Vitality and Growth of the Threatened Lichen Lobaria pulmonaria (L.) Hoffm. in Response to Logging and Implications for Its Conservation in Mediterranean Oak Forests
Abstract
1. Introduction
2. Materials and Methods
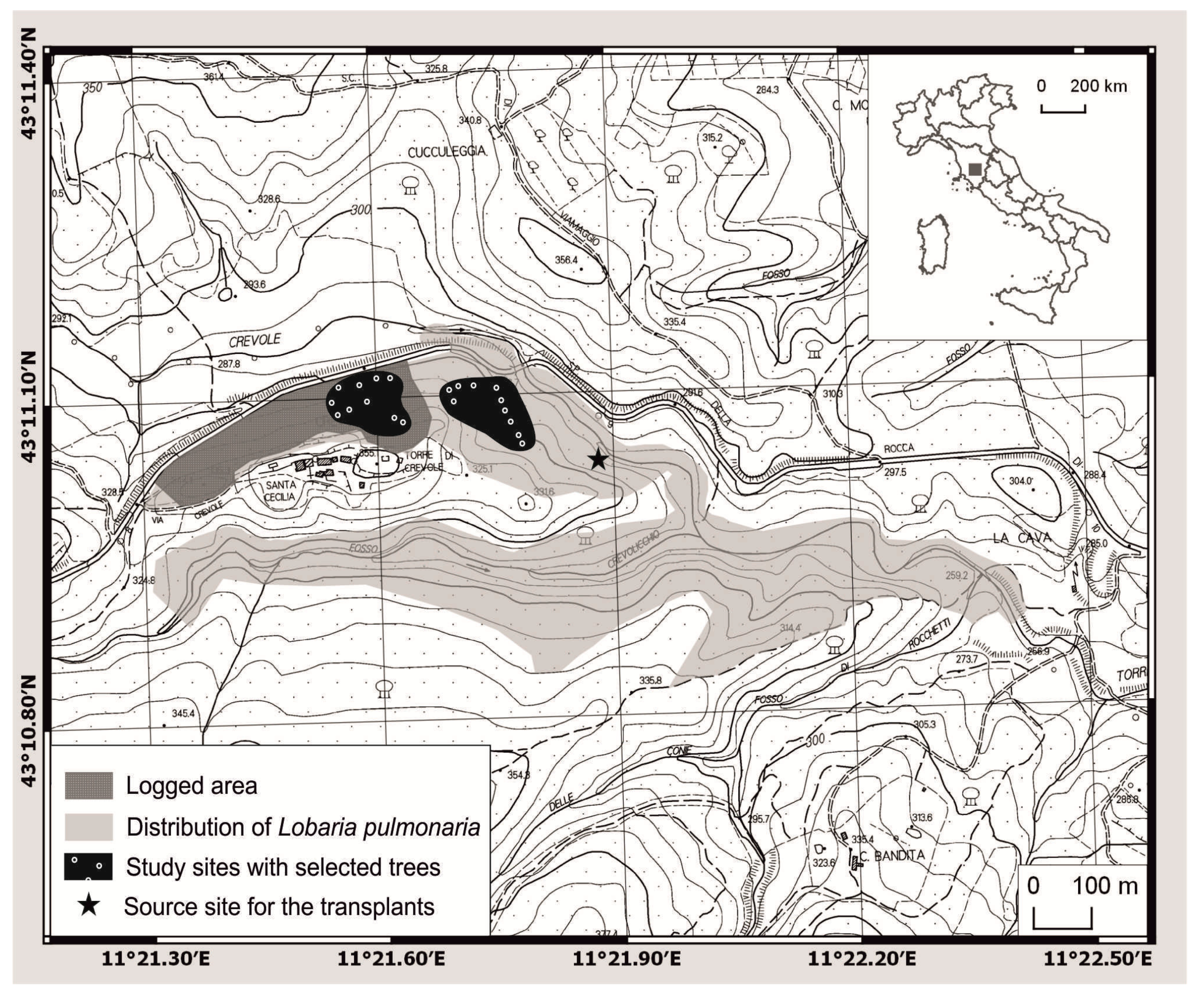
2.1. Study Sites
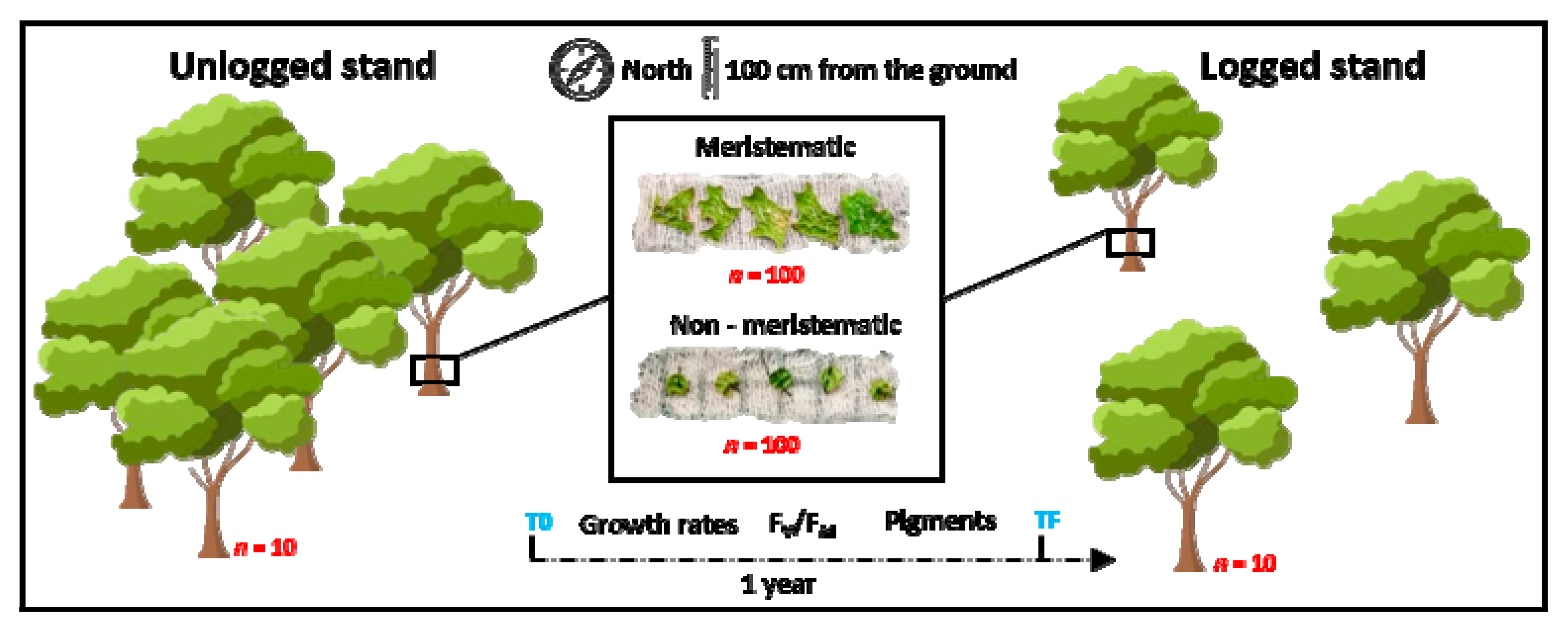
2.2. Experimental Design
2.3. Vitality of the Lichen Photobiont
2.4. Lichen Growth
2.5. Data Analysis
- Growth rate = (area TF − area T0) area T0−1, where:
- area TF: area of the transplanted thallus fragment at the end of the exposure.
