Differentiation in Leaf Physiological Traits Related to Shade and Drought Tolerance Underlies Contrasting Adaptations of Two Cyclobalanopsis (Fagaceae) Species at the Seedling Stage
Abstract
1. Introduction
2. Materials and Methods
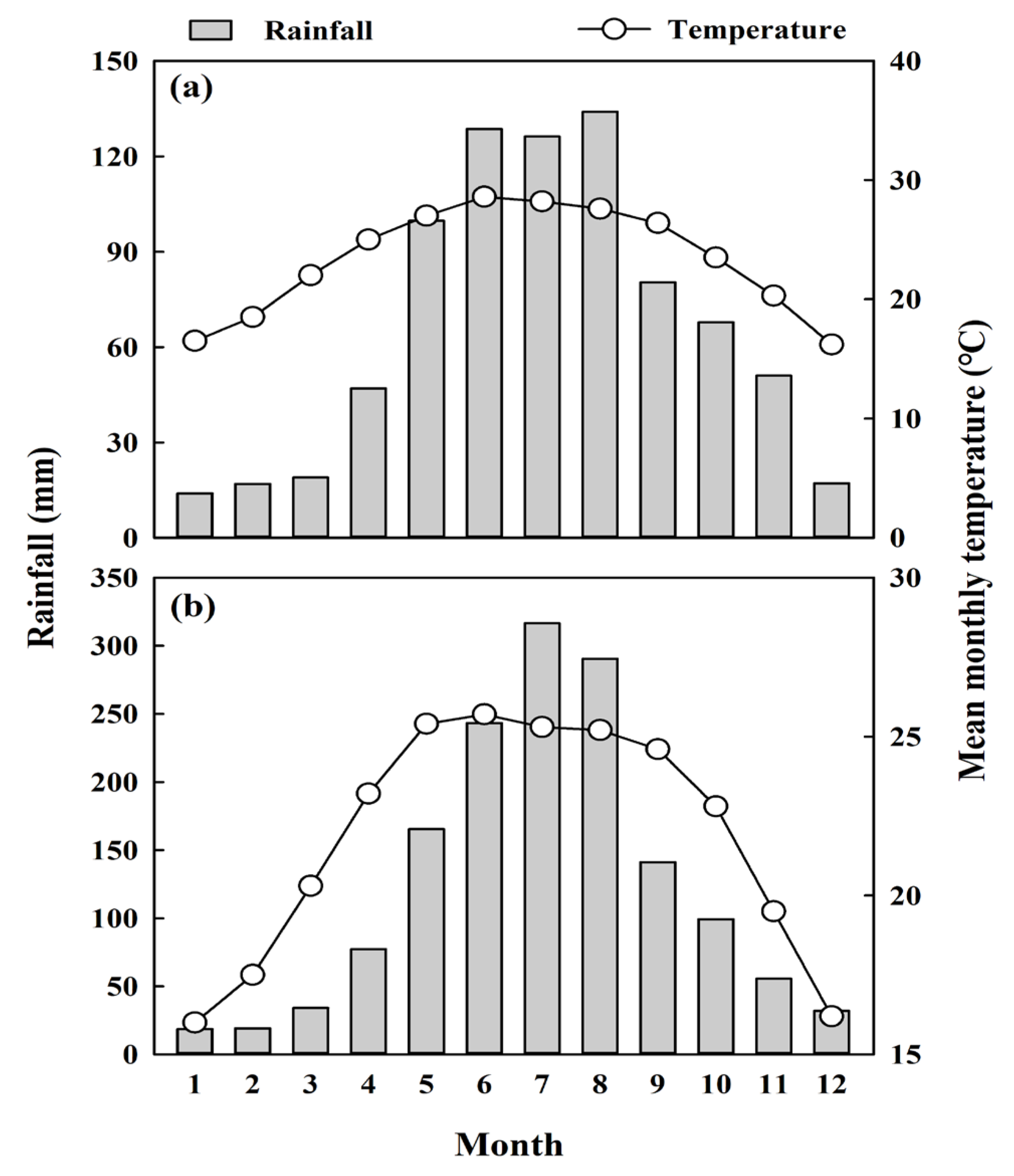
2.1. Overview of the Study Site and Species
2.2. Experimental Treatments
2.3. Measurement of Leaf Photosynthetic Parameters
2.4. Measurement of Leaf Anatomical Characteristics
2.5. Determination of Maximum Leaf Hydraulic Conductance (Kleaf)
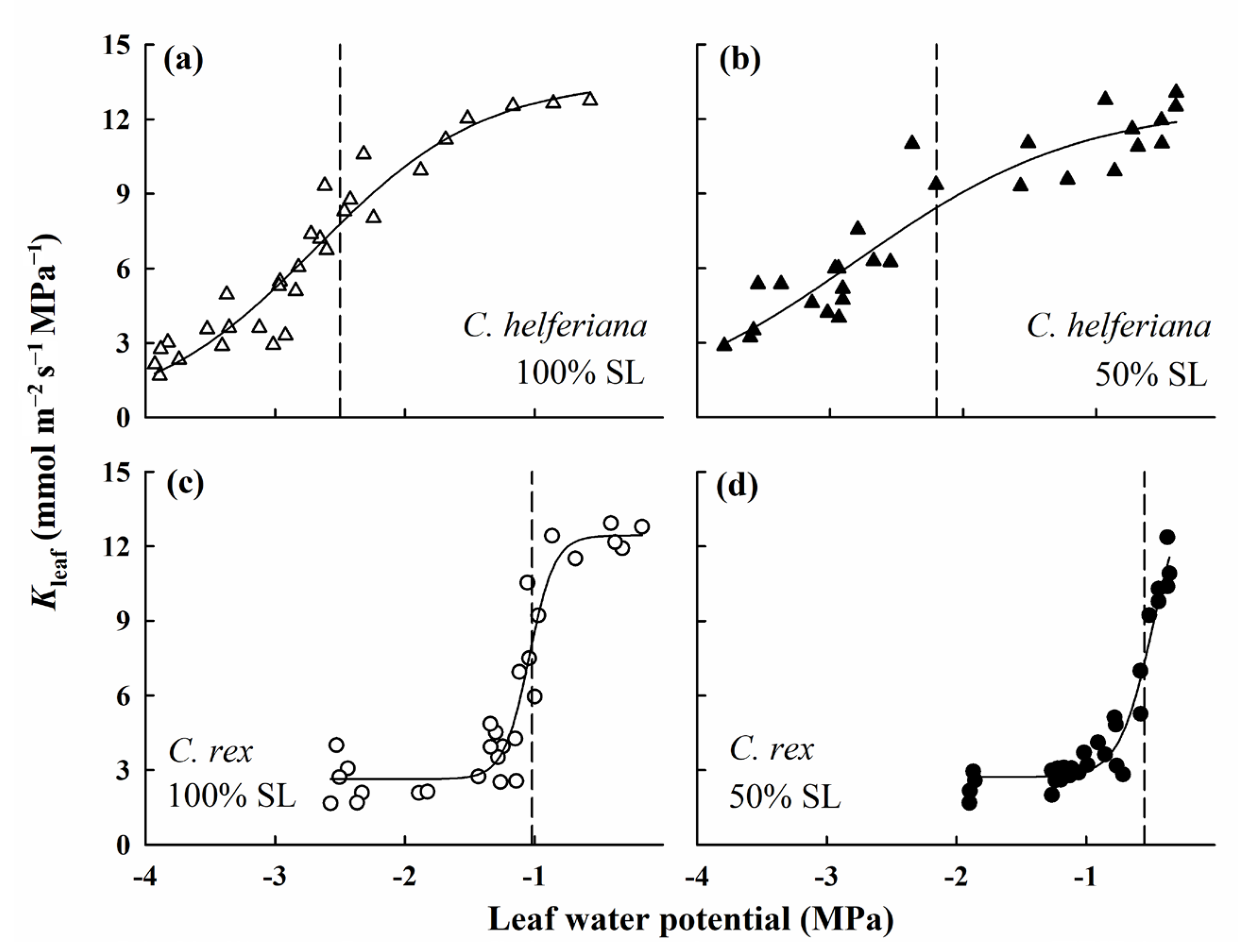
2.6. Drought Treatment and Construction of Leaf Hydraulic Vulnerability Curves
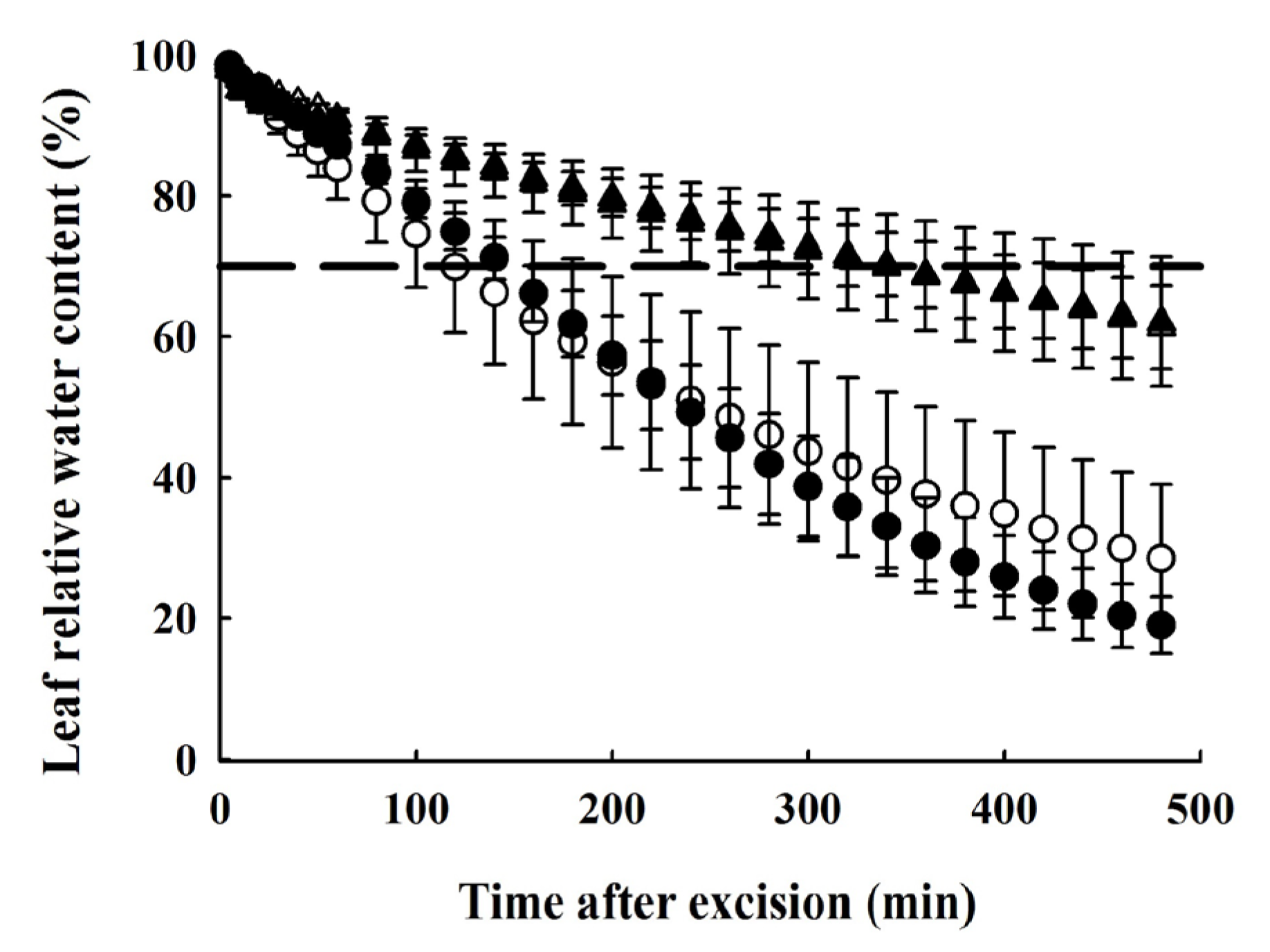
2.7. Determination of Leaf Dehydration Curves
2.8. Data Analysis
3. Results
4. Discussion
4.1. Differences in Leaf Traits and Responses to Variation in Growth Light Conditions between the Two Species
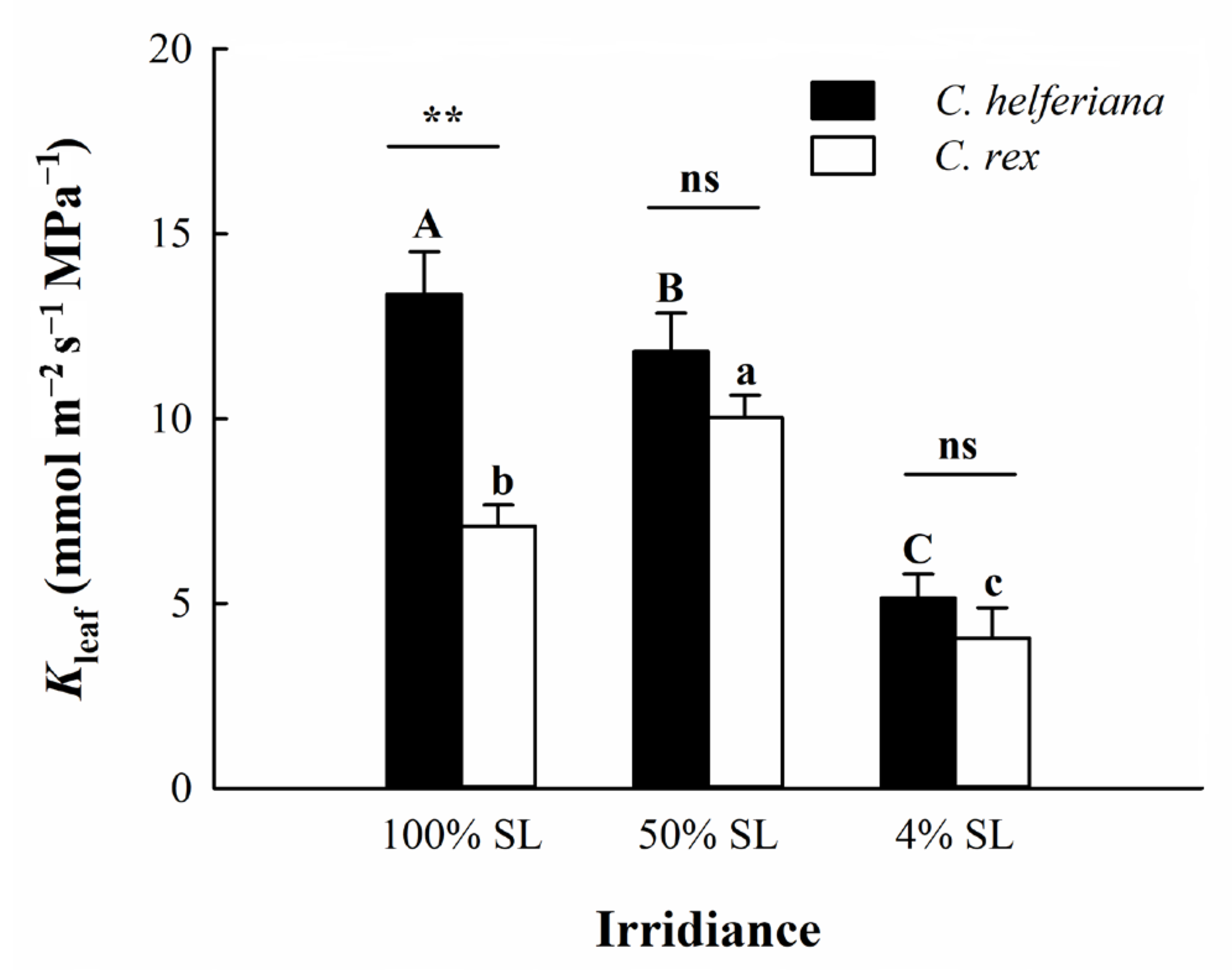
4.2. Difference in Leaf Hydraulic Conductance between the Two Species
4.3. Coordination between Leaf Hydraulic Conductance and Photosynthetic Gas Exchange
4.4. Difference in Drought Resistance and Water-Holding Capacity between the Two Species
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cavender, B.J.; Ackerly, D.D.; Baum, D.A.; Bazzaz, F.A. Phylogenetic overdispersion in Floridian oak communities. Am. Nat. 2004, 163, 823–843. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.-Y.; Hoffmann, W.A.; Scholz, F.G.; Bucci, S.J.; Meinzer, F.C.; Franco, A.C.; Meinzer, F.C.; Franco, A.C.; Cao, K.-F.; Goldstein, G. Stem and leaf hydraulics of congeneric tree species from adjacent tropical savanna and forest ecosystems. Oecologia 2008, 155, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.-Y.; Goldstein, G.; Sack, L.; Holbrook, N.M.; Liu, Z.H.; Wang, A.-Y.; Harrison, R.D.; Su, Z.H.; Cao, K.