Estimation of Chilling and Heat Accumulation Periods Based on the Timing of Olive Pollination
Abstract
1. Introduction
2. Material and Methods
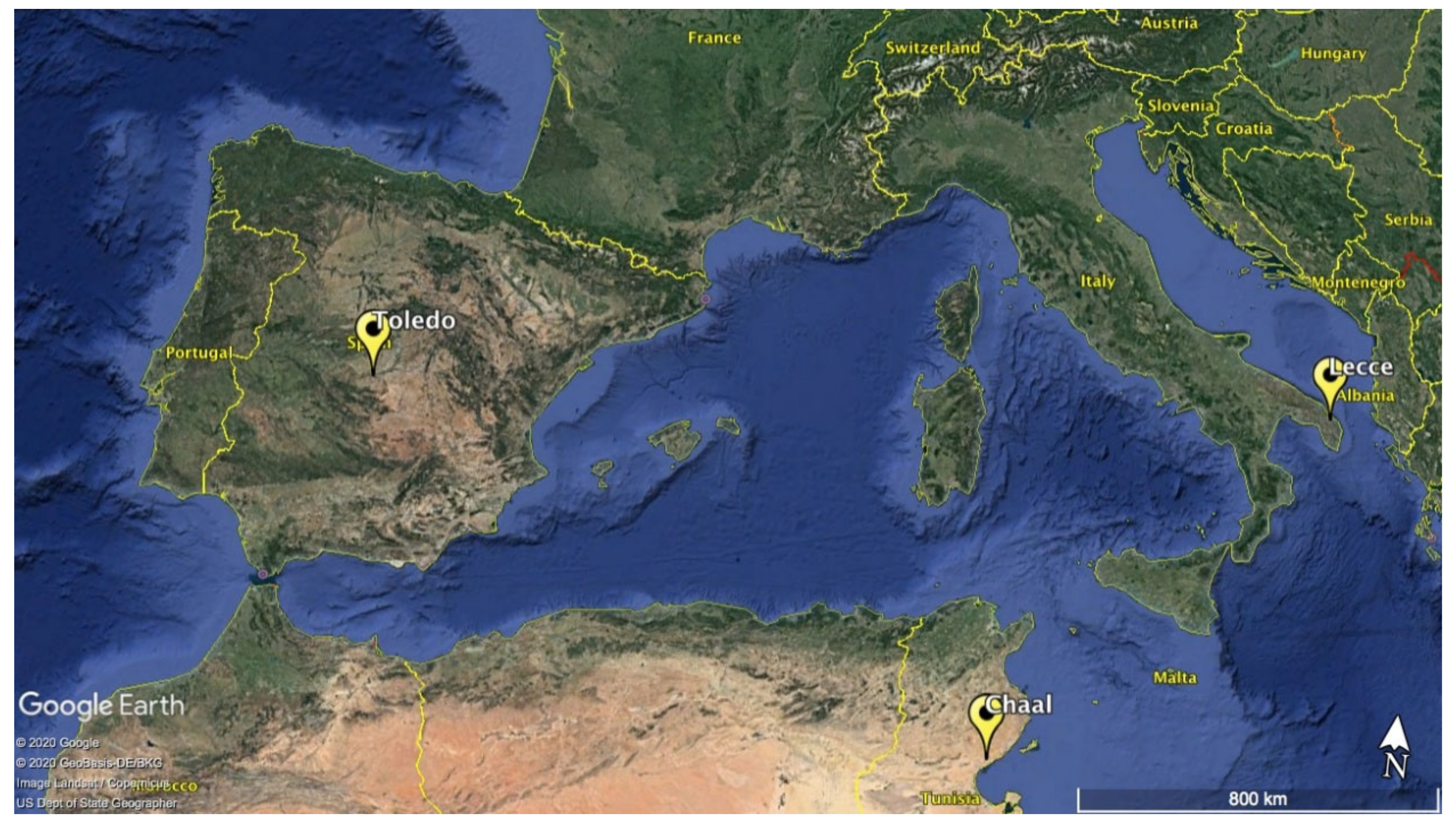
2.1. Phenological Data
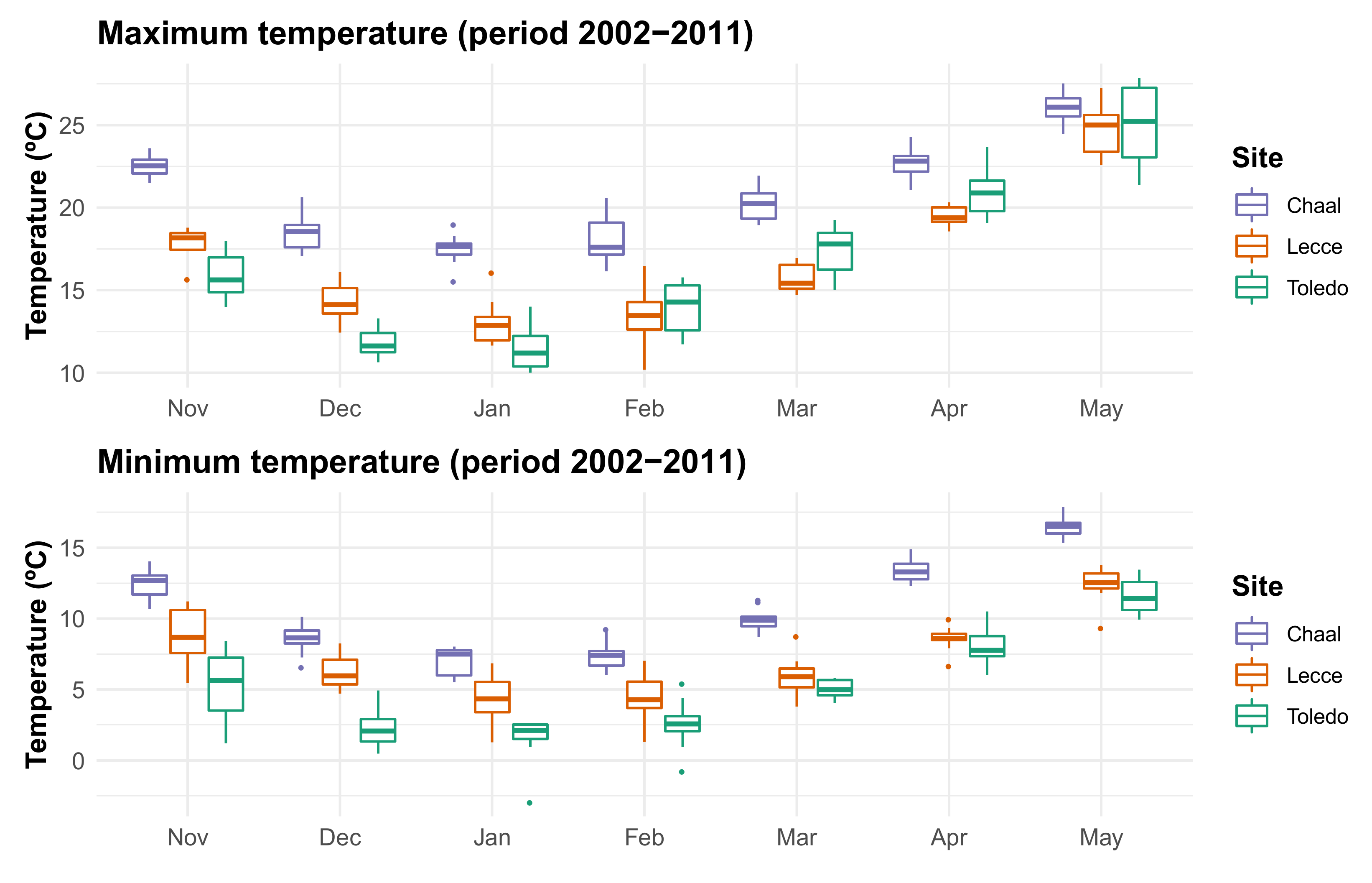
2.2. Meteorological Data
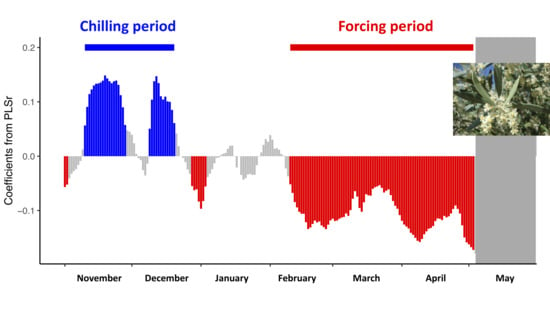
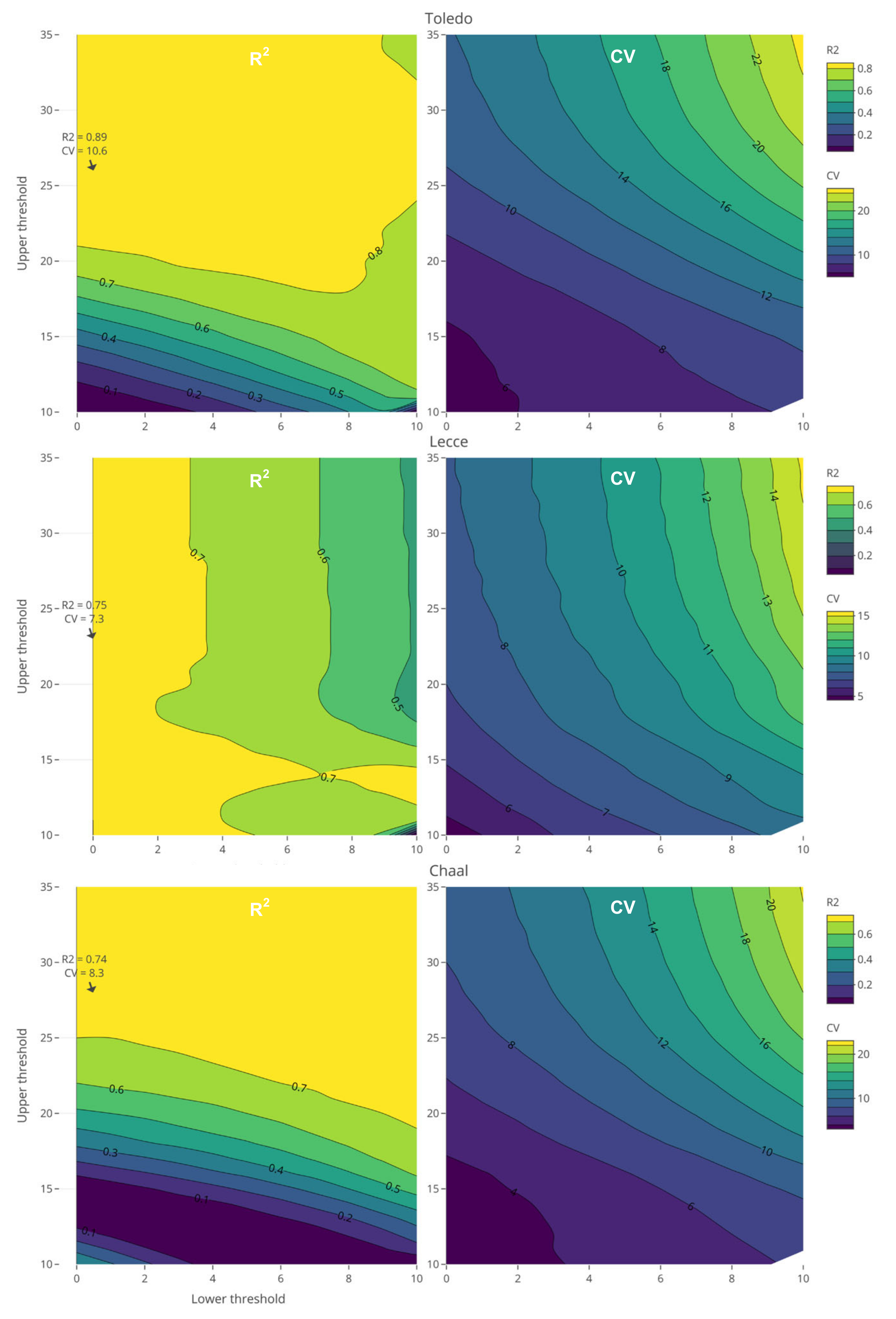
2.3. Chilling and Heat Accumulation Periods
2.4. Chilling and Heat Models
3. Results and Discussion
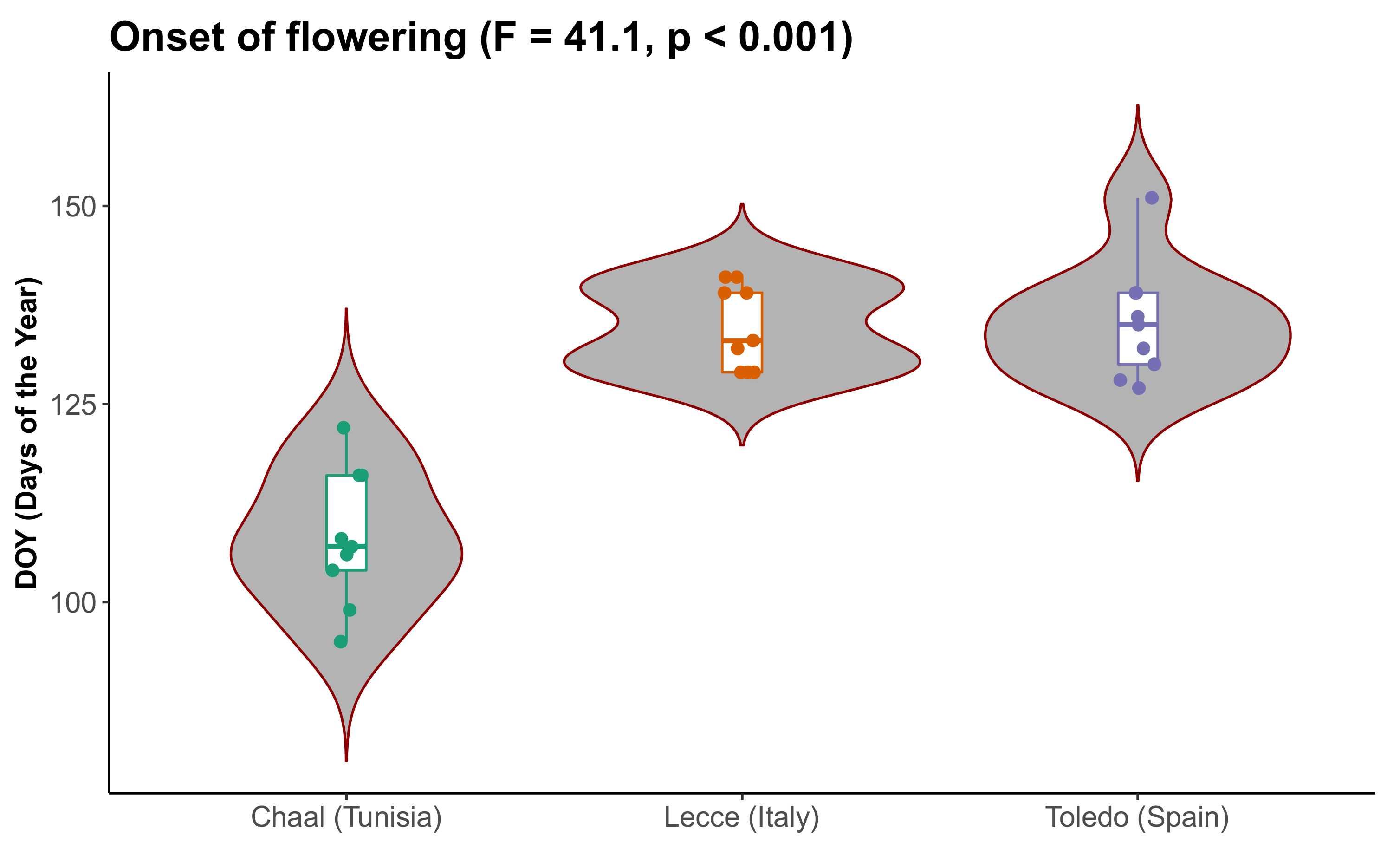
3.1. Flowering of Olive Trees
3.2. Estimation of the Thermal Accumulation Periods
3.3. Calculation of the Thermal Requirements
3.4. Predictive Models and Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bonofiglio, T.; Orlandi, F.; Ruga, L.; Romano, B.; Fornaciari, M. Climate change impact on the olive pollen season in Mediterranean areas of Italy: Air quality in late spring from an allergenic point of view. Environ. Monit. Assess. 2013, 185, 877–890. [Google Scholar] [CrossRef]
- Rojo, J.; Pérez-Badia, R. Models for forecasting the flowering of Cornicabra olive groves. Int. J. Biometeorol. 2015, 59, 1547–1556. [Google Scholar] [CrossRef]
- Orlandi, F.; Garcia-Mozo, H.; Dhiab, A.B.; Galán, C.; Msallem, M.; Romano, B.; Abichou, M.; Dominguez-Vilches, E.; Fornaciari, M. Climatic indices in the interpretation of the phenological phases of the olive in mediterranean areas during its biological cycle. Clim. Chang. 2013, 116, 263–284. [Google Scholar] [CrossRef]
- Ramos, A.; Rapoport, H.F.; Cabello, D.; Rallo, L. Chilling accumulation, dormancy release temperature, and the role of leaves in olive reproductive budburst: Evaluation using shoot explants. Sci. Hortic. 2018, 231, 241–252. [Google Scholar] [CrossRef]
