, M.; Urrea, V.; Boulesteix, A.; Malats, N. AUC-RF: A New Strategy for Genomic Profiling with Random Forest. Hum. Hered. 2011, 72, 121–132. [Google Scholar] [CrossRef]
- Kukunda, C.; Duque-Lazo, J.; González-Ferreiro, E.; Thaden, H.; Kleinn, C. Ensemble classification of individual Pinus crowns from multispectral satellite imagery and airborne LiDAR. Int. J. Appl. Earth Obs. 2018, 65, 12–23. [Google Scholar] [CrossRef]
- Naimi, B. Uncertainty Analysis for Species Distribution Models. R Package Version 1.1-15. Available online: https://cran.r-project.org/web/packages/usdm/index.html (accessed on 15 May 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; The R Foundation: Vienna, Austria, 2016; Available online: https://www.R-project.org/. (accessed on 30 May 2020).
- Duque-Lazo, J.; Navarro-Cerrillo, R.M.; Van Hils, H.; Groen, T. Forecasting oak decline caused by Phytophthora cinnamomi in Andalusia: Identification of priority areas for intervention. For. Ecol. Manag. 2018, 417, 122–136. [Google Scholar] [CrossRef]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Thuiller, W.; Valorel, S.; Araújo, M.; Sykes, M.; Prentice, I. Climate change threats to plant diversity in Europe. PNAS. 2005, 102, 8245–8250. [Google Scholar] [CrossRef] [PubMed]
- Duque-Lazo, J.; Navarro-Cerrillo, R.M. What to save, the host or the pest? The spatial distribution of xylophage insects within the Mediterranean oak woodlands of Southwestern Spain. For. Ecol. Manag. 2017, 392, 90–104. [Google Scholar] [CrossRef]
- Quinto, L.; Navarro-Cerrillo, R.M.; Palacios-Rodriguez, G.; Ruiz-Gomez, F.; Duque-Lazo, J. The current situation and future perspectives of Quercus ilex and Pinus halepensis afforestation on agricultural land in Spain under climate change scenarios. New For. 2020, 1–22. [Google Scholar] [CrossRef]
- Bourne, A.; Haigh, A.; Ellsworth, D. Stomatal sensitivity to vapour pressure deficit relates to climate of origin in Eucalyptus species. Tree Physiol. 2015, 35, 266–278. [Google Scholar] [CrossRef]
- Souza, T.; Ramalho, M.; Marco, B.; Gabriel, D.; Sampaio, D. Performance of Eucalyptus clones according to environmental conditions Desempenho de clones de eucalipto em função de condições ambientais. Sci. For. 2017, 45, 601–610. [Google Scholar] [CrossRef]
- Rutherford, S.; Bonser, S.; Wilson, P.; Rossetto, M. Seedling response to environmental variability: The relationship between phenotypic plasticity and evolutionary history in closely related Eucalyptus species. Am. J. Bot. 2017, 104, 840–857. [Google Scholar] [CrossRef]
- Bell, D.; Williams, J. Eucalypt Ecophysiology. In Eucalypt Ecology: Individuals to Ecosystem; Cambridge University Press: Cambridge, UK, 1997; pp. 168–196. [Google Scholar]
- Rachid Castani, A. Hybrid Mensurational-Physiological Models for Pinus taeda and Eucalyptus grandis in Uruguay. Ph.D. Thesis, Department of Forest Science, University of Canterbury, Canterbury, New Zealand, 2016. [Google Scholar]
- Escudero, R.; Sganga, J.; Sayagués, L.; Graf, E.; Pedochi, R.; Petrini, L.; Munka, C.; Iirisity, F.; Morás, G. Análisis de los efectos de algunos factores ambientales sobre la productividad de Eucalyptus globulus ssp. globulus Labill. Série Act. Difusión INIA 2002, 289, 48–54. [Google Scholar]
- González de León, S. BioInvasiones. Rev. Invasiones Biológicas Am. Lat. El Caribe. 2015, 1, 1–38. [Google Scholar]
- Lafetá, B.; Santana, R.; Penido, T.; Machado, E.; Vierira, D. Climatic suitability for Euaclyptus cloeziana cultivation in four Brazilian States. Floresta 2018, 48, 77–86. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, M.; Li, F.; Weng, Q.; Zhou, C.; Li, M.; Li, J.; Huang, H.; Mo, X.; Gan, S. Genome scans for divergent selection in natural populations of the widespread hardwood species Eucalyptus grandis (Myrtaceae) using microsatellites. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Menegol, O.; Alexandre, J. Soil Attributes Related to Eucalypt and Pine Plantations Productivity in the South of Brazil. J. Sust. For. 2014, 24, 61–82. [Google Scholar] [CrossRef]
