Abstract
Fire exclusion has dramatically altered historically fire adapted forests across western North America. In response, forest managers reduce forest fuels with mechanical thinning and/or prescribed burning to alter fire behavior, with additional objectives of restoring forest composition, structure, and ecosystem processes. There has been extensive research on the effects of fuel reduction and restoration treatments on trees, fuels, regeneration, and fire behavior; but less is known about how these treatments influence understory vegetation, which contains the majority of vascular plant diversity in many dry conifer forests. Of particular interest is how understory vegetation may respond to the season and interval of prescribed burning. The season and interval of prescribed burning is often determined by operational constraints rather than historical fire regimes, potentially resulting in fire conditions and burn intervals to which native plants are poorly adapted. In this study, we examined how understory vegetation has responded to season and interval of prescribed burning in ponderosa pine (Pinus ponderosa) forests in the Blue Mountains of northeastern Oregon, USA. Using over a decade (2002–2015) of understory vegetation data collected in stands with different intervals (5 versus 15 year) and seasons (spring versus fall) of prescribed burning, we quantified how season and interval of prescribed burning has influenced understory vegetation compositional trajectories and indicator species over time. Season of prescribed burning resulted in different understory communities and distinct trajectories of understory composition over time, but interval of burning did not. Indicator species analysis suggests fall burning is facilitating early seral species, with native annual forbs displaying ephemeral responses to frequent burning, while invasive cheatgrass (Bromus tectorum) increased in abundance and frequency across all treatments over time. These findings indicate that understory vegetation in these ecosystems are sensitive to seasonality of burning, but the responses are subtle. Our findings suggest season and interval of prescribed burning used in this study do not result in large changes in understory vegetation community composition, a key consideration as land managers increase the pace and scale of prescribed fire in these forests.
1. Introduction
Fire plays a critical role in terrestrial ecosystems across the globe, shaping ecosystem composition, structure, and function [1,2,3,4]. In western North America, fire exclusion has fundamentally altered the structure and function of fire adapted forests, particularly forest types such as ponderosa pine and dry mixed conifer forests historically characterized by frequent low and mixed severity fire regimes [5,6,7]. Frequent fire in these forest types reduced overall stand densities and fuel loading [5,6,7], while maintaining heterogeneous spatial patterns of vegetation structure that promoted resilience and moderated fire behavior [8,9,10]. In combination with the changing climate, livestock overgrazing, and timber harvesting, fire exclusion in these forests has dramatically altered vegetation composition and structure, increased fuel loading and spatial continuity, and increased the risk of high severity fire. In response, fuel reductions via mechanical thinning and/or prescribed burning are widely applied in fire adapted forests to reduce wildfire severity and reintroduce fire as an ecological process [11,12]. National policies have emerged to incentivize changes away from prioritization of fire suppression to more holistic approaches to wildfire management [13]. Yet, the pace and scale of fuel reduction treatments lags behind what is suggested to affect meaningful landscape change [14], while persistent operational and administrative constraints hinder more widespread application of fuel reduction treatments and restoration of fire as an ecological process [15,16,17].
Reducing the probability of high severity fire and extreme fire behavior is often the primary objective of fuel reduction treatments [11], with concurrent goals to restore ecosystem composition, structure, and function. Increasing native species biodiversity is a common objective of ecological restoration [18,19,20], following the central tenant that native biodiversity benefits from restoration of natural environmental conditions and processes [21]. Most plant diversity in conifer forests of western North America is found in the understory [22,23], so understanding how understory plant communities respond to fuel reduction and forest restoration treatments is critical to evaluate restoration effectiveness. In fire adapted landscapes, plant species can persist via fire resistant and resilient traits [24,25,26]. For example, some species resprout from belowground parts that survive a fire, others have seeds stimulated by fire resulting in species persistence even when the adults are killed by a fire, while yet others avoid fire by growing on sites that are less likely to have fire. Understory vegetation responses can be highly variable across forest types, time since disturbance, and disturbance intensities [27], highlighting the continuing need to understand how understory vegetation communities respond to specific restoration treatments and forest conditions, especially if adverse understory responses potentially constrain application of fuel reduction and restoration treatments.
