Abstract
Emerald ash borer is an invasive pest in North American forests. Ecological impacts of ash mortality from emerald ash borer are wide-ranging, including shifts in insect communities and wildlife behavior. Additionally, loss of ash from forests may have important implications regarding plant succession. Surveys of overstory, midstory, and understory trees within forests in northeastern Indiana, Lower Peninsula of Michigan, and northwestern Ohio were conducted to quantify the change in forest composition over a 10 year period. Interpolation of ash dominance illustrated inversion of live and dead ash values between 2007 and 2017. Even though more than 83% of overstory live ash basal area was lost across the study area, green ash was the most abundant midstory and understory species representing regeneration. Additionally, loss of ash from many of the sites resulted in compositional changes that were greater than merely the subtraction of ash. Due to the relatively large number of forest types with which ash species are associated, loss of ash will have broad ecological consequences, including on community composition.
1. Introduction
Introduced in the 1990s from Asia, emerald ash borer (Agrilus planipennis Fairmaire) has become a well-established insect pest in North American forests [1,2]. Since that introduction and subsequent discovery in 2002, emerald ash borer has spread throughout much of eastern Canada and the United States causing widespread decline and mortality in ash (Fraxinus spp.) [3]. Efforts have been made to improve biological and silvicultural control methods, as well as urban focused chemical control techniques, e.g., [4,5,6]. However, those improvements have had little impact on emerald ash borer, as evidenced by its continued spread and infestation, as well as continued ash mortality.
Ecological impacts of emerald ash borer in infested forests are potentially broad, ranging from changes in wildlife behavior and insect abundances to successional trajectories and nutrient cycling [7,8,9]. The breadth of these impacts is an indication of the relative importance of ash species within North American forests. Ash (mostly black [F. nigra Marshall], green [F. pennsylvanica Marshall], and white [F. americana L.]) are defining species for eight different forest types and are commonly associated with 33 other forest types in North America [10]. Advance regeneration (i.e., seedlings in place before a disturbance) in those forests infested with emerald ash borer will be key to the subsequent recruitment of individuals. Densities of ash and other species within forests are variable and will likely relate to overstory composition [11,12].
Similar losses of a single genus or species have occurred with major consequence in eastern North American forests. The introductions of chestnut blight resulted in removal of American chestnut (Castanea dentata [Marshall] Borkh.) from much of North America and Dutch elm disease led to the loss of North American elms as a genus (Ulmus spp.) from many areas [13]. Forest ecosystems are dynamic with the scale and intensity of genus or species loss variable by forest location, composition, and other external factors. Additionally, gap formation and structural changes as a result of group or individual tree losses are essential to defining the vegetation mosaic within a forest [14]. However, genus removal from a forest may extend the impact beyond forest mosaic maintenance.
Since ash as a genus is an important or associated component in many different forest types, the density of ash in those forests will vary. Density of emerald ash borer in any given forest is variable as well [15]. The objectives of this study were to (1) survey and characterize forest composition at sites in Indiana, Michigan, and Ohio, (2) quantify forest changes over a 10 year period of emerald ash borer infestation, and (3) test the hypothesis that forests with greater densities of both ash and emerald ash borer in 2007 will exhibit greater compositional changes over that decade.
2. Materials and Methods
Forty-four sites were originally selected and established in 2007 based on collaboration with state agencies (state parks, forests, and game areas), accessibility (permission to use and physically access), visual dominance of ash (rapid visual surveys conducted at numerous sites), and presence of emerald ash borer (prior trapping studies or state agency detection) [15]. Sites were in northeastern Indiana (n = 7), Lower Peninsula of Michigan (n = 30), and northwestern Ohio (n = 7), USA. After the 2007 surveys were conducted, sites were categorized as low emerald ash borer density (≤87 adults) and high density (>87 adults) based on number of adults captured on eight different trap types, as defined as a natural break in the data by Marshall et al. [15] (Figure 1). In both 2007 and 2017, five locations were randomly selected within a forest stand and used as center points for surveys. Available center point locations for random selection were originally associated with emerald ash borer traps used in 2007 to quantify densities [15]. Selection of locations for plot center points included avoiding overlap during basal area measurements.

Figure 1.
Survey locations in Indiana, Michigan, and Ohio. Emerald ash borer density in 2007 from [15].
