Improving Fungal Decay Resistance of Less Durable Sapwood by Impregnation with Scots Pine Knotwood and Black Locust Heartwood Hydrophilic Extractives with Antifungal or Antioxidant Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Material
2.3. Extraction
2.4. Chemical Analysis of Extractives
2.5. Fungal Tests
2.5.1. Preparation of Treating Solutions for Antifungal and Decay Testing
2.5.2. Fungal Cultures
2.5.3. Antifungal Assay
2.5.4. Impregnation of Wood Blocks for Decay Assay
2.5.5. Decay Test
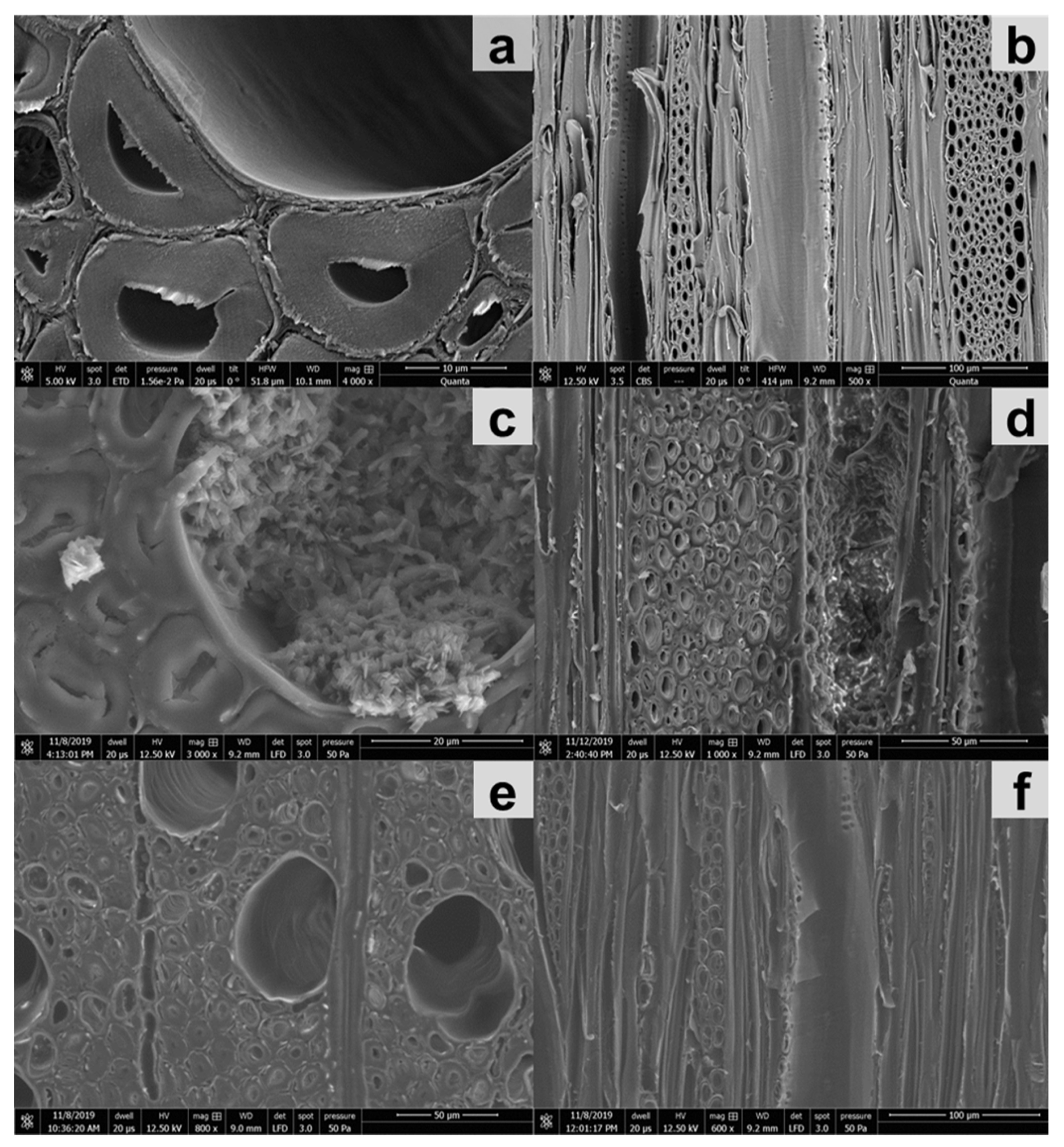
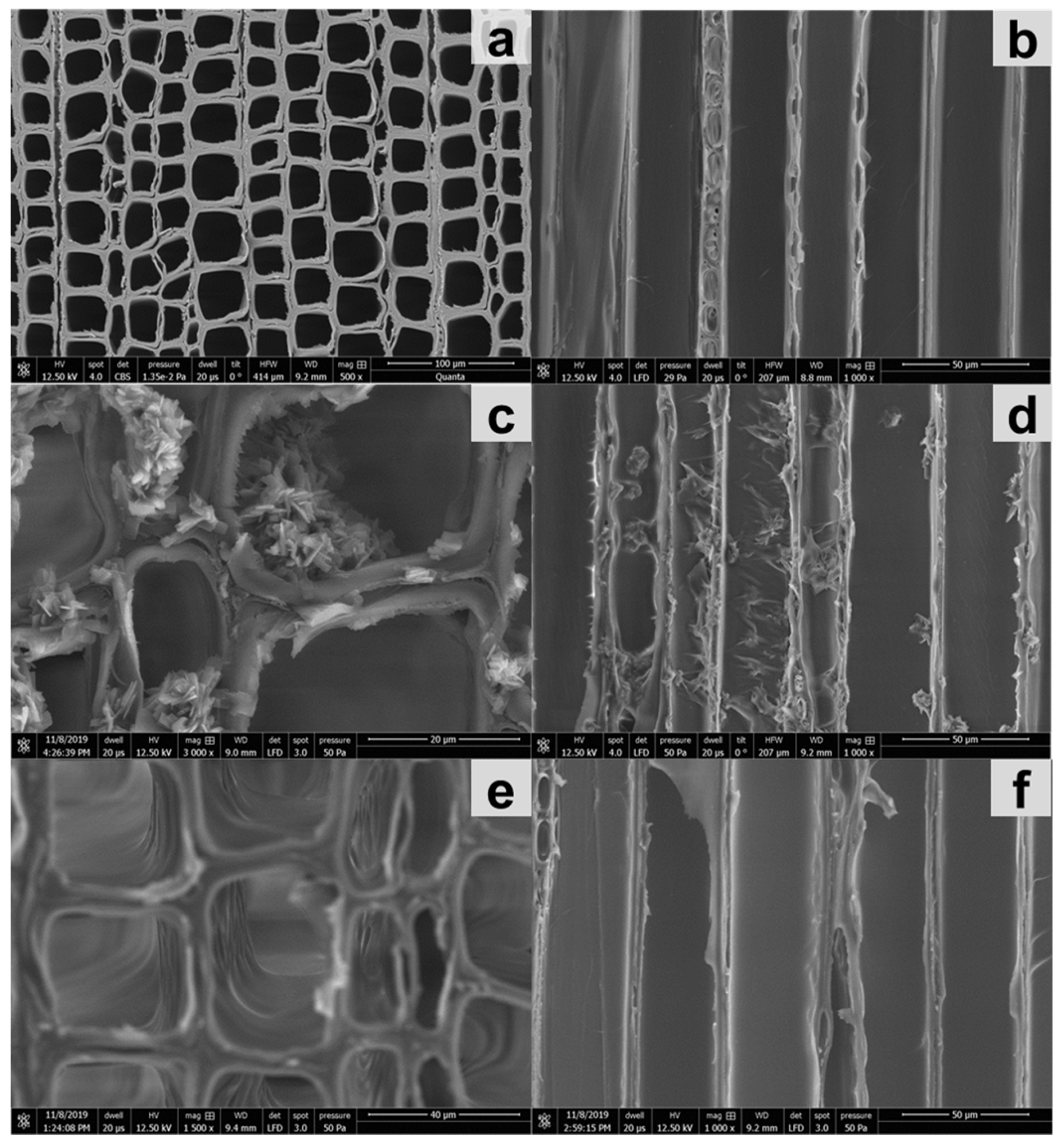
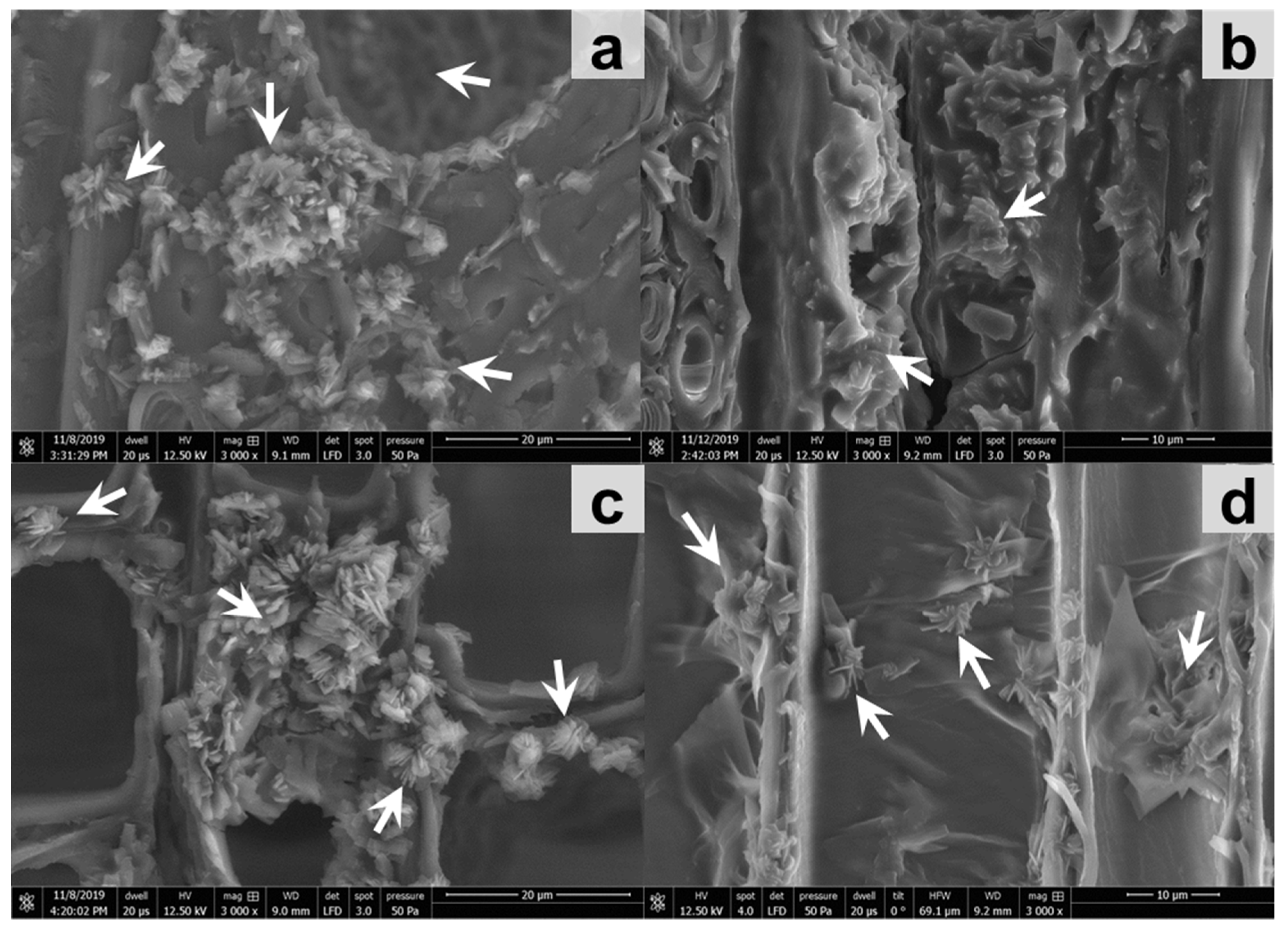
2.6. Assessment of Impregnation Efficiency with Microscopy
2.6.1. Sample Preparation
2.6.2. Confocal Laser Scanning Microscope (CLSM)
2.6.3. Scanning Electron Microscopy (SEM)
2.7. Assessment of Impregnation Efficiency with Gravimetry
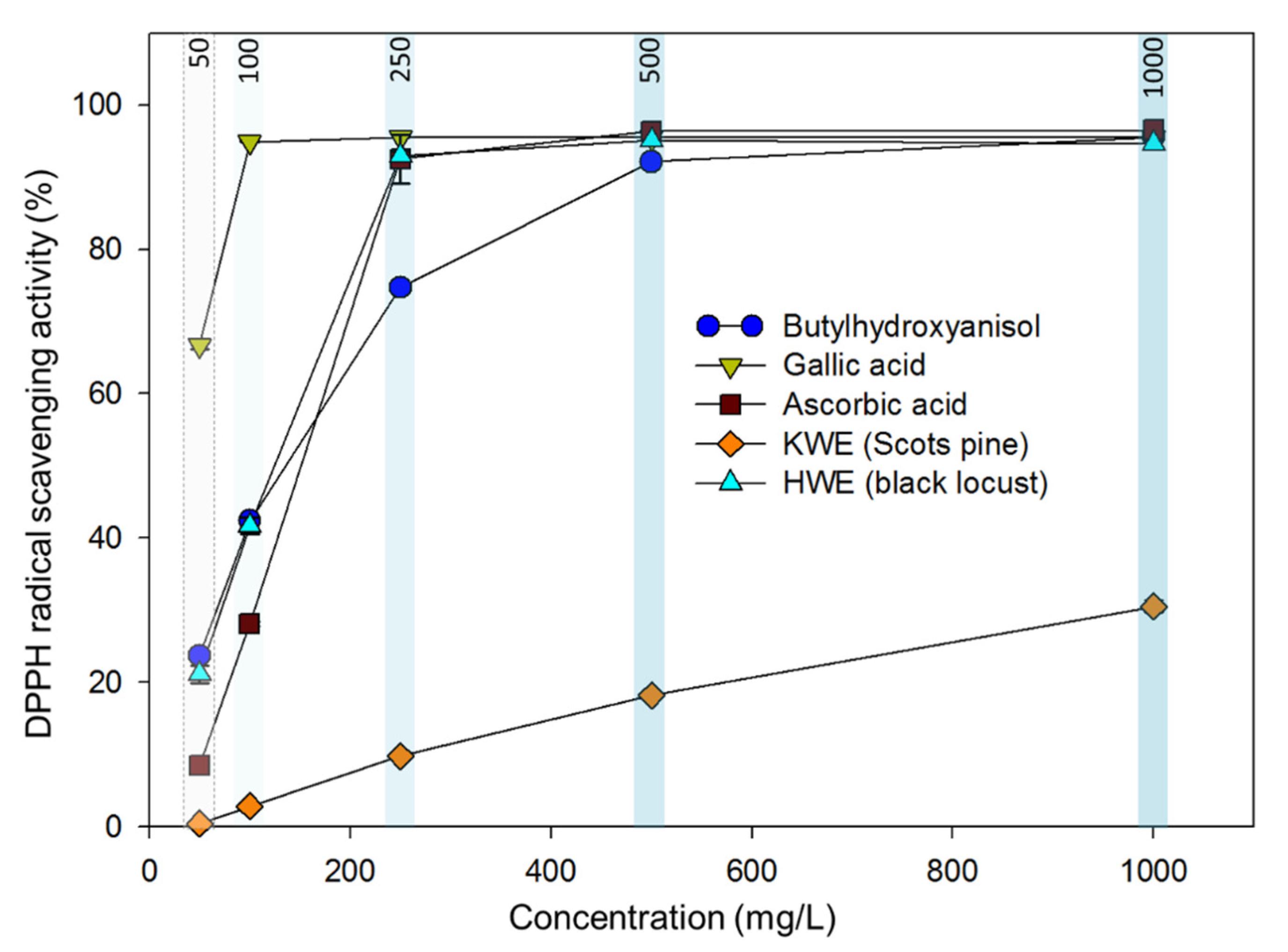
2.8. Antioxidant Assay (a DPPH Radical Scavenging Activity)
2.9. Statistics
3. Results and Discussion
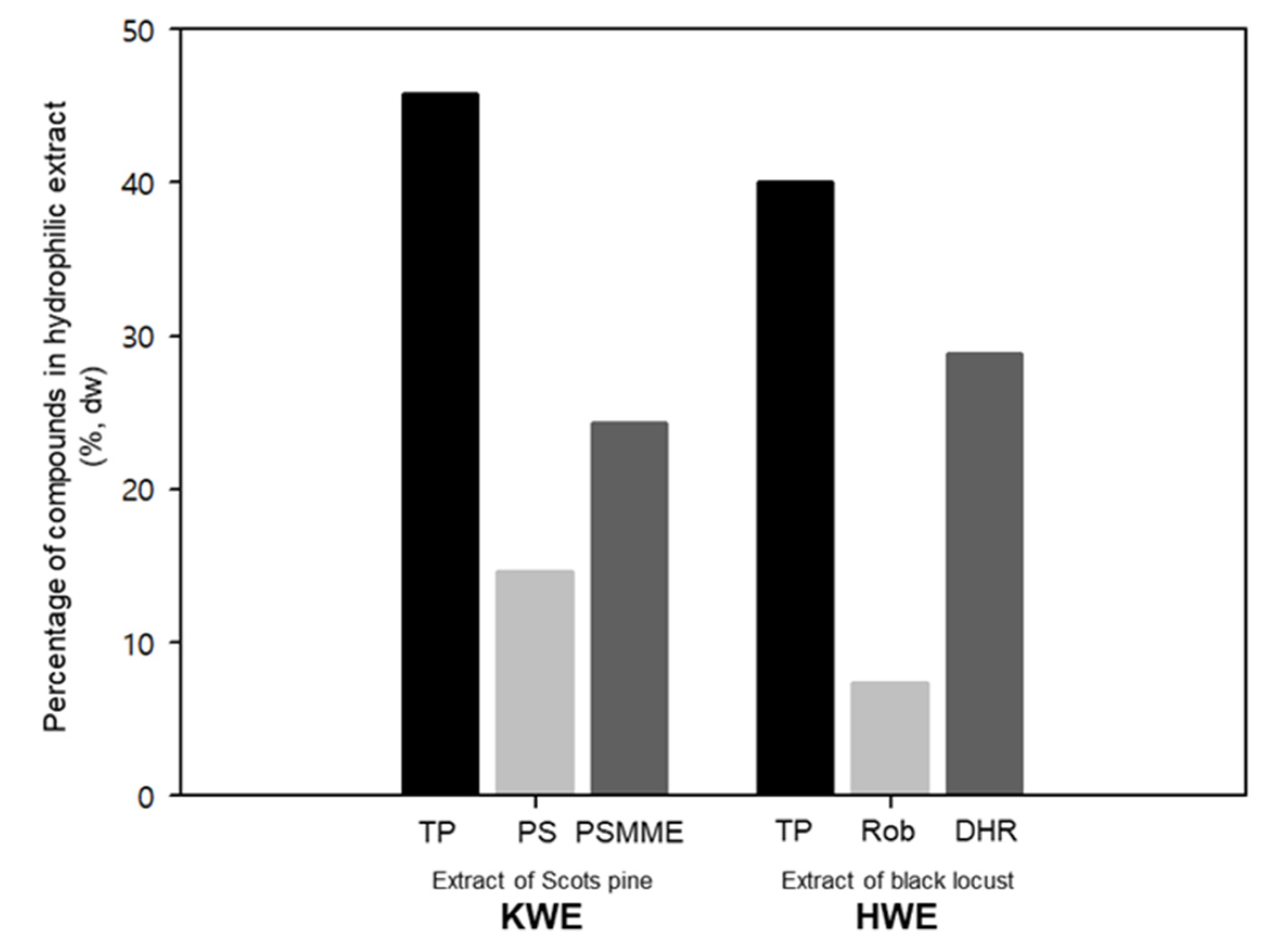
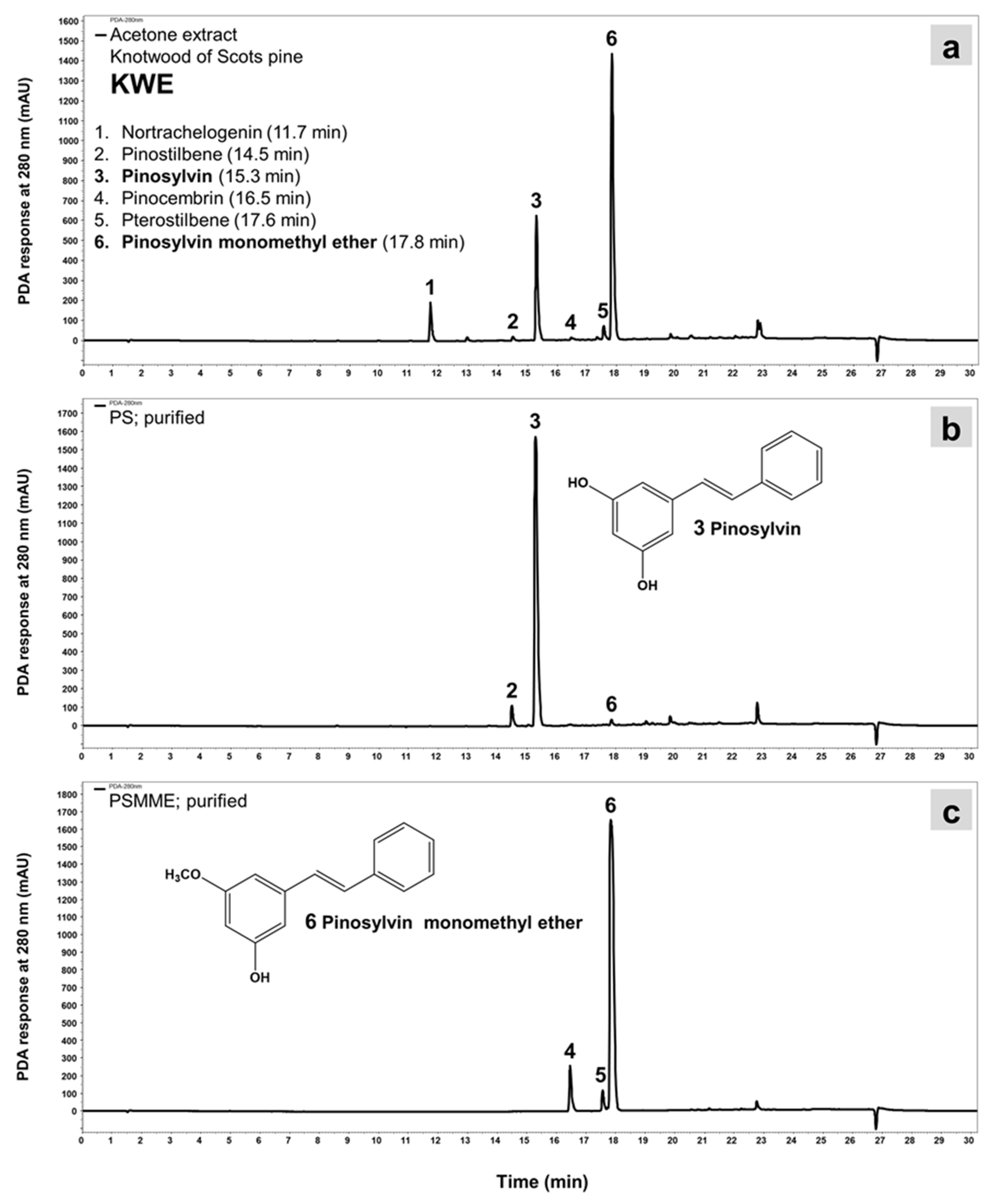
3.1. Extraction Yields and Chemical Composition of Extracts
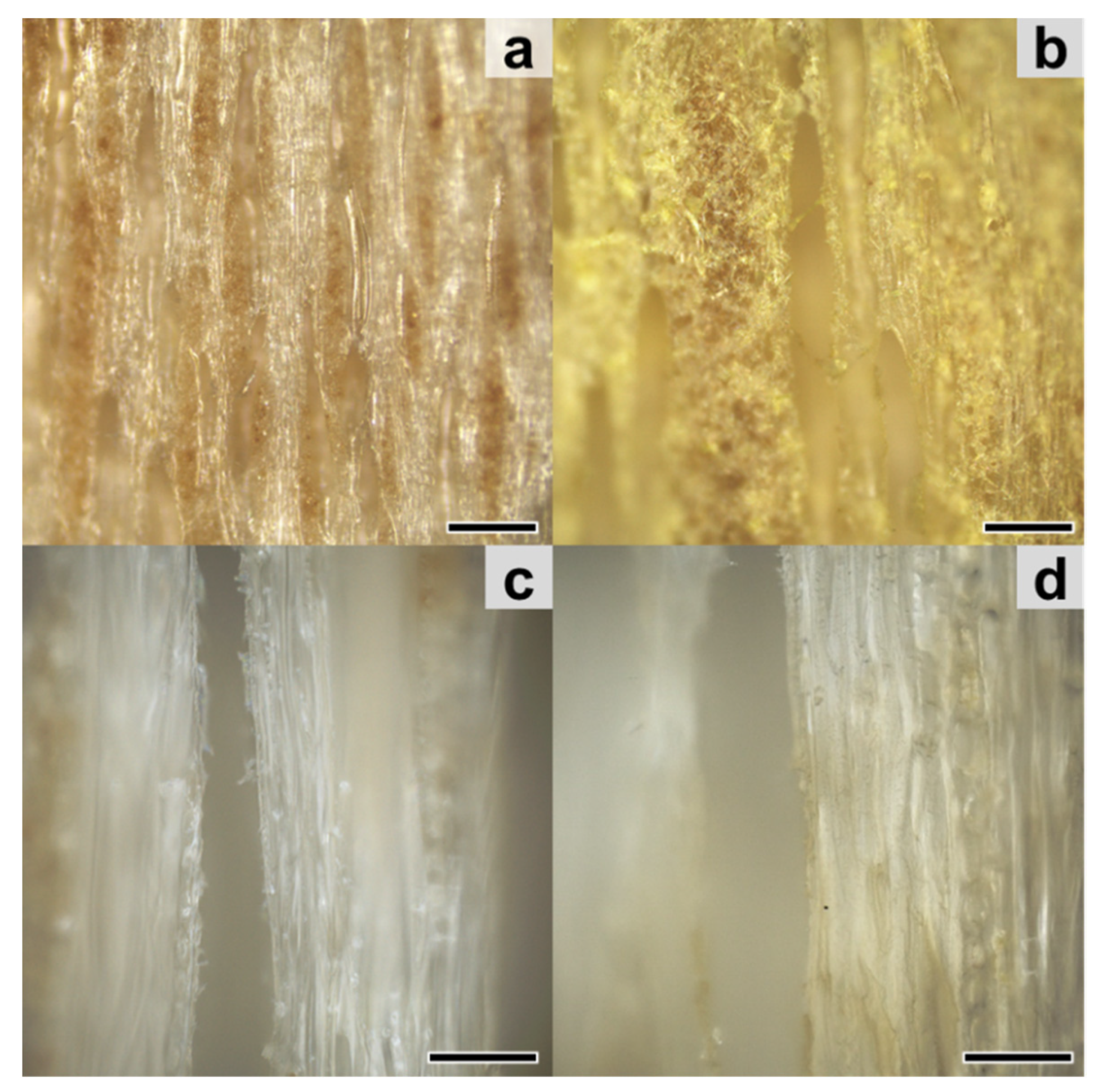
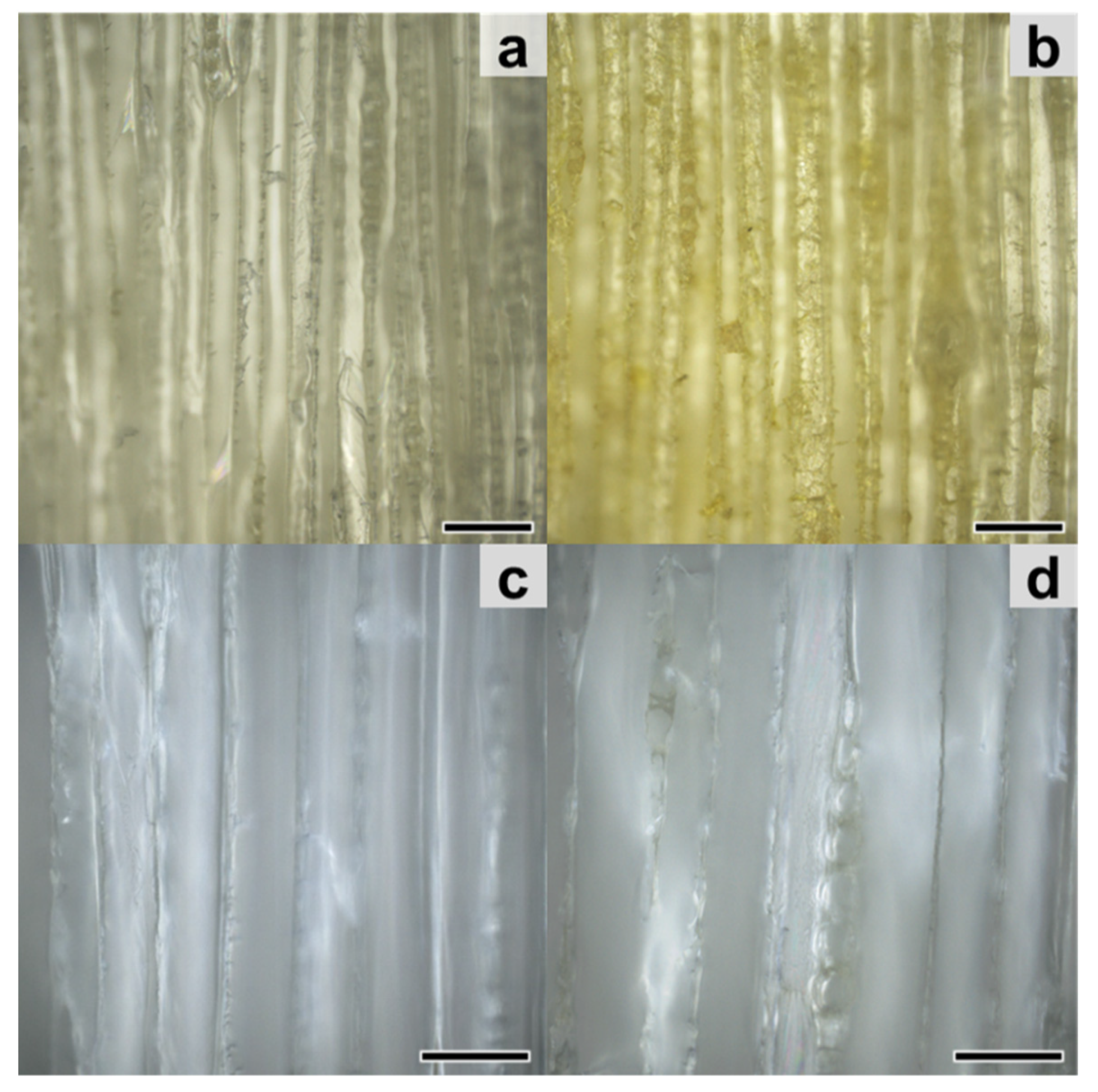
3.2. Occurrence of Extractives and Gained Weight after Impregnation of Wood Blocks
3.3. Fungal Growth Inhibition of Scots Pine and Black Locust Extractives
3.4. Decay Resistance of Wood Impregnated with Scots Pine and Black Locust Extractives
3.5. Radical Scavenging Activity of Wood Extracts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 1989; p. 613. [Google Scholar]
- Oleson, K.R.; Schwartz, D.T. Extractives in Douglas-fir forestry residue and considerations for biofuel production. Phytochem. Rev. 2016, 15, 985–1008. [Google Scholar] [CrossRef]
- Belgacem, N.; Pizzi, A. Lignocellulosic Fibers and Wood Handbook: Renewable Materials for Today’s Environment; Scrivener Publishing: Salem, MA, USA, 2016; p. 669. [Google Scholar]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Humar, M.; Kržišnik, D.; Lesar, B.; Brischke, C. The Performance of Wood Decking after Five Years of Exposure: Verification of the Combined Effect of Wetting Ability and Durability. Forests 2019, 10, 903. [Google Scholar] [CrossRef]
- Miebach, F. Design ideas for solid timber bridges. Wood Mater. Sci. Eng. 2018, 13, 184–189. [Google Scholar] [CrossRef]
- Hill, C.A.S. Wood modification: An update. BioResources 2011, 6, 918–919. [Google Scholar]
- Petrič, M. Surface Modification of Wood. Rev. Adhes. Adhes. 2013, 1, 216–247. [Google Scholar] [CrossRef]
- Pitman, A. Deterioration and protection of sustainable biomaterials. Int. Wood Prod. J. 2017, 8, 56. [Google Scholar] [CrossRef]
