Surface-Based Analysis of Leaf Microstructures for Adsorbing and Retaining Capability of Airborne Particulate Matter in Ten Woody Species
Abstract
1. Introduction
2. Materials and Methods
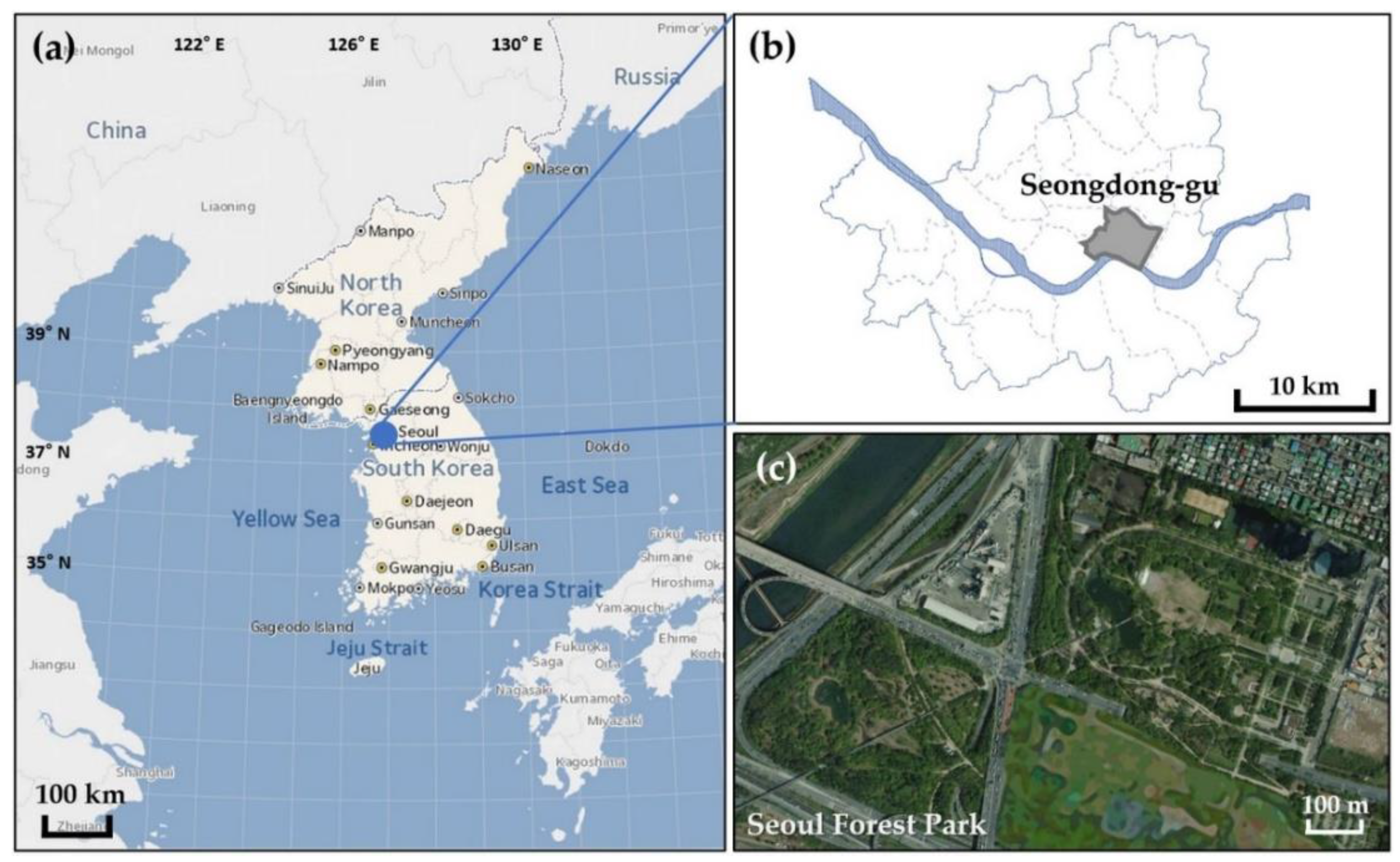
2.1. Study Site Description
2.2. Plant Material and Sample Collection
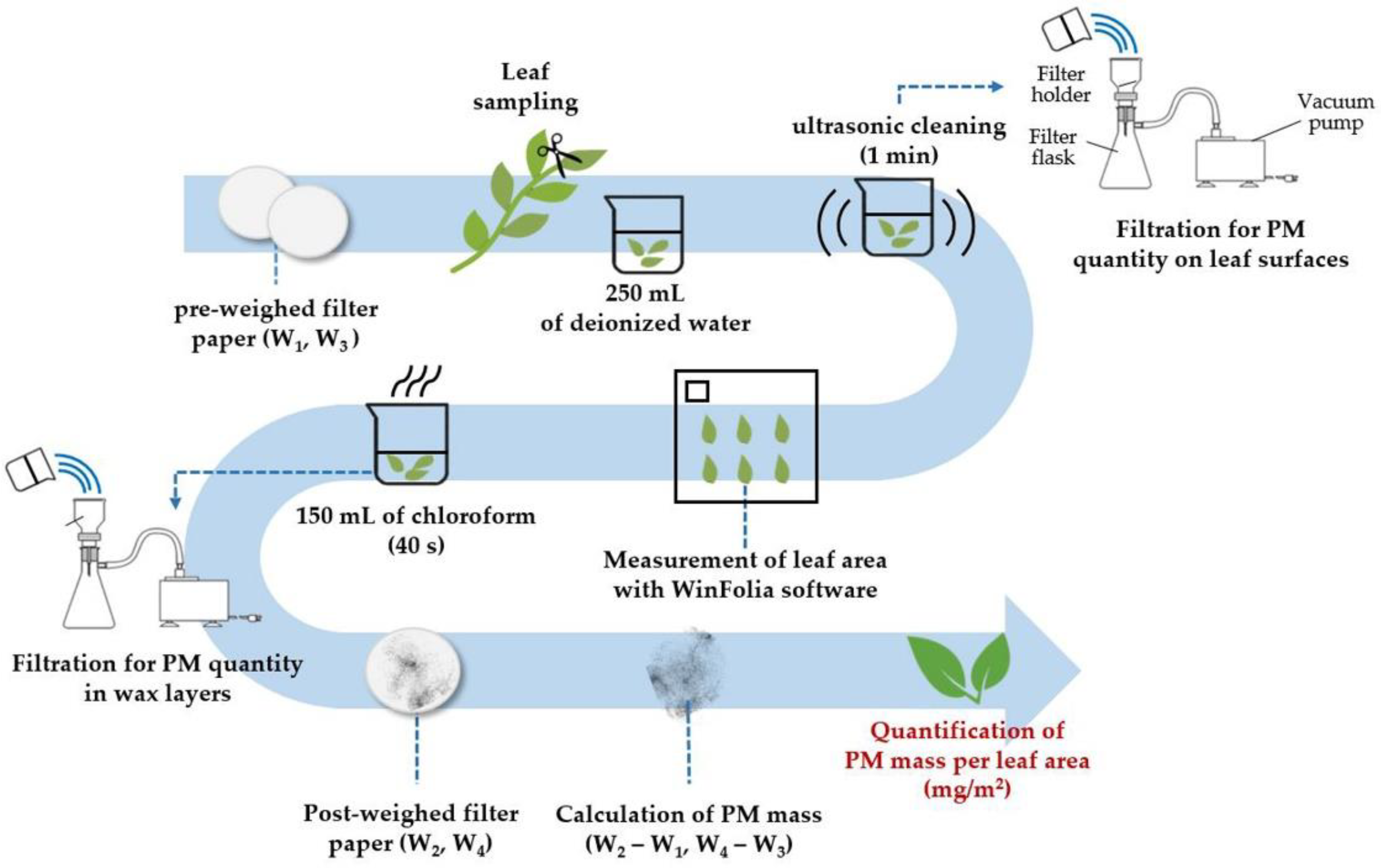
2.3. Determination of PM10 and PM2.5 Adsorption Capacity on Leaf Surfaces
2.4. Determination of PM2.5 Adsorption Capacity in Epicuticular Wax Layers
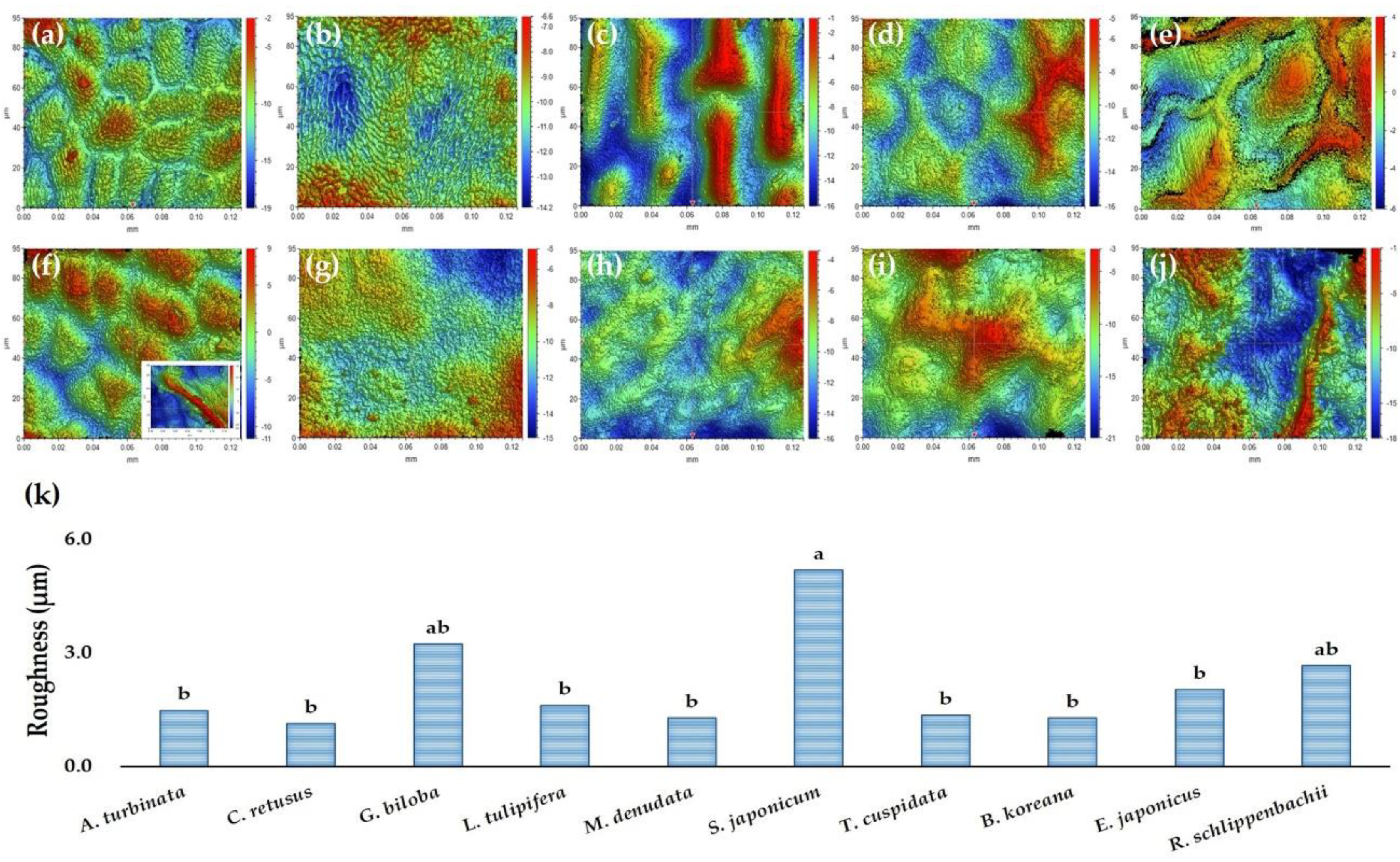
2.5. Micro-Morphological Characteristics on Leaf Surfaces
2.6. Measurement of the Contact Angle on Leaf Surfaces
2.7. Statistical Analysis
3. Results
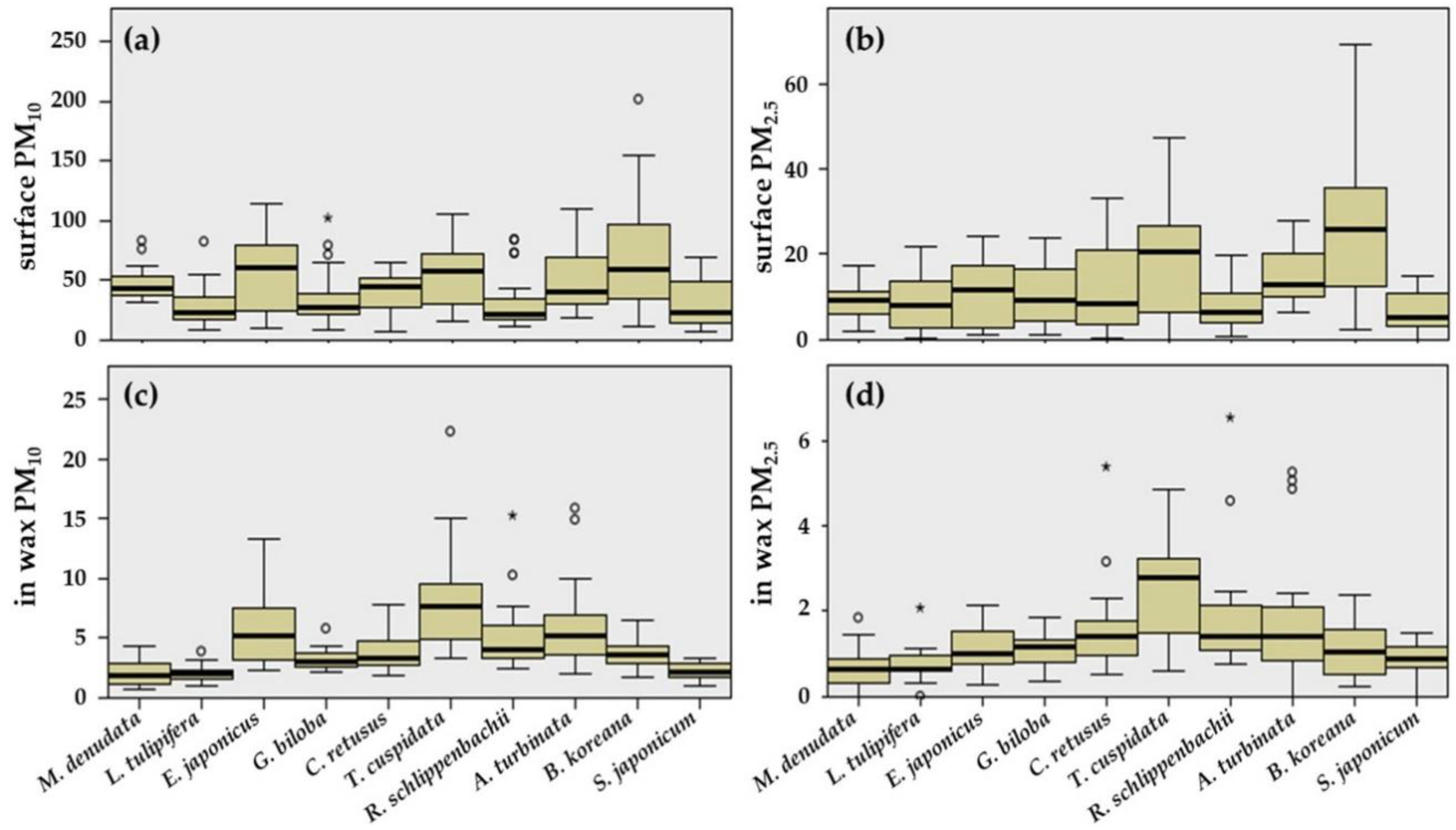
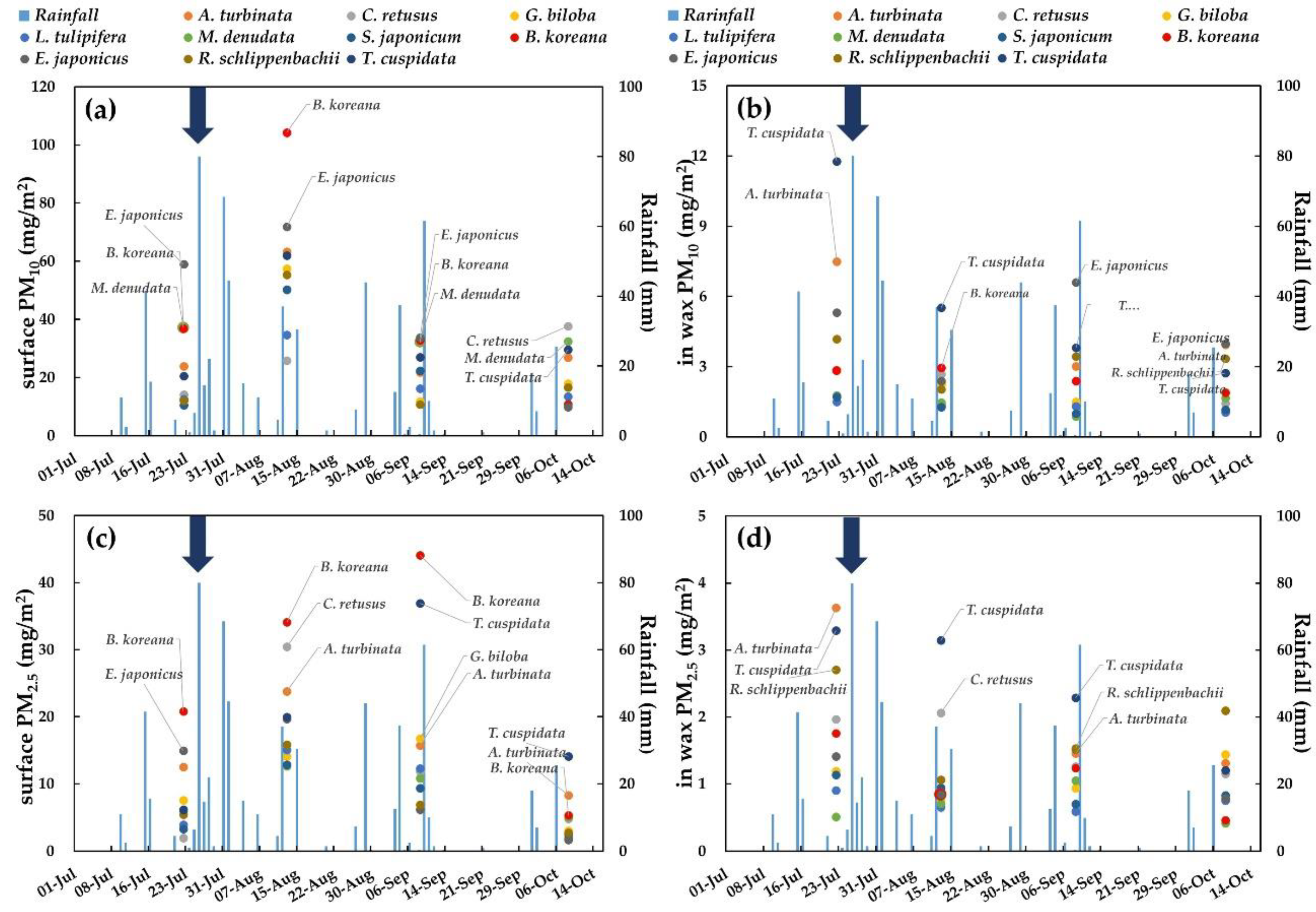
3.1. Adsorption Capacity on Leaf Surfaces and in Epicuticular Wax Layers
3.2. Micro-Morphological Characteristics on Leaf Surfaces
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dzierżanowski, K.; Popek, R.; Gawrońska, H.; Saebø, A.; Gawroński, S.W. Deposition of particulate matter of different size fractions on leaf surfaces and in waxes of urban forest species. Int. J. Phytoremediat. 2011, 13, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.L.; de Melo, E.J.T.; Miguens, F.C. Tillandsia stricta Sol (Bromeliaceae) leaves as monitors of airborne particulate matter-A comparative SEM methods evaluation: Unveiling an accurate and odd HP-SEM method. Microsc. Res. Tech. 2016, 79, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Urošević, M.A.; Jovanović, G.; Stević, N.; Deljanin, I.; Nikolić, M.; Tomašević, M.; Samson, R. Leaves of common urban tree species (Aesculus hippocastanum, Acer platanoides, Betula pendula and Tilia cordata) as a measure of particle and particle-bound pollution: A 4-year study. Air Qual. Atmos. Health 2019, 12, 1081–1090. [Google Scholar] [CrossRef]