-F. Ecology of hemiepiphytism in fig species is based on evolutionary correlation of hydraulics and carbon economy. Ecology 2011, 92, 2117–2130. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.-Y.; Lucero, M.E.; Sanderson, S.C.; Zacharias, E.H.; Holbrook, N.M. Polyploidy enhances the occupation of heterogeneous environments through hydraulic related trade-offs in Atriplex canescens (Chenopodiaceae). New Phytol. 2013, 197, 970–978. [Google Scholar] [CrossRef]
- Zhang, W.-W.; Song, J.; Wang, M.; Liu, Y.Y.; Li, N.; Zhang, Y.J.; Holbrook, N.M.; Hao, G.-Y. Divergences in hydraulic architecture form an important basis for niche differentiation between diploid and polyploid Betula species in NE China. Tree Physiol. 2017, 37, 604–616. [Google Scholar] [CrossRef]
- Holmgren, M. Combined effects of shade and drought on tulip poplar seedlings: Trade-off in tolerance or facilitation? Oikos 2000, 90, 67–78. [Google Scholar] [CrossRef]
- Poorter, L.; Kitajima, K. Carbohydrate storage and light requirements of tropical moist and dry forest tree species. Ecology 2007, 88, 1000–1011. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching: A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Wang, A.-Y.; Jiang, Y.-J.; Hao, G.-Y.; Cao, K.-F. The effect of seasonal drought to plant hydraulics and photosynthesis of three dominant evergreen tree species in seasonal tropical rainforest of Xishuangbanna limestone area. Acta Botanica Yunnanica. 2008, 30, 325–332, (In Chinese with English Abstract). [Google Scholar]
- Zhang, J.L.; Hao, G.-Y.; Cao, K.-F. Phenology of woody species in Yunnan dry-hot valley in Yunnan province. J. Wuhan Bot. Res. 2009, 27, 76–82, (In Chinese with English Abstract). [Google Scholar]
