Abstract
Norway spruce trees weakened by soil drought and progressive die-off of mycorrhizas in root systems become susceptible to infection by rhizomorphs of Armillaria spp. The developing mycelium of this necrotroph induces resin channels in wood, and the induced resin releases some volatile compounds which falsely signal bark beetles that it is safe to invade the host. As a result of the developing beetle outbreak, host trees die, becoming a long-term stock of substrate for the fungus in its saprotrophic stage. This hypothesis is discussed as a fungal survival strategy.
1. Introduction
Survival strategies are connected with producing offspring and ensuring the persistence of future generations, that is, the survival of the species. The survival strategies of plants and fungi, as well as other micro-organisms, differ from animals in several ways, including reproductive allocation—the production of seeds and spores, and in contrast in animals, the mechanisms of escape from threats [1,2,3]. Trees, with the inability to consciously choose their habitat and in the absence of a mechanism to escape stressful conditions, must express survival mechanisms in the place they germinate. This is done through plant structures that have been genetically encoded to provide passive resistance to stress, and through physiological and biochemical mechanisms of stress resistance that are induced by exposure to stress. Stresses drive ongoing phylogenetic development of plant species through sexual reproduction [4,5].
For phytopathologists, the world of fungi is of great interest, especially fungal pathogens that attack trees. The behavior of root pathogens is the least understood, owing to the difficulties in making direct observations of fungal infection and host responses. At the moment of fungus-tree contact, enzymatic reactions between tree tissues and pathogen mycelium occur, at which point there is either rejection of the fungal intruder or infection by the disease [6,7]. Some authors consider members of the genus Armillaria to be secondary pathogens, infecting a host together with or after infection by a primary pathogen [8,9]. However, Armillaria spp. may in some instances be the lone source of infection, and play an important aggravating role in stressed trees [10]. One particular fungal pathogen species, Armillaria ostoyae (Romagn.) Herink, is the largest recorded organism in the world [11], yet it still needs a partner (a tree) to carry out its ontogenetic development. Armillaria can complete its ontogenetic development whether the host is dead or living [12]. The life cycle of Armillaria is quite complicated—it is characterized by development as a necrotroph and saprotroph. The infectious organs are rhizomorphs, subcortical mycelium and basidiospores. It is a long-lived organism in inhabited substrate [13,14,15]. The optimal survival strategy for the fungus is to use infectious mechanisms (enzymes, secondary metabolites) to build the fungus’ complicated structures. To accomplish this, it uses “energy outsourcing”, obtaining nutrients from its host. As a heterotroph in the parasitic phase, it uses energy contained in the organ it killed (e.g., tree roots), and in the saprotrophic phase it obtains organic substances contained in the tissues of the dead tree [8,16]. As organic matter decomposes within the tissues in which a saprotroph is residing, the saprotroph enzymatically breaks such matter down to simpler compounds that contain more digestible carbon [6].
Questions exist about the relationships between Armillaria and Ips typographus (L.), an insect pest of commercial spruce forests. Furthermore, there is interest in what happens when environmental stress affects all three parties—host tree, pathogen and insect—as is increasingly being observed and attributed to climate change (or more accurately, to weather anomalies made more common by climate change). The nature of pest outbreaks in the “Weather-Armillaria-Ips-Spruce” syndrome, which has recently caused large areas of declining spruce stands in Europe [17,18], is an important area of discussion.
2. Hypothesis
This review addresses the hypothesis that “Nature” itself (the term Nature in the authors’ interpretation is the entirety of species, natural phenomena and the mechanisms and dependencies connecting them, both internal, at all functional levels, and external creating reality in time and space that is either perceived or not by humans) causes changes in species composition, an example being spruce stands growing in unsuitable habitats (e.g., in monocultures or stands on post-agricultural lands), and/or under conditions of water stress (e.g., long-term drought and disappearance of mycorrhiza from the soil). In the presence of Armillaria in coniferous forests, roots of trees weakened by drought undergo infection by fungal rhizomorphs. Infection triggers a chemical signal arising from increased resin production in attacked roots, and the formation of metabolites from decomposition [19]. These chemical signals are received by bark beetle pioneers, maybe indicating that “there is food, there are breeding bases, it is time for reproduction”. However, the beetles are actually being deceived; such signals indicate to beetles that fungus has affected the entire tree (including mycorrhiza and extrametrical mycelium) and that the tree is dying. In coniferous forests, in the case of the initial phase of Armillaria infection, roots are gradually infected, and the false chemical signal deceives bark beetles into attacking the tree. This chemical signaling can also work backward in some instances, with some chemicals triggered by bark beetles reducing fungal growth [20,21,22], although such a mechanism is not recognized in the case of I. typographus. The developing insect outbreak terminates when live trees, the beetles’ food resource, have been killed, and natural enemies that reduce the population of the beetle appear. In the end, tree death caused by Ips benefits Armillaria, providing dead trees that are the substrate for the fungus for years.