- area T0: area of the transplanted thallus fragment at the beginning of the experiment.
- Delta fluo = [(FV/FM)TF − (FV/FM)T0)] (FV/FM)T0−1, where:
- (FV/FM) TF: FV/FM of the transplanted thallus fragment at the end the exposure.
- (FV/FM) T0: FV/FM of the transplanted thallus fragment at the beginning of the experiment.
- Delta Chl = (ChlTF − ChlT0) Chl T0−1, where:
- Chl TF: chlorophyll content of the transplanted thallus fragment at the final time of the exposure.
- Chl T0: chlorophyll content of the transplanted thallus fragment at the beginning of the experiment.
3. Results
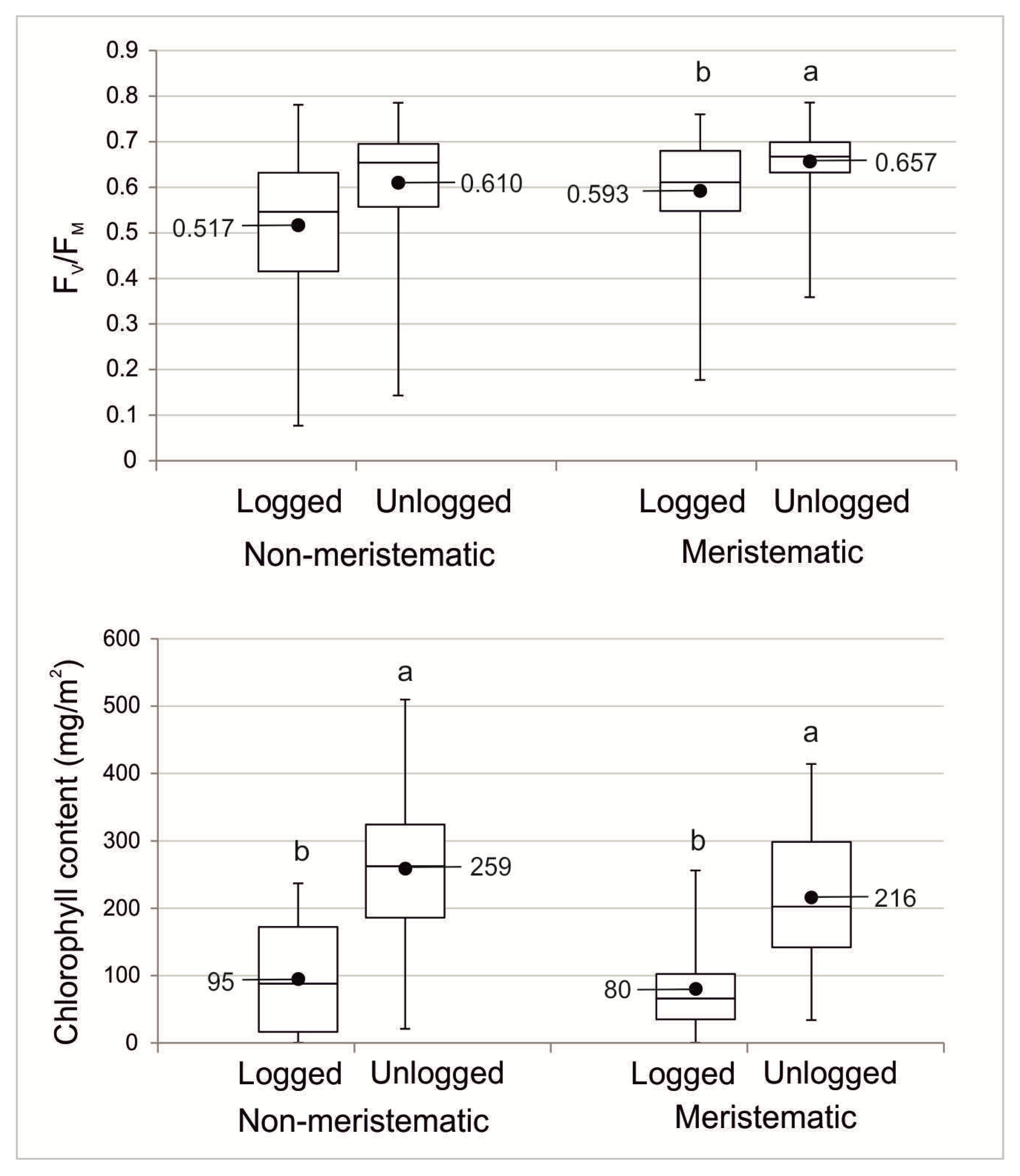
3.1. Lichen Vitality
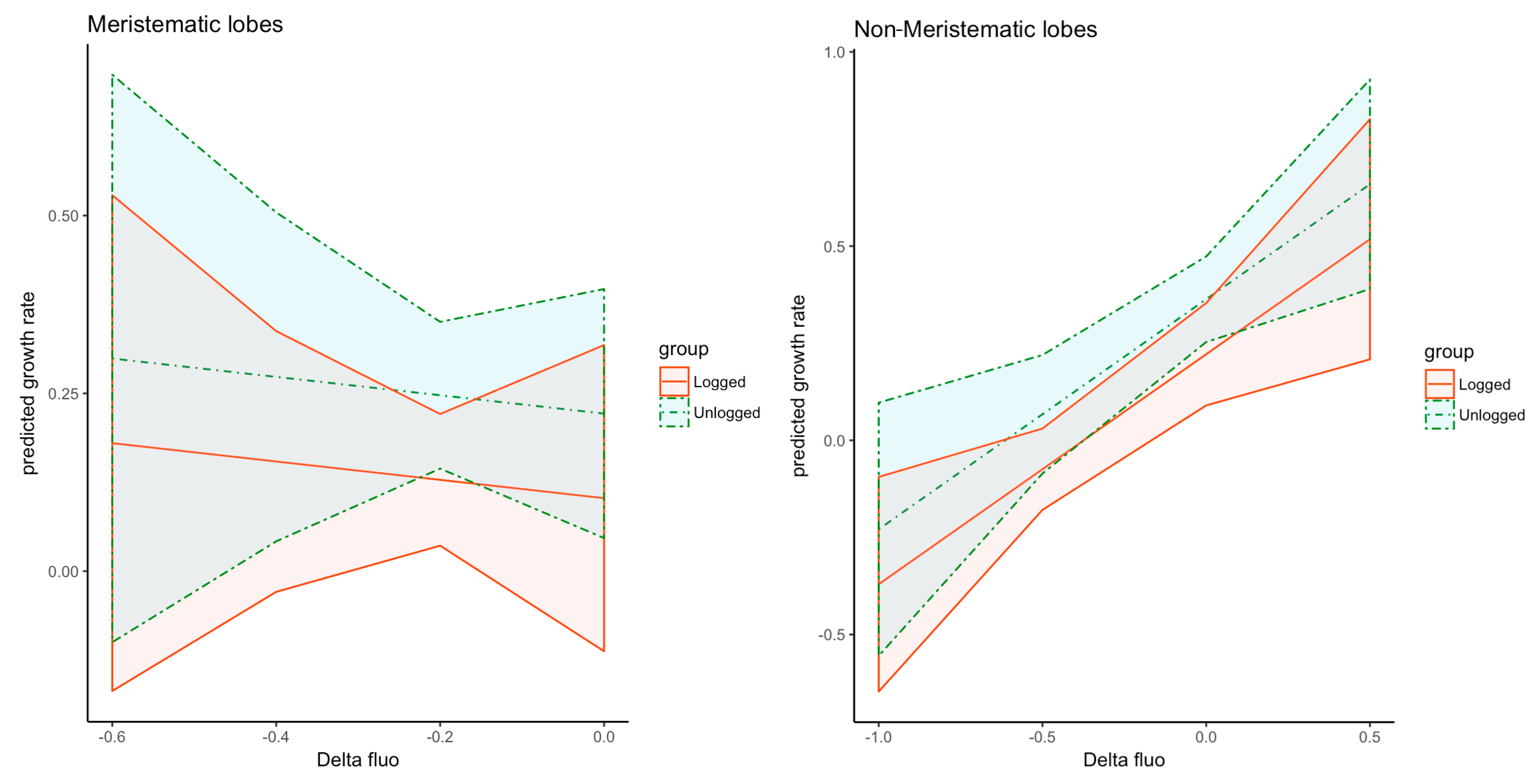
3.2. Thallus Growth
3.3. Principal Component Analysis (PCA)
3.4. Multiple Linear Regression Models
4. Discussion
- They can be directly destroyed/lost due to the logging of the trunks for timber production, as already occurred in the investigated oak forest (loss of 40% of L. pulmonaria biomass) [48].
