The Variation in Water Consumption by Transpiration of Qinghai Spruce among Canopy Layers in the Qilian Mountains, Northwestern China
Abstract
:1. Introduction
2. Materials and Methods
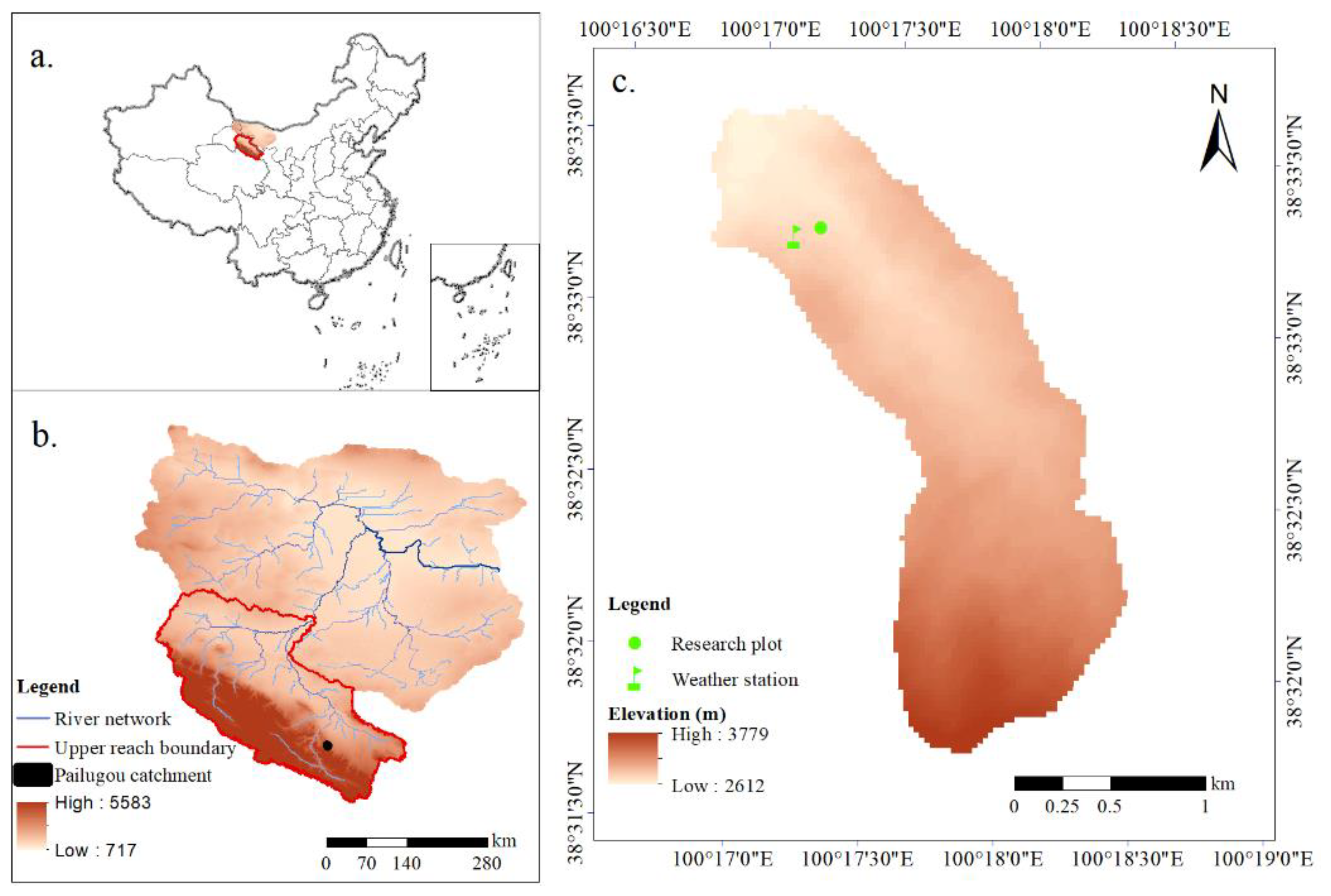
2.1. Study Area
2.2. Research Plot
2.3. Sap Flow Measurement and Transpiration Calculation
2.4. Weather and Soil Moisture Measurements
2.5. Data Analysis
3. Results
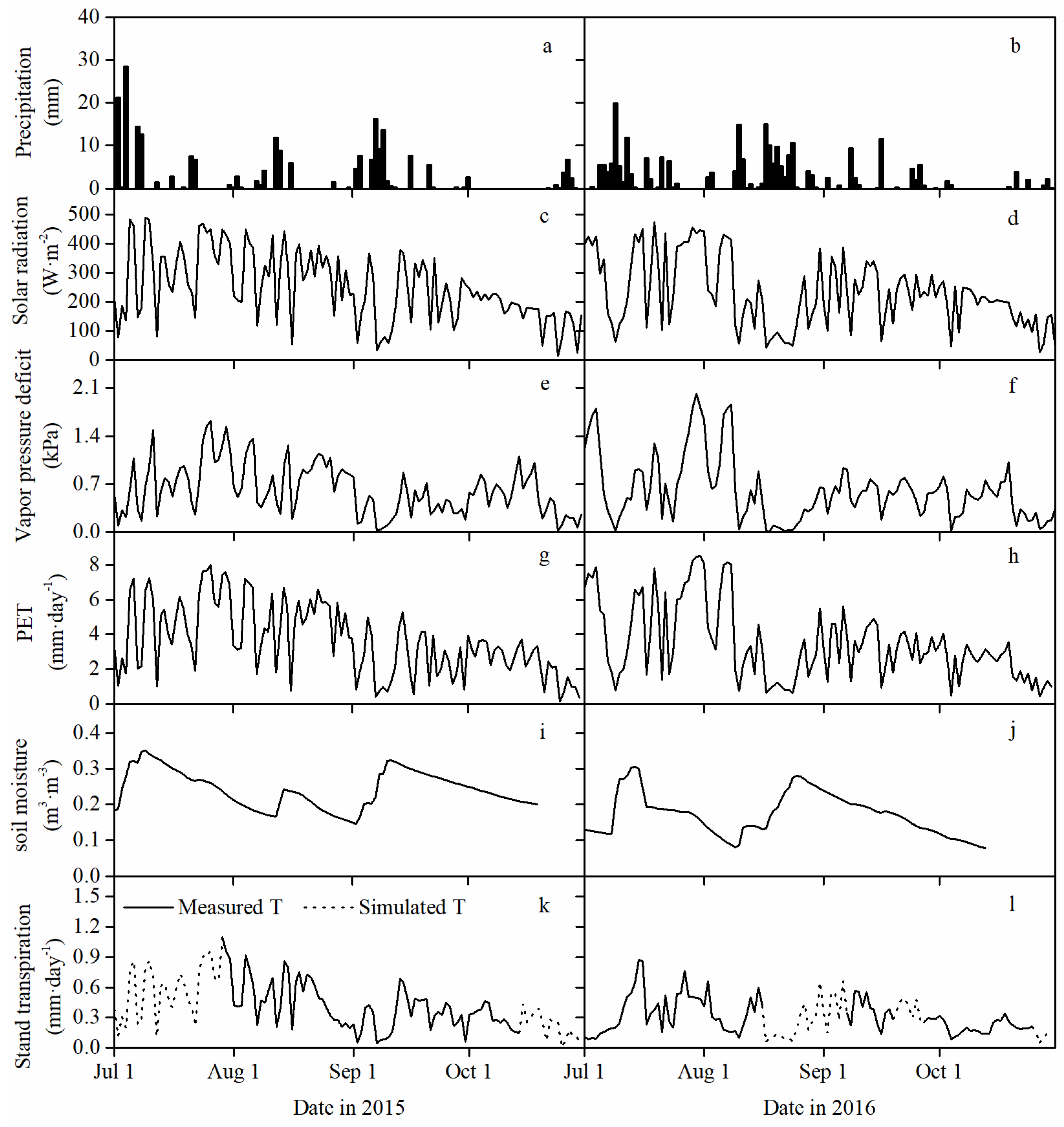
3.1. Environmental Characteristics during the Growing Season
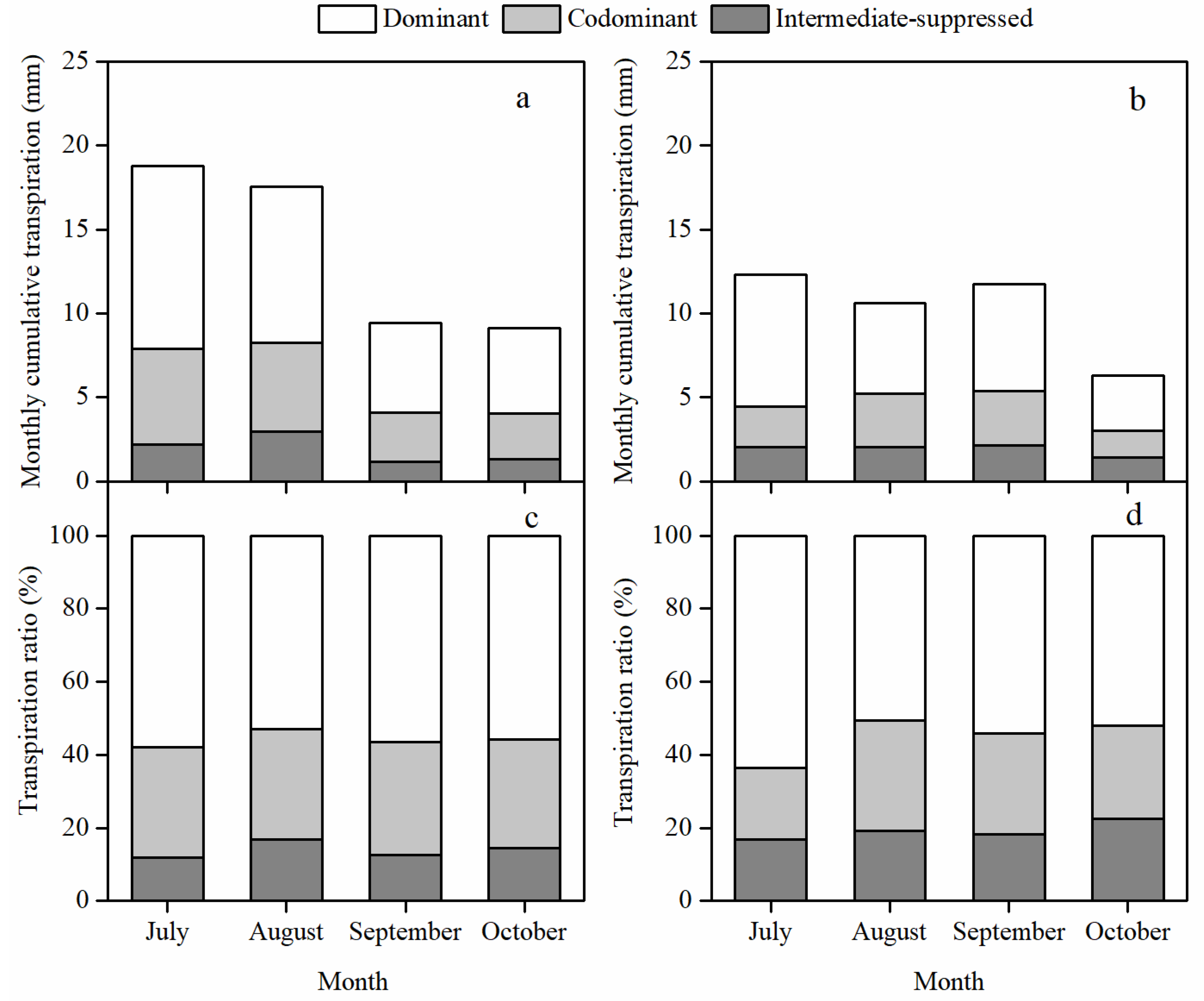
3.2. Stand Transpiration and Its Vertical Distribution among Canopy Layers
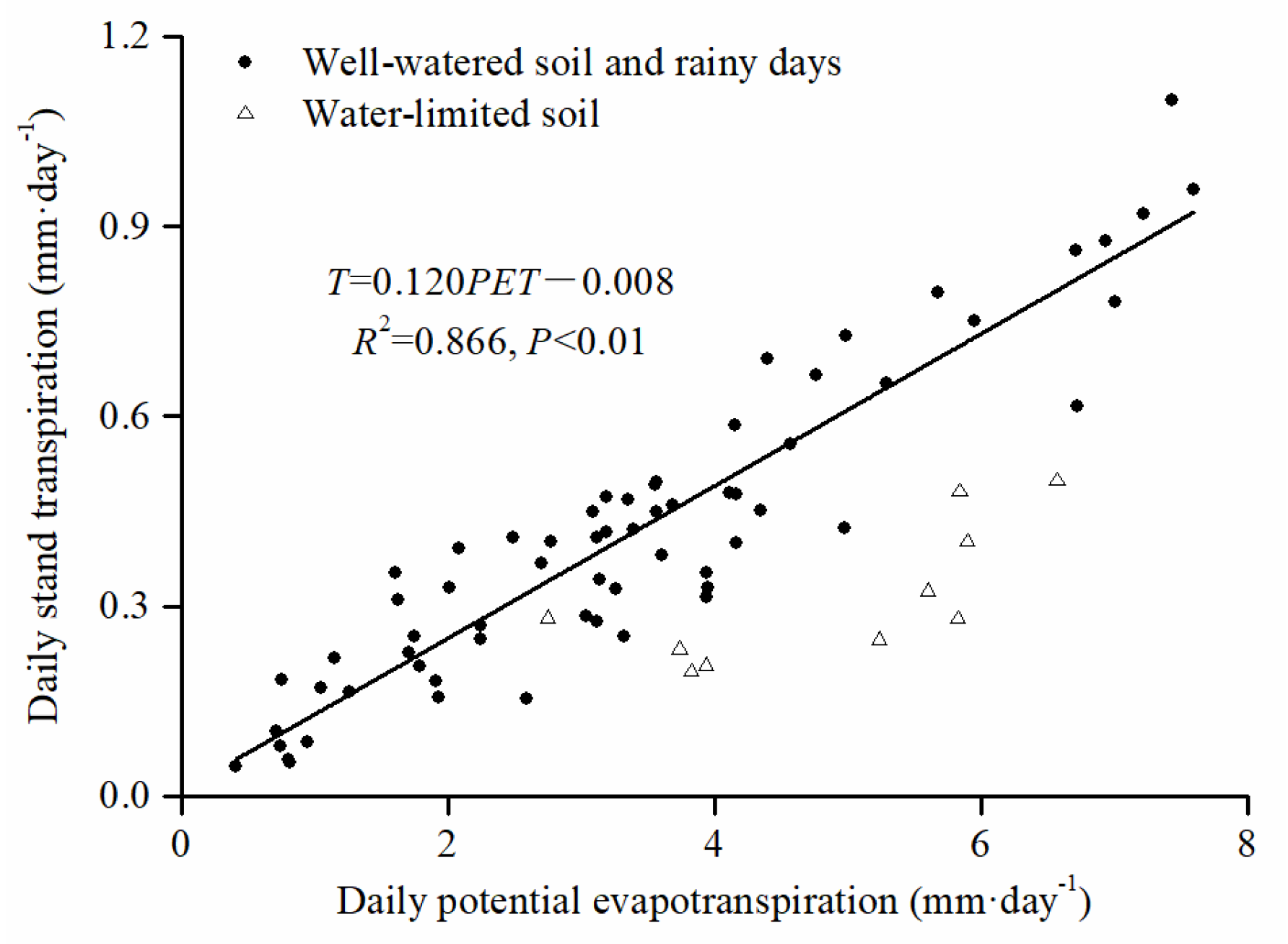
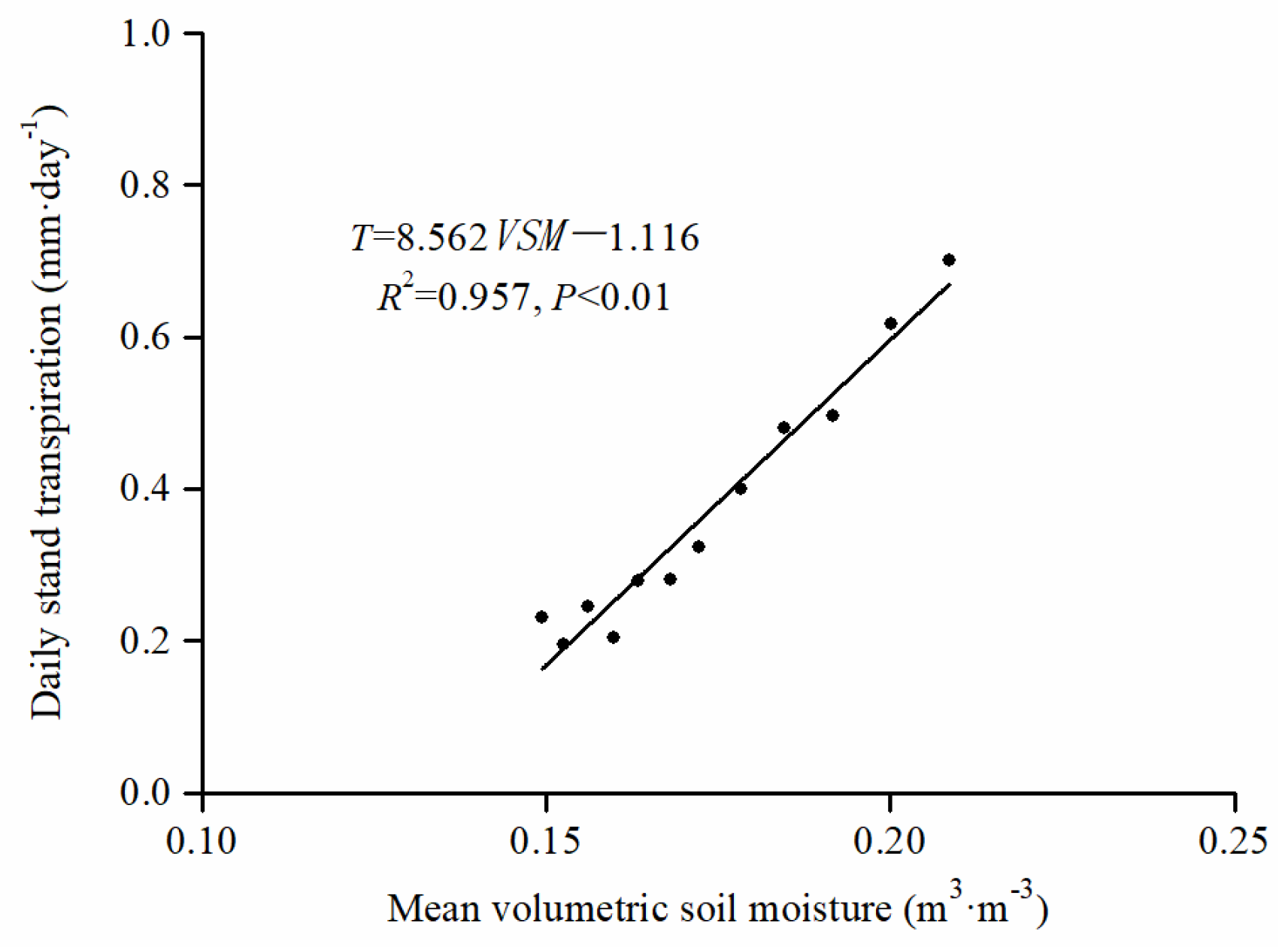
3.3. Daily Stand Transpiration Responses to PET and VSM
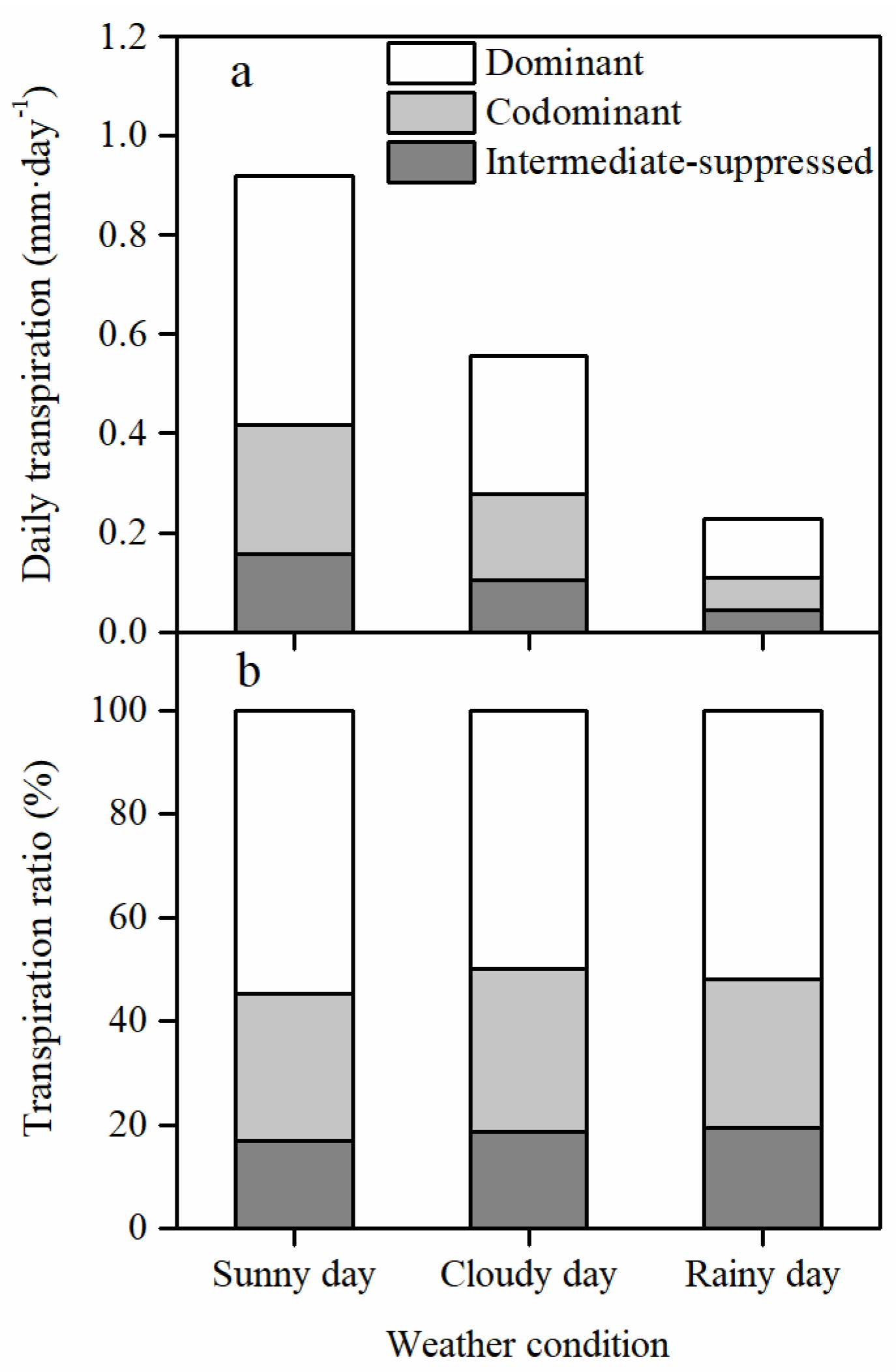
3.4. Different Responses of the Daily Transpiration to PET and VSM among Canopy Layers
4. Discussion
4.1. Tree Dominance Determines Its Role in Forest Transpiration
4.2. Effects of PET and VSM on the Daily Transpiration among Canopy Layers
4.3. Implications for Tree Water Use
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.H.; Yu, P.T.; Xiong, W.; Shen, Z.X.; Guo, M.C.; Shi, Z.J.; Du, A.P.; Wang, L.M. Water-yield reduction after afforestation and related processes in the Semiarid Liupan Mountains, Northwest China. J. Am. Water Resour. Assoc. 2008, 44, 1086–1097. [Google Scholar] [CrossRef]
- Wallace, J.; McJannet, D. Processes controlling transpiration in the rainforests of north Queensland, Australia. J. Hydrol. 2010, 384, 107–117. [Google Scholar] [CrossRef]
- Razyaseef, N.; Yakir, D.; Schiller, G.; Cohen, S. Dynamics of evapotranspiration partitioning in a semi-arid forest as affected by temporal rainfall patterns. Agric. For. Meteorol. 2012, 157, 77–85. [Google Scholar] [CrossRef]
- Li, Z.H.; Yu, P.T.; Wang, Y.H.; Webb, A.A.; He, C.; Wang, Y.B.; Yang, L.L. A model coupling the effects of soil moisture and potential evaporation on the tree transpiration of a semi-arid larch plantation. Ecohydrology 2017, 1–11. [Google Scholar] [CrossRef]