- Alburquerque, N.; García-Montiel, F.; Carrillo, A.; Burgos, L. Chilling and heat requirements of sweet cherry cultivars and the relationship between altitude and the probability of satisfying the chill requirements. Environ. Exp. Bot. 2008, 64, 162–170. [Google Scholar] [CrossRef]
- Darbyshire, R.; Pope, K.; Goodwin, I. An evaluation of the chill overlap model to predict flowering time in apple tree. Sci. Hortic. 2016, 198, 142–149. [Google Scholar] [CrossRef]
- Guo, L.; Dai, J.; Ranjitkar, S.; Yu, H.; Xu, J.; Luedeling, E. Chilling and heat requirements for flowering in temperate fruit trees. Int. J. Biometeorol. 2014, 58, 1195–1206. [Google Scholar] [CrossRef]
- Wenden, B.; Mariadassou, M. Sweet cherry phenology in the context of climate change: A systems biology approach. Acta Hortic. 2017, 31–38. [Google Scholar] [CrossRef]
- Fabbri, A.; Benelli, C. Review Article Flower bud induction and differentiation in olive. J. Hortic. Sci. Biotechnol. 2000, 75, 131–141. [Google Scholar] [CrossRef]
- Fernandez-Escobar, R.; Benlloch, M.; Navarro, C.; Martin, G.C. The time of floral induction in the olive. J. Am. Soc. Hortic. Sci. 1992, 117, 304–307. [Google Scholar] [CrossRef]
- Connor, D.J.; Fereres, E. The Physiology of Adaptation and Yield Expression in Olive. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Oxford, UK, 2010; ISBN 978-0-470-65088-2. [Google Scholar]
- Campoy, J.A.; Ruiz, D.; Egea, J. Dormancy in temperate fruit trees in a global warming context: A review. Sci. Hortic. 2011, 130, 357–372. [Google Scholar] [CrossRef]