- Liu, L.; Guan, D.; Peart, M.R. The morphological structure of leaves and the dust-retaining capability of afforested plants in urban Guangzhou, South China. Environ. Sci. Pollut. Res. 2012, 19, 3440–3449. [Google Scholar] [CrossRef]
- Kroeger, T.; McDonald, R.I.; Boucher, T.; Zhang, P.; Wang, L. Where the people are: Current trends and future potential targeted investments in urban trees for PM10 and temperature mitigation in 27 U.S. cities. Landsc. Urban. Plan. 2018, 177, 227–240. [Google Scholar] [CrossRef]
- Zhai, S.; Jacob, D.J.; Wang, X.; Shen, L.; Li, K.; Zhang, Y.; Gui, K.; Zhao, T.; Liao, H. Fine particulate matter (PM2.5) trends in China, 2013–2018: Separating contributions from anthropogenic emissions and meteorology. Atmos. Chem. Phys. 2019, 19, 11031–11041. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Yi, M.; Wang, Y.; Wang, P.; Xu, J.; Niu, F.; Lin, F. High-performance inertial impaction filters for particulate matter removal. Sci. Rep. 2018, 8, 4757. [Google Scholar] [CrossRef]
- Weagle, C.L.; Snider, G.; Li, C.; Van Donkelaar, A.; Philip, S.; Bissonnette, P.; Burke, J.; Jackson, J.; Latimer, R.; Stone, E.; et al. Global sources of fine particulate matter: Interpretation of PM2.5 chemical composition observed by SPARTAN using a global chemical transport model. Environ. Sci. Technol. 2018, 52, 11670–11681. [Google Scholar] [CrossRef]
- Sosa, B.S.; Porta, A.; Colman Lerner, J.E.; Banda Noriega, R.; Massolo, L. Human health risk due to variations in PM10–PM2.5 and associated PAHs levels. Atmos. Environ. 2017, 160, 27–35. [Google Scholar] [CrossRef]
- Zanoletti, A.; Bilo, F.; Borgese, L.; Depero, L.E.; Fahimi, A.; Ponti, J.; Valsesia, A.; Spina, R.L.; Montini, T.; Bontempi, E. SUNSPACE, a porous material to reduce air particulate matter (PM). Front. Chem. 2018, 6, 534. [Google Scholar] [CrossRef]
- Wei, X.; Lyu, S.; Yu, Y.; Wang, Z.; Liu, H.; Pan, D.; Chen, J. Phylloremediation of air pollutants: Exploiting the potential of plant leaves and leaf-associated microbes. Front. Plant Sci. 2017, 8, 1318. [Google Scholar] [CrossRef] [PubMed]