- Humar, M.; Lesar, B.; Thaler, N.; Krzisnik, D.; Kregar, N.; Drnovsek, S. Quality of copper impregnated wood in slovenian hardware stores. Drvna Ind. 2018, 69, 121–126. [Google Scholar] [CrossRef]
- Karppanen, O.; Venäläinen, M.; Harju, A.M.; Willför, S.; Pietarinen, S.; Laakso, T.; Kainulainen, P. Knotwood as a window to the indirect measurement of the decay resistance of Scots pine heartwood. Holzforschung 2007, 61, 600–604. [Google Scholar] [CrossRef]
- Freeman, M.H.; McIntyre, C.R. A comprehensive review of copper-based wood preservatives. For. Prod. J. 2008, 58, 6–27. [Google Scholar]
- Schultz, T.P.; Nicholas, D.D. Efficacy of two organic biocides with co-added antioxidants. Holzforschung 2011, 65, 771. [Google Scholar] [CrossRef]
- Ekeberg, D.; Flćte, P.O.; Eikenes, M.; Fongen, M.; Naess-Andresen, C.F. Qualitative and quantitative determination of extractives in heartwood of Scots pine (Pinus sylvestris L.) by gas chromatography. J. Chromatogr. A 2006, 1109, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Singh, A.P. A review on natural products as wood protectant. Wood. Sci. Technol. 2012, 46, 851–870. [Google Scholar] [CrossRef]
- Barbero-Lopez, A.; Chibily, S.; Tomppo, L.; Salami, A.; Ancin-Murguzur, F.J.; Venalainen, M.; Lappalainen, R.; Haapala, A. Pyrolysis distillates from tree bark and fibre hemp inhibit the growth of wood-decaying fungi. Ind. Crops. Prod. 2019, 129, 604–610. [Google Scholar] [CrossRef]
- Dian-Qing, Y. Potential utilization of plant and fungal extracts for wood protection. For. Prod. J. 2009, 59, 97–103. [Google Scholar]
- Kadir, R.; Hale, M. Biocidal potential of the extractives of four Malaysian timbers against subterranean termites and wood decay fungi. Eur. J. Wood Wood Prod. 2019, 77, 147–155. [Google Scholar] [CrossRef]
- Valette, N.; Perrot, T.; Sormani, R.; Gelhaye, E.; Morel-Rouhier, M.; Morel-Rouhier, M. Antifungal activities of wood extractives. Fungal Biol. Rev. 2017, 31, 113–123. [Google Scholar] [CrossRef]
- Poljanšek, I.; Oven, P.; Vek, V.; Raitanen, J.E.; Hemming, J.; Willför, S. Isolation of pure pinosylvins from industrial knotwood residue with non-chlorinated solvents. Holzforschung 2019, 73, 475–484. [Google Scholar] [CrossRef]
- Willför, S.M.; Ahotupa, M.O.; Hemming, J.E.; Reunanen, M.H.T.; Eklund, P.C.; Sjoholm, R.E.; Eckerman, C.S.E.; Pohjamo, S.P.; Holmbom, M.R. Antioxidant activity of knotwood extractives and phenolic compounds of selected tree species. J. Agric. Food Chem. 2003, 51, 7600–7606. [Google Scholar] [CrossRef]
- Belt, T.; Hanninen, T.; Rautkari, L. Antioxidant activity of Scots pine heartwood and knot extractives and implications for resistance to brown rot. Holzforschung 2017, 71, 527–534. [Google Scholar] [CrossRef]