Whether this hypothesis is only speculation based on an anthropomorphic approach to understanding the functioning of nature, or a real functional relationship, has been difficult to discern through research [9,23]. To review the evolution of views on this subject in Norway spruce stands, and relationships between Armillaria and Ips, databases in Scopus, Google Scholar and Web of Science were browsed for the keywords: “Armillaria in Norway spruce stands” (~2.86 thousand records were identified), “Ips typographus in Norway spruce stands” (~3.63 thousand records), and “drought and Armillaria and Ips in Norway spruce stands” (only ~370 records). For the present paper, only publications referring to these keywords since 2000 were analyzed.
3. Fungal Infection and Pest Invasion
Post factum it is known that many of the trees infested by bark beetles have advanced infection by Armillaria mycelium in their roots or stumps [24]. Cellulose–lignin complexes are decomposed by fungal enzymes and by non-enzymatic cell disruption by –OH groups; peptide bonds are decayed by proteases to decompose proteins into amino acids and starch is decomposed to simple disaccharides by fungal amylases, etc., which then allows the metabolites of decayed wood to be absorbed by the mycelium [25,26]. The developing mycelia also stimulate the formation of traumatic resin canals in early and late wood of the annual ring [27]. All these reactions, necessary for fungal development, also affect the behavior of insects. Resin contains terpenoids of the volatile kairomones type, which for insects—depending on many external factors—can constitute aggregation or repellent signals [28,29,30,31].
Olfactory recognition of host tree semiochemical signals is crucial in spruce bark beetle biology, in order to distinguish P. abies from non-host tree species [32]. However, it has been demonstrated that I. typographus is only weakly [33,34] or not at all [35] attracted to host species emissions of volatile compounds. Nevertheless, the mechanism of how bark beetles find weakened and susceptible host trees that are “easier” for colonization remains unknown [35]. This initial semiochemical signal attracts “pioneer” beetles, those that are the first to attack trees. After initial infestation by pioneers, semiochemical-based aggregation of bark beetles takes place [29]. Differences in phenolic composition were found between “susceptible” and “resistant” trees [36,37], suggesting that weakened spruce trees might also release specific volatile signals that are attractive to beetles [29]. Some bark beetles, including I. typographus, may find susceptible weak host trees by following volatiles produced by competing insect species during colonization (e.g., [38]), but no evidence exists that weakened trees release specific compounds that are recognized by bark beetles as a signal for the selection of the host tree. In Tomicus piniperda (L.), differences were found between attacks on trees of different vigor (less on those with lower vigor) [39], but, according to the TSA (Threshold of Successful Attack) model [28], lower numbers of attacking beetles are necessary to overcome the defenses of weakened trees (and vice versa). Thus, even if weakened trees produce a chemical signal, it is unknown if I. typographus is able to detect and respond to it.
Such a signal is believed to be produced in trees infected by Armillaria, but not by Armillaria itself. It has long been recognized that differences in chemical composition of volatile oils are found in the needles between healthy spruces and those affected by this pathogen [40]. Nevertheless, Armillaria infection is associated with changes in terpenoids and phenyl propanoids in the phloem of Pinus contorta Dougl. ex Loud. trees attacked by mountain pine beetles, Dendroctonus ponderosae Hopkins, suggesting a biochemical basis for host selection by the beetle [41]. There is, however, no evidence that such differences are found in P. abies cambium (an important host species for I. typographus), although many Armillaria metabolites have been identified in infected pine trees [42,43]. Armillaria root disease is a long process—the pathogen can be present in a host tree for many years without visible disease symptoms or insect infestation. How then is the point of tree weakening defined? Is there a specific semiochemical signal produced at that time? When does the release of the chemical signal begin? Is I. typographus able to detect/recognize it and respond? On the other hand, it is generally known that Armillaria is a crucial factor in Norway spruce decline [9,44], and infections by this pathogen are considered an important predisposing factor for bark beetle attack [24,45,46].