- Neinhuis, C. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef]
- Kirkwood, R.C. Recent developments in our understanding of the plant cuticle as a barrier to the foliar uptake of pesticides. Pestic. Sci. 1999, 55, 69–77. [Google Scholar] [CrossRef]
- Ristic, Z.; Jenks, M.A. Leaf cuticle and water loss in maize lines differing in dehydration avoidance. J. Plant Physiol. 2002, 159, 645–651. [Google Scholar] [CrossRef]
- Jetter, R.; Kunst, L.; Samuels, A.L. Composition of plant cuticular waxes. In Annual Plant Reviews, Biology of the Plant Cuticle; Riederer, M., Müller, C., Eds.; Blackwell Publishing: Oxford, UK, 2007; Volume 23, pp. 145–181. [Google Scholar]
- Shaheenuzzamn, M.; Liu, T.; Shi, S.; Wu, H.; Wang, Z. Research advances on cuticular waxes biosynthesis in crops: A review. Int. J. Agric. Biol. 2019, 21, 911–921. [Google Scholar] [CrossRef]
- Tian, N.; Liu, F.; Wang, P.; Zhang, X.; Li, X.; Wu, G. The molecular basis of glandular trichome development and secondary metabolism in plants. Plant Gene 2017, 12, 1–12. [Google Scholar] [CrossRef]
- Pandey, S.; Goel, R.; Bhardwaj, A.; Asif, M.H.; Sawant, S.V.; Misra, P. Transcriptome analysis provides insight into prickle development and its link to defense and secondary metabolism in Solanum viarum Dunal. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, I.J.; Rathore, D. Suspended particulate matter deposition and its impact on urban trees. Atmos. Pollut. Res. 2018, 9, 1072–1082. [Google Scholar] [CrossRef]
- Badach, J.; Dymnicka, M.; Baranowski, A. Urban Vegetation in Air Quality Management: A Review and Policy Framework. Sustainability 2020, 12, 1258. [Google Scholar] [CrossRef]
- Song, Y.; Maher, B.A.; Li, F.; Wang, X.; Sun, X.; Zhang, H. Particulate matter deposited on leaf of five evergreen species in Beijing, China: Source identification and size distribution. Atmos. Environ. 2015, 105, 53–60. [Google Scholar] [CrossRef]