- They can remain attached to the bark of retained trees and face a gradient of more or less stressing conditions according to their cardinal exposure, position on the trunk, and distance among the trees.
- (a)
- Some of the thalli show visible symptoms of damage consisting of discoloration and bleaching of the surfaces. Curling can occur as a strategy to limit the damage [65]. The thalli can experience a significant reduction of photosynthetic performance (decreased vitality) and the absence of growth. They become thinner due to the significant reduction (by 35%) of the algal layer [49]. Most of them likely will be lost in the long term.
- (b)
- Some of the thalli try to acclimate to ongoing stresses by melanization of the fungal cortex and reduction of the photosynthetic activity. To a certain extent, forest lichens are able to produce melanin to screen excess solar radiation, they increase their thickness in situations with more light, as well as their water holding capacity to acclimate for limited water availability and rare occurrences of hydration events [56,66]. However, in the long term, they still can be at risk.
- (c)
- Some of the thalli are not negatively affected; they maintain their vitality and growth rates thanks to suitable microclimatic conditions that locally persist, even after logging. They can be preserved in the long term. This is also the case for our transplant experiment (in particular for meristematic fragments, likely due to their situation and the north side of the trunk).
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Economic Forum. The Global Risks Report 2020. 2020. Available online: http://www3.weforum.org/docs/WEF_Global_Risk_Report_2020.pdf (accessed on 21 July 2020).
- Leadley, P. Biodiversity scenarios: Projections of 21st century change in biodiversity and associated ecosystem services. In Secretariat of the Convention on Biological Diversity; Diversity SotCoB, Ed.; Technical Series no. 50; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2010; pp. 1–132. [Google Scholar]
- Pacifici, M.; Foden, W.; Visconti, P.; Watson, J.E.M.; Butchart, S.H.M.; Kovacs, K.M.; Scheffers, B.R.; Hole, D.G.; Martin, T.G.; Akçakaya, H.R.; et al. Assessing species vulnerability to climate change. Nat. Clim. Chang. 2015, 5, 215–224. [Google Scholar] [CrossRef]
- Van der Putten, W.H.; Macel, M.; Visser, M.E. Predicting species distribution and abundance responses to climate change: Why it is essential to include biotic interactions across trophic levels. Philos. Trans. R. Soc. B 2010, 365, 2025–2034. [Google Scholar] [CrossRef]
- Urban, M.C. Accelerating extinction risk from climate change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef]
- Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES). Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Unedited Advance Version; IPBES: Bonn, Germany, 2019. [Google Scholar]
- Pimm, S.L.; Raven, P. Extinction by numbers. Nature 2000, 403, 843–845. [Google Scholar] [CrossRef]
- Thomas, J.A.; Telfer, M.G.; Roy, D.B.; Preston, C.D.; Greenwood, J.J.D.; Asher, J.; Fox, R.; Clarke, R.T.; Lawton, J.H. Comparative losses of British butterflies, birds, and plants and the global extinction crisis. Science 2004, 303, 1879–1881. [Google Scholar] [CrossRef] [PubMed]
- Román-Palacios, C.; Wiens, J.J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. USA 2020, 117, 4211–4217. [Google Scholar] [CrossRef] [PubMed]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, J.; Svenning, J.C. Climate-related range shifts—A global multidimensional synthesis and new research directions. Ecography 2015, 38, 15–28. [Google Scholar] [CrossRef]
- Sintayehu, D.W. Impact of climate change on biodiversity and associated key ecosystem services in Africa: A systematic review. Ecosyst. Health Sustain. 2018, 4, 225–239. [Google Scholar] [CrossRef]
- Zotz, G.; Bader, M.Y. Epiphytic plants in a changing world-global: Change effects on vascular and non-vascular epiphytes. In Progress in Botany; Lüttge, U., Beyschlag, W., Büdel, B., Francis, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 70. [Google Scholar]
- Gauslaa, Y. Rain, dew, and humid air as drivers of morphology, function and spatial distribution in epiphytic lichens. Lichenologist 2014, 46, 1–16. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Goward, T.; Pypker, T. Canopy settings shape elemental composition of the epiphytic lichen Lobaria pulmonaria in unmanaged conifer forests. Ecol. Indic. 2020, 113, 106–294. [Google Scholar] [CrossRef]
- Porada, P.; Weber, B.; Elbert, W.; Pöschl, U.; Kleidon, A. Estimating impacts of lichens and bryophytes on global biogeochemical cycles. Glob. Biogeochem. Cycles 2014, 28, 71–85. [Google Scholar] [CrossRef]
- Rikkinen, J. Cyanolichens. Biodivers. Conserv. 2015, 24, 973–993. [Google Scholar] [CrossRef]
- Zedda, L.; Rambold, G. The diversity of lichenised fungi: Ecosystem functions and ecosystem services. In Recent Advances in Lichenology; Upreti, D.K., Divakar, P.K., Shukla, V., Bajpai, R., Eds.; Springer: New Delhi, India, 2015; pp. 121–145. [Google Scholar] [CrossRef]