- Picornell, A.; Buters, J.; Rojo, J.; Traidl-Hoffmann, C.; Menzel, A.; Bergmann, K.C.; Werchan, M.; Schmidt-Weber, C.; Oteros, J. Predicting the start, peak and end of the Betula pollen season in Bavaria, Germany. Sci. Total Environ. 2019, 690, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Hänninen, H.; Tanino, K. Tree seasonality in a warming climate. Trends Plant Sci. 2011, 16, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Luedeling, E. Climate change impacts on winter chill for temperate fruit and nut production: A review. Sci. Hortic. 2012, 144, 218–229. [Google Scholar] [CrossRef]
- Lang, G.A. Endo-, para-, and ecodormancy: Physiological terminology and classification for dormancy research. HortScience 1987, 22, 371–377. [Google Scholar]
- Orlandi, F.; Garcia-Mozo, H.; Ezquerra, L.V.; Romano, B.; Dominguez, E.; Galan, C.; Fornaciari, M. Phenological olive chilling requirements in Umbria (Italy) and Andalusia (Spain). Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2004, 138, 111–116. [Google Scholar] [CrossRef]
- Rallo, L.; Martin, G.C. The role of chilling and releasing olive floral buds from dormancy. HortScience 1991, 26, 1058–1062. [Google Scholar] [CrossRef]
- de Melo-Abreu, J.P.; Barranco, D.; Cordeiro, A.M.; Tous, J.; Rogado, B.M.; Villalobos, F.J. Modelling olive flowering date using chilling for dormancy release and thermal time. Agric. For. Meteorol. 2004, 125, 117–127. [Google Scholar] [CrossRef]
- Alcalá, A.R.; Barranco, D. Prediction of Flowering Time in Olive for the Cordoba Olive Collection. HortScience 1992, 27, 1205–1207. [Google Scholar] [CrossRef]
- Aguilera, F.; Fornaciari, M.; Ruiz-Valenzuela, L.; Galán, C.; Msallem, M.; Dhiab, A.B.; la Guardia, C.D.; del Mar-Trigo, M.; Bonofiglio, T.; Orlandi, F. Phenological models to predict the main flowering phases of olive (Olea europaea L.) along a latitudinal and longitudinal gradient across the Mediterranean region. Int. J. Biometeorol. 2015, 59, 629–641. [Google Scholar] [CrossRef]