- Harju, A.M.; Venäläinen, M. Measuring the decay resistance of Scots pine heartwood indirectly by the Folin-Ciocalteu assay. Can. J. For. Res. 2006, 36, 1797–1804. [Google Scholar] [CrossRef]
- Lindberg, L.E.; Willför, S.M.; Holmbom, B.R. Antibacterial effects of knotwood extractives on paper mill bacteria. J. Ind. Microbiol. Biotechnol. 2004, 31, 137–147. [Google Scholar] [CrossRef]
- Pietarinen, S.; Willför, S.; Ahotupa, M.; Hemming, J.; Holmbom, B. Knotwood and bark extracts: Strong antioxidants from waste materials. J. Wood. Sci. 2006, 52, 436–444. [Google Scholar] [CrossRef]
- Välimaa, A.L.; Honkalampi-Hämäläinen, U.; Pietarinen, S.; Willför, S.; Holmbom, B.; von Wright, A. Antimicrobial and cytotoxic knotwood extracts and related pure compounds and their effects on food-associated microorganisms. Int. J. Food Microbiol. 2007, 115, 235–243. [Google Scholar] [CrossRef]
- Lu, J.R.; Venalainen, M.; Julkunen-Tiitto, R.; Harju, A.M. Stilbene impregnation retards brown-rot decay of Scots pine sapwood. Holzforschung 2016, 70, 261–266. [Google Scholar] [CrossRef]
- Harju, A.M.; Venäläinen, M.; Anttonen, S.; Viitanen, H.; Kainulainen, P.; Saranpää, P.; Vapaavuori, E. Chemical factors affecting the brown-rot decay resistance of Scots pine heartwood. Trees 2003, 17, 263–268. [Google Scholar] [CrossRef]
- Venäläinen, M.; Harju, A.M.; Kainulainen, P.; Viitanen, H.; Nikulainen, H. Variation in the decay resistance and its relationship with other wood characteristics in old Scots pines. Ann. For. Sci. 2003, 60, 409–417. [Google Scholar] [CrossRef]
- Celimene, C.C.; Micales, J.A.; Ferge, L.; Young, R.A. Efficacy of pinosylvins against white-rot and brown-rot fungi. Holzforschung 1999, 53, 491–497. [Google Scholar] [CrossRef]
- Wijayanto, A.; Dumarçay, S.; Gérardin-Charbonnier, C.; Sari, R.K.; Syafii, W.; Gérardin, P. Phenolic and lipophilic extractives in Pinus merkusii Jungh. et de Vries knots and stemwood. Ind. Crops. Prod. 2015, 69, 466–471. [Google Scholar] [CrossRef]
- Seppänen, S.K.; Syrjälä, L.; von Weissenberg, K.; Teeri, T.H.; Paajanen, L.; Pappinen, A. Antifungal activity of stilbenes in in vitro bioassays and in transgenic Populus expressing a gene encoding pinosylvin synthase. Plant. Cell. Rep. 2004, 22, 584–593. [Google Scholar] [CrossRef]
- Vítková, M.; Müllerová, J.; Sádlo, J.; Pergl, J.; Pyšek, P. Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe. For. Ecol. Manage. 2017, 384, 287–302. [Google Scholar] [CrossRef]
- Bahor, B.; Klopcic, M. Black locust (Robinia pseudoacacia L.) in Bela krajina: Distribution, growth, regeneration and management. Acta Silvae Ligni 2019, 120, 13–28. [Google Scholar] [CrossRef]
- Torelli, N. Robinija (Robinia pseudoacacia L.) in njen les. Les 2003, 54, 6–10. [Google Scholar]