- Kimsey, M.; Moore, J.; McDaniel, P. A geographically weighted regression analysis of Douglas-fir site index in north central Idaho. Forest Sci. 2008, 54, 356–366. [Google Scholar]
- Weiskittel, A.; Gould, P.; Temesgen, H. Sources of variation in the self-thinning boundary line for three species with varying levels of shade tolerance. Forest Sci. 2009, 55, 84–93. [Google Scholar]
- Verbyla, D.; Fisher, R. Effect of aspect on ponderosa pine height and diameter growth. For. Ecol. Manag. 1989, 27, 93–98. [Google Scholar] [CrossRef]
- Rachid-Casnati, A.; Mason, E.; Woollons, R. Using soil-based and physiographic variables to improve stand growth equations in Uruguayan forest plantations. iForest 2019, 12, 237–245. [Google Scholar] [CrossRef]
- Castaño, J.; Ceroni, A.; Furest, M.; Aunchayna, J.; Bidegain, R. Caracterización agroclimática del Uruguay 1980–2009. Serie Técnica INIA 2011, 193, 33. [Google Scholar]
- Zimmermann, N.; Yoccoz, N.; Edwards, T.; Meier, E.; Thuiller, W.; Guisan, A.; Schmatz, D.; Pearman, P. Climatic extremes improve predictions of spatial patterns of tree species. Proc. Natl. Acad. Sci. USA 2009, 106, 19723–19728. [Google Scholar] [CrossRef]
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef]
- Marmion, M.; Parviainen, M.; Luoto, M.; Heikkinen, R.; Thuiller, W. Evaluation of consensus methods in predictive species distribution modelling. Divers. Distrib. 2009, 15, 59–69. [Google Scholar] [CrossRef]
- Koo, K.; Park, S.; Seo, C. Effects of climate change on the climatic niches of warm-adapted evergreen plants: Expansion or contraction? Forests 2017, 8, 500. [Google Scholar] [CrossRef]
- Koo, K.; Park, S.; Kong, W.; Hong, S.; Jang, I.; Seo, C. Potential climate change effects on tree distributions in the Korean Peninsula: Understanding model & climate uncertainties. Ecol. Model. 2017, 353, 17–27. [Google Scholar] [CrossRef]
- Shabani, F.; Kumar, L.; Ahmadi, M. Climate Modelling Shows Increased Risk to Eucalyptus sideroxylon on the Eastern Coast of Australia Compared to Eucalyptus albens. Plants 2017, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Quintana, M.; Salomón, O.; Guerra, O.; Lizarralde, M.; De Grosso, A.; Fuenzalida, A. Phlebotominae of epidemiological importance in cutaneous leishmaniasis in northwestern Argentina: Risk maps and ecological niche models. Med. Vet. Entomol. 2013, 27, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Van Gils, H.; Westinga, E.; Carafa, M.; Antonucci, A. Where the bears roam in Majella National Park, Italy. J. Nat. Conserv. 2014, 22, 23–34. [Google Scholar] [CrossRef]
- FAO. El Eucalipto en la Repoblación Forestal; Food and Agriculture Organization of the United Nations, FAO: Roma, Italy, 1981. [Google Scholar]
- Almeida, A.; Landsberg, J.; Sands, P. Parameterisation of 3-PG model for fast-growing Eucalyptus grandis plantations. For. Ecol. Manag. 2004, 193, 179–195. [Google Scholar] [CrossRef]
- Pinkard, E.; Wardlaw, T.; Kriticos, D.; Ireland, K.; Bruce, J. Climate change and pest risk in temperate eucalypt and radiata pine plantations: A review. Aust. For. 2017, 80, 1–14. [Google Scholar] [CrossRef]
- Baesso, R.; Ribeiro, A.; Silva, M. Impacto das mudanças climáticas na produtividade do eucalipto na região norte do Espírito Santo e sul da Bahia. Ciência Florest. 2010, 20, 335–344. [Google Scholar] [CrossRef][Green Version]