- Ellis, C.J. Lichen epiphyte diversity: A species, community and trait-based review. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 131–152. [Google Scholar] [CrossRef]
- Asplund, J.; Wardle, D.A. How lichens impact on terrestrial community and ecosystem properties. Biol. Rev. 2017, 92, 1720–1738. [Google Scholar] [CrossRef] [PubMed]
- Otálora, M.A.G.; Martínez, I.; Belinchón, R.; Widmer, I.; Aragón, G.; Escudero, A.; Scheidegger, C. Remnant fragments preserve genetic diversity of the old forest lichen Lobaria pulmonaria in a fragmented Mediterranean mountain forest. Biodivers. Conserv. 2011, 20, 1239–1254. [Google Scholar] [CrossRef]
- Whittet, R.; Ellis, C.J. Critical tests for lichen indicators of woodland ecological continuity. Biol. Conserv. 2013, 168, 19–23. [Google Scholar] [CrossRef]
- Scheidegger, C.; Werth, S. Conservation strategies for lichens: Insights from population biology. Fungal Biol. Rev. 2009, 23, 55–66. [Google Scholar] [CrossRef]
- Nilsson, S.G.; Arup, U.; Baranowski, R.; Ekman, S. Tree-dependent lichens and beetles as indicators in conservation forests. Biol. Conserv. 1995, 9, 1208–1215. [Google Scholar]
- Thüs, H.; Licht, W. Oceanic and hygrophytic lichens from the Gargano-Peninsula (Puglia/South-Eastern-Italy). Herzogia 2006, 19, 149–153. [Google Scholar]
- Edman, M.; Eriksson, A.M.; Villard, M.A. Effects of selection cutting on the abundance and fertility of indicator lichens Lobaria pulmonaria and Lobaria quercizans. J. Appl. Ecol. 2008, 45, 26–33. [Google Scholar] [CrossRef]
- Nascimbene, J.; Brunialti, G.; Ravera, S.; Frato, L.; Caniglia, G. Testing Lobaria pulmonaria (L.) Hoffm. as an indicator of lichen conservation importance of Italian forests. Ecol. Indic. 2010, 10, 353–360. [Google Scholar] [CrossRef]
- Campbell, J.; Fredeen, L. Lobaria pulmonaria abundance as an indicator of macrolichen diversity in Interior Cedar-Hemlock forests of east-central British Columbia. Can. J. Bot. 2004, 82, 970–982. [Google Scholar] [CrossRef]
- Matteucci, E.; Benesperi, R.; Giordani, P.; Piervittori, R.; Isocrono, D. Epiphytic lichen communities in chestnut stands in Central-North Italy. Biology 2012, 67, 61–70. [Google Scholar] [CrossRef]
- Nascimbene, J.; Thor, G.; Nimis, P.L. Effects of forest management on epiphytic lichens in temperate deciduous forests of Europe—A review. For. Ecol. Manag. 2013, 298, 27–38. [Google Scholar] [CrossRef]
- Brunialti, G.; Frati, L.; Ravera, S. Structural variables drive the distribution of the sensitive lichen Lobaria pulmonaria in Mediterranean old-growth forests. Ecol. Indic. 2015, 53, 37–42. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Solhaug, K.A. High-light-intensity damage to the foliose lichen Lobaria pulmonaria within a natural forest: The applicability of chlorophyll fluorescence methods. Lichenologist 2000, 32, 271–289. [Google Scholar] [CrossRef]
- Rubio-Salcedo, M.; Merinero, S.; Martínez, I. Tree species and microhabitat influence the population structure of the epiphytic lichen Lobaria pulmonaria. Fungal Ecol. 2015, 18, 1–9. [Google Scholar] [CrossRef]
- Benesperi, R.; Nascimbene, J.; Lazzaro, L.; Bianchi, E.; Tepsich, A.; Longinotti, S.; Giordani, P. Successful conservation of the endangered forest lichen Lobaria pulmonaria requires knowledge of fine-scale population structure. Fungal Ecol. 2018, 33, 65–71. [Google Scholar] [CrossRef]
- Nascimbene, J.; Benesperi, R.; Casazza, G.; Chiarucci, A.; Giordani, P. Range shifts of native and invasive trees exacerbate the impact of climate change on epiphyte distribution: The case of lung lichen and black locust in Italy. Sci. Total Environ. 2020, 735, 139537. [Google Scholar] [CrossRef]
- Ódor, P.; Király, I.; Tinya, F.; Bortignon, F.; Nascimbene, J. Patterns and drivers of species composition of epiphytic bryophytes and lichens in managed temperate forests. For. Ecol. Manag. 2013, 306, 256–265. [Google Scholar] [CrossRef]
- Scheidegger, C.; Frey, B.; Zoller, S. Transplantation of symbiotic propagules and thallus fragments: Methods for the conservation of threatened epiphytic lichen populations. Mitt. Eidgenöss. Forsch. Wald Schnee Landsch. 1995, 70, 41–62. [Google Scholar]
- Scheidegger, C. Early development of transplanted isidioid soredia of Lobaria pulmonaria in an endangered population. Lichenologist 1995, 27, 361–374. [Google Scholar] [CrossRef]
- Öckinger, E.; Niklasson, M.; Nilsson, S.G. Is local distribution of the epiphytic lichen Lobaria pulmonaria limited by dispersal capacity or habitat quality? Biodivers. Conserv. 2005, 14, 759–773. [Google Scholar] [CrossRef]
- Zoller, S.; Lutzoni, F.; Scheidegger, C. Genetic variation within and among populations of the threatened lichen Lobaria pulmonaria in Switzerland and implications for its conservation. Mol. Ecol. 1999, 8, 2049–2059. [Google Scholar] [CrossRef]