- Tian, A.; Wang, Y.H.; Webb, A.A.; Liu, Z.B.; Yu, P.T.; Xiong, W.; Wang, X. Partitioning the causes of spatiotemporal variation in the sunny day sap flux density of a larch plantation on a hillslope in northwest China. J. Hydrol. 2019, 571, 503–515. [Google Scholar] [CrossRef]
- Chang, X.X.; Zhao, W.Z.; Liu, H.; Wei, X.; Liu, B.; He, Z.B. Qinghai spruce (Picea crassifolia) forest transpiration and canopy conductance in the upper Heihe River basin of arid northwestern China. Agric. For. Meteorol. 2014, 198–199, 209–220. [Google Scholar] [CrossRef]
- Piao, S.L.; Ciais, P.; Huang, Y.; Shen, Z.; Peng, S.; Li, J.; Zhou, L.; Liu, L.; Liu, H.; Ma, Y.; et al. The impacts of climate change on water resources and agriculture in China. Nature 2010, 467, 43–51. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Farahani, E., Kadner, S., Seyboth, K., Adler, A., Baum, I., Brunner, S., Eickemeier, P., et al., Eds.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Lyon, B.; Christie-Blick, N.; Gluzberg, Y. Water shortages, development, and drought in Rockland County, New York. J. Am. Water Resour. Assoc. 2005, 41, 1457–1469. [Google Scholar] [CrossRef] [Green Version]
- Li, J.B.; Cook, E.R.; D’arrigo, R.; Chen, F.H.; Gou, X.H. Moisture variability across China and Mongolia: 1951–2005. Clim. Dyn. 2009, 32, 1173–1186. [Google Scholar] [CrossRef]
- Sheffield, J.; Andreadis, K.M.; Wood, E.F.; Lettenmaier, D.P. Global and continental drought in the second half of the twentieth century: Severity-area-duration analysis and temporal variability of large-scale events. J. Clim. 2009, 22, 1962–1981. [Google Scholar] [CrossRef]
- Jackson, R.B.; Jobbagy, E.G.; Nosetto, M.D. Ecohydrology in a human-dominated landscape. Ecohydrology 2009, 2, 383–389. [Google Scholar] [CrossRef]
- Liu, Z.B.; Wang, Y.H.; Tian, A.; Webb, A.A.; Yu, P.T.; Xiong, W.; Xu, L.H.; Wang, Y.R. Modeling the response of daily evapotranspiration and its components of a larch plantation to the variation of weather, soil moisture, and canopy leaf area index. J. Geophys. Res. Atmos. 2018, 123, 7354–7374. [Google Scholar] [CrossRef]