2.1. Overstory Survey
Basal area was measured in July 2007 with a 10-factor Cruz-All angle gauge (Forestry Suppliers, Jackson, MS, USA) [15] and in August and September 2017 with a 10-factor cruising prism (Forestry Suppliers, Jackson, MS, USA). Individuals ≥ 8 cm dbh were identified to species. Basal area (m/ha) for live ash, dead ash, and total forest were calculated, as well as percent of total forest basal area comprised of live and dead ash.
2.2. Understory and Midstory Survey
In August and September 2017, understory and midstory tree individuals were counted. At each plot center, a 1 m quadrat and a 5 m quadrat were established. Within the 1 m quadrat, all understory trees (≤2 m in height) were counted and identified to species. Within the 5 m quadrat, all midstory trees (>2 m in height, <8 cm dbh) were counted and identified to species.
2.3. Data Analysis
Relative dominance was calculated as basal area of each ash species at a site divided by total basal area of the site. An analysis of variance (ANOVA) was used to compare the relative dominance of black, green, and white ash blocked on state and nested in sampling years with a Tukey’s HSD post-hoc test. Relative importance values were calculated for each species a site as the sum of relative frequency (number of plots/total number of plots), relative density (number of individuals/total number of individuals), and relative dominance (basal area/total basal area) divided by 3. Inverse distance weighting (IDW) interpolation was used to map the distribution of relative dominance (percent basal area) of live and dead ash from 2007 and 2017 surveys. Pearson’s correlation was used to test for relationships between understory and midstory counts (ash and pooled other species) and 2017 overstory live ash, dead ash, and pooled other species basal area measures (m/ha). A dissimilarity ratio was calculated for each forest as
where was the Bray-Curtis dissimilarity of forest overstory composition between 2007 and 2017, and was the Bray-Curtis dissimilarity between 2007 with ash removed and 2017. Bray-Curtis dissimilarity provided a robust measure of ecological “distance” and represented a compositional “distance” between sample years at a given site [16]. Dissimilarity ratio values greater than 1.0 represented dissimilarity (i.e., distance) in forest community composition between sample years that was greater than the subtraction of ash, while values of 1.0 or less represent community compositional change as merely the subtraction of ash. Bray-Curtis dissimilarity values were calculated using vegdist function in the vegan package of R [17]. Chi-square tests were used to test for independence in shifts of the most important overstory species related to ash and emerald ash borer density categories. All statistical analyses were conducted with = 0.05 in R version 4.0.2 [18].
3. Results
While five plot locations were selected and surveyed in each forest stand, data analysis was conducted with stand as the experimental unit and plot values were pooled within each stand (labeled as site due to single stand per forest). ANOVA identified differences in the relative dominance of the three ash species (F = 7.70, P < 0.001), however, the difference with a post-hoc test was only between green and black ash dominance. While this difference did exist, further analysis pooled ash as a genus because (1) green-white and black-white relative dominance did not differ, and (2) black ash was the single ash species at only one site. Across all sites, ash ranked in the top-5 of relative importance values at 37 of the 44 sites (84.1%). However, ash was the top-ranked species at only 14 sites (31.8%). There was a reduction in mean live ash basal area from 3.29 to 0.54 m/ha across the entire study area, with an increase in mean dead ash basal area from 0.23 to 4.91 m/ha.
IDW interpolation map for portions of Indiana, Michigan, and Ohio illustrate the patchy relative dominance of ash across the sampling sites in 2007 (Figure 2A,B). For most of the study area, live ash accounted for less than 40% of basal area in 2007 with relatively low numbers of standing dead ash in 2007 (Figure 2B, Table 1). These values switched in 2017 with relatively low numbers of live ash and standing dead ash becoming much more common (Figure 2C,D, Table 1). No sites were included in southwest Michigan, which limits the accuracy and interpretation of the IDW interpolation in that portion of study area (Figure 1 and Figure 2).