In the context of ecosystem restoration, prescribed fire can approximate the disturbance historically created by wildfires, but there are reasons to question this assumption. The first prescribed burn after decades of fire suppression and fuel accumulation may occur with higher severity and negative ecological effects than would be expected under the natural fire regime [28,29,30]. Ecosystem restoration relies on reference conditions as a fundamental premise [31], implying that prescribed burning frequency should approximate a forest’s historical fire return interval. However, knowledge of the appropriate fire return interval for a given forest type and location is often lacking. Fire scar data used to quantify historical fire return intervals are often not available for specific locations, while interpretation and inference from fire scar data have been a subject of debate [32,33]. Furthermore, climate change is likely to alter future wildfire frequency and severity [34,35], potentially reducing the relevance of historical fire regimes as reference conditions to guide future restoration objectives [36] and adding uncertainty as to the appropriate prescribed fire return interval best suited for a given forest type, location, and desired outcomes.
In addition to uncertainties regarding appropriate prescribed fire frequency, operational considerations (e.g., air quality, weather, fuel loading and moisture, fire control, available personnel and resources, etc.) often constrain the seasonality of prescribed fires in the western United States to an early season after cessation of spring precipitation and snow melt, and a late season before the onset of winter precipitation. Yet in the Pacific Northwest, wildfires historically burned in the late summer and fall [3]. Application of prescribed fire outside the historical wildfire season may have unintended consequences for understory vegetation, as season of burn can differentially stimulate or damage plants depending on species-specific traits and developmental stages. High moisture content in plant tissue during the spring can make plants more susceptible to fire [37]. Additionally, many perennial plant species are most sensitive to fire when their carbohydrate reserves are lowest [38], and the timing of this varies by species. Fires during the growing season can result in higher mortality and reduced biomass of grasses and forbs, in comparison to dormant season prescribed burns [39,40]. The seedbank response to fire can also be altered by seasonal changes in soil moisture, with seeds of some species being tolerant of fire if the soil is dry [41], while others being stimulated by heat under higher moisture soil conditions [42]. In addition to species adaption traits, the season of burning can influence fuel consumption and plant mortality, with early season burns often occurring in high fuel moisture conditions, resulting in reduced fuel consumption and fire severity [43,44], lower plant mortality [45,46], and more unburned patches where fire sensitive species are likely to persist [44,47]. Lastly, invasive species such as cheatgrass (Bromus tectorum) pose a serious threat to ecosystem composition, structure, and function in many fire adapted forests in western North America [48,49]. Cheatgrass invasion is associated with changing fire frequency and seasonality [50], so conversely the seasonality and frequency of prescribed fire may inhibit or exacerbate cheatgrass invasion [51]. Taken in combination, it is unclear how changes in fire season and interval influence understory vegetation, and therefore restoration success.
This study focuses on how season and interval of prescribed burning influence the composition and compositional trajectories of understory vegetation, using a unique long-term (18 yr) experiment in ponderosa pine (Pinus ponderosa) dominated forests of northeastern Oregon, USA. Previously, this experiment examined the effects of season and interval of prescribed burning on tree growth and mortality [46,52,53], butterfly defoliation [54], tree regeneration and fuels [55], and understory vegetation [56,57,58]. Focusing on functional groups, vegetation cover, and diversity, Kerns and Day [57] found most native perennial functional groups resisted or recovered from different seasons or intervals of burning, but did not display strong responses to any specific combination of burn season or interval. Here we focus on overall vegetation composition, temporal trajectories, and specific indicator species of understory vegetation. First, we asked if species composition differs among treatments. Second, we asked whether compositional trajectories differ over time in response to spring or fall burning and 5- vs. 15-yr interval prescribed burning. Third, we asked if any individual species were indicators of different seasons and intervals of prescribed burning, and how did the importance of these indicator species vary over time.