- Sack, L.; Cowan, P.D.; Holbrook, N.M. The ‘hydrology’ of leaves: Co-ordination of structure and function in temperate woody species. Plant Cell Environ. 2003, 26, 1343–1356. [Google Scholar] [CrossRef]
- Myers, J.A.; Kitajima, K. Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest. J. Ecol. 2007, 95, 383–395. [Google Scholar] [CrossRef]
- Smith, T.; Huston, M. A theory of the spatial and temporal dynamics of plant communities. Vegetatio 1989, 83, 49–69. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, Ü. Shade tolerance, a key plant feature of complex nature and consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef]
- Denslow, J.S.; Hartshorn, G.S. Treefall gap environments and forest dynamic process. In LaSelva: Ecology and Natural History of a Neotropical Rain Forest; McDade, L.A., Bawa, K., Hespenheide, H., Hartshorn, G.S., Eds.; University of Chicago Press: Chicago, IL, USA, 1994; pp. 120–127. [Google Scholar] [CrossRef]
- Fetcher, N.; Oberbauer, S.F.; Chazdon, R.L. Physiological ecology of plants at La Selva. In La Selva: Ecology and Natural History of a Neotropical Forest; McDale, L., Bawa, K.S., Hespenheide, H., Hartson, G., Eds.; University of Chicago Press: Chicago, IL, USA, 1994; pp. 128–141. [Google Scholar] [CrossRef]
- Chazdon, R.L. Sunflecks in the forest understory. Adv. Ecol. Res. 1988, 18, 1–63. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Pearcy, R.; Lee, D.; Fetcher, D. Photosynthetic responses of tropical plants to contrasting light environments. In Tropical Forest Plant Ecophysiology; Mulkey, S.S., Chazdon, R.L., Smith, A.P., Eds.; Springer: Boston, MA, USA, 1996; pp. 5–55. [Google Scholar] [CrossRef]