- Rojo, J.; Pérez-Badia, R. Effects of topography and crown-exposure on olive tree phenology. Trees 2014, 28, 449–459. [Google Scholar] [CrossRef]
- Kumral, N.A.; Kovanci, B.; Akbudak, B. Pheromone trap catches of the olive moth, Prays oleae (Bern.) (Lep., Plutellidae) in relation to olive phenology and degree-day models. J. Appl. Entomol. 2005, 129, 375–381. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Hadley, P.; Ordidge, M.; Xu, X.; Luedeling, E. Delayed chilling appears to counteract flowering advances of apricot in southern UK. Agric. For. Meteorol. 2017, 237, 209–218. [Google Scholar] [CrossRef]
- Orlandi, F.; Vazquez, L.M.; Ruga, L.; Bonofiglio, T.; Fornaciari, M.; Garcia-Mozo, H.; Domínguez, E.; Romano, B.; Galan, C. Bioclimatic requirements for olive flowering in two Mediterranean regions located at the same latitude (Andalucia, Spain, and Sicily, Italy). Ann. Agric. Environ. Med. 2005, 12, 47. [Google Scholar] [PubMed]
- Fadón, E.; Rodrigo, J.; Herrero, M. Is there a specific stage to rest? Morphological changes in flower primordia in relation to endodormancy in sweet cherry (Prunus avium L.). Trees 2018, 32, 1583–1594. [Google Scholar] [CrossRef]
- Luedeling, E.; Kunz, A.; Blanke, M.M. Identification of chilling and heat requirements of cherry trees—A statistical approach. Int. J. Biometeorol. 2013, 57, 679–689. [Google Scholar] [CrossRef]
- Luedeling, E.; Gassner, A. Partial Least Squares Regression for analyzing walnut phenology in California. Agric. For. Meteorol. 2012, 158, 43–52. [Google Scholar] [CrossRef]