- Giordani, P.; Brunialti, G.; Bacaro, G.; Nascimbene, J. Functional traits of epiphytic lichens as potential indicators of environmental conditions in forest ecosystems. Ecol. Indic. 2012, 18, 413–420. [Google Scholar] [CrossRef]
- Belinchón, R.; Martínez, I.; Aragón, G.; Escudero, A.; De la Cruz, M. Fine spatial pattern of an epiphytic lichen species is affected by habitat conditions in two forest types in the Iberian Mediterranean region. Fungal Biol. 2011, 115, 1270–1278. [Google Scholar] [CrossRef]
- Nascimbene, J.; Casazza, G.; Benesperi, R.; Catalano, I.; Cataldo, D.; Grillo, M.; Isocrono, D.; Matteucci, E.; Ongaro, S.; Potenza, G.; et al. Climate change fosters the decline of epiphytic Lobaria species in Italy. Biol. Conserv. 2016, 201, 377–384. [Google Scholar] [CrossRef]
- Paoli, L.; Guttová, A.; Sorbo, S.; Lackovičová, A.; Ravera, S.; Landi, S.; Landi, M.; Basile, A.; Sanità di Toppi, L.; Vannini, A.; et al. Does air pollution influence the success of species translocation? Trace elements, ultrastructure and photosynthetic performances in transplants of a threatened forest macrolichen. Ecol. Indic. 2020, 117, 106666. [Google Scholar] [CrossRef]
- Atienza, V.; Segarra, J.G. Preliminary Red List of the lichens of the Valencian Community (eastern Spain). Snow Landsc. Res. 2000, 75, 391–400. [Google Scholar]
- Scheidegger, C.; Clerc, P. Lista Rossa delle Specie Minacciate in Svizzera. Licheni Epifiti e Terricoli; Birmensdorf e Conservatoire et Jardin botaniques de la Ville de Genève CJBG, Ed.; Istituto federale di ricerca WSL; Collana dell’UFAFP «Ambiente Esecuzione»; UFAFP: Berna, Switzerland, 2002; p. 122. [Google Scholar]
- Roux, C.; Monnat, J.-Y.; Poumarat, S.; Esnault, J.; Bertrand, M.; Gardiennet, A.; Masson, D.; Bauvet, C.; Lagrandie, J.; Derrein, M.-C.; et al. Catalogue des Lichens et Champignons Lichénicoles de France Métropolitaine, 3rd ed.; Association Française de Lichénologie (AFL): Fontainebleau, France, 2020; p. 1769. [Google Scholar]
- Paoli, L.; Benesperi, R.; Fačkovcová, Z.; Nascimbene, J.; Ravera, S.; Marchetti, M.; Anselmi, B.; Landi, M.; Landi, S.; Bianchi, E.; et al. Impact of forest management on threatened epiphytic macrolichens: Evidence from a Mediterranean mixed oak forest (Italy). iForest-Biogeosci. For. 2019, 12, 383–388. [Google Scholar] [CrossRef]
- Fačkovcová, Z.; Guttová, A.; Benesperi, R.; Loppi, S.; Bellini, E.; Sanità di Toppi, L.; Paoli, L. Retaining unlogged patches in Mediterranean oak forests may preserve threatened forest macrolichens. iForest-Biogeosci. For. 2019, 12, 187–192. [Google Scholar] [CrossRef]
- Hilmo, O.; Gauslaa, Y.; Rocha, L.; Lindmo, S.; Holien, H. Vertical gradients in population characteristics of canopy lichens in boreal rainforests of Norway. Botany 2013, 91, 814–821. [Google Scholar] [CrossRef]
- Available online: https://www.regione.toscana.it/-/regolamento-d-attuazione-della-legge-forestale-della-toscana-l-r-39-00- (accessed on 27 August 2020).
- Giordani, P.; Brunialti, G. Anatomical and histochemical differentiation in lobes of the lichen Lobaria pulmonaria. Mycol. Prog. 2002, 1, 131–136. [Google Scholar] [CrossRef]
- Gauslaa, Y. Trade-off between reproduction and growth in the foliose old forest lichen Lobaria pulmonaria. Basic Appl. Ecol. 2006, 7, 455–460. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Solhaug, K.A. High-light damage in air-dry thalli of the old forest lichen Lobaria pulmonaria—Interactions of irradiance, exposure duration and high temperature. J. Exp. Bot. 1999, 50, 697–705. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Lie, M.; Solhaug, K.A.; Ohlson, M. Growth and ecophysiological acclimation of the foliose lichen Lobaria pulmonaria in forests with contrasting light climates. Oecologia 2006, 147, 406–416. [Google Scholar] [CrossRef]
- Mafole, T.C.; Chiang, C.; Solhaug, K.A.; Beckett, R.P. Melanisation in the old forest lichen Lobaria pulmonaria reduces the efficiency of photosynthesis. Fungal Ecol. 2017, 29, 103–110. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Solhaug, K.A. Fungal melanins as a sun screen for symbiotic green algae in the lichen Lobaria pulmonaria. Oecologia 2001, 126, 462–471. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Solhaug, K.A. Photoinhibition in lichens depends on cortical characteristics and hydration. Lichenologist 2004, 36, 133–143. [Google Scholar] [CrossRef]
- McEvoy, M.; Gauslaa, Y.; Solhaug, K.A. Changes in pools of depsidones and melanins, and their function, during growth and acclimation under contrasting natural light in the lichen Lobaria pulmonaria. New Phytol. 2007, 175, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, S.; Fan, X.Y.; Yuan, G.D.; Hu, T.; Shi, X.M.; Wu, C.S. Comparison of two noninvasive methods for measuring the pigment content in foliose macrolichens. Photosynth. Res. 2019, 141, 245–257. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development for R; RStudio Inc.: Boston, MA, USA, 2016; Available online: http://www.rstudio.com/ (accessed on 30 June 2020).