- Almeida, A.; Sands, P.; Bruce, J.; Siggins, A.; Leriche, A.; Battaglia, M.; Batista, T. Use of a spatial process-based model to quantify forest plantation productivity and water use efficiency under climate change scenarios. In Proceedings of the18th World IMACS/MODSIM Congress, Cairns, Australia, 13–17 July 2009; pp. 1816–1822. [Google Scholar]
- Hamer, J.; Veneklaas, E.; Renton, M.; Poot, P. Links between soil texture and root architecture of Eucalyptus species may limit distribution ranges under future climates. Plant Soil 2015, 403, 217–229. [Google Scholar] [CrossRef]
- Fensham, R.; Bouchard, D.; Catterall, C.; Dwyer, J. Do local moisture stress responses across tree species reflect dry limits of their geographic ranges? Austral Ecol. 2014, 39, 612–618. [Google Scholar] [CrossRef]
- Shirk, A.J.; Cushman, S.A.; Waring, K.M.; Wehenkel, C.A.; Leal-Sáenz, A.; Toney, C.; Lopez-Sanchez, C. Southwestern white pine (Pinus strobiformis) species distribution models project a large range shift and contraction due to regional climatic changes. For. Ecol. Manag. 2018, 411, 176–186. [Google Scholar] [CrossRef]
- Jovanovic, T.; Arnold, R.; Booth, T. Determining the climatic suitability of Eucalyptus dunnii for plantations in Australia, China and Central and South America. New For. 2000, 19, 215–226. [Google Scholar] [CrossRef]
- Booth, T.H.; Jovanovic, T.; Arnold, R.J. Planting domains under climate change for Eucalyptus pellita and Eucalyptus urograndis in parts of China and South East Asia. Aust. For. 2017, 80, 1–9. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.; Duque-Lazo, J.; Rios-Gil, N.; Guerrero-Alvarez, J.; Lopez-Quintanilla, J.; Palacios-Rodriguez, G. Can habitat prediction models contribute to the restoration and conservation of the threatened tree Abies pinsapo Boiss. in Southern Spain? New For. 2020, 1–24. [Google Scholar] [CrossRef]

| Code | Variables | Units |
|---|---|---|
| Bioclimatic | ||
| Bio1 | Annual Mean Temperature | °C |
| Bio2 | Mean Diurnal Range (Mean of monthly (max temp-min temp)) | °C |
| Bio3 | Isothermality (BIO2/BIO7) (×100) | °C |
| Bio4 | Temperature Seasonality (standard deviation ×100) | |
| Bio5 | Max Temperature of Warmest Month | °C |
| Bio6 | Min Temperature of Coldest Month | °C |
| Bio7 | Temperature Annual Range (BIO5-BIO6) | °C |
| Bio8 | Mean Temperature of Wettest Quarter | °C |
| Bio9 | Mean Temperature of Driest Quarter | °C |
| Bio10 | Mean Temperature of Warmest Quarter | °C |
| Bio11 | Mean Temperature of Coldest Quarter | °C |
| Bio12 | Annual Precipitation | mm |
| Bio13 | Precipitation of Wettest Month | mm |
| Bio14 | Precipitation of Driest Month | mm |
| Bio15 | Precipitation Seasonality (Coefficient of Variation) | |
| Bio16 | Precipitation of Wettest Quarter | mm |
| Bio17 | Precipitation of Driest Quarter | mm |
| Bio18 | Precipitation of Warmest Quarter | mm |
| Bio19 | Precipitation of Coldest Quarter | mm |
| Climatic | ||
| # Tmin 1–12 | Minimum monthly temperature | °C |
| # Tmean 1–12 | Medium monthly temperature | °C |
| # Tmax 1–12 | Maximum monthly temperature | °C |
| Prep 1–12 | Monthly precipitation | mm |
| Edaphic | ||
| Depth. Hor. A | Depth of Horizon A | cm |
| %Sand | Sand content | % |
| %Silt | Silt content | % |
| %Clay | Clay content | % |
| Ph-water | Acidity | |
| C | Carbon content | % |
| Org.carbon | Organic matter content | % |
| N | Nitrogen | % |
| Ca | Calcium content | % |
| Mg | Magnesium content | % |
| K | Potassium content | % |
| B | Exchangeable Bases | % |
| Al | Aluminum content | meq/100 g |
| CEC7 | Cation exchange capacity at pH7 | meq/100 g |
| Vph7 | Bases Saturation pH7 | % |
| Na_int. | Exchangeable sodium | % |
| Al_int. | Exchangeable aluminum | % |
| Topographic | ||
| RN 1–12 | Monthly solar radiation | Joules/m2 |
| A | Aspect | Degrees |
| S | Slope | percentage |
| E | Elevation | m |
| E. dunnii | E. grandis | ||||||
|---|---|---|---|---|---|---|---|