- Yu, W.; Wang, Y.; Wang, Y.; Li, B.; Liu, Y.; Liu, X. Application of a coupled model of photosynthesis and stomatal conductance for estimating plant physiological response to pollution by fine particulate matter (PM2.5). Environ. Sci. Pollut. Res. 2018, 25, 19826–19835. [Google Scholar] [CrossRef] [PubMed]
- Risom, L.; Møller, P.; Loft, S. Oxidative stress-induced DNA damage by particulate air pollution. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2005, 592, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Øvrevik, J.; Refsnes, M.; Låg, M.; Holme, J.; Schwarze, P. Activation of proinflammatory responses in cells of the airway Mucosa by particulate matter: Oxidant-and non-oxidant-mediated triggering mechanisms. Biomolecules 2015, 5, 1399–1440. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K. Impacts of particulate matter pollution on plants: Implications for environmental biomonitoring. Ecotoxicol. Environ. Saf. 2016, 129, 120–136. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.; Hsu, L.F.; Lee, C.W.; Chiang, Y.C.; Lee, M.H.; How, J.M.; Wu, C.M.; Huang, C.L.; Lee, I.T. Resveratrol inhibits urban particulate matter-induced COX-2/PGE2 release in human fibroblast-like synoviocytes via the inhibition of activation of NADPH oxidase/ROS/NF-κB. Int. J. Biochem. Cell Biol. 2017, 88, 113–123. [Google Scholar] [CrossRef]
- Šuškalo, N.; Hasanagić, D.; Topalić-Trivunović, L.; Kukrić, Z.; Samelak, I.; Savić, A.; Kukavica, B. Antioxidative and antifungal response of woody species to environmental conditions in the urban area. Ecotoxicology 2018, 27, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Environmental Research. Air Quality Monitoring Station; AQMS. Available online: http://www.airkorea.or.kr/web/last_amb_hour_data (accessed on 6 August 2019).