- De Filippis, L.; Magel, E. Identification of biochemical differences between the sapwood and transition zone in Robinia pseudoacacia L. by differential display of proteins. Holzforschung 2012, 66, 543–549. [Google Scholar] [CrossRef]
- Bostyn, S.; Destandau, E.; Charpentier, J.P.; Serrano, V.; Seigneuret, J.M.; Breton, C. Optimization and kinetic modelling of robinetin and dihydrorobinetin extraction from Robinia pseudoacacia wood. Ind. Crops. Prod. 2018, 126, 22–30. [Google Scholar] [CrossRef]
- Meszaros, E.; Jakab, E.; Varhegyi, G. TG/MS, Py-GOMS and THM-GIC/MS study of the composition and thermal behavior of extractive components of Robinia pseudoacacia. J. Anal. Appl. Pyrolysis 2007, 79, 61–70. [Google Scholar] [CrossRef]
- Sanz, M.; de Simon, B.F.; Esteruelas, E.; Munoz, A.M.; Cadahia, E.; Hernandez, T.; Estrella, I.; Pinto, E. Effect of toasting intensity at cooperage on phenolic compounds in acacia (Robinia pseudoacacia) heartwood. J. Agric. Food Chem. 2011, 59, 3135–3145. [Google Scholar] [CrossRef]
- Sergent, T.; Kohnen, S.; Jourez, B.; Beauve, C.; Schneider, Y.J.; Vincke, C. Characterization of black locust (Robinia pseudoacacia L.) heartwood extractives: Identification of resveratrol and piceatannol. Wood Sci. Technol. 2014, 48, 1005–1017. [Google Scholar] [CrossRef]
- Magel, E.; Jayallemand, C.; Ziegler, H. Formation of heartwood substances in the stemwood of Robinia pseudoacacia L. II. Distribution of nonstructural carbohydrates and wood extractives across the trunk. Trees 1994, 8, 165–171. [Google Scholar] [CrossRef]
- Vek, V.; Vivod, B.; Poljanšek, I.; Oven, P. Vsebnost ekstraktivov v skorji in lesu robinije (Robinia pseudoacacia L.)/Content of extractives in bark and wood of black locust (Robinia pseudoacacia L.). Acta Silvae Ligni 2019, 119, 13–25. [Google Scholar] [CrossRef]
- Smith, A.L.; Campbell, C.L.; Walker, D.B.; Hanover, J.W. Extracts from black locust as wood preservatives: Extraction of decay resistance from black locust heartwood. Holzforschung 1989, 43, 293–296. [Google Scholar] [CrossRef]
- Sablik, P.; Giagli, K.; Paril, P.; Baar, J.; Rademacher, P. Impact of extractive chemical compounds from durable wood species on fungal decay after impregnation of nondurable wood species. Eur. J. Wood Wood Prod. 2016, 74, 231–236. [Google Scholar] [CrossRef]
- Rademacher, P.; Rousek, R.; Fodor, F.; Baar, J.; Koch, G.; Németh, R.; Pařil, P.; Paschová, Z.; Sablík, P.; Paul, D.; et al. Robinia Wood Research—New innovations for a traditional material. In Proceedings of the Eco-Efficient Resource Wood with Special Focus on Hardwoods, Hardwood Conference, Sopron, Hungary, 8–9 September 2016; pp. 80–108. [Google Scholar]