2. Materials and Methods
2.1. Study Area
Initiated in 1997, the study is located in the Malheur National Forest in the Blue Mountains Ecoregion of northeastern Oregon, USA (Figure 1). The study consists of five upland stands (Driveway stands 14, 26, and 28 to the east; Kidd Flat and Trout stands to the west) ranging in size from 35–56 ha and 1600–1700 m in elevation. Soils are dominated by Mollisols and Inceptisols, although Alfisols are also present, and soil textures are similar among stands [59]. The climate is Mediterranean with an interior continental influence. Mean precipitation is 464 mm per year (1981–2012), with only 24% falling during the growing season, the remainder mostly falling as snow between October and April. Summers are dry, with hot days and cold nights, while winter temperatures are consistently low.
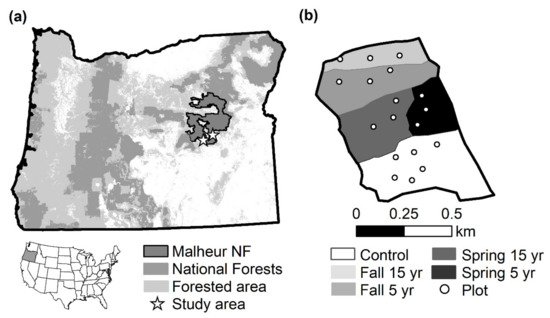
Figure 1.
Study area locator map: (a) Study area within Oregon, USA; (b) Example study design for one stand (Kid Flat) with season (Control = no burning, spring, or fall) and interval (5-yr, 15-yr) of prescribed burning, and plot locations.
The overstory is dominated by mixed age ponderosa pine (Pinus ponderosa), with minor components of western juniper (Juniperus occidentalis), curl-leaf mountain mahogany (Cercocarpus ledifolius), grand fir (Abies grandis), and Douglas-fir (Pseudotsuga menziesii). Individual ponderosa pines in the stands were approximately 80–100 years old, with occasional individuals that were nearly 200 years old. Understory vegetation composition differed between the eastern and western stands [56]. The more xeric eastern stands were dominated by bunchgrasses (Elymus elymoides, Achnatherum occidentale, Poa wheeleri, P. secunda, Bromus carinatus), Carex rossii, and Chrysothamnus spp. Exotic species were primarily Bromus tectorum and Cirsium vulgare. The more productive western stands were dominated by Carex geyeri, Arnica cordifolia, and Kelloggia galioides (data from untreated stands in 2002). Exotic species were less abundant in these western stands, while shrubs such as Berberis repens, Purshia tridentata, Symphoricarpus albus, S. oreophilus, Prunus emarginata, and P. virginiana var. melanocarpus were more common compared to the eastern stands.
No fire reconstruction exists for the immediate study area, but a recent fire scar based history of ponderosa pine dominated forests in the southern Blue Mountains had a historical mean fire return interval of 10–18 years, and fire was largely excluded from the region by 1900 [7,60]. The study area has a long history of livestock grazing. Records from the Emigrant Creek Ranger District indicate the area has been grazed almost continuously since at least 1946 [56], and in every year of this study. The stands were more heavily grazed from the 1940s until the 1990s, after which cattle numbers were greatly reduced. In addition, cattle grazing likely had occurred for a much longer period prior to the formation of the National Forest Reserves in the late 1880s. Prior to initiation of this study, all of the stands (including unburned controls) were evenly thinned from below in 1994 and 1995, resulting in residual tree densities of 181–252 trees ha−1, and residual mean diameters at breast height of 25.5–31.8 cm.