- Herron, N.; Davis, R.; Jones, R. The effects of large-scale afforestation and climate change on water allocation in the Macquarie River catchment, NSW, Australia. J. Environ. Manag. 2002, 65, 369–381. [Google Scholar] [CrossRef]
- Wang, Y.H.; Xiong, W.; Yu, P.T.; Shen, Z.X.; Guo, M.C.; Guan, W.; Ma, C.M.; Ye, B.; Guo, H. Study on the evapotranspiration of forest and vegetation in dryland. Sci. Soil Water Conserv. 2006, 4, 19–26. [Google Scholar] [CrossRef]
- Granier, A. Evaluation of transpiration in a douglas-fir stand by means of sap flow measurements. Tree Physiol. 1987, 3, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, F.; Lindroth, A. Variation in sap flow and stem growth in relation to tree size, competition and thinning in a mixed forest of pine and spruce in Sweden. Forest Ecol. Manag. 2004, 188, 51–63. [Google Scholar] [CrossRef]
- Kume, T.; Tsuruta, K.; Komatsu, H.; Kumagai, T.; Higashi, N.; Shinohara, Y.; Otsuki, K. Effects of sample size on sap flux-based stand-scale transpiration estimates. Tree Physiol. 2010, 30, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.W.; Ouyang, L.; Zhao, P.; Zhang, C.F. Effects of size and microclimate on whole-tree water use and hydraulic regulation in Schima superba trees. PeerJ 2018, 6, e5164. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.H.; Wang, Y.H.; Yu, P.T.; Wang, Y.B.; Han, X.S.; Cao, G.X. Variation of sap flow density of Larix principis-rupprechtii with dominances and its impact on stand transpiration estimation. Forest Res. 2015, 28, 8–16. [Google Scholar] [CrossRef]
- Wan, Y.F.; Yu, P.T.; Liu, X.D.; Wang, S.L.; Wang, Y.H.; Xiong, W. Variation in sap flow density among levels of tree dominance in Picea crassifolia in the Qilian Mountains. Acta Ecol. Sin. 2017, 37, 3106–3114. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, Y.J.; Wang, Y.Q.; Zhang, S.H.; Zhao, X.L. Effects of social position and competition on tree transpiration of a natural mixed forest in Chongqing, China. Trees 2019, 33, 719–732. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, Y.H.; Yu, P.T.; Liu, H.L.; Xu, L.H.; Shi, Z.J.; Mo, F. Variation of sap flow among individual trees and scaling-up for estimation of transpiration of Larix principis-rupprechtii stand. Sci. Silva. Sin. 2008, 44, 34–40. [Google Scholar]
- Xiong, W.; Oren, R.; Wang, Y.H.; Yu, P.T.; Liu, H.L.; Cao, G.X.; Xu, L.H.; Wang, Y.N.; Zuo, H.J. Heterogeneity of competition at decameter scale: Patches of high canopy leaf area in a shade-intolerant larch stand transpire less yet are more sensitive to drought. Tree Physiol. 2015, 35, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Gartner, K.; Nadezhdina, N.; Englisch, M.; Čermak, J.; Leitgeb, E. Sap flow of birch and Norway spruce during the European heat and drought in summer 2003. Forest Ecol. Manag. 2009, 258, 590–599. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.H.; Liu, X.D.; Li, G.; Wang, S.L.; Zhao, W.J.; Ma, J. Phenology of five shrub communities along an elevation gradient in the Qilian Mountains, China. Forests 2018, 9, 58. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Yu, P.T.; Zhang, L.; Wang, Y.H.; Yu, P.T.; Wang, S.L. Differential trends of Qinghai spruce growth with elevation in Northwestern China during the recent warming hiatus. Forests 2019, 10, 712. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.F.; Yu, P.T.; Li, X.Q.; Wang, Y.H.; Wang, B.; Yu, Y.P.; Zhang, L.; Liu, X.D.; Wang, S.L. Seasonal pattern of stem diameter growth of Qinghai spruce in the Qilian mountains, northwestern China. Forests 2020, 11, 494. [Google Scholar] [CrossRef]
- Chang, X.X.; Zhao, W.Z.; He, Z.B. Radial pattern of sap flow and response to microclimate and soil moisture in Qinghai spruce (Picea crassifolia) in the upper Heihe River basin of arid northwestern china. Agric. For. Meteorol. 2014, 187, 14–21. [Google Scholar] [CrossRef]