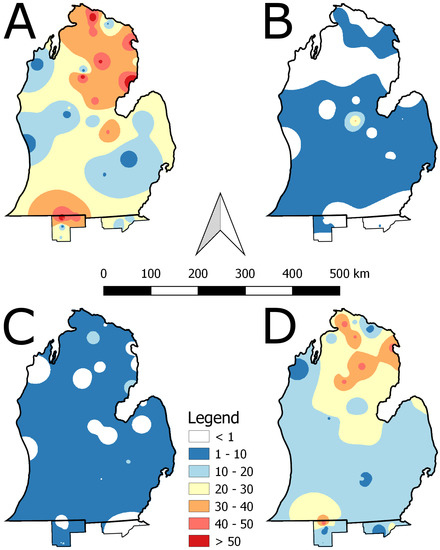
Figure 2.
Inverse distance weighting interpolation of relative dominance (percent basal area) of (A) live ash in 2007, (B) dead ash in 2007, (C) live ash in 2017, and (D) dead ash in 2017.

Table 1.
Mean percent forest basal area comprised of live and dead ash at sites in Indiana, Michigan, and Ohio, (standard error) with range values.
In the understory, green ash was the most abundant species accounting for 52.8% of all seedlings counted across the study area. Black and white ash were substantially less abundant, accounting for 4.1% and 1.9%, respectively. All plots surveyed had ash seedlings. The top-3 non-ash understory species were sugar maple (Acer saccharum Marshall) as 19.1% of individuals, black cherry (Prunus serotina Ehrh.) as 2.2%, and American elm (Ulmus americana L.) as 2.1%. Green ash was also the most abundant midstory species, accounting for 30.4% of individuals. As with the understory, black and white ash were far less abundant, accounting for 3.1% and 0.7% of midstory individuals, respectively. Black cherry and American elm were in the top-3 non-ash species in the midstory (5.1% and 4.2% of individuals, respectively). Red maple (A. rubrum L.) was the most abundant non-ash midstory species (6.8% of individuals). Counts of understory ash and other tree species pooled were positively correlated (r = 0.31, p = 0.044). However, counts of midstory ash and other tree species were not correlated (r = −0.07, p = 0.630). Understory ash counts were not correlated with basal area measurements in 2017. However, understory species pooled other than ash were positively correlated with the 2017 pooled other species basal area measurement (r = 0.32, p = 0.035). Midstory ash counts were positively correlated with 2017 dead ash basal area (r = 0.37, p = 0.014).
In 18 of the surveyed forests, the overstory composition did not change beyond the subtraction of ash (dissimilarity ratio ≤ 1.0), while shifting of the composition greater than merely the subtraction of ash (dissimilarity ratio > 1.0) occurred at 26 forests (59%) (Figure 3). However, the shift was independent of the 2007 ash density categories (X = 0.70, p = 0.402) and emerald ash borer density categories (X = 3.13, p = 0.077). In eight single forest stands, the most dominant overstory species were boxelder (A. negundo L.), red maple, sugar maple, standing dead black ash, black walnut (Juglans nigra L.), swamp white oak (Quercus bicolor Willd.), and sassafras (Sassafras albidum [Nutt.] Nees). In two stands each, the most dominant overstory species were Balsam fir (Abies balsamea [L.] Mill.), silver maple (A. saccharinum L.), shagbark hickory (Carya ovata [Mill.] K. Koch), quaking aspen (Populus tremuloides Michx.), and black cherry. Standing dead green ash became the most dominant in nine forests. Removing standing dead ash from the relative importance value calculations, those dead ash dominated forests added one forest each of balsam fir, eastern cottonwood (P. deltoides W. Bartram ex Marshall), quaking aspen, black cherry, and arborvitae (Thuja occidentalis L.); two forests of black walnut; and three forests of red maple. Seventy-one percent of forests with ash as the most important species in 2007 shifted to standing dead ash, with most of those becoming dominated by standing dead green ash. In one forest, the 2017 dominant species did not occur in the five most relatively important species in 2007, shifting from green ash to sugar maple; however, sugar maple was in the ten most abundant species originally (Supplmentary Tables S1 and S2).
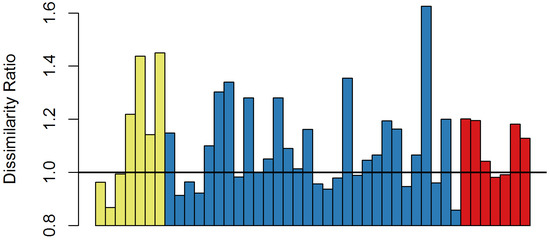
Figure 3.