- Clark, D.B.; Clark, D.A.; Rich, P.M.; Weiss, S.; Oberbauer, S.F. Landscape-scale evaluation of understory light and canopy structures: Methods and application in a neotropical lowland rain forest. Can. J. For. Res. 1996, 26, 747–757. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Physiological Plant Ecology, 4th ed.; Springer: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Poorter, L. Growth and carbon partitioning of tropical tree seedlings in contrasting light environments. In Inherent Variation in Plant Growth: Physiological Mechanisms and Ecological Consequences; Lambers, H., Poorter, H., Van Vuuren, M.M.I., Eds.; Backhuys Publsihers: Leiden, The Netherlands, 1998; pp. 337–362. [Google Scholar]
- Walters, M.B.; Reich, P.B. Low-light carbon balance and shade tolerance in the seedlings of woody plants: Do winter deciduous and broad-leaved evergreen species differ? New Phytol. 1999, 143, 143–154. [Google Scholar] [CrossRef]
- Urli, M.; Porte, A.J.; Cochard, H.; Guengant, Y.; Burlett, R.; Delzon, S. Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiol. 2013, 33, 672–683. [Google Scholar] [CrossRef]
- Lucani, C.J.; Brodribb, T.J.; Jordan, G.J.; Mitchell, P.J. Juvenile and adult leaves of heteroblastic Eucalyptus globulus vary in xylem vulnerability. Trees-Struct. Funct. 2019, 33, 1167–1178. [Google Scholar] [CrossRef]
- Nardini, A.; Salleo, S. Limitation of stomatal conductance by hydraulic traits: Sensing or preventing xylem cavitation? Trees-Struct. Funct. 2000, 15, 14–24. [Google Scholar] [CrossRef]
- Sack, L.; Tyree, M.T. Leaf hydraulics and its implications in plant structure and function. In Vascular Transport in Plants; Holbrook, N.M., Zweiniecki, M.A., Eds.; Elsevier/Academic Press: Oxford, UK, 2005; pp. 93–114. [Google Scholar] [CrossRef]
- Zhu, S.-D.; Liu, H.; Xu, Q.-Y.; Cao, K.-F.; Ye, Q. Are leaves more vulnerable to cavitation than branches? Funct. Ecol. 2016, 30, 1740–1744. [Google Scholar] [CrossRef]