- Benmoussa, H.; Luedeling, E.; Ghrab, M.; Ben-Yahmed, J.; Ben-Mimoun, M. Performance of pistachio (Pistacia vera L.) in warming Mediterranean orchards. Environ. Exp. Bot. 2017, 140, 76–85. [Google Scholar] [CrossRef]
- Benmoussa, H.; Ghrab, M.; Ben-Mimoun, M.; Luedeling, E. Chilling and heat requirements for local and foreign almond (Prunus dulcis Mill.) cultivars in a warm Mediterranean location based on 30 years of phenology records. Agric. For. Meteorol. 2017, 239, 34–46. [Google Scholar] [CrossRef]
- Guo, L.; Dai, J.; Ranjitkar, S.; Xu, J.; Luedeling, E. Response of chestnut phenology in China to climate variation and change. Agric. For. Meteorol. 2013, 180, 164–172. [Google Scholar] [CrossRef]
- Luedeling, E.; Guo, L.; Dai, J.; Leslie, C.; Blanke, M.M. Differential responses of trees to temperature variation during the chilling and forcing phases. Agric. For. Meteorol. 2013, 181, 33–42. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Kizildeniz, T.; Vučetić, V.; Dai, Z.; Luedeling, E.; van Leeuwen, C.; Gomès, E.; Pascual, I.; Irigoyen, J.J.; Morales, F.; et al. Sensitivity of Grapevine Phenology to Water Availability, Temperature and CO2 Concentration. Front. Environ. Sci. 2016, 4. [Google Scholar] [CrossRef]
- el Yaacoubi, A.; Malagi, G.; Oukabli, A.; Hafidi, M.; Legave, J.-M. Global warming impact on floral phenology of fruit trees species in Mediterranean region. Sci. Hortic. 2014, 180, 243–253. [Google Scholar] [CrossRef]
- Navas-Lopez, J.F.; León, L.; Trentacoste, E.R.; de la Rosa, R. Multi-environment evaluation of oil accumulation pattern parameters in olive. Plant Physiol. Biochem. 2019, 139, 485–494. [Google Scholar] [CrossRef]