Dissimilarity ratio ([Bray-Curtis dissimilarity between 2007 site data and 2017 site data]/[Bray-Curtis dissimilarity between 2007 site data with ash individuals removed and 2017 site data]) for sites in Indiana (yellow), Michigan (blue), and Ohio (red). Values greater than 1.0 represent change in forest community composition greater than simply subtraction of ash.
4. Discussion
The introduction of emerald ash borer to North America has resulted in the major loss of ash individuals within numerous forests, e.g., [12]. Loss of these stand defining species would be expected to lead to compositional and structural changes within those forests. Many of the forests in the study presented here had compositional changes greater than merely the subtraction of ash, even though ash was the top-ranked species by basal area at only 32% of sites. Such losses of stand defining species can have a variety of effects on biotic and abiotic characteristics of forests [19].
IDW interpolation provided a graphical representation of the change that occurred across the study sites. The inversion of abundance between live and dead ash is striking, however, there was not an exact conversion from live to dead. While not measured, at many sites there was an abundance of coarse woody debris made up of downed ash individuals. This likely reduced the standing dead basal area measures at several sites. If an intermediate measurement between 2007 and 2017 had been collected, there likely would have been a clearer pattern of ash loss and subsequent accumulation of coarse woody debris as there are interactions between disturbance and time across different forest types [20,21]. The locations with maximum live ash basal area in 2017 were isolated in the IDW map, with ash reduced to well below 10% of forest basal area. Across the study area, live ash was reduced by approximately 84%. This value of loss for the entire study site is lower than 2008 values presented by Klooster et al. [12]. Additionally, Marshall et al. [22] estimated 7.8% of standing ash were dead individuals in 2006 across a similar study area as presented here, which is reasonable that in 2007 4.4% of standing ash basal area was dead with the potential falling of trees to become coarse woody debris.
Since understory and midstory counts were not performed in 2007, there is no measure of regeneration change between years. However, other studies have quantified the changes in ash understories, which provide context in what was observed in these 44 sites. Rapid decreases in ash seedling densities over the course of several years were reported in previously published studies performed in 2008 and 2009, where no new seedlings were observed in most sites [12,23]. However, all sites and plots within sites surveyed in 2017 had ash seedlings. Even with different survey methods, these values are inconsistent with those presented by those previous studies [12,23]. The results presented here from 2017 were similar to those reported by Burr and McCullough from [11] who found ash seedlings in relatively high densities at sites close to the introduction epicenter for emerald ash borer in southeast Michigan. Also, the most abundant understory and midstory non-ash species were similar to Burr and McCullough [11], with sugar maple, black cherry, and American elm dominating. Flower et al. [8] reported maple and elm species had high growth rates in forests where ash was lost.
While ash regeneration is still occurring and individuals are established in the understory and midstory, overstory compositional changes have occurred with the loss of ash. Removing a genus from the forest will inherently lead to compositional change, shifting dominance to different species. However, the intensity of that change varied across study sites. At 18 sites, dissimilarity between 2007 and 2017 was accounted for by merely subtracting ash from the overstory. However, the remaining sites had substantial dissimilarity ratios that indicated a compositional change greater than the subtraction of ash. These forests did shift to different dominant species. While the understory species were similar to previous studies, the overstory composition shifts were to a variety of species.
With over 80% of live ash lost to emerald ash borer between the 10 years of surveys, there was a clear change in forest composition. Whether costs are calculated in economic or ecological terms, e.g., [8,24,25], this loss has resulted in a substantial and devastating impact on the forests within the North American Great Lakes region. Ash has been retained in the overstory at many sites surveyed, but those areas with ash basal area accounting for more than 10% of the forest are isolated. Due to the dominance and importance of ash in several different forest types, the loss of ash will have long-term successional and functional repercussions [8]. Forests will respond to the loss of ash, however, the inherent resiliency of each forest to disturbance (e.g., silvicultural activities, ice damage, wind-throw, disaease) will be weakened [26,27].