- Sack, L.; Tyree, M.T.; Holbrook, N.M. Leaf hydraulic architecture correlates with regeneration irradiance in tropical rainforest trees. New Phytol. 2005, 167, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Sack, L.; Frole, K. Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. Ecology 2006, 87, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.-Y.; Sack, L.; Wang, A.-Y.; Cao, K.-F.; Goldstein, G. Differentiation of leaf water flux and drought tolerance traits in hemiepiphytic and non-hemiepiphytic Ficus tree species. Funct. Ecol. 2010, 24, 731–740. [Google Scholar] [CrossRef]
- Huang, C.C.; Zhang, Y.T.; Bartholomew, B. Flora of China; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 1999; Volume 4. [Google Scholar]
- Bassman, J.H.; Zwier, J.C. Gas exchange characteristics of Populus trichocarpa, Populus deltoides and Populus trichocarpa × P. deltoides clones. Tree Physiol. 1991, 8, 145–159. [Google Scholar] [CrossRef]
- Franks, P.J. Higher rates of leaf gas exchange are associated with higher leaf hydrodynamic pressure gradients. Plant Cell Environ. 2006, 29, 584–592. [Google Scholar] [CrossRef]
- Scholander, P.F.; Hammel, H.T.; Brandstreet, E.D. Sap pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Muchow, R.C.; Sinclair, T.R. Epidermal conductance, stomatal density and stomatal size among genotypes of Sorghum bicolor (L.) Moench. Plant Cell Environ. 1989, 12, 425–431. [Google Scholar] [CrossRef]
- Zhang, J.L. Phenology, Leaf Structure and Function, and Seasonal Variation in Photosynthesis of Woody Plants in a Dry-hot Valley of Yuanjiang, Southwestern China. Ph.D. Thesis, Chinese Academy of Sciences, Beijing, China, 2007. (In Chinese with English Abstract). [Google Scholar]
- Pathare, V.S.; Koteyeva, N.; Cousins, A.B. Increased adaxial stomatal density is associated with greater mesophyll surface area exposed to intercellular air spaces and mesophyll conductance in diverse C-4 grasses. New Phytol. 2020, 225, 169–182. [Google Scholar] [CrossRef]