- Gajbhiye, T.; Pandey, S.K.; Lee, S.S.; Kim, K.H. Size fractionated phytomonitoring of airborne particulate matter (PM)and speciation of PM bound toxic metals pollution through Calotropis procera in an urban environment. Ecol. Indic. 2019, 104, 32–40. [Google Scholar] [CrossRef]
- Zampieri, M.C.T.; Sarkis, J.E.S.; Pestana, R.C.B.; Tavares, A.R.; Melo-de-Pinna, G.F.A. Characterization of Tibouchina granulosa (Desr.) Cong. (Melastomataceae) as a biomonitor of air pollution and quantification of particulate matter adsorbed by leaves. Ecol. Eng. 2013, 61, 316–327. [Google Scholar] [CrossRef]
- Bickford, C.P. Ecophysiology of leaf trichomes. Funct. Plant Biol. 2016, 43, 807. [Google Scholar] [CrossRef]
- Neinhuis, C.; Barthlott, W. Seasonal changes of leaf surface contamination in beech, oak, and ginkgo in relation to leaf micromorphology and wettability. New Phytol. 1998, 138, 91–98. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Zhu, L.; Xing, B.; Chen, B. Dependence of plant uptake and diffusion of polycyclic aromatic hydrocarbons on the leaf surface morphology and micro-structures of cuticular waxes. Sci. Rep. 2017, 7, 46235. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Shahinnia, F.; Le Roy, J.; Laborde, B.; Sznajder, B.; Kalambettu, P.; Mahjourimajd, S.; Tilbrook, J.; Fleury, D. Genetic association of stomatal traits and yield in wheat grown in low rainfall environments. BMC Plant Biol. 2016, 16, 150. [Google Scholar] [CrossRef] [PubMed]
- Dow, G.J.; Berry, J.A.; Bergmann, D.C. The physiological importance of developmental mechanisms that enforce proper stomatal spacing in Arabidopsis thaliana. New Phytol. 2014, 201, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.J.; Lee, J.; Kim, H.; Park, S.; Lim, Y.; Kim, J.E.; Baek, S.G.; Seo, S.M.; Kim, K.N.; Woo, S.Y. The removal efficiencies of several temperate tree species at adsorbing airborne particulate matter in urban forests and roadsides. Forests 2019, 10, 960. [Google Scholar] [CrossRef]
- Shukla, S.; Sharma, R.; Sahu, M. Dust pollution affect morphophysiological traits of plant Mangifera indica linn. Int. J. Bot. 2019, 15, 1–4. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Chen, Q. Potential of thirteen urban greening plants to capture particulate matter on leaf surfaces across three levels of ambient atmospheric pollution. Int. J. Environ. Res. Public Health 2019, 16, 402. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, H.; Wang, X.; Zhou, X.; Zhang, K.; Chen, X.; Liu, J.; Han, J.; Wang, A. The roles of different types of trichomes in tomato resistance to cold, drought, whiteflies, and botrytis. Agronomy 2020, 10, 411. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Wang, B.; Wang, Y.; Yu, W. The response of plant photosynthesis and stomatal conductance to fine particulate matter (PM2.5) based on leaf factors analyzing. J. Plant Biol. 2019, 62, 120–128. [Google Scholar] [CrossRef]
- Popek, R.; Gawrońska, H.; Wrochna, M.; Gawroński, S.W.; Sæbø, A. Particulate matter on foliage of 13 woody species: Deposition on surfaces and phytostabilisation in waxes–a 3-year study. Int. J. Phytoremediat. 2013, 15, 245–256. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Z.; Chen, Y.; Chen, Z.; Xu, S. Contamination characteristics and possible sources of PM10 and PM2.5 in different functional areas of Shanghai, China. Atmos. Environ. 2013, 68, 221–229. [Google Scholar] [CrossRef]