2.2. Experimental Design
Prior to burning, the five stands were each divided into three units averaging 15 ha in area (range 5–25 ha), and randomly assigned a season of burn treatment (spring, fall, or unburned control). Initial burns occurred in the fall of 1997 and spring of 1998. In 2002, the study was expanded to include interval of burning by dividing each seasonal unit, and randomly assigning the divisions to be reburned every five or fifteen years. The experimental design resulted in five treatments at each of the five stands: unburned control (UNBURNED), fall five year reburn (F5YR), fall 15 year reburn (F15YR), spring five year reburn (S5YR), and spring 15 year reburn (S15YR). Control treatments averaged 10 ha in area (range 5–15 ha) and all other treatment combinations averaged 8 ha in area (range 3–18 ha). Prescribed burns were applied with drip torches using a multi-strip head-fire pattern with an average flame length of approximately 60 cm. Spring and fall burns had similar relative humidity, wind speed, and flame length, but spring burns consistently occurred during warmer conditions [55]. The 5-yr interval reburns were conducted in the fall of 2002, 2007, and 2012/2013 and the spring of 2003, 2008, and 2013/2014. The 15-yr interval reburns were conducted in the fall of 2012/2013 and spring of 2012/2013. In 2012, only one stand (Driveway 14) was burned due to adverse burn conditions, and the other four stands were burned in the fall of 2013 and spring of 2014. See Figure 2 for a timeline of thinning, prescribed burning, and field sampling.
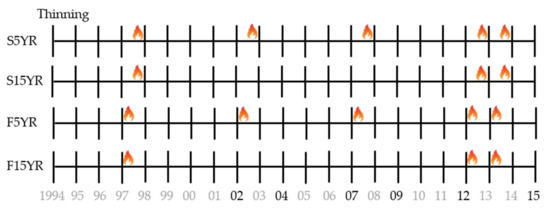
Figure 2.
Study timeline of thinning and burning by treatment. Flames denote prescribed burning. S5YR = Spring 5 year burns, S15YR = Spring 15 year burns, F5YR = Fall 5 year burns, and F15YR = Fall 15 year burns. Years in black text denote field sampling dates. Note that the fourth 5 year and first 15 year interval reburns occurred across multiple years (2012–2014) due to adverse burn conditions as noted in the experimental design description.
2.3. Field Sampling
The study was originally designed to examine only season of prescribed burning, with six 10 m radius vegetation plots located in each seasonal treatment unit. Seasonal treatment units were later split into two burn interval units, resulting in three plots per split, except for unburned controls which maintained six plots each. Plots were established along systematic transects as described by Thies and others [46]. On each plot, understory vegetation cover (percent) was visually estimated by species on eight 1 m2 quadrats using a marked PVC sampling square. Quadrats were located 5 m and 6 m from the plot center in each cardinal direction. To improve consistency of cover estimates, standardization exercises were periodically performed throughout the field season. For additional details regarding field sampling, see Kerns and Day [57]. In this study, we analyze the understory vegetation cover data collected in six sampling years (2002, 2004, 2007, 2009, 2012, 2015).
2.4. Statistical Analyses
Understory vegetation cover was averaged across quadrats by species at each plot. Due to inconsistencies in species identification caused by nomenclature changes and difficultly identifying species complexes, species names were harmonized across sample years before analysis, resulting in 138 species. Species present in less than 5% of plots were excluded, resulting in 55 species used in analyses (Table A1 and Table A2). We removed rare species because they may provide limited interpretive value [61], reflect stochastic environmental and sampling effects that add noise to statistical analyses [62,63,64,65], and because common species may provide better information in bioassessments [66]. In our study, 60% of all species occurred on fewer than 5% of plots, suggesting that stochastic and sampling effects would dominate analyses if rare species were retained. Given the potential management and policy relevance of our findings, we erred on the side of caution to avoid findings driven by rare species whose trends may be highly stochastic. Lastly, none of the “rare” species in this study were legally protected, threatened, or endangered with management implications. Understory vegetation cover was greater than zero on all plots in each sampling year, so a dummy variable to facilitate inclusion of samples lacking plant cover was not required. Data transformations are commonly used for proportional data in ecology [67,68], but we did not transform data prior to analyses. In the community trajectory analysis described below, commonly used transformations such as square root may distort the angles between consecutive segments and the overall trajectory shapes [69]. Additionally, in the indicator species analysis described below [70], the indicator value for each species is the product of its relative abundance and relative frequency among categories, so transforming to account for large differences in abundance between species would have little value.