- McCulloh, K.A.; Sperry, J.S.; Adler, F.R. Water transport in plants obeys Murray’s law. Nature 2003, 421, 939–942. [Google Scholar] [CrossRef]
- Song, J.; Yang, D.; Niu, C.-Y.; Zhang, W.-W.; Wang, M.; Hao, G.-Y. Correlation between leaf size and hydraulic architecture in five compound-leaved tree species of a temperate forest in NE China. For. Ecol. Manag. 2018, 418, 63–72. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, Y.-J.; Song, J.; Niu, C.-Y.; Hao, G.-Y. Compound leaves are associated with high hydraulic conductance and photosynthetic capacity: Evidence from trees in Northeast China. Tree Physiol. 2019, 39, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Meinzer, F.C. Co-ordination of vapour and liquid phase water transport properties in plants. Plant Cell Environ. 2002, 25, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Santiago, L.S.; Goldstein, G.; Meinzer, F.C.; Fisher, J.B.; Machado, K.; Woodruff, D.; Jones, T. Leaf photosynthetic traits scale with hydraulic conductivity and wood density in Panamanian forest canopy trees. Oecologia 2004, 140, 543–550. [Google Scholar] [CrossRef]
- Hao, G.-Y.; Wang, A.-Y.; Liu, Z.-H.; Franco, A.C.; Goldstein, G.; Cao, K.-F. Differentiation in light energy dissipation between hemiepiphytic and non-hemiepiphytic Ficus species with contrasting xylem hydraulic conductivity. Tree Physiol. 2011, 31, 626–636. [Google Scholar] [CrossRef]
- Zhu, J.-J.; Zhang, J.-L.; Liu, H.-C.; Cao, K.-F. Photosynthesis, non-photochemical pathways and activities of antioxidant enzymes in a resilient evergreen oak under different climatic conditions from a valley-savanna in Southwest China. Physiol. Plantarum. 2009, 135, 62–72. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Prat, E.; Oliveras, I.; Piñol, J. Xylem hydraulic properties of roots and stems of nine Mediterranean woody species. Oecologia 2002, 133, 19–29. [Google Scholar] [CrossRef]