- He, X.; Ruan, Y.; Chen, W.; Lu, T. Responses of the anti-oxidative system in leaves of Ginkgo biloba to elevated ozone concentration in an urban area. Bot. Stud. 2006, 47, 409–416. [Google Scholar]
- Xu, X.; Xia, J.; Gao, Y.; Zheng, W. Additional focus on particulate matter wash-off events from leaves is required: A review of studies of urban plants used to reduce airborne particulate matter pollution. Urban For. Urban Green 2020, 48, 126559. [Google Scholar] [CrossRef]
- Wang, H.; Shi, H.; Wang, Y. The wetting of leaf surfaces and its ecological significances. In Wetting and Wettability; Intech: Rijeka, Croatia, 2015; pp. 295–321. [Google Scholar] [CrossRef]
- Xie, C.; Yan, L.; Liang, A.; Che, S. Understanding the washoff processes of PM2.5 from leaf surfaces during rainfall events. Atmos. Environ. 2019, 214, 116844. [Google Scholar] [CrossRef]
- Rai, A.; Kulshreshtha, K.; Srivastava, P.K.; Mohanty, C.S. Leaf surface structure alterations due to particulate pollution in some common plants. Environmentalist 2010, 30, 18–23. [Google Scholar] [CrossRef]
- Liu, L.; Fang, Y.M.; Wang, S.C.; Xie, Y.; Yang, D.D. Leaf micro-morphology and features in adsorbing air suspended particulate matter and accumulating heavy metals in seven tress species. Huanjing Kexue/Environ. Sci. 2013, 34, 2361–2367. [Google Scholar]
- Ram, S.S.; Majumder, S.; Chaudhuri, P.; Chanda, S.; Santra, S.C.; Maiti, P.K.; Sudarshan, M.; Chakraborty, A. Plant canopies: Bio-monitor and trap for re-suspended dust particulates contaminated with heavy metals. Mitig. Adapt. Strateg. Glob. Chang. 2014, 19, 499–508. [Google Scholar] [CrossRef]
- Yan, G.; Liu, J.; Zhu, L.; Zhai, J.; Cong, L.; Ma, W.; Wang, Y.; Wu, Y.; Zhang, Z. Effectiveness of wetland plants as biofilters for inhalable particles in an urban park. J. Clean. Prod. 2018, 194, 435–443. [Google Scholar] [CrossRef]
- Baraldi, R.; Chieco, C.; Neri, L.; Facini, O.; Rapparini, F.; Morrone, L.; Rotondi, A.; Carriero, G. An integrated study on air mitigation potential of urban vegetation: From a multi-trait approach to modeling. Urban For. Urban Green 2019, 41, 127–138. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, G.; An, H.; Yin, W.; Xia, X. Quantifying the particulate matter accumulation on leaf surfaces of urban plants in Beijing, China. Atmos. Pollut. Res. 2017, 8, 836–842. [Google Scholar] [CrossRef]
- Zhao, X.; Yan, H.; Liu, M.; Kang, L.; Yu, J.; Yang, R. Relationship between PM2.5 adsorption and leaf surface morphology in ten urban tree species in Shenyang, China. Energy Sources Part A Recover. Util. Environ. Eff. 2019, 41, 1029–1039. [Google Scholar] [CrossRef]
- Wang, H.; Shi, H.; Li, Y.; Yu, Y.; Zhang, J. Seasonal variations in leaf capturing of particulate matter, surface wettability and micromorphology in urban tree species. Front. Environ. Sci. Eng. 2013, 7, 579–588. [Google Scholar] [CrossRef]
- Weerakkody, U.; Dover, J.W.; Mitchell, P.; Reiling, K. Evaluating the impact of individual leaf traits on atmospheric particulate matter accumulation using natural and synthetic leaves. Urban For. Urban Green 2018, 30, 98–107. [Google Scholar] [CrossRef]
- Terzaghi, E.; Wild, E.; Zacchello, G.; Cerabolini, B.E.L.; Jones, K.C.; Di Guardo, A. Forest Filter Effect: Role of leaves in capturing/releasing air particulate matter and its associated PAHs. Atmos. Environ. 2013, 74, 378–384. [Google Scholar] [CrossRef]