All statistical analysis were conducted in R version 3.5.2. [71]. To test for differences in understory community composition between treatments, we used a randomized block split-plot permutation multivariate analysis of variance (PERMANOVA) in the package vegan 2.5-6 [72]. PERMANOVA was run on plot-level species cover, with season and treatment as fixed effects (no season x interval interaction due to split-plot design), and Euclidean distance metric. PERMANOVA was run for each sampling year individually.
To quantify understory community trajectories over time, we followed the community trajectory analysis (CTA) methods of De Cáceres et al. [69] using the package vegclust 1.7.7 [73]. CTA was conducted at the treatment level (i.e., plot-level cover values averaged across stands by treatment). A Euclidean distance matrix was calculated for the treatment-level understory vegetation cover values. Understory community trajectories were visualized in ordination space using Principle Coordinates Analysis (PCoA) [74]. We used uncentered trajectories, which emphasize differences in position between trajectories and reflect different starting points (initial community composition between treatments), rather than focusing on relative changes over time, as community composition for our first sampling point represents five years after initial entry burns. The resemblance between treatment trajectories was also calculated for uncentered trajectories by conducting a non-metric multi-dimensional scaling (nMDS) ordination on the trajectory distances [75]. In addition to visualizing trajectories over time at the treatment level, multiple metrics were calculated for uncentered trajectories. To quantify the relative community change (both overall and from year to year), trajectory lengths, angles, and overall directionality were calculated. Trajectory convergence/divergence was tested using the Mann-Whitney trend test on the sequences of distances between points of paired trajectories. Values of the trend test statistic (‘tau’) greater than zero correspond to trajectories that are diverging, whereas values less than zero correspond to trajectories that are converging [69].
To quantify which species are indicators of specific treatments over time, we used Indicator Species Analysis (ISA) following Dufrêne and Legendre [70]. ISA was conducted using the package labdsv 1.8 [76]. ISA was conducted at the treatment only (i.e., plot-level cover values averaged across stands by treatment). For species with significant indicator values (p ≤ 0.05) in any sample year, the relative abundance (RA), relative frequency (RF), and indicator values (IV = 100 × (RA × RF)) were calculated for all treatments and all sample years.
3. Results
3.1. Treatment-Level Differences in Community Composition
PERMANOVA analyses found strong evidence community composition differed between season of burn for all sample years, except 2012 which was only suggestive (Table 1). However, there was no evidence that community composition differed between burn intervals.

Table 1.
Summary statistics for Permutation Multivariate Analysis of Variance (PERMANOVA) of understory species composition in relation to season and interval of burning for each sampling year.
3.2. Treatment-Level Community Trajectories over Time
Treatment-level CTA indicated two distinct patterns over time (Figure 3). First, trajectories for unburned controls and spring burns appear to have different starting positions in 2002 compared to fall burns, indicative of pre-sampling differences in season as a result of the initial entry burns also evident in PERMANOVA results. Following three reburns, however, the spring 5-yr treatment diverges from the spring 15-yr treatment and moves towards fall burn community composition. Note that in 2002, all plots had still received only a single seasonal burn. Trajectory angles (indicative of direction of compositional change) displayed changes in 2004 and 2012, temporally corresponding to prescribed burns in 2002–2003 and 2012–2013 (Figure 3, Table 2). The fall 5-yr and fall 15-yr burn treatments have the greatest overall trajectory lengths (Table 3), indicating the greatest overall composition change, while the spring 5-yr treatment has the next greatest overall trajectory length. Fall burns generally had the greatest increase in trajectory length during the year following burning. Trajectory directionality (sensitive to both year triplet angles and segment lengths) indicates that all treatments follow relatively inconsistent trajectories (a value of 1 would indicate a straight path), with spring 5-yr burning having the most consistent trajectory (0.44), and both unburned (0.37) and fall 5-yr (0.38) having the least consistent path (Table 3). Trend analysis found that community trajectories tended to diverge more than converge, with 8 out of 10 treatment trajectory pairs diverging over time (Table 4). There was strong evidence that both fall and spring 5 year reburns diverged in community trajectories from unburned controls (tau = 0.73, p = 0.06), and in particular the spring 5 year 2002 composition was similar to unburned and its ending composition was more similar to fall burns.