- Gustafsson, L.; Fedrowitz, K.; Hazell, P. Survival and vitality of a macrolichen 14 years after transplantation on aspen trees retained at clearcutting. For. Ecol. Manag. 2013, 291, 436–441. [Google Scholar] [CrossRef]
- Larsson, P.; Solhaug, K.A.; Gauslaa, Y. Winter—The optimal logging season to sustain growth and performance of retained epiphytic lichens in boreal forests. Biol. Conserv. 2014, 180, 108–114. [Google Scholar] [CrossRef]
- Coxson, D.S.; Stevenson, S.K. Growth rate responses of Lobaria pulmonaria to canopy structure in even-aged and old-growth cedar-hemlock forests of central-interior British Columbia, Canada. For. Ecol. Manag. 2007, 242, 5–16. [Google Scholar] [CrossRef]
- Barták, M.; Solhaug, K.A.; Vráblíková, H.; Gauslaa, Y. Curling during desiccation protects the foliose lichen Lobaria pulmonaria against photoinhibition. Oecologia 2006, 149, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Merinero, S.; Martínez, I.; Rubio-Salcedo, M.; Gauslaa, Y. Epiphytic lichen growth in Mediterranean forests: Effects of proximity to the ground and reproductive stage. Basic Appl. Ecol. 2015, 16, 220–230. [Google Scholar] [CrossRef]
- Hazell, P.; Gustafsson, L. Retention of trees at final harvest—Evaluation of a conservation technique using epiphytic bryo phyte and lichen transplants. Biol. Conserv. 1999, 90, 133–142. [Google Scholar] [CrossRef]
- Shirazi, A.M.; Muir, P.S.; McCune, B. Environmental factors influencing the distribution of the lichens Lobaria oregana and L. pulmonaria. Bryologist 1996, 99, 12–18. [Google Scholar] [CrossRef]
- Nimis, P.L. The Lichens of Italy. A Second Annotated Catalogue; EUT Edizioni Università di Trieste: Trieste, Italy, 2016; p. 739. [Google Scholar]
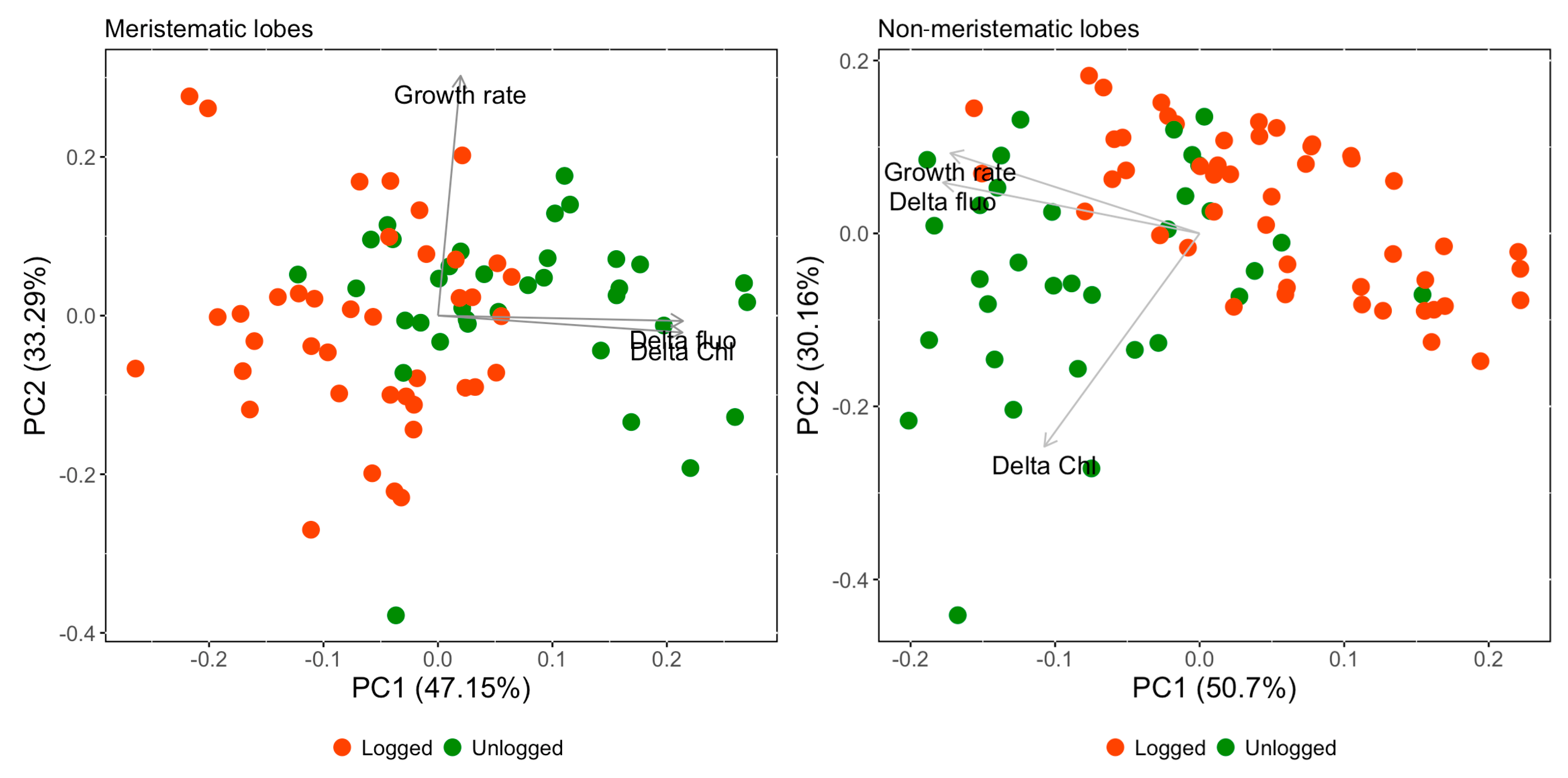
| PCA with Meristematic Dataset | |||
| PC1 (47.15%) | PC2 (33.29%) | PC3 (19.56%) | |
| Growth rate | 0.065 | 0.997 | −0.034 |
| Delta fluo | 0.706 | −0.022 | 0.707 |
| Delta Chl | 0.705 | −0.070 | −0.706 |
| PCA with Non-Meristematic Dataset | |||
| PC1 (50.70%) | PC2 (30.16%) | PC3 (19.14%) | |
| Growth rate | −0.639 | 0.344 | −0.688 |
| Delta fluo | −0.658 | 0.218 | 0.721 |
| Delta Chl | −0.398 | −0.913 | −0.087 |
| Type | Estimate | Std. Error | t Value | Summary Statistics | |