- Hosseinihashemi, S.K.; HosseinAshrafi, S.K.; Goldeh, A.J.; Salem, M.Z.M. Antifungal and antioxidant activities of heartwood, bark, and leaf extracts of Robinia pseudoacacia. BioResources 2016, 11, 1634–1646. [Google Scholar] [CrossRef]
- Schultz, T.P.; Nicholas, D.D. Naturally durable heartwood: Evidence for a proposed dual defensive function of the extractives. Phytochemisrty 2000, 54, 47–52. [Google Scholar] [CrossRef]
- Hsu, F.L.; Chen, P.S.; Chang, H.T.; Chang, S.T. Effects of alkyl chain length of gallates on their antifungal property and potency as an environmentally benign preservative against wood-decay fungi. Int. Biodeterior. Biodegrad. 2009, 63, 543–547. [Google Scholar] [CrossRef]
- Chen, P.S.; Chen, Y.H.; Yeh, T.F.; Chang, S.T. Mechanism of decay resistance of heartwood extracts from Acacia confusa against the brown-rot fungus Laetiporus sulphureus. Wood Sci. Technol. 2014, 48, 451–465. [Google Scholar] [CrossRef]
- Schultz, T.P.; Harms, W.B.; Fisher, T.H.; McMurtrey, K.D.; Minn, J.; Nicholas, D.D. Durability of angiosperm heartwood: The importance of extractives. Holzforschung 1995, 49, 29–34. [Google Scholar] [CrossRef]
- Schultz, T.P.; Nicholas, D.D.; Prewitt, M.L. Environmentally-benign wood preservatives based on an organic biocide: Antioxidant combination: Ground-contact efficacy ratings and BHT depletion after four years of exposure. Holzforschung 2004, 58, 300–304. [Google Scholar] [CrossRef]
- Humar, M.; Petrič, M.; Pohleven, F. Changes of the pH value of impregnated wood during exposure to wood-rotting fungi. Holz Roh Werkst. 2001, 59, 288–293. [Google Scholar] [CrossRef]
- Schmidt, O. Wood and Tree Fungi; Biology, Damage, Protection, and Use; Springer: Berlin/Heidelberg, Germany, 2006; p. 334. [Google Scholar]
- Green, F.; Clausen, C. Copper tolerance of brown-rot fungi: Oxalic acid production in southern pine treated with arsenic-free preservatives. Int. Biodeterior. Biodegrad. 2005, 56, 75–79. [Google Scholar] [CrossRef]
- Humar, M.; Lesar, B. Fungicidal properties of individual components of copper–ethanolamine-based wood preservatives. Int. Biodeterior. Biodegrad. 2008, 62, 46–50. [Google Scholar] [CrossRef]
- Vek, V. Application of Pinewood Extractives for Wood Preservation; Final Project Report; Republic of Slovenia, Ministry of Education, Science and Sport: Ljubljana, Slovenia, 2015.
- Vek, V.; Poljanšek, I.; Oven, P. Variability in content of hydrophilic extractives and individual phenolic compounds in black locust stem. Eur. J. Wood Wood Prod. 2020, 78, 501–511. [Google Scholar] [CrossRef]
- Willför, S.; Reunanen, M.; Eklund, P.; Sjoholm, R.; Kronberg, L.; Fardim, P.; Pietarinen, S.; Holmbom, B. Oligolignans in Norway spruce and Scots pine knots and Norway spruce stemwood. Holzforschung 2004, 58, 345–354. [Google Scholar] [CrossRef]