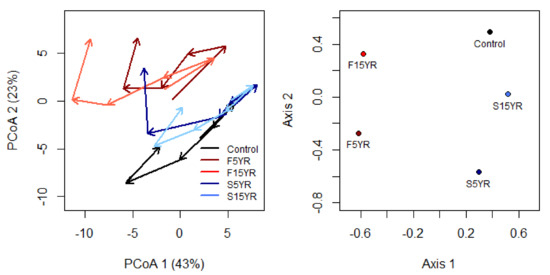
Figure 3.
Treatment-level uncentered understory community trajectories (left panel) and dissimilarities between trajectories (right panel). Principle Coordinates Analysis (PCoA) ordination of community trajectories indicated by arrows, with 2002 as the starting year, 2004, 2007, 2009, and 2012 as midpoints, and 2015 as the end year of vectors. Percentages on PCoA axes are the percentage of variance described for those ordination axes. Non-metric multi-dimensional scaling (nMDS) ordinations of dissimilarities between uncentered trajectories, with treatments closer together indicating more similar community trajectories over time. Control = Unburned, S15YR = Spring 5 year reburns, S15YR = Spring 15 year reburns, F5YR = Fall 5 year reburns, and F15YR = Fall 15 year reburns.

Table 2.
Treatment-level angles of understory community trajectories.

Table 3.
Treatment-level annual trajectory length, overall trajectory lengths, and overall directionality.

Table 4.
Treatment-level trajectory convergence and divergence trends.
3.3. Treatment-Level Indicator Species Analysis (ISA)
Ten species were significant indicators (i.e., had significant differences in indicator values between treatments for at least one sample year). Across these ten species, responses to treatments over time varied considerably (Figure 4). Some species (Ceanothus velutinus, Eriogonum heracleiodes, Ericameria spp., Phlox longifolia) displayed strong treatment fidelity over time. Both the exotic grass Bromus tectorum and native grass Elymus elymoides became more abundant and frequent across treatments over time, while the exotic forb Cirsium vulgare was only abundant and frequent in the fall burns in 2002 and declined over time, suggesting a more ephemeral population. Known fire responders, B. tectorum, C. velutinus and Ericameria spp., were indicators primarily in fall burn treatments, although their importance waned through time. Annual native forbs (Montia perfoliata, Polygonum douglasii and to a lesser extent Onagraceae spp.) displayed high interannual variability in importance values over time with clear pulses following burning. M. perfoliata values were slightly higher in fall and spring 5 year burns until 2015. E. heracleiodes, a large woody perennial forb, consistently had the highest indicator values in unburned controls, while Onagraceae spp. had slightly higher importance values in any burn treatment, but in particular for 5 year reburns.
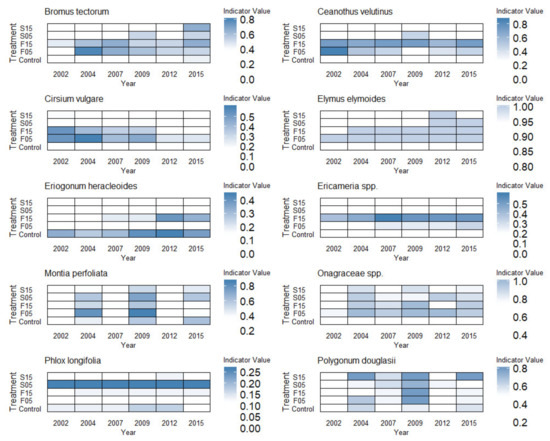
Figure 4.