In the case of spruce, when bark beetles aggregate in trees and lay eggs, the resulting larvae carry out their developmental cycle in the phloem (Figure 1). Bark beetles, especially in the first stage of infestation, attack trees considered susceptible to the insect [46], and most of these trees were previously infected by Armillaria near the base of the stem [24]. It cannot be ruled out that mycelium growing under spruce bark facilitate colonization by other species of fungi associated with the bark beetle, e.g., blue stain fungi [47,48,49,50], although fungal presence, necessary for reproduction, is not a prerequisite for successful colonization by I. typographus [51,52]. The role of bacteria, nematodes and mites in colonization by I. typographus can also be questioned; after all, Westra et al. [53] states that bacteria can drive evolution of defense mechanisms against infection risk.
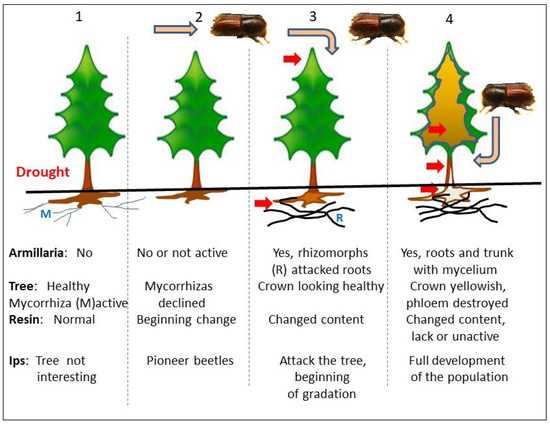
Figure 1.
The phases (1–4) of colonization of stressed Norway spruce trees by Armillaria and Ips (orig.).
The phenomenon of aggregation of aggressive bark beetle individuals (so-called pioneer beetles, or pathfinders) in favorable weather conditions lasts no more than a few days. The insect’s receptors receive signals in the form of volatile compounds emitted by a “target” tree (volatile phenolic compounds, oleoresins). They can also differentiate enzymes synthesized by the tree and/or secondary metabolites activated during cellular metabolism, initiated as a result of infection of the root tissues by fungal hyphae [54]. Fungal metabolites, produced in the roots, act as stimulator on the beetles; the metabolites are transported upwards to the crown in the xylem stream. In addition to metabolites, there are compounds produced by decomposition of wood and phloem by the hyphae, that may turn out to be elicitors triggering subsequent cause-and-effect reactions between fungus, tree and insect. So far, however, no clear and direct connection between bark beetles and Ophiostomatoid fungi (as well as with root rot fungi or bacteria) has been found [28,36,47,55,56,57], especially when beetles attack trees in the early stage of Armillaria infection. It is known that different structures of bark beetle-associated fungi produce some attractive, sometimes crucial metabolites [58], among them are nitrogen compounds and sterols [59,60]. Fungi developing in phloem of roots may participate in delivery of produced sterols to upper parts of the tree, especially since insects are not able to use plant-produced sterols [61]. The example of relations between the root feeding bark beetle Hylastes spp. and staining fungi was described by Zang et al. [62]. Even CO2 or methane CH4 produced by saprotrophic basidiomycetes during wood decay may stimulate insect growth [63,64,65].
The smell of mycelium volatile secretions, of Ophiostomatales, is associated with olfactory receptors of beetles, and the volatile organic compounds (VOCs) emitted by symbiotic fungi may act as recognition signals for bark beetles [65]. However, Zhao et al. [66] report that methyl jasmonate, which induces extensive biochemical and anatomical changes in Norway spruce, Picea abies (L.) Karst., similar to those caused by pathogens [67], reduces the emission of aggregation pheromones by I. typographus. Therefore, it is quite probable that I. typographus is able to detect such a signal released also by an Armillaria-infected tree, possibly in a manner similar to the response of beetles to the olfactory-like signals related to tree age [68].