- Kraft, G. Beiträge zur Lehre Von Den Durchforstungen, Schlagstellungen und Lichtungshieben; Klindsworth’s Verlag: Hannover, Germany, 1884. [Google Scholar]
- Kaartinen, H.; Hyyppä, J.; Yu, X.; Vastaranta, M.; Hyyppä, H.; Kukko, A. An international comparison of individual tree detection and extraction using airborne laser scanning. Remote Sens. 2012, 4, 245–273. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.S.; Hyyppä, J.; Liang, X.L.; Kaartinen, H.; Yu, X.; Lindberg, E. International benchmarking of the individual tree detection methods for modeling 3-d canopy structure for silviculture and forest ecology using airborne laser scanning. IEEE Trans. Geosci. Remote Sens. 2016, 54, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yu, P.T.; Wang, Y.H.; Wang, S.L.; Liu, X.D. Biomass change of middle aged forest of Qinghai spruce along an altitudinal gradient on the north slope of Qilian Mountains. Sci. Silva. Sin. 2015, 51, 4–10. [Google Scholar] [CrossRef]
- Wang, B.; Yu, P.T.; Wang, S.L.; Wang, Y.H.; Liu, X.D.; Jin, M.; Zhang, X.L. Response of soil water storage to slope position of forestland in Qilian Mountains. Sci. Soil Water Conserv. 2016, 14, 101–108. [Google Scholar] [CrossRef]
- López-Bernal, Á.; Alcántara, E.; Testi, L.; Villalobos, F.J.; Phillips, N. Spatial sap flow and xylem anatomical characteristics in olive trees under different irrigation regimes. Tree Physiol. 2010, 30, 1536–1544. [Google Scholar] [CrossRef] [Green Version]
- Oren, R.; Phillips, N.; Ewers, B.; Pataki, D.; Megonigal, J. Sap-flux-scaled transpiration responses to light, vapor pressure deficit, and leaf area reduction in a flooded Taxodium distichum forest. Tree Physiol. 1999, 6, 337–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinohara, Y.; Tsuruta, K.; Ogura, A.; Noto, F.; Komatsu, H.; Otsuki, K.; Maruyama, T. Azimuthal and radial variations in sap flux density and effects on stand-scale transpiration estimates in a Japanese cedar forest. Tree Physiol. 