Treatment-level indicator values of selected species by treatment over time. Selected species are those with significant p-values (p ≤ 0.05) in indicator species analysis for at least one sampling year. Indicator values = (relative abundance x relative frequency) × 100. Control = Unburned, S15YR = Spring 5 year burns, S15YR = Spring 15 year burns, F5YR = Fall 5 year burns, and F15YR = Fall 15 year burns. Reference Figure 2 for timing of burns relevant to sampling years.
4. Discussion
In this study with six years of vegetation measurement spanning 13 years, we found strong evidence that season of burn affected understory composition, and limited evidence that interval of burning mattered. However, compositional trajectory analysis shows that the combination of season and interval was important in determining overall compositional trends. Compositional trajectory analysis showed that after four burns the spring 5 year treatments were compositionally more similar to fall burns than 15 year spring reburns or unburned. However, only two pairwise comparisons (spring 5 yr vs. unburned control and fall 5 yr vs. unburned control) displayed strong evidence of diverging compositional trajectories, and no treatment pairs displayed strongly converging trajectories, suggesting understory community responses to seasonal and varying intervals of reburning are subtle, and initial entry burns were likely important drivers of initial trajectories. Indicator species analysis suggests, however, that species specific responses may be more nuanced, which may have been obscured by compositional analyses and the functional group approach of previous work [58]. Indicator species analysis found increased importance in fall burns of early successional species that are typical “fire increasers” such as Ceonothus velutinus, Ericameria spp., and Elymus elymoides. At the same time, some early successional native forbs such as Montia perfoliata and Polygonum douglasii appear to have strong but very ephemeral and episodic responses to burning, although post burn pulses appear to have weakened through time. However, the ephemeral temporal trend of Montia perfoliata was also somewhat present in unburned controls, suggesting other drivers such as climate, grazing, or seed source availability could play a role across treatments. The exotic species Bromus tectorum increased in importance across all seasons and intervals of burning, which is a management concern due to this exotic grass’s negative influence on ecosystem composition, structure, and function [49]. Below we pose multiple, non-exclusive explanations for the understory vegetation responses we found to season and interval of burn, and place our findings in the context of fire and fuel management of fire adapted forests in western North America.
Our results suggest that subtle differences in season of burn are important drivers of understory vegetation composition. Given the historical mean fire return interval of 10–18 years in the region [7,60], it is likely that native understory species are adapted to the range of fire intervals in this study, although we have some limited evidence that five year interval reburning may be too frequent. For example, pulses of short lived native early seral species (Montia perfoliata and Polygonum douglasii) characteristic of immediate post-fire years appear to be waning in their burn response after multiple reburns. Differences in understory vegetation due to season of burning are likely due to a combination of fire intensity and species-specific traits. Fall burns tended to have greater fuel consumption [55], indicating high fire intensity. Ceanothus velutinus in particular was strongly associated with fall burning, which may reflect a combination of vegetative resprouting and seed germination, both of which are encouraged by higher intensity fire [77,78]. One important deviation from season of burn differences in community trajectories is after multiple burns, spring 5 year burns trended towards fall burns in community composition. Only one indicator species (Elymus elymoides) clearly displayed increased importance in spring 5 year burns. In combination with compound effects of multiple burns on surface fuels [55], this may indicate that over decadal time frames multiple reburns may have comparable effects on understory vegetation to different seasons of burns. However, it is important to note that overall community trajectories for all treatments were rather similar; implying that a driver such as regional climate variability may have had a top-down effect on vegetation composition and obscured treatment effects [57].