- Chen, L.; Liu, C.; Zhang, L.; Zou, R.; Zhang, Z. Variation in tree species ability to capture and retain airborne fine particulate matter (PM2.5). Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Yli-Pelkonen, V.; Scott, A.A.; Viippola, V.; Setälä, H. Trees in urban parks and forests reduce O3, but not NO2 concentrations in Baltimore, MD, USA. Atmos. Environ. 2017, 167, 73–80. [Google Scholar] [CrossRef]
- Nowak, D.J.; Hirabayashi, S.; Doyle, M.; McGovern, M.; Pasher, J. Air pollution removal by urban forests in Canada and its effect on air quality and human health. Urban. For. Urban. Green. 2018, 29, 40–48. [Google Scholar] [CrossRef]
- Valotto, G.; Zannoni, D.; Guerriero, P.; Rampazzo, G.; Visin, F. Characterization of road dust and resuspended particles close to a busy road of Venice mainland (Italy). Int. J. Environ. Sci. Technol. 2019, 16, 6513–6526. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Niazi, N.K.; Xiong, T.T.; Farooq, A.B.U.; Khalid, S. Ecotoxicology of Heavy Metal(loid)-Enriched Particulate Matter: Foliar Accumulation by Plants and Health Impacts. In Reviews of Environmental Contamination and Toxicology (Continuation of Residue Reviews); Springer: New York, NY, USA, 2019; pp. 1–49. [Google Scholar]
- Liu, Z.; Zhou, J.; Zhang, J.; Mao, Y.; Huang, X.; Qian, G. Evaluation for the heavy metal risk in fine particulate matter from the perspective of urban energy and industrial structure in China: A meta-analysis. J. Clean. Prod. 2020, 244, 118597. [Google Scholar] [CrossRef]
- Yin, H.; Mu, S.; Zhao, L.; Qi, X.; Pan, X. Microscopic morphology and elemental composition of size distributed atmospheric particulate matter in Urumqi, China. Environ. Earth Sci. 2013, 69, 2139–2150. [Google Scholar] [CrossRef]
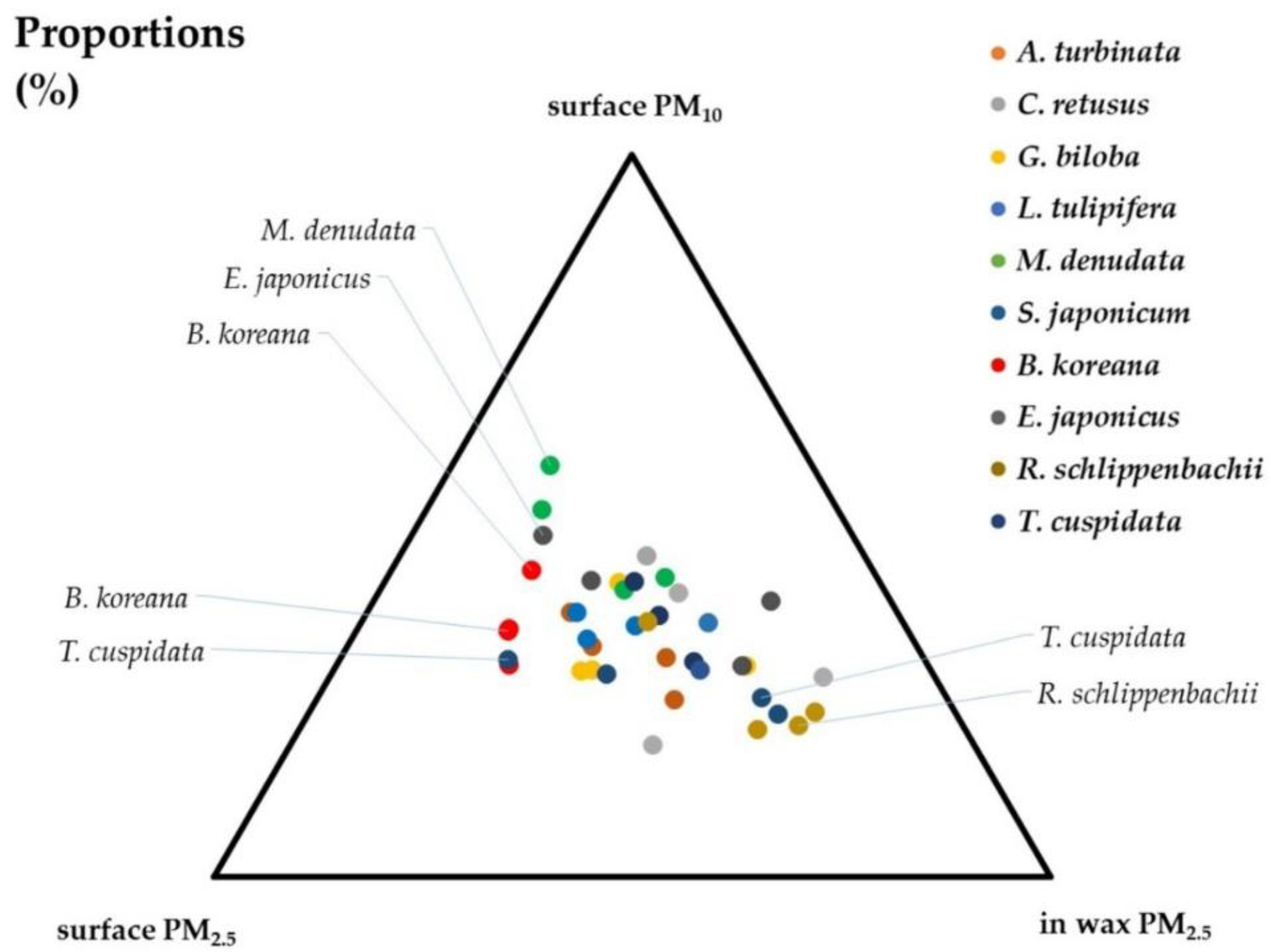
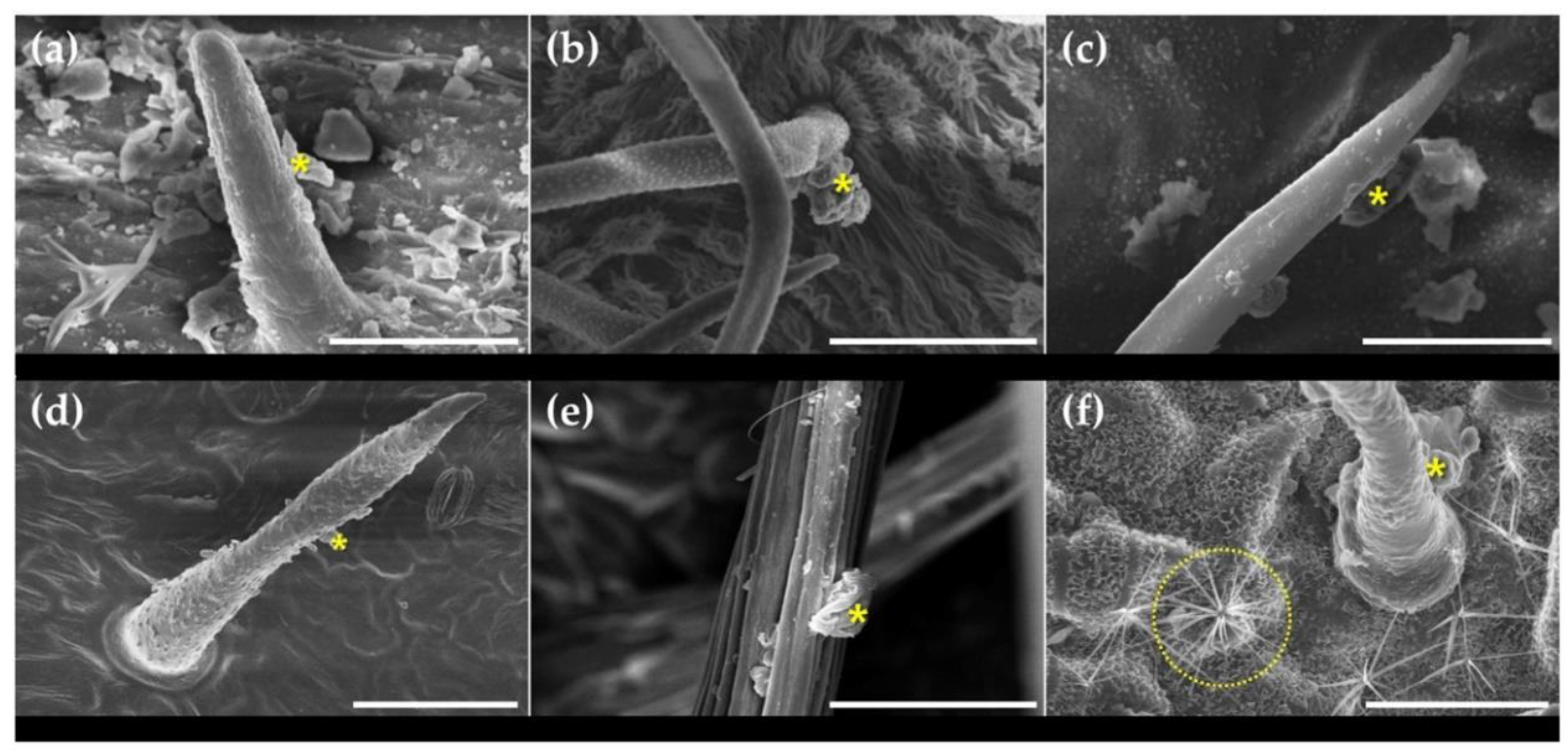
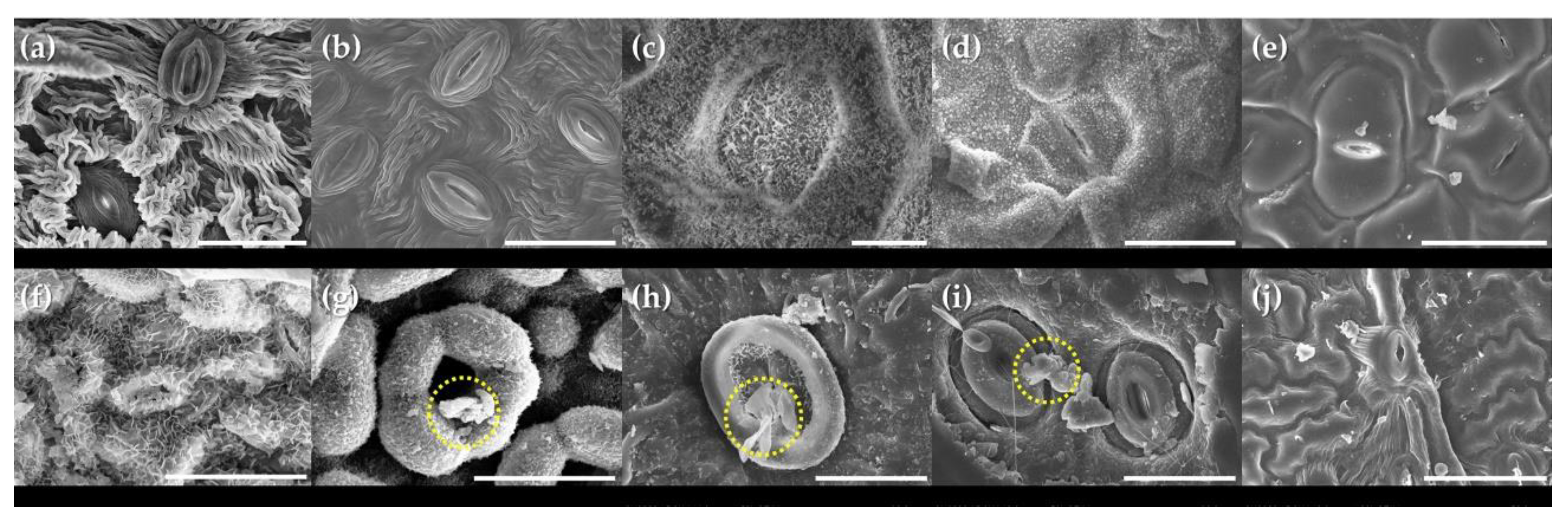
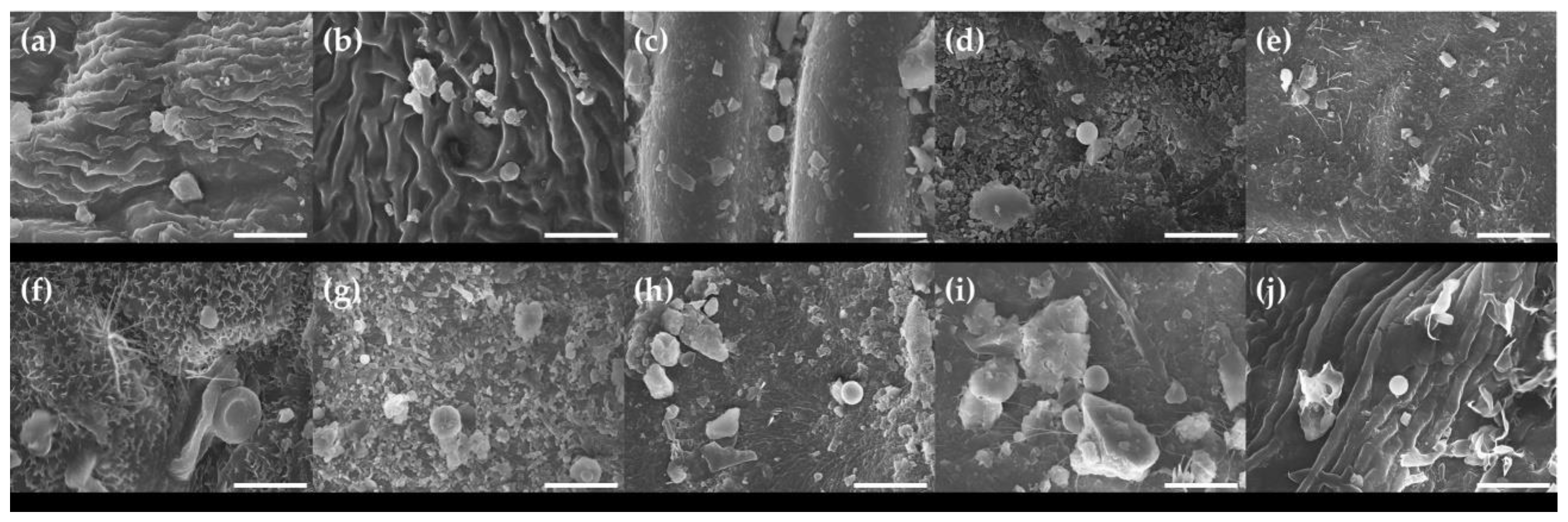
| Scientific Name | Common Name | Family | Height | Diameter at Breast Height | Type |
|---|---|---|---|---|---|
| Aesculus turbinata | Japanese Horse Chestnut | Sapindaceae | 10.1 m | 29 cm | Deciduous broadleaf tree |
| Buxus koreana | Korean boxwood | Buxaceae | 0.8 m | − | Evergreen broadleaf shrub |
| Chionanthus retusus | Retusa fringetree | Oleaceae | 8.9 m | 15.1 cm | Deciduous broadleaf tree |
| Euonymus japonicus | Evergreen spindle | Celastraceae | 1.2 m | 6.2 cm | Evergreen broadleaf shrub |
| Ginkgo biloba | Maidenhair Tree | Ginkgoaceae | 12.4 m | 15.7 cm | Deciduous conifer tree |
| Liriodendron tulipifera | Tuliptree | Magnoliaceae | 11.0 m | 21.3 cm | Deciduous broadleaf tree |
| Magnolia denudata | Yulan Magnolia | Magnoliaceae | 8.0 m | 14 cm | Deciduous broadleaf tree |
| Rhododendron schlippenbachii | Royal azalea | Ericaceae | 1.0 m | − | Deciduous broadleaf shrub |
| Styphnolobium japonicum | Pagoda tree | Leguminosae | 11.1 m | 22.9 cm | Deciduous broadleaf tree |
| Taxus cuspidata | Japanese yew | Taxaceae | 3.8 m | 8.5 cm | Evergreen coniferous shrub or small tree |
| Particulate Matter | Kruskal Wallis Test | Seasonal Rainfall Events | Mann-Whitney U | |||||
|---|---|---|---|---|---|---|---|---|
| Chi-Square | Df | p Value | U | Z | Asymp. Sig. | Significant Difference | ||
| Surface PM10 | 91.089 | 3 | <0.001 | July–Aug. | 223.0 | −7.080 | 0.000 | Yes |
| July–Sep. | 839.0 | −2.833 | 0.005 | Yes | ||||
| July–Oct. | 1015.0 | −1.620 | 0.105 | No | ||||
| Aug.–Sep. | 331.0 | −6.335 | 0.000 | Yes | ||||
| Aug.–Oct. | 90.0 | −7.997 | 0.000 | Yes | ||||
| Sep.–Oct. | 609.0 | −4.419 | 0.000 | Yes | ||||