- Sperry, J.S.; Meinzer, F.C.; McCulloh, K.A. Safety and efficiency conflicts in hydraulic architecture: Scaling from tissues to trees. Plant Cell Environ. 2008, 31, 632–645. [Google Scholar] [CrossRef]
- Manzoni, S.; Vico, G.; Katul, G.; Palmroth, S.; Jackson, R.B.; Porporato, A. Hydraulic limits on maximum plant transpiration and the emergence of the safety-efficiency trade-off. New Phytol. 2013, 198, 169–178. [Google Scholar] [CrossRef]
- Chen, J.-W.; Zhang, Q.; Li, X.-S.; Cao, K.-F. Independence of stem and leaf hydraulic traits in six Euphorbiaceae tree species with contrasting leaf phenology. Planta 2009, 230, 459–468. [Google Scholar] [CrossRef]


| Title | Irradiance | Amax (μmol m−2 s−1) | LCP (μmol m−2 s−1) | LSP (μmol m−2 s−1) | Rd (μmol m−2 s−1) |
|---|---|---|---|---|---|
| C. helferiana | 100% | 9.98 ± 1.65Aa | 19.4 ± 2.64Aa | 517.1 ± 35.2Aa | 0.47 ± 0.10Ba |
| 50% | 7.52 ± 0.98Ab | 7.13 ± 0.76Ab | 336.7 ± 15.5Ab | 0.33 ± 0.03Aa | |
| 4% | 5.52 ± 0.34Ac | 7.70 ± 0.65Ab | 232.4 ± 9.4Ac | 0.45 ± 0.02Aa | |
| C. rex | 100% | 4.82 ± 0.68Bb | 12.3 ± 0.90Ba | 325.4 ± 8.2Ba | 0.96 ± 0.10Aa |
| 50% | 7.05 ± 0.44Aa | 6.58 ± 1.27Ab | 227.6 ± 7.2Bb | 0.40 ± 0.03Ab | |
| 4% | 1.78 ± 0.12Bc | 5.98 ± 1.01Ab | 59.7 ± 3.3Bc | 0.45 ± 0.01Ab |
| Title | Irradiance | Palisade Thickness (μm) | Spongy Thickness (μm) | Palisade/ Spongy | SD (mm−2) | GCL (μm) | LMA (g m−2) |
|---|---|---|---|---|---|---|---|
| C. helferiana | 100% | 32.91 ± 1.43Aa | 26.7 ± 2.2Aab | 0.81 ± 0.03Bb | 386 ± 27.1Ba | 28.2 ± 0.9Ab | 159 ± 8.1Aa |
| 50% | 29.54 ± 0.62Aa | 28.4 ± 2.7Aa | 0.96 ± 0.18Bab | 487 ± 60.1Ba | 22.8 ± 0.9Aa | 116 ± 6.3Ab | |