These phenomena are widely known and described, both in classical phytopathological literature and in many ecological and entomological studies (e.g., [69]. Referring to hologenome theory [70,71], it can be assumed that, in a managed ecosystem with a larger than natural proportion of spruce, which is the case in many commercial stands, there is natural selection of trees of this species. Selection is performed jointly not only by Armillaria and bark beetles, by affecting the genomes of symbiotic partners, but appears to be a part of a multifunctional temporal network [72]. Has it created a peculiar strategy of survival, or is it even more “clever” than imagined? It remains to be seen, as “Nothing is difficult for nature” (Democritus).
4. Final Remarks
Spruce, weakened by soil water deficit, becomes a target of pioneer bark beetles after primary infection of the roots by Armillaria (by rhizomorphs). The information obtained by Ips beetles that a source of energy and space is available, results from changes in resin content caused by infection by the pathogen (rhizomorphs and mycelium). However, this signal actually misrepresents the state of the tree to the affected insects. This false information provided by volatile compounds emitted from resin and phloem, indicates to bark beetles that a tree is weakened and is in a susceptible state (i.e., resin will not flood the insect). Armillaria, therefore, employs an individual specimen development, whereas Ips plays an outsourcing and ontogenetic role. The deadwood constitutes the substrate in the saprotrophic stage of development of this fungus. This provocative hypothesis is discussed as a fungus-specific and purposeful survival strategy. The question remains: Is this truly a fungal survival strategy that developed over time, or is it simply coincidence? The arguments presented here may provide impetus to consider whether modifications of management for economic objectives is needed for threatened stands (e.g., whether to protect biologically or to remove vulnerable stands?) to ensure their sustainable development.
Author Contributions
Z.S. and W.G. substantially contributed to conceptualization, resources, writing the original draft, review and editing the text. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was partially supported by the State Forest Holding in Poland (Project No. 500426, and by statutory funds of the Warmia and Mazury University in Olsztyn, Poland.
Acknowledgments
The authors thank the anonymous reviewers for their valuable comments and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mattheck, M.; Bethge, C. The mechanical survival strategy of trees. Arbor. J. 1998, 22, 369–386. [Google Scholar] [CrossRef]
- Gadagkar, R. Survival Strategies. Cooperation and Conflict in Animal Societies; Harvard University Press: Massachusetts, MA, USA, 2001. [Google Scholar]
- Casadevall, A. Determinants of virulence in the pathogenic fungi. Fungal. Biol. Rev. 2007, 21, 130–132. [Google Scholar] [CrossRef]
- Merrill, W. Mechanisms of Resistance to Fungi in Woody Plants: A Historical Perspective. In Defense Mechanisms of Woody Plants Against Fungi, Springer Series in Wood Science; Blanchette, R.A., Biggs, A.R., Eds.; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Heitman, J.; Sun, S.; James, T.Y. Evolution of fungal sexual reproduction. Mycologia 2013, 105, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Stenlid, J. Infection of roots of Norway spruce (Picea abies) by Heterobasidion annosum. Eur. J. For. Path. 1985, 15, 32–45. [Google Scholar] [CrossRef]