- Rivas-Martínez, S.; Penas, Á.; del Río, S.; González, T.E.D.; Rivas-Sáenz, S. Bioclimatology of the Iberian Peninsula and the Balearic Islands. In The Vegetation of the Iberian Peninsula; Springer: Berlin, Germany, 2017; pp. 29–80. [Google Scholar]
- Rivas-Martínez, S.; Rivas-Saenz, S.; Penas, A. Worldwide bioclimatic classfication system. Glob. Geobot. 2011, 1, 1–634. [Google Scholar]
- Oteros, J.; García-Mozo, H.; Hervás, C.; Galán, C. Biometeorological and autoregressive indices for predicting olive pollen intensity. Int. J. Biometeorol. 2013, 57, 307–316. [Google Scholar] [CrossRef]
- Hirst, J.M. An automatic volumetric spore trap. Ann. Appl. Biol. 1952, 39, 257–265. [Google Scholar] [CrossRef]
- Cour, P. Nouvelles techniques de d’etection des flux et des retombées polliniques: Étude de la sedimentation des pollens et des spores à la surface du sol. Pollen Spores 1974, 16, 103–141. [Google Scholar]
- Orlandi, F.; Oteros, J.; Aguilera, F.; Ben-Dhiab, A.; Msallem, M.; Fornaciari, M. Design of a downscaling method to estimate continuous data from discrete pollen monitoring in Tunisia. Environ. Sci. Process. Impacts 2014, 16, 1716–1725. [Google Scholar] [CrossRef]
- Rojo, J.; Pérez-Badia, R. Spatiotemporal analysis of olive flowering using geostatistical techniques. Sci. Total Environ. 2015, 505, 860–869. [Google Scholar] [CrossRef]
- Ribeiro, H.; Cunha, M.; Abreu, I. Definition of Main Pollen Season Using a Logistic Model. Ann. Agric. Environ. Med. 2007, 14, 259–264. [Google Scholar] [PubMed]
- Cunha, M.; Ribeiro, H.; Costa, P.; Abreu, I. A comparative study of vineyard phenology and pollen metrics extracted from airborne pollen time series. Aerobiologia 2015, 31, 45–56. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 16 October 2019).