- Dünisch, O.; Richter, H.G.; Koch, G. Wood properties of juvenile and mature heartwood in Robinia pseudoacacia L. Wood Sci. Technol. 2010, 44, 301–313. [Google Scholar] [CrossRef]
- Vek, V. Extractives in Wounded Wood and Knots of Common Beech (Fagus sylvatica L.). Ph.D. Thesis, University of Ljubljana, Ljubljana, Slovenia, 2013; p. 162. [Google Scholar]
- Vek, V.; Poljanšek, I.; Oven, P. Efficiency of three conventional methods for extraction of dihydrorobinetin and robinetin from wood of black locust. Eur. J. Wood Wood Prod. 2019, 77, 891–901. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Scalbert, A.; Monties, B.; Janin, G. Tannins in wood: Comparison of different estimation methods. J. Agric. Food Chem. 1989, 37, 1324–1329. [Google Scholar] [CrossRef]
- Pearce, R.B. Antimicrobial defences in the wood of living trees. New Phytol. 1996, 132, 203–233. [Google Scholar] [CrossRef]
- Raspor, P.; Smole-Možina, S.; Podjavoršek, J.; Pohleven, F.; Gogala, N.; Nekrep, F.V.; Rogelj, I.; Hacin, J. Culture collection of industrial microorganisms (ZIM). Biotech. Fac. Food Sci. Technol. Dept. 1995, 65, 65–72. [Google Scholar]
- Humar, M.; Pohleven, F. Experiences with non-standard test methods for estimation of fungicidal properties and mode of fungicidal action (Experienţe în utilizarea unor metode de testare nestandardizate de evaluare a proprietâţilor fungicidelor şi a modului lor de acţiune). Pro Ligno 2007, 3, 17–25. [Google Scholar]
- Zimmer, K.; Melcher, E. A screening study on extractive content and composition of Scots pine heartwood of three stands with close proximity and their resistance against basidiomycetes. Int. Wood Prod. J. 2017, 8, 45–49. [Google Scholar] [CrossRef]
- Humar, M.; Thaler, N. Performance of copper treated utility poles and posts used in service for several years. Int. Biodeterior. Biodegrad. 2017, 116, 219–226. [Google Scholar] [CrossRef]
- Pietrzak, W.; Nowak, R.; Olech, M. Effect of extraction method on phenolic content and antioxidant activity of mistletoe extracts from Viscum album subsp. abietis. Chem. Pap. 2014, 68, 976–982. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Ferreira, I.C.F.R. Effect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measured by several biochemical assays. Food Chem. Toxicol. 2007, 45, 1731–1737. [Google Scholar] [CrossRef]
- Benković, E.T.; Žigon, D.; Mihailović, V.; Petelinc, T.; Jamnik, P.; Kreft, S. Identification, in vitro and in vivo antioxidant activity, and gastrointestinal stability of lignans from silver fir (Abies alba) wood extract. J. Wood Chem. Technol. 2017, 467–477. [Google Scholar] [CrossRef]