- Robinson, R.M.; Sturrock, R.N.; Davidson, J.J. Detection of a chitinase-like protein in the roots of Douglas-fir trees infected with Armillaria ostoyae and Phellinus weirii. Tree Phys. 2000, 20, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Prospero, S.; Holdenrieder, O.; Rigling, D. Comparison of the virulence of Armillaria cepistipes and Armillaria ostoyae on four Norway spruce provenances. For. Pathol. 2004, 34, 1–14. [Google Scholar] [CrossRef]
- Holuša, J.; Lubojacký, J.; Čurn, V.; Tonka, T.; Lukášová, K.; Horák, J. Combined effects of drought stress and Armillaria infection on tree mortality in Norway spruce plantations. For. Ecol. Manag. 2018, 427, 434–445. [Google Scholar] [CrossRef]
- Sipos, G.; Prasanna, A.N.; Walter, M.C.; O’Connor, E.; Bálint, B.; Krizsán, K.; Kiss, B.; Hess, J.; Varga, T.; Slot, J.; et al. Genome expansion and lineage-specific genetic innovations in the forest pathogenic fungi. Armillaria. Nat. Ecol. Evol. 2017, 1, 1931–1941. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.A.; Dreisbach, T.A.; Parks, C.G.; Filip, G.M.; Schmitt, C.L. Coarse-scale population structure of pathogenic Armillaria species in a mixed-conifer forest in the Blue Mountains of northeast Oregon. Can. J. For. Res. 2003, 33, 612–623. [Google Scholar] [CrossRef]
- Baumgartner, K.; Coetzee, M.P.; Hoffmeister, D. Secrets of the subterranean pathosystem of Armillaria. Mol. Plant. Pathol. 2011, 12, 515–534. [Google Scholar] [CrossRef]
- Morrison, D.J. Rhizomorph growth habit, saprophytic ability and virulence of 15 Armillaria species. For. Pathol. 2004, 34, 15–26. [Google Scholar] [CrossRef]
- Kubiak, K.; Żółciak, A.; Damszel, M.; Lech, P.; Sierota, Z. Armillaria Pathogenesis under Climate Changes. Forests 2017, 8, 100. [Google Scholar] [CrossRef]
- Heizelmann, R.; Dutech, C.; Tsykun, T.; Labbe, F.; Soularule, J.-P. Latest advances and future perspectives in Armillaria research. Can. J. Plant. Path. 2019, 41, 1–23. [Google Scholar] [CrossRef]
- Legrand, P.; Ghahari, S.; Guillaumin, J.J. Occurrence of genets of Armillaria spp. In four mountain forests in Central France: The colonization strategy of Armillaria ostoyae. New Phytol. 1996, 133, 321–332. [Google Scholar] [CrossRef]
- Schelhaas, M.-J.; Nabuurs, G.-J.; Schuck, A. Natural disturbances in the European forests in the 19th and 20th centuries. Glob. Chang. Biol. 2003, 9, 1620–1633. [Google Scholar] [CrossRef]
- Grégoire, J.-C.; Evans, H. Damage and Control of Bawbilt Organisms, An Overview. In Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis; Lieutier, F., Day, K.R., Battisti, A., Grégoire, J.-C., Evans, H., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 2004; pp. 19–37. [Google Scholar]
- Misiek, M.; Hoffmeister, D. Sesquiterpene aryl ester natural products in North American Armillaria species. Mycol. Progress 2012, 11, 7–15. [Google Scholar] [CrossRef]
- Raffa, K.F.; Klepzig, K.D. Tree Defense Mechanisms Against Fungi Associated with Insects. In Defense Mechanisms of Woody Plants Against Fungi; Blanchette, R.A., Biggs, A.R., Eds.; Springer Series in Wood Science: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Six, D.L.; Wingfield, M.J. The role of phytopathogenicity in bark beetle–Fungus Symbioses: A challenge to the classic paradigm. Ann. Rev. Ent. 2011, 56, 255–272. [Google Scholar] [CrossRef]
- Lombardero, M.J.; Solla, A.; Ayres, M.P. Pine defenses against the pitch canker disease are modulated by a native insect newly associated with the invasive fungus. For. Ecol. Manag. 2019, 437, 253–262. [Google Scholar] [CrossRef]
- Fettig, C.J.; Hilszczanski, J. Management strategies for bark beetles in conifer forests. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Springer: London, UK, 2015. [Google Scholar]