|---|---|---|---|---|---|
| Meristematic | (Intercept) | 0.055 | 0.131 | 0.419 | RSE: 0.272 (71 df) Multiple R2: 0.033 Adjusted R2: −0.008 F: 0.809 (3 and 71 df) p-value: 0.493 |
| Unlogged stand | 0.119 | 0.077 | 1.537 | ||
| Delta fluo | −0.129 | 0.452 | −0.285 | ||
| Delta Chl content | −0.077 | 0.124 | −0.621 | ||
| Non-meristematic | (Intercept) | 0.184 | 0.099 | 1.854 | RSE: 0.229 (78 df) Multiple R2: 0.227 Adjusted R2: 0.197 F: 7.628 (3 and 78 df) p-value: <0.001 |
| Unlogged stand | 0.142 | 0.067 | 2.109 * | ||
| Delta fluo | 0.592 | 0.193 | 3.063 ** | ||
| Delta Chl content | −0.060 | 0.093 | −0.650 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, E.; Benesperi, R.; Brunialti, G.; Di Nuzzo, L.; Fačkovcová, Z.; Frati, L.; Giordani, P.; Nascimbene, J.; Ravera, S.; Vallese, C.; et al. Vitality and Growth of the Threatened Lichen Lobaria pulmonaria (L.) Hoffm. in Response to Logging and Implications for Its Conservation in Mediterranean Oak Forests. Forests 2020, 11, 995. https://doi.org/10.3390/f11090995
Bianchi E, Benesperi R, Brunialti G, Di Nuzzo L, Fačkovcová Z, Frati L, Giordani P, Nascimbene J, Ravera S, Vallese C, et al. Vitality and Growth of the Threatened Lichen Lobaria pulmonaria (L.) Hoffm. in Response to Logging and Implications for Its Conservation in Mediterranean Oak Forests. Forests. 2020; 11(9):995. https://doi.org/10.3390/f11090995
Chicago/Turabian StyleBianchi, Elisabetta, Renato Benesperi, Giorgio Brunialti, Luca Di Nuzzo, Zuzana Fačkovcová, Luisa Frati, Paolo Giordani, Juri Nascimbene, Sonia Ravera, Chiara Vallese, and et al. 2020. "Vitality and Growth of the Threatened Lichen Lobaria pulmonaria (L.) Hoffm. in Response to Logging and Implications for Its Conservation in Mediterranean Oak Forests" Forests 11, no. 9: 995. https://doi.org/10.3390/f11090995
APA StyleBianchi, E., Benesperi, R., Brunialti, G., Di Nuzzo, L., Fačkovcová, Z., Frati, L., Giordani, P., Nascimbene, J., Ravera, S., Vallese, C., & Paoli, L. (2020). Vitality and Growth of the Threatened Lichen Lobaria pulmonaria (L.) Hoffm. in Response to Logging and Implications for Its Conservation in Mediterranean Oak Forests. Forests, 11(9), 995. https://doi.org/10.3390/f11090995