- Siau, J.F. Wood: Influence of Moisture on Physical Properties; Polytechnic Institute and State University: Blacksburg, VA, USA, 1995; pp. 39–63. [Google Scholar]
- Fang, W.; Hemming, J.; Reunanen, M.; Eklund, P.; Conde, E.; Poljanšek, I.; Oven, P.; Willför, S. Evaluation of selective extraction methods for recovery of polyphenols from pine. Holzforschung 2013, 67, 843–851. [Google Scholar] [CrossRef]
- Willför, S.; Hemming, J.; Reunanen, M.; Eckerman, C.; Holmbom, B. Lignans and lipophilic extractives in Norway spruce knots and stemwood. Holzforschung 2003, 57, 27–36. [Google Scholar] [CrossRef]
- Willför, S.; Hemming, J.; Reunanen, M.; Holmbom, B. Phenolic and lipophilic extractives in Scots pine knots and stemwood. Holzforschung 2003, 57, 359–372. [Google Scholar] [CrossRef]
- Lekounougou, S.; Mounguengui, S.; Dumarçay, S.; Rose, C.; Courty, P.E.; Garbaye, J.; Gérardin, P.; Jacquot, J.P.; Gelhaye, E. Initial stages of Fagus sylvatica wood colonization by the white-rot basidiomycete Trametes versicolor: Enzymatic characterization. Int. Biodeterior. Biodegrad. 2008, 61, 287–293. [Google Scholar] [CrossRef]
- Schultz, T.P.; Nicholas, D.D. Development of environmentally-benign wood preservatives based on the combination of organic biocides with antioxidants and metal chelators. Phytochemistry 2002, 61, 555–560. [Google Scholar] [CrossRef]
- Ioannidis, K.; Melliou, E.; Alizoti, P.; Magiatis, P. Identification of black pine (Pinus nigra Arn.) heartwood as a rich source of bioactive stilbenes by qNMR. J. Sci. Food. Agric. 2017, 97, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Arantes, V.; Jellison, J.; Goodell, B. Peculiarities of brown-rot fungi and biochemical Fenton reaction with regard to their potential as a model for bioprocessing biomass. Appl. Microbiol. Biotechnol. 2012, 94, 323–338. [Google Scholar] [CrossRef]
- Reinprecht, L. Wood Deterioration, Protection and Maintenance; John Wiley & Sons: Hoboken, NJ, USA, 2016; p. 376. [Google Scholar]
- Backa, S.; Gierer, J.; Reitberger, T.; Nilsson, T. Hydroxyl radical activity associated with the growth of white-rot fungi. Holzforschung 1993, 47, 181–187. [Google Scholar] [CrossRef]

| Mean | SD | |
|---|---|---|
| (mg g−1, dw) | (mg g−1 dw) | |
| Knotwood Samples of Scots Pine | ||
| Total hydrophilic extractives | 72.8 | 31.56 |
| Total phenols | 33.3 * | 5.71 |
| Pinosylvin | 10.6 | 4.02 |
| Pinosylvin monomethyl ether | 17.7 | 3.82 |
| Heartwood Samples of Black Locust | ||
| Total hydrophilic extractives | 61.8 | 21.60 |
| Total phenols | 24.8 | 12.58 |
| Robinetin | 4.5 | 2.38 |
| Dihydrorobinetin | 17.8 | 7.51 |
| Sapwood Blocks | GW (%) KWE | GW (%) HWE |
|---|---|---|
| Fagus sylvatica | 0.52 ± 0.07 a | 0.84 ± 0.09 b |
| Pinus sylvestris | 0.61 ± 0.14 a | 1.06 ± 0.14 c |