2013, 5, 550–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.N.; Zhu, J.J.; Zhang, X.; Wang, K.; Lü, L.Y.; Zhang, X.L.; Hao, G.Y. Transpiration and canopy conductance dynamics of Pinus sylvestris var. mongolica in its natural range and in an introduced region in the sandy plains of Northern China. Agric. For. Meteorol. 2020, 281. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration-Guidelines for Computing Crop Water Requirements-FAO Irrigation and Drainage Paper 56; FAO: Roma, Italy, 1998; pp. 1–15. [Google Scholar]
- Johnson, J.W.; Lebreton, J.M. History and use of relative importance indices in organizational research. Organ. Res. Methods 2004, 7, 238–257. [Google Scholar] [CrossRef]
- Lane, D.M.; Murphy, K.R.; Marques, T.E. Measuring the importance of cues in policy capturing. Organ. Behav. Hum. Perform. 1982, 30, 231–240. [Google Scholar] [CrossRef]
- Liu, J.L.; Man, X.L.; Hu, Y. Response of tree sap flow of Larix gmelinii with various differentiation classes to multiple environmental factors. For. Res. 2016, 29, 726–734. [Google Scholar] [CrossRef]
- Small, E.E.; Mcconnell, J.R. Comparison of soil moisture and meteorological controls on pine and spruce transpiration. Ecohydrology 2010, 1, 205–214. [Google Scholar] [CrossRef]
- Fiora, A.; Cescatti, A. Diurnal and seasonal variability in radial distribution of sap flux density: Implications for estimating stand transpiration. Tree Physiol. 2006, 26, 1217–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez, M.S.; Nadezhdina, N.; Cermák, J.; Morales, D. Radial variation in sap flow in five laurel forest tree species in Tenerife, Canary Islands. Tree Physiol. 2000, 20, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Kume, T.; Takizawa, H.; Yoshifuji, N.; Tanaka, K.; Tantasirin, C.; Tanaka, N.; Suzuki, M. Impact of soil drought on sap flow and water status of evergreen trees in a tropical monsoon forest in northern Thailand. For. Ecol. Manag. 2007, 238, 220–230. [Google Scholar] [CrossRef]