- Rojo, J.; Picornell, A.; Oteros, J. AeRobiology: The computational tool for biological data in the air. Methods Ecol. Evol. 2019, 1371–1376. [Google Scholar] [CrossRef]
- Almorox, J.; Hontoria, C.; Benito, M. Statistical validation of daylength definitions for estimation of global solar radiation in Toledo, Spain. Energy Convers. Manag. 2005, 46, 1465–1471. [Google Scholar] [CrossRef]
- Linvill, D.E. Calculating chilling hours and chill units from daily maximum and minimum temperature observations. HortScience 1990, 25, 14–16. [Google Scholar] [CrossRef]
- Linvill, D.E. Using maximum and minimum temperatures to determine chilling completion. In Proceedings of the II International Peach Symposium 254, Clemson, SC, USA, 19–23 June 1988; pp. 249–254. [Google Scholar]
- Weinberger, J.H. Chilling requirements of peach varieties. In Proceedings of the American Society for Horticultural Science; American Society for Horticultural Science: Alexandria, VA, USA, 1950; Volume 56, pp. 122–128. [Google Scholar]
- Gabaldón-Leal, C.; Ruiz-Ramos, M.; de la Rosa, R.; León, L.; Belaj, A.; Rodríguez, A.; Santos, C.; Lorite, I.J. Impact of changes in mean and extreme temperatures caused by climate change on olive flowering in southern Spain. Int. J. Climatol. 2017, 37, 940–957. [Google Scholar] [CrossRef]
- Richardson, E.A. A model for estimating the completion of rest for ’Redhaven’ and ’Elberta’ peach trees. Hortscience 1974, 9, 331–332. [Google Scholar]
- Fishman, S.; Erez, A.; Couvillon, G.A. The temperature dependence of dormancy breaking in plants: Mathematical analysis of a two-step model involving a cooperative transition. J. Theor. Biol. 1987, 124, 473–483. [Google Scholar] [CrossRef]
- Darbyshire, R.; Webb, L.; Goodwin, I.; Barlow, S. Winter chilling trends for deciduous fruit trees in Australia. Agric. For. Meteorol. 2011, 151, 1074–1085. [Google Scholar] [CrossRef]
- Anderson, J.L.; Richardson, E.A.; Kesner, C.D. Validation of chill unit and flower bud phenology models for “Montmorency” sour cherry. Acta Hortic. 1986, 71–78. [Google Scholar] [CrossRef]
- Aguilera, F.; Ruiz, L.; Fornaciari, M.; Romano, B.; Galán, C.; Oteros, J.; Dhiab, A.B.; Msallem, M.; Orlandi, F. Heat accumulation period in the Mediterranean region: Phenological response of the olive in different climate areas (Spain, Italy and Tunisia). Int. J. Biometeorol. 2014, 58, 867–876. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa, R.; Rallo, L.; Rapoport, H.F. Olive Floral Bud Growth and Starch Content During Winter Rest and Spring Budbreak. HortScience 2000, 35, 1223–1227. [Google Scholar] [CrossRef]
- Fabbri, A.; Alerci, L. Reproductive and vegetative bud differentiation in Olea europaea L. J. Hortic. Sci. Biotechnol. 1999, 74, 522–527. [Google Scholar] [CrossRef]
- Pinney, K.; Polito, V.S. Flower initiation in “Manzanillo” Olive. Acta Hortic. 1990, 203–206. [Google Scholar] [CrossRef]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavská, O.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Chang. Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- el Yaacoubi, A.; Oukabli, A.; Legave, J.-M.; Ainane, T.; Mouhajir, A.; Zouhair, R.; Hafidi, M. Response of almond flowering and dormancy to Mediterranean temperature conditions in the context of adaptation to climate variations. Sci. Hortic. 2019, 257, 108687. [Google Scholar] [CrossRef]
- González-Naharro, R.; Quirós, E.; Fernández-Rodríguez, S.; Silva-Palacios, I.; Maya-Manzano, J.M.; Tormo-Molina, R.; Pecero-Casimiro, R.; Monroy-Colin, A.; Gonzalo-Garijo, Á. Relationship of NDVI and oak (Quercus) pollen including a predictive model in the SW Mediterranean region. Sci. Total Environ. 2019, 676, 407–419. [Google Scholar] [CrossRef]
- Testa, S.; Soudani, K.; Boschetti, L.; Borgogno-Mondino, E. MODIS-derived EVI, NDVI and WDRVI time series to estimate phenological metrics in French deciduous forests. Int. J. Appl. Earth Obs. Geoinf. 2018, 64, 132–144. [Google Scholar] [CrossRef]