| Surface PM2.5 | 94.998 | 3 | <0.001 | July–Aug. | 280.0 | −6.687 | 0.000 | Yes |
| July–Sep. | 636.0 | −4.233 | 0.000 | Yes | ||||
| July–Oct. | 758.0 | −3.392 | 0.001 | Yes | ||||
| Aug.–Sep. | 794.0 | −3.144 | 0.002 | Yes | ||||
| Aug.–Oct. | 117.0 | −7.811 | 0.000 | Yes | ||||
| Sep.–Oct. | 258.0 | −6.839 | 0.000 | Yes | ||||
| In wax PM10 | 14.294 | 3 | 0.003 | July–Aug. | 818.0 | −2.978 | 0.003 | Yes |
| July–Sep. | 849.0 | −2.764 | 0.006 | Yes | ||||
| July–Oct. | 761.0 | −3.371 | 0.001 | Yes | ||||
| Aug.–Sep. | 1216.5 | −0.231 | 0.817 | No | ||||
| Aug.–Oct. | 1198.0 | −0.358 | 0.720 | No | ||||
| Sep.–Oct. | 1159.5 | −0.624 | 0.533 | No | ||||
| In wax PM2.5 | 13.101 | 3 | 0.004 | July–Aug. | 877.0 | −2.571 | 0.010 | No |
| July–Sep. | 1006.5 | −1.679 | 0.093 | No | ||||
| July–Oct. | 761.0 | −3.371 | 0.001 | Yes | ||||
| Aug.–Sep. | 1069.0 | −1.248 | 0.212 | No | ||||
| Aug.–Oct. | 1179.5 | −0.486 | 0.627 | No | ||||
| Sep.–Oct. | 976.5 | −1.885 | 0.059 | No | ||||
| Species | Stomata on Leaf Surfaces | Trichomes on Leaf Surfaces | ||||||
|---|---|---|---|---|---|---|---|---|
| Density | Size (µm) | Trichome Types | Distribution | Size (µm) | ||||
| (No. mm−2) | Length | Width | Adaxial | Abaxial | Adaxial | Abaxial | ||
| Aesculus turbinata | 637 ± 190 | 12 ± 3 | 5 ± 2 | non-glandular | – | ++ | 182 ± 108 | |
| Chionanthus retusus | 316 ± 142 | 20 ± 3 | 8 ± 2 | non-glandular | – | + | 195 ± 75 | |
| peltate glandular | + | ++ | 37 ± 3 | |||||
| Ginkgo biloba | 99 ± 8 | 22 ± 3 | 15 ± 3 | – | – | |||
| Liriodendron tulipifera | 166 ± 13 | 20 ± 5 | 8 ± 2 | – | – | |||
| Magnolia denudata | 275 ± 52 | 15 ± 2 | 6 ± 1 | non-glandular | + | + | 192 ± 38 | 397 ± 76 |
| Styphnolobium japonicum | 178 ± 19 | 12 ± 2 | 6 ± 2 | non-glandular | + | ++ | 213 ± 29 | 459 ± 141 |
| stellate | + | 33 ± 8 | ||||||
| Buxus koreana | 127 ± 9 | 42 ± 3 | 33 ± 3 | glandular | + | – | 54 ± 14 | |
| Euonymus japonicus | 243 ± 36 | 27 ± 1 | 21 ± 1 | – | – | |||
| Rhododendron schlippenbachii | 347 ± 35 | 17 ± 2 | 8 ± 1 | non-glandular | + | + | 981 ± 315 | 1328 ± 428 |
| Taxus cuspidata | 187 ± 24 | 42 ± 4 | 34 ± 4 | – | – | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, M.J.; Lee, J.K.; Park, S.; Kim, H.; Lim, Y.J.; Lee, K.-A.; Son, J.-a.; Oh, C.-Y.; Kim, I.; Woo, S.Y. Surface-Based Analysis of Leaf Microstructures for Adsorbing and Retaining Capability of Airborne Particulate Matter in Ten Woody Species. Forests 2020, 11, 946. https://doi.org/10.3390/f11090946
Kwak MJ, Lee JK, Park S, Kim H, Lim YJ, Lee K-A, Son J-a, Oh C-Y, Kim I, Woo SY. Surface-Based Analysis of Leaf Microstructures for Adsorbing and Retaining Capability of Airborne Particulate Matter in Ten Woody Species. Forests. 2020; 11(9):946. https://doi.org/10.3390/f11090946
Chicago/Turabian StyleKwak, Myeong Ja, Jong Kyu Lee, Sanghee Park, Handong Kim, Yea Ji Lim, Keum-Ah Lee, Joung-a Son, Chang-Young Oh, Iereh Kim, and Su Young Woo. 2020. "Surface-Based Analysis of Leaf Microstructures for Adsorbing and Retaining Capability of Airborne Particulate Matter in Ten Woody Species" Forests 11, no. 9: 946. https://doi.org/10.3390/f11090946
APA StyleKwak, M. J., Lee, J. K., Park, S., Kim, H., Lim, Y. J., Lee, K.-A., Son, J.-a., Oh, C.-Y., Kim, I., & Woo, S. Y. (2020). Surface-Based Analysis of Leaf Microstructures for Adsorbing and Retaining Capability of Airborne Particulate Matter in Ten Woody Species. Forests, 11(9), 946. https://doi.org/10.3390/f11090946