- Ceulemans, R.; Rombold, J.S.; Kucera, J.; Brown, K.J.; Hinckley, T.M.; Sprugel, D.G. Crown conductance and tree and stand transpiration in a second growth Abies amabilis forest. Can. J. For. Res. 1997, 27, 797–808. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.K.; Yang, G.T.; Zhang, Q.Z.; Zhou, X.F. Sapflow flux of Pinus koraiensis plantation measured by thermal dissipation probes. Sci. Silva. Sin. 2007, 43, 8–14. [Google Scholar] [CrossRef]
- Tian, Q.Y.; He, Z.B.; Xiao, S.C.; Peng, X.M.; Ding, A.J.; Lin, P.F. Response of stem radial growth of Qinghai spruce (Picea crassifolia) to environmental factors in the Qilian Mountains of China. Dendrochronologia 2017, 44, 76–83. [Google Scholar] [CrossRef]


| Canopy Layers | Number of Trees | Average Tree Height (m) | Average Diameter at Breast Height (cm) | Average Leaf Biomass (kg) | Total Sapwood Area (cm2) | Percent of Plot Sapwood Area (%) |
|---|---|---|---|---|---|---|
| Dominant | 21 | 18.1 ± 2.9 | 31.1 ± 6.2 | 24.7 ± 5.7 | 8735.32 | 41.7 |
| Codominant | 35 | 13.9 ± 2.9 | 20.1 ± 3.1 | 14.8 ± 2.3 | 6747.06 | 32.2 |
| Intermediate-suppressed | 84 | 6.7 ± 2.8 | 9.3 ± 4.1 | 5.4 ± 3.1 | 5455.45 | 26.1 |
| Canopy Layers | Tree No. | Relative Tree Height | Tree Height (m) | Diameter at Breast Height (cm) | Canopy Width (m) | Canopy Thickness (m) | Sapwood Thickness (mm) | Sapwood Area (mm2) | Leaf Biomass (kg) |
|---|---|---|---|---|---|---|---|---|---|
| Dominant | 1 | 1.93 | 20.5 | 33.55 | 4.9 | 14.3 | 51.34 | 42,277 | 29.81 |
| 2 | 1.93 | 20.5 | 22.95 | 4.8 | 17.7 | 39.39 | 21,519 | 20.86 | |
| 3 | 1.79 | 19.0 | 37.65 | 6.7 | 15.3 | 56.88 | 53,296 | 32.06 | |
| 4 | 1.74 | 18.5 | 23.45 | 5.1 | 14.5 | 39.88 | 22,296 | 20.28 | |
| 5 | 1.74 | 18.5 | 23.31 | 4.8 | 10.2 | 39.74 | 22,076 | 20.17 | |
| Codominant | 6 | 1.56 | 16.5 | 15.68 | 3.6 | 11.7 | 32.84 | 11,965 | 13.89 |
| 7 | 1.55 | 16.4 | 18.11 | 3.8 | 14.5 | 34.90 | 14,824 | 15.03 | |
| 8 | 1.51 | 16.0 | 17.61 | 4.8 | 10.8 | 34.46 | 14,211 | 14.47 | |
| 9 | 1.43 | 15.2 | 15.16 | 2.8 | 15.6 | 32.42 | 11,392 | 12.27 | |