| 4% | 19.94 ± 1.27Ab | 22.7 ± 8.4Abc | 1.14 ± 0.17Ba | 411 ± 4.6 Ba | 23.6 ± 0.9Aa | 62.5 ± 1.8Ac | |
| C. rex | 100% | 26.64 ± 1.20Aa | 15.2 ± 1.3Ba | 0.57 ± 0.01Ab | 955 ± 44.2Aa | 13.9 ± 0.5Ba | 104 ± 2.1Ba |
| 50% | 26.29 ± 1.18Aa | 15.8 ± 1.7Ba | 0.60 ± 0.07Ab | 743 ± 29.1Ab | 13.6 ± 0.2Ba | 96.9 ± 3.3Aa | |
| 4% | 18.21 ± 0.92Ab | 15.8 ± 2.0Aa | 0.87 ± 0.03Aa | 535 ± 17.7Ac | 13.9 ± 0.5Ba | 82.3 ± 4.8Ab |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.-Y.; Hao, G.-Y.; Guo, J.-J.; Liu, Z.-H.; Zhang, J.-L.; Cao, K.-F. Differentiation in Leaf Physiological Traits Related to Shade and Drought Tolerance Underlies Contrasting Adaptations of Two Cyclobalanopsis (Fagaceae) Species at the Seedling Stage. Forests 2020, 11, 844. https://doi.org/10.3390/f11080844
Wang A-Y, Hao G-Y, Guo J-J, Liu Z-H, Zhang J-L, Cao K-F. Differentiation in Leaf Physiological Traits Related to Shade and Drought Tolerance Underlies Contrasting Adaptations of Two Cyclobalanopsis (Fagaceae) Species at the Seedling Stage. Forests. 2020; 11(8):844. https://doi.org/10.3390/f11080844
Chicago/Turabian StyleWang, Ai-Ying, Guang-You Hao, Jing-Jing Guo, Zhi-Hui Liu, Jiao-Lin Zhang, and Kun-Fang Cao. 2020. "Differentiation in Leaf Physiological Traits Related to Shade and Drought Tolerance Underlies Contrasting Adaptations of Two Cyclobalanopsis (Fagaceae) Species at the Seedling Stage" Forests 11, no. 8: 844. https://doi.org/10.3390/f11080844
APA StyleWang, A.-Y., Hao, G.-Y., Guo, J.-J., Liu, Z.-H., Zhang, J.-L., & Cao, K.-F. (2020). Differentiation in Leaf Physiological Traits Related to Shade and Drought Tolerance Underlies Contrasting Adaptations of Two Cyclobalanopsis (Fagaceae) Species at the Seedling Stage. Forests, 11(8), 844. https://doi.org/10.3390/f11080844