5. Conclusions
Loss of ash to emerald ash borer from many North American forests has been a growing concern since the pest insect was discovered. Interpolation of ash dominance between 2007 and 2017 surveys illustrates a clear loss of ash and isolation of few areas with ash basal area accounting for more the 10% of the forest. Additionally, regeneration of ash has occurred in these forests, but with removal of over 83% of ash basal area, the seed source is in peril. The substantial loss of ash across the Great Lakes region has caused overstory compositional shifts that will likely result in changes in successional trajectories. Several different species have moved into the dominant position in these communities, however, in many of the forests standing dead ash is now the dominant basal area component. Those new dominant species were not consistent across the study area. These forests are responding to the removal of ash as a key component of the forest type. Potentially, such a loss of a key genus will reduce the inherent resiliency each forest has to future disturbances.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4907/11/9/949/s1, Table S1: Forest overstory relative importance values (summed relative frequency, relative density, and relative dominance, divided by 3) by species in 2007 survey; Table S2: Forest overstory relative importance values (summed relative frequency, relative density, and relative dominance, divided by 3) by species in 2017 survey.
Funding
Funding for the 2007 data collection was provided by the USDA-APHIS Accelerated Emerald Ash Borer Research Program. Data collection in 2017 received no external funding.
Acknowledgments
I would like to thank Janet Fredrick, Michelle Freeman, Rita Koch, Mike Rietz, Nicole Smith, and Andrew Storer for assistance in 2007 data collection.
Conflicts of Interest
The author declares no conflict of interest.
References
- Poland, T.M.; McCullough, D.G. Emerald ash borer: Invasion of the urban forest and the threat to North America’s ash resource. J. For. 2006, 104, 118–124. [Google Scholar]
- Siegert, N.W.; McCullough, D.G.; Liebhold, A.M.; Telewski, F.W. Dendrochronological reconstruction of the epicenter and early spread of emerald ash borer in North America. Divers. Distrib. 2014, 20, 847–858. [Google Scholar] [CrossRef]
- Herms, D.A.; McCullough, D.G. Emerald ash borer invasion of North America: History, biology, ecology, impacts, and management. Annu. Rev. Entomol. 2014, 59, 13–30. [Google Scholar] [CrossRef]
- Duan, J.J.; Bauer, L.S.; Hansen, J.A.; Abell, K.J.; Van Driesche, R. An improved method for monitoring parasitism and establishment of Oobius agrili (Hymenoptera: Encyrtidae), an egg parasitoid introduced for biological control of the emerald ash borer (Coleoptera: Buprestidae) in North America. Biol. Control 2012, 60, 255–261. [Google Scholar] [CrossRef]
- Duan, J.J.; Bauer, L.S.; Abell, K.J.; Lelito, J.P.; Van Driesche, R. Establishment and abundance of Tetrastichus planipennisi (Hymenoptera: Eulophidae) in Michigan: Potential for success in classical biocontrol of the invasive emerald ash borer (Coleoptera: Buprestidae). J. Econ. Entomol. 2013, 106, 1145–1154. [Google Scholar] [CrossRef]
- McCullough, D.G.; Mercader, R.J. Evaluation of potential strategies to SLow Ash Mortality (SLAM) caused by emerald ash borer (Agrilus planipennis): SLAM in an urban forest. Int. J. Pest Manag. 2012, 58, 9–23. [Google Scholar] [CrossRef]
- Gandhi, K.J.K.; Herms, D.A. North American arthropods at risk due to widespread Fraxinus mortality caused by the alien emerald ash borer. Biol. Invasions 2010, 12, 1839–1846. [Google Scholar] [CrossRef]
- Flower, C.E.; Knight, K.S.; Gonzalez-Meler, M.A. Impacts of the emerald ash borer (Agrilus planipennis Fairmaire) induced ash (Fraxinus spp.) mortality on forest carbon cycling and successional dynamics in the eastern United States. Biol. Invasions 2013, 15, 931–944. [Google Scholar] [CrossRef]