- Jankovský, L.; Cudlín, P.; Moravec, I. Root decays as a potential predisposition factor of a bark beetle disaster in the Šumava Mts. J. For. Sci. 2003, 49, 125–132. [Google Scholar] [CrossRef]
- Clegg, C.J.; Mackean, D.G. Advanced Biology: Principles and Applications; John Murray: London, UK, 2006. [Google Scholar]
- Eastwood, D.C.; Floudas, D.; Binder, M.; Majcherczyk, A.; Schneider, P.; Aerts, A.; Baker, S.E.; Barry, K.; Bendiksby, M.; Blumentritt, M.; et al. The Plant Cell Wall–Decomposing Machinery Underlies the Functional Diversity of Forest Fungi. Science 2011, 333, 762. [Google Scholar] [CrossRef]
- Cruickshank, M.G.; Lejour, D.; Morrison, D.J. Traumatic resin canals as markers of infection events in Douglas fir roots infected with Armillaria root disease. For. Pathol. 2006, 36, 372–384. [Google Scholar] [CrossRef]
- Christiansen, E.; Waring, R.H.; Berryman, A.A. Resistance of conifers to bark beetle attack: Searching for general relationships. For. Ecol. Manag. 1987, 22, 89–106. [Google Scholar] [CrossRef]
- Byers, J.A. Chemical ecology of bark beetles in a complex olfactory landscape. In Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis; Lieutier, F., Day, K.R., Battisti, A., Grégoire, J.-C., Evans, H., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 2004; pp. 89–134. [Google Scholar]
- Wermelinger, B. Ecology and management of the spruce bark beetle Ips typographus—A review of recent research. For. Ecol. Manag. 2004, 202, 67–82. [Google Scholar] [CrossRef]
- Donnelly, D.M.X.; Abe, F.; Coveney, D.; Fukuda, N.; O’Reilly, J.; Polonsky, J.; Prange, T. Antibacterial sesquiterpene aryl esters from Armillaria mellea. J. Nat. Prod. 1985, 48, 10–16. [Google Scholar] [CrossRef]
- Zhang, Q.-H.; Schlyter, F. Olfactory recognition and behavioral avoidance of angiosperm non host volatiles by conifer-inhabiting bark beetles. Agric. For. Entom. 2004, 6, 1–19. [Google Scholar] [CrossRef]
- Austarå, O.; Bakke, A.; Midtgaard, F. Response in Ips typographus to logging waste odors and synthetic pheromones. J. Appl. Entom. 1986, 101, 194–198. [Google Scholar] [CrossRef]
- Lindelöw, A.; Risberg, B.; Sjodin, K. Attraction during flight of Scolytids and other bark and wood-dwelling beetles to volatiles from fresh and stored spruce wood. Can. J. For. Res. 1992, 22, 224–228. [Google Scholar] [CrossRef]
- Schlyter, F.; Birgersson, G.; Byers, J.A.; Löfqvist, J.; Bergström, G. Field response of spruce bark beetle, Ips typographus, to aggregation pheromone candidates. J. Chem. Ecol. 1987, 13, 701–716. [Google Scholar] [CrossRef]
- Brignolas, F.; Lieutier, F.; Sauvard, D.; Christiansen, E.; Berrymann, A.A. Phenolic predictors for Norway spruce resistance to bark beetle Ips typographus and an associated fungus Ceratocystis polonica. Can. J. For. Res. 1998, 28, 720–728. [Google Scholar] [CrossRef]
- Lieutier, F.; Brignolas, F.; Sauvard, D.; Yart, A.; Galet, C.; Brunet, M.; Van de Sype, H. Intra- and inter-provenance variability in phloem polyphenols of Picea abies (L.) Karst. and relation with resistance to a bark-beetle-associated fungus. Tree Physiol. 2003, 23, 247–256. [Google Scholar] [CrossRef]
- Tommerås, B.A.; Mustaparta, H.; Grégoire, J.-C. Receptor cells in Ips typographus and Dendroctonus micans specific to pheromones of the reciprocal genus. J. Chem. Ecol. 1984, 10, 759–769. [Google Scholar] [CrossRef]
- Schroeder, L.M. Attraction of the bark beetle Tomicus piniperda to Scots pine trees in relation to tree vigor and attack density. Entom. Exp. Applic. 1987, 44, 53–58. [Google Scholar] [CrossRef]