- Santos, J.A.; Costa, R.; Fraga, H. Climate change impacts on thermal growing conditions of main fruit species in Portugal. Clim. Chang. 2017, 140, 273–286. [Google Scholar] [CrossRef]
- Benlloch-González, M.; Sánchez-Lucas, R.; Bejaoui, M.A.; Benlloch, M.; Fernández-Escobar, R. Global warming effects on yield and fruit maturation of olive trees growing under field conditions. Sci. Hortic. 2019, 249, 162–167. [Google Scholar] [CrossRef]
- Moriondo, M.; Ferrise, R.; Trombi, G.; Brilli, L.; Dibari, C.; Bindi, M. Modelling olive trees and grapevines in a changing climate. Environ. Model. Softw. 2015, 72, 387–401. [Google Scholar] [CrossRef]
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Guiot, J.; Cramer, W. Climate change: The 2015 Paris Agreement thresholds and Mediterranean basin ecosystems. Science 2016, 354, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Karatzas, K.D. Informing the public about atmospheric quality: Air pollution and pollen. Allergo J. 2009, 18, 212–217. [Google Scholar] [CrossRef]



| Toledo (Spain) | Lecce (Italy) | Chaal (Tunisia) | ||||||
|---|---|---|---|---|---|---|---|---|
| Seasons | CH | GDH | Seasons | CH | GDH | Seasons | CH | GDH |
| 1998/1999 | 91.0 | 27,283.8 | 1998/1999 | 44.0 | 21,105.2 | |||
| 1999/2000 | 51.0 | 26,583.4 | 1999/2000 | 97.0 | 25,863.9 | |||
| 2000/2001 | 74.0 | 34,348.6 | 2000/2001 | 26.0 | 25,589.7 | |||
| 2001/2002 | 140.0 | 30,364.1 | 2001/2002 | 67.0 | 25,914.5 | |||
| 2002/2003 | 291.0 | 20,306.4 | 2002/2003 | 2.0 | 26,165.0 | 2002/2003 | 39.0 | 26,287.2 |
| 2003/2004 | 407.0 | 15,657.4 | 2003/2004 | 39.0 | 26,735.2 | 2003/2004 | 90.0 | 25,392.7 |
| 2004/2005 | 477.0 | 19,246.8 | 2004/2005 | 10.0 | 25,639.3 | 2004/2005 | 17.0 | 26,445.2 |
| 2005/2006 | 414.0 | 21,110.1 | 2005/2006 | 61.0 | 26,560.8 | 2005/2006 | 25.0 | 26,388.6 |
| 2006/2007 | 330.0 | 19,253.0 | 2006/2007 | 67.0 | 30,706.6 | 2006/2007 | 3.0 | 30,186.0 |
| 2007/2008 | 451.0 | 20,848.8 | 2007/2008 | 48.0 | 29,016.2 | 2007/2008 | 18.0 | 28,153.6 |
| 2008/2009 | 537.0 | 19,723.3 | 2008/2009 | 49.0 | 27,870.8 | 2008/2009 | 15.0 | 25,421.2 |
| 2009/2010 | 374.0 | 19,344.0 | 2009/2010 | 23.0 | 31,526.8 | 2009/2010 | 0.0 | 25,137.1 |
| 2010/2011 | 416.0 | 22,419.0 | 2010/2011 | 32.0 | 28,621.0 | 2010/2011 | 63.0 | 23,998.3 |
| 2011/2012 | 368.0 | 17,636.9 | 2011/2012 | 0.0 | 25,447.6 | |||
| 2012/2013 | 324.0 | 16,604.6 | 2012/2013 | 44.0 | 29,177.1 | |||
| 2013/2014 | 482.0 | 22,149.6 | 2013/2014 | 92.0 | 27,302.5 | |||
| 2014/2015 | 287.0 | 21,473.5 | ||||||
| 2015/2016 | 385.0 | 18,119.2 | ||||||
| Mean | 395.9 | 19,563.7 | Mean | 52.8 | 28,570.9 | Mean | 39.6 | 26,320.5 |
| SD | 74.2 | 2022.3 | SD | 36.2 | 2549.3 | SD | 29.9 | 2195.4 |
| CV% | 18.7 | 10.3 | CV% | 68.5 | 8.9 | CV% | 75.6 | 8.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojo, J.; Orlandi, F.; Ben Dhiab, A.; Lara, B.; Picornell, A.; Oteros, J.; Msallem, M.; Fornaciari, M.; Pérez-Badia, R. Estimation of Chilling and Heat Accumulation Periods Based on the Timing of Olive Pollination. Forests 2020, 11, 835. https://doi.org/10.3390/f11080835
Rojo J, Orlandi F, Ben Dhiab A, Lara B, Picornell A, Oteros J, Msallem M, Fornaciari M, Pérez-Badia R. Estimation of Chilling and Heat Accumulation Periods Based on the Timing of Olive Pollination. Forests. 2020; 11(8):835. https://doi.org/10.3390/f11080835
Chicago/Turabian StyleRojo, Jesús, Fabio Orlandi, Ali Ben Dhiab, Beatriz Lara, Antonio Picornell, Jose Oteros, Monji Msallem, Marco Fornaciari, and Rosa Pérez-Badia. 2020. "Estimation of Chilling and Heat Accumulation Periods Based on the Timing of Olive Pollination" Forests 11, no. 8: 835. https://doi.org/10.3390/f11080835
APA StyleRojo, J., Orlandi, F., Ben Dhiab, A., Lara, B., Picornell, A., Oteros, J., Msallem, M., Fornaciari, M., & Pérez-Badia, R. (2020). Estimation of Chilling and Heat Accumulation Periods Based on the Timing of Olive Pollination. Forests, 11(8), 835. https://doi.org/10.3390/f11080835