- Flower, C.E.; Long, L.C.; Knight, K.S.; Rebbeck, J.; Brown, J.S.; Gonzalez-Meler, M.A.; Whelan, C.J. Native bark-foraging birds preferentially forage in infected ash (Fraxinus spp.) and prove effective predators of the invasive emerald ash borer (Agrilus planipennis Fairmaire). For. Ecol. Manag. 2014, 313, 300–306. [Google Scholar] [CrossRef]
- Eyre, F.H. Forest Cover Types of the United States and Canada; Society of American Foresters: Washington, DC, USA, 1980. [Google Scholar]
- Burr, S.J.; McCullough, D.G. Condition of green ash (Fraxinus pennsylvanica) overstory and regeneration at three stages of the emerald ash borer invasion wave. Can. J. For. Res. 2014, 44, 768–776. [Google Scholar] [CrossRef]
- Klooster, W.S.; Herms, D.A.; Knight, K.S.; Herms, C.P.; McCullough, D.G.; Smith, A.; Gandhi, K.J.K.; Cardina, J. Ash (Fraxinus spp.) mortality, regeneration, and seed bank dynamics in mixed hardwood forests following invasion by emerald ash borer (Agrilus planipennis). Biol. Invasions 2014, 16, 859–873. [Google Scholar] [CrossRef]
- Castello, J.D.; Leopold, D.J.; Smallidge, P.J. Pathogens, patterns, and processes in forest ecosystems. BioScience 1995, 45, 16–24. [Google Scholar] [CrossRef]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics; John Wiley & Sons, Inc.: New York, NY, USA, 1996. [Google Scholar]
- Marshall, J.M.; Storer, A.J.; Fraser, I.; Beachy, J.A.; Mastro, V.C. Effectiveness of differing trap types for the detection of emerald ash borer (Coleoptera: Buprestidae). Environ. Ent. 2009, 38, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Faith, D.P.; Minchin, P.R.; Belbin, L. Compositional dissimilarity as a robust measure of ecological distance. Vegetatio 1987, 69, 57–68. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-6. Available online: https://CRAN.R-project.org/package=vegan (accessed on 11 August 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: https://www.R-project.org/ (accessed on 11 August 2020).
- Ellison, A.M.; Bank, M.S.; Clinton, B.D.; Colburn, E.A.; Elliott, K.; Ford, C.R.; Foster, D.R.; Kloeppel, B.D.; Knoepp, J.D.; Lovett, G.M.; et al. Loss of foundation species: Consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ. 2005, 3, 479–486. [Google Scholar] [CrossRef]
- Sturtevant, B.R.; Bissonette, J.A.; Long, J.N.; Roberts, D.W. Coarse woody debris as a function of age, stand structure, and disturbance in boreal Newfoundland. Ecol. Appl. 1997, 7, 702–712. [Google Scholar] [CrossRef]
- Cobb, R.C.; Chan, M.N.; Meentemeyer, R.K.; Rizzo, D.M. Common factors drive disease and coarse woody debris dynamics in forests impacted by sudden oak death. Ecosystems 2012, 15, 242–255. [Google Scholar] [CrossRef]
- Marshall, J.M.; Smith, E.L.; Mech, R.; Storer, A.J. Estimates of Agrilus planipennis infestation rates and potential survival of ash. Am. Midl. Nat. 2013, 169, 179–193. [Google Scholar] [CrossRef]
- Kashain, D.M.; Witter, J.A. Assessing the potential for ash canopy tree replacement via current regeneration following emerald ash borer-caused mortality on southeastern Michigan landscapes. For. Ecol. Manag. 2011, 261, 480–488. [Google Scholar] [CrossRef]
- Sydnor, T.D.; Bumgardner, M.; Todd, A. The potential economic impacts of emerald ash borer (Agrilus planipennis) on Ohio, U.S., communities. Arboric. Urban For. 2007, 33, 48–54. [Google Scholar]
- Kovac, K.F.; Haight, R.G.; McCullough, D.G.; Mercader, R.J.; Siegert, N.W.; Liebhold, A.M. Cost of potential emerald ash borer damage in U.S. communities, 2009–2019. Ecol. Econ. 2010, 69, 569–578. [Google Scholar] [CrossRef]
- Abrams, M.D.; Scott, M.L. Disturbance-mediated accelerated succession in two Michigan forest types. For. Sci. 1989, 35, 42–49. [Google Scholar]
- Folke, C.; Carpenter, S.; Walker, B.; Scheffer, M.; Elmqvist, T.; Gunderson, L.; Holling, C.S. Regime shifts, resilience, and biodiversity in ecosystem management. Ann. Rev. Ecol. Evol. Syst. 2004, 35, 557–581. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).