- Madziara-Borusiewicz, K.; Strzelecka, H. Conditions of spruce (Picea excelsa Lk.) infestation by the engraver beetle (Ips typographus L.) in mountains of Poland. Z. Angew. Entom. 1977, 83, 409–415. [Google Scholar] [CrossRef]
- Nebeker, T.E.; Schmitz, R.F.; Tisdale, R.A.; Hobson, K.R. Chemical and nutritional status of dwarf mistletoe, Armillaria root rot, and comandra blister rust infected trees which may influence tree susceptibility to bark beetle attack. Can. J. Bot. 1995, 73, 360–369. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Lech, P.; Żółciak, A.; Rusak, M.; Szczepaniak, L. Gas chromatographic-mass spectrometric investigation of metabolites from the needles and roots of pine seedlings at early stages of pathogenic fungi Armillaria ostoyae attack. Trees 2008, 22, 531–542. [Google Scholar] [CrossRef]
- Dörfer, M.; Gressler, M.; Hoffmeister, D. Diversity and bioactivity of Armillaria sesquiterpene aryl ester natural products. Mycol. Prog. 2019, 18, 1027–1037. [Google Scholar] [CrossRef]
- Longauerová, V.; Leontovyč, R.; Krajmerová, D.; Vakula, J.; Grodzki, W. Fungal pathogens–hidden agents of decline. In Spruce Forests Decline in the Beskids–Hynutie smrekových porastov v Beskydoch; Hlásny, T., Sitková, Z., Eds.; National Forest Centre–Forest Research Institute Zvolen: Zvolen, Slovakia; Czech University of Life Sciences Prague: Prague, Czech Republic; Forestry and Game Management Research Institute Jíloviště–Strnady: Jíloviště, Czech Republic, 2010; pp. 93–105. [Google Scholar]
- Hertert, H.D.; Miller, D.L.; Partridge, A.D. Interaction of bark beetles (Coleoptera: Scolytidae) and root rot pathogens in grand fir in northern Idaho. Can. Entom. 1975, 107, 899–904. [Google Scholar] [CrossRef]
- Christiansen, E.; Huse, K.J. Infestation ability of Ips typographus in Norway spruce, in relation to butt rot, tree vitality, and increment. Medd. NISK 1980, 35, 469–482. [Google Scholar]
- Krokene, P.; Solheim, H. Fungal associates of five bark beetle species colonizing Norway spruce. Can. J. For. Res. 1997, 26, 2115–2122. [Google Scholar] [CrossRef]
- Jankowiak, R. Fungi associated with Ips typographus on Picea abies in southern Poland and their succession into the phloem and sapwood of beetle-infested trees and logs. For. Path. 2005, 35, 37–55. [Google Scholar] [CrossRef]
- Jankowiak, R.; Hilszczański, J. Ophiostomatoid fungi associated with Ips typographus L. on Picea abies (L.) H. Karst. and Pinus sylvestris L. in North-Eastern Poland. Acta Soc. Bot. Pol. 2005, 74, 345–350. [Google Scholar] [CrossRef]
- Kirisits, T. Fungi isolated from Picea abies infested by the bark beetle Ips typographus in the Białowieża forest in north-eastern Poland. For. Path. 2010, 40, 100–110. [Google Scholar] [CrossRef]
- Linnakoski, R.; de Beer, Z.W.; Niemelä, P.; Wingfield, M.J. Associations of conifer-infesting bark beetles and fungi in fennoscandia. Insects 2012, 3, 200–227. [Google Scholar] [CrossRef]
- Six, D.L. Ecological and evolutionary determinants of bark beetle—Fungus Symbioses. Insects 2012, 3, 339–366. [Google Scholar] [CrossRef] [PubMed]
- Westra, E.R.; van Houte, S.; Oyesiku-Blakemore, S.; Makin, B.; Broniewski, J.M.; Best, A.; Bondy-Denomy, J.; Davidson, A.; Boots, M.; Buckling, A. Parasite exposure drives selective evolution of constitutive versus inducible defense. Curr. Biol. 2015, 25, 1043–1049. [Google Scholar] [CrossRef]
- Raffa, K.F. Mixed messages across multiple trophic levels: The ecology of bark beetle chemical communication systems. Chemoecology 2001, 11, 49–65. [Google Scholar] [CrossRef]