| Trametes Versicolor (%) | Schizophyllum Commune (%) | Gloeophyllum Trabeum (%) | Fibroporia Vaillantii (%) | |
|---|---|---|---|---|
| Control (DMSO) | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Extractives of Scots Pine | ||||
| PS (Ref 1) | 14.05 ± 1.02 c | 42.38 ± 5.80 c | 42.06 ± 0.71 b | 36.91±11.34 b,c |
| PSMME (Ref 2) | 25.76 ± 1.77 d | 57.67 ± 5.20 d | 67.62 ± 2.85 d | 48.95± 20.15 c |
| KWE1 | 10.85 ± 2.83 b | 37.03 ± 5.26 b | 42.40 ± 1.43 b | 21.97 ± 18.47 b |
| KWE5 | 12.79 ± 4.22 b,c | 41.18 ± 5.19 c | 50.64 ± 3.86 c | 24.43 ± 18.02 b |
| Extractives of Black Locust | ||||
| DHR | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 3.12 ± 3.63 a |
| Rob | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 6.20 ± 2.46 a |
| HWE1 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 3.52 ± 4.37 a |
| HWE5 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 5.10 ± 6.94 a |
| Sapwood Blocks of | Fungal Culture | ML (%) C | ML (%) KWE | ML (%) HWE |
|---|---|---|---|---|
| Fagus sylvatica | ||||
| Trametes versicolor | 9.17 ± 1.72 c,d | 7.75 ± 1.48 b,c,d | 7.04 ± 1.04 b,c | |
| Gloeophyllum trabeum | 10.18 ± 2.23 d | 3.09 ± 1.61 a | 5.23 ± 2.03 a,b | |
| Pinus sylvestris | ||||
| Trametes versicolor | 7.30 ± 0.84 b | 5.42 ± 0.87 a,b | 4.89 ± 1.16 a,b | |
| Gloeophyllum trabeum | 22.46 ± 3.87 c | 3.36 ± 3.84 a | 2.17 ± 2.58 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vek, V.; Balzano, A.; Poljanšek, I.; Humar, M.; Oven, P. Improving Fungal Decay Resistance of Less Durable Sapwood by Impregnation with Scots Pine Knotwood and Black Locust Heartwood Hydrophilic Extractives with Antifungal or Antioxidant Properties. Forests 2020, 11, 1024. https://doi.org/10.3390/f11091024
Vek V, Balzano A, Poljanšek I, Humar M, Oven P. Improving Fungal Decay Resistance of Less Durable Sapwood by Impregnation with Scots Pine Knotwood and Black Locust Heartwood Hydrophilic Extractives with Antifungal or Antioxidant Properties. Forests. 2020; 11(9):1024. https://doi.org/10.3390/f11091024
Chicago/Turabian StyleVek, Viljem, Angela Balzano, Ida Poljanšek, Miha Humar, and Primož Oven. 2020. "Improving Fungal Decay Resistance of Less Durable Sapwood by Impregnation with Scots Pine Knotwood and Black Locust Heartwood Hydrophilic Extractives with Antifungal or Antioxidant Properties" Forests 11, no. 9: 1024. https://doi.org/10.3390/f11091024
APA StyleVek, V., Balzano, A., Poljanšek, I., Humar, M., & Oven, P. (2020). Improving Fungal Decay Resistance of Less Durable Sapwood by Impregnation with Scots Pine Knotwood and Black Locust Heartwood Hydrophilic Extractives with Antifungal or Antioxidant Properties. Forests, 11(9), 1024. https://doi.org/10.3390/f11091024