| Intermediate-suppressed | 10 | 1.24 | 13.2 | 10.43 | 2.4 | 9.4 | 28.80 | 6741 | 8.36 |
| 11 | 1.06 | 11.2 | 12.43 | 3.8 | 6.7 | 30.28 | 8590 | 8.82 | |
| 12 | 0.72 | 7.6 | 14.32 | 3.9 | 5.3 | 31.74 | 10,494 | 8.40 | |
| 13 | 0.58 | 6.1 | 10.52 | 3.6 | 4.0 | 28.87 | 6821 | 5.67 |
| Factors | Canopy Layers | Regression Equation | Kd | R2 | P |
|---|---|---|---|---|---|
| PET | Dominant | F1 = 0.069PET − 0.003 | 0.938 | 0.880 | 0.000 |
| Codominant | F2 = 0.037PET + 0.002 | 0.893 | 0.797 | 0.000 | |
| Intermediate-suppressed | F3 = 0.014PET + 0.010 | 0.783 | 0.613 | 0.000 | |
| VSM | Dominant | F1 = 3.738VSM − 0.451 | 0.965 | 0.931 | 0.000 |
| Codominant | F2 = 2.680VSM − 0.355 | 0.954 | 0.910 | 0.000 | |
| Intermediate-suppressed | F3 = 2.143VSM − 0.310 | 0.991 | 0.980 | 0.000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, Y.; Yu, P.; Wang, Y.; Wang, B.; Yu, Y.; Wang, X.; Liu, Z.; Liu, X.; Wang, S.; Xiong, W. The Variation in Water Consumption by Transpiration of Qinghai Spruce among Canopy Layers in the Qilian Mountains, Northwestern China. Forests 2020, 11, 845. https://doi.org/10.3390/f11080845
Wan Y, Yu P, Wang Y, Wang B, Yu Y, Wang X, Liu Z, Liu X, Wang S, Xiong W. The Variation in Water Consumption by Transpiration of Qinghai Spruce among Canopy Layers in the Qilian Mountains, Northwestern China. Forests. 2020; 11(8):845. https://doi.org/10.3390/f11080845
Chicago/Turabian StyleWan, Yanfang, Pengtao Yu, Yanhui Wang, Bin Wang, Yipeng Yu, Xiao Wang, Zebin Liu, Xiande Liu, Shunli Wang, and Wei Xiong. 2020. "The Variation in Water Consumption by Transpiration of Qinghai Spruce among Canopy Layers in the Qilian Mountains, Northwestern China" Forests 11, no. 8: 845. https://doi.org/10.3390/f11080845
APA StyleWan, Y., Yu, P., Wang, Y., Wang, B., Yu, Y., Wang, X., Liu, Z., Liu, X., Wang, S., & Xiong, W. (2020). The Variation in Water Consumption by Transpiration of Qinghai Spruce among Canopy Layers in the Qilian Mountains, Northwestern China. Forests, 11(8), 845. https://doi.org/10.3390/f11080845