- Harding, S. The Influence of Mutualistic Blue Stain Fungi on Bark Beetle Population Dynamics. Ph.D. Thesis, Department of Zoology, Royal Veterinary & Agricultural University, Copenhagen, Denmark, 1989. [Google Scholar]
- Hammerbacher, A.; Kandasamy, D.; Ullah, C.; Schmidt, A.; Wright, L.; Gershenzon, J. Flavanone-3-hydroxylase plays an important role in the biosynthesis of spruce phenolic defenses against bark beetles and their fungal associates. Front. Plant. Sci. 2019, 10, 208. [Google Scholar] [CrossRef]
- Malloch, D.; Blackwell, M. Dispersal biology of the Ophiostomatid fungi. In Ceratocystis and Ophiostoma: Taxonomy, Ecology, and Pathogenicity; Wingfield, M.J., Seifert, K.A., Webber, J.F., Eds.; APS Press: St. Paul, MN, USA, 1993; pp. 195–206. [Google Scholar]
- Ayres, M.P.; Wilkens, R.T.; Ruel, J.J.; Vallery, E. Fungal relationships and the nitrogen budget of phloem-feeding bark beetles (Coleoptera:Scolytidae). Ecology 2000, 81, 2198–2210. [Google Scholar] [CrossRef]
- Mondy, N.; Corio-Coster, M.F. The response of the grape berry moth (Lobesia botrana) to a dietary phytopathogenic fungus (Botrytis cinerea): The significance of fungus sterols. J. Ins. Physiol. 2000, 46, 1557–1564. [Google Scholar] [CrossRef]
- Clayton, R.B. The utilization of sterols by insects. J. Lipid Res. 1964, 5, 3–19. [Google Scholar]
- Kleipzig, K.D.; Six, D.L. Bark beetle-funga;l symbiosis: Contex dependency in complex associations. Symbiosis 2004, 37, 189–205. [Google Scholar]
- Zang, W.; Song, H.; Ding, Q. Application of methane fermenative residues in control of crop diseases and insect pests. Res. Agric. Modern. 2001, 22, 167–170. [Google Scholar]
- Lenhart, K.; Bunge, M.; Ratering, S.; Neu, T.R.; Schüttmann, I.; Greule, M.; Kammann, C.; Schnell, S.; Müller, C.; Zorn, H. Evidence for methane production by saprotrophic fungi. Nat. Comm. 2012, 3, 1046. [Google Scholar] [CrossRef]
- Weissteiner, S.; Huetteroth, W.; Kollmann, M.; Weißbecker, B.; Romani, R.; Schachtner, J.; Schütz, S. Cockchafer larvae smell host root scents in soil. PLoS ONE 2012, 7, e45827. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, D.; Gershenzon, J.; Andersson, M.N.; Hammerbacher, A. Volatile organic compounds influence the interaction of the Eurasian spruce bark beetle (Ips typographus) with its fungal symbionts. ISME 2019, 13, 1788–1800. [Google Scholar] [CrossRef]
- Zhao, T.; Borg-Karlson, A.-K.; Erbilgin, N.; Krokene, P. Host resistance elicited by methyl jasmonate reduces emission of aggregation pheromones by the spruce bark beetle, Ips typographus. Oecologia 2011, 167, 691–699. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Krekling, T.; Christiansen, E. Picea abies (Pinaceae) stems induces defense-related responses in phloem and xylem. Am. J. Bot. 2002, 89, 602–610. [Google Scholar] [CrossRef]
- Blažytė-Čereškienė, L.; Apšegaitė, V.; Radžiutė, S.; Mozūraitis, R.; Būda, V.; Pečiulytė, D. Electrophysiological and behavioural responses of Ips typographus (L.) to trans-4-thujanol—A host tree volatile compound. Ann. For. Sci. 2016, 73, 247–256. [Google Scholar] [CrossRef]
- Vega, F.E.; Blackwell, M. (Eds.) Insect–Fungal Associations: Ecology and Evolution; Oxford University Press: Oxsford, UK, 2005. [Google Scholar]
- Rosenberg, E.; Zilber-Rosenberg, I. Symbiosis and development: The hologenome concept. Birth Defects Res. (Part C) 2011, 93, 56–66. [Google Scholar] [CrossRef]
- Rosenberg, E.; Zilber-Rosenberg, I. The hologenome concept of evolution after 10 years. Microbiome 2018, 6, 78. [Google Scholar] [CrossRef]
- Pilosof, S.; Porter, M.A.; Pascual, M.; Kéfi, S. The multilayer nature of ecological networks. Nat. Ecol. Evol. 2017, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).