| Order | Variables | Importance (Mean Decrease Gini (MDG)) | Probability of Selection | Order | Variables | Importance (MDG) | Probability of Selection |
| 1 | Depth Hor. A | 8.49 | 1.00 | 1 | % Clay | 8.20 | 0.98 |
| 2 | Temp.max in April | 6.96 | 1.00 | 2 | Depth Hor. A | 7.06 | 0.99 |
| 3 | Temp.min in May | 5.12 | 0.98 | 3 | Isothermality | 4.11 | 0.65 |
| 4 | Bio 9 | 4.69 | 0.95 | 4 | % Silt | 4.10 | 0.91 |
| 5 | Aspect | 2.20 | 0.57 | ||||
| Ensemble Model | Kappa | TSS | AUC | Threshold | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Mean | 0.771 | 0.987 | 0.980 | 0.511 | 1.00 | 0.987 |
| Confidence interval inferior | 0.771 | 0.891 | 0.981 | 0.500 | 1.00 | 0.891 |
| Confidence interval superior | 0.77 | 0.887 | 0.980 | 0.557 | 1.00 | 0.887 |
| Median | 0.741 | 0.887 | 0.980 | 0.502 | 1.00 | 0.887 |
| Committee averaging | 0.801 | 0.878 | 0.982 | 0.500 | 1.00 | 0.878 |
| Probability mean weight decay | 0.771 | 0.891 | 0.981 | 0.500 | 1.00 | 0.887 |
| Ensemble Model | Kappa | TSS | AUC | Threshold | Sensitivity | Specificity |
| Mean | 0.807 | 0.866 | 0.978 | 0.651 | 0.944 | 0.922 |
| Confidence interval inferior | 0.812 | 0.869 | 0.979 | 0.636 | 0.944 | 0.922 |
| Confidence interval superior | 0.807 | 0.866 | 0.978 | 0.675 | 0.944 | 0.922 |
| Median | 0.788 | 0.864 | 0.975 | 0.712 | 0.944 | 0.919 |
| Committee averaging | 0.830 | 0.867 | 0.979 | 0.843 | 0.917 | 0.949 |
| Probability mean weight decay | 0.807 | 0.866 | 0.978 | 0.500 | 9.44 | 0.920 |
| (A) | ||||||
| Present | Year | Area | % | Area | % | |
| 2000 | 28,891 | 100.00 | 30,537 | 100.00 | ||
| RCP | CA | WD | ||||
| Future | 2050 | 2.6 | 1214 | 4.20 | 794 | 2.60 |
| 4.5 | 0 | 0.00 | 0 | 0.00 | ||
| 6.0 | 10 | 0.03 | 0 | 0.00 | ||
| 8.5 | 0 | 0.00 | 0 | 0.00 | ||
| 2070 | 2.6 | 1134 | 3.93 | 990 | 3.24 | |
| 4.5 | 3 | 0.01 | 1 | 0.00 | ||
| 6.0 | 0 | 0.00 | 0 | 0.00 | ||
| 8.5 | 0 | 0.00 | 0 | 0.00 | ||
| (B) | ||||||
| Present | Year | Area | % | Area | % | |
| 2000 | 16,070 | 100 | 12,105 | 100 | ||
| RCP | CA | WD | ||||
| Future | 2050 | 2.6 | 16,471 | 102.50 | 12,107 | 100.02 |
| 4.5 | 16,211 | 100.88 | 12,183 | 100.64 | ||
| 6.0 | 15,518 | 96.57 | 11,496 | 94.97 | ||
| 8.5 | 16,198 | 100.80 | 12,285 | 101.49 | ||
| 2070 | 2.6 | 16,121 | 100.32 | 11,901 | 98.31 | |
| 4.5 | 16,401 | 102.06 | 12,129 | 100.20 | ||
| 6.0 | 15,292 | 95.16 | 11,168 | 92.26 | ||
| 8.5 | 12,215 | 76.01 | 10,013 | 82.72 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resquin, F.; Duque-Lazo, J.; Acosta-Muñoz, C.; Rachid-Casnati, C.; Carrasco-Letelier, L.; Navarro-Cerrillo, R.M. Modelling Current and Future Potential Habitats for Plantations of Eucalyptus grandis Hill ex Maiden and E. dunnii Maiden in Uruguay. Forests 2020, 11, 948. https://doi.org/10.3390/f11090948
Resquin F, Duque-Lazo J, Acosta-Muñoz C, Rachid-Casnati C, Carrasco-Letelier L, Navarro-Cerrillo RM. Modelling Current and Future Potential Habitats for Plantations of Eucalyptus grandis Hill ex Maiden and E. dunnii Maiden in Uruguay. Forests. 2020; 11(9):948. https://doi.org/10.3390/f11090948
Chicago/Turabian StyleResquin, Fernando, Joaquín Duque-Lazo, Cristina Acosta-Muñoz, Cecilia Rachid-Casnati, Leonidas Carrasco-Letelier, and Rafael M. Navarro-Cerrillo. 2020. "Modelling Current and Future Potential Habitats for Plantations of Eucalyptus grandis Hill ex Maiden and E. dunnii Maiden in Uruguay" Forests 11, no. 9: 948. https://doi.org/10.3390/f11090948
APA StyleResquin, F., Duque-Lazo, J., Acosta-Muñoz, C., Rachid-Casnati, C., Carrasco-Letelier, L., & Navarro-Cerrillo, R. M. (2020). Modelling Current and Future Potential Habitats for Plantations of Eucalyptus grandis Hill ex Maiden and E. dunnii Maiden in Uruguay. Forests, 11(9), 948. https://doi.org/10.3390/f11090948