While this study found differences in understory composition in relation to season of burning, the results are subtle, consistent with prior findings at this site [57]. There are multiple non-exclusive explanations for the subtle understory vegetation responses in this study. The study was conducted in a relatively low productivity ponderosa pine forest in northeastern Oregon, and multispecies dynamics can result from interactions between diversity, disturbance, and productivity [79]. Other studies of prescribed burning have found comparatively weak vegetation responses in lower productivity southwestern ponderosa pine forests [80], and stronger responses in productive mixed-conifer forests of the California Sierra Nevada [81,82,83]. The lack of strong understory responses in our study may reflect an inherent limited capacity of compositional change possible in low productivity forests subject to decades of fire exclusion. This limited capacity to change could also reflect ecological inertia of forest composition, structure, and seed sources constrained by forest conditions that after initial thinning still deviated greatly from pre-settlement conditions [84,85]. Additionally, both the mechanical thinning, grazing and prescribed burning in our study may have homogenized environmental conditions. Mechanical thinning from below resulted in a fairly even spatial distribution of residual trees, and did not result in stands with high variation in canopy cover. Likewise, prescribed burns were very low severity, and applied with drip torches using a multi-strip head-fire pattern with an average flame length of approximately 60 cm. The operational implementation of very low severity prescribed burning was likely effective in consuming surface fuels [55], but may not have promoted heterogeneous fire behavior and ecological effects (Figure 5), and potentially limited active fire spread that is in part driven by fuel moisture that varies seasonally. Having said that, it is important to note the number, size, and geographic placement of vegetation plots was not designed explicitly for quantifying spatial heterogeneity.

Figure 5.
Photos of prescribed burning and vegetation response of study experimental units on the Malheur National Forest, Oregon USA: (a) Example from more mesic Trout experimental unit of multi-strip ignition with drip torches designed for combustion of surface fuels and low flame lengths; (b) More eastern and xeric Driveway fall 5 year plot unit in 2013 with fairly homogenous surface fuels, understory vegetation, and overstory canopy conditions; (c) Fall 2018 prescribed burn in Trout experimental unit with more active fire behavior, resulting in torching of smaller tree patches; (d) Fall 2018 prescribed burn in Trout experimental unit with more intense fire and combustion of heavier fuels. Photos by D.J. Westlind, M.A. Day, B.K. Kerns, and T. Boyce.
Lastly, these stands have a long history of livestock grazing, and the plots used in this study continued to be grazed by cattle during this study. Livestock grazing can have short-term effects on vegetation response in ponderosa pine forests by reducing overall vegetation cover and altering the relative abundance of plant functional groups [56]. In grassland ecosystems, long-term cattle grazing can affect the composition of the seed bank [86], but it is unclear how strong a role the seed bank has in shaping understory vegetation responses in ponderosa pine forests [87].
5. Conclusions
In the context of thinned ponderosa pine forests, we found that the season of prescribed burn subtly altered the understory vegetation community in our study. However, there is evidence that the combination of season and interval was important in determining overall compositional trends. Our findings suggest fall burning, which is more consistent with the seasonality of the historical fire regime in these forests, resulted in different understory vegetation compared to spring burning, likely associated with more early seral species. However, there was some evidence that native early seral species may be unable to continue to respond to repeated very frequent (5 year) burning. This would suggest fire managers should give more consideration to the seasonality and interval of prescribed burning in the context of desired outcomes. The 5 year fire regime tested in this study is at the low end of historical fire regimes in the area. While spring burning is less consistent with the seasonality of historical fire regimes, we found no specific negative impacts related to spring burning.
There is a call to increase the pace and scale of prescribed fire and fuel reduction treatments in many fire adapted forests of western North America that have experienced decades of fire exclusion. Our results, coupled with earlier findings from this project, demonstrate that season and interval of prescribed burning may not be a strong ecological constraint for implementing prescribed burn programs, although fire intensity and resultant severity remain important considerations. That is, strict adherence to mimicking historical fire seasons may not be necessary to achieve desired outcomes and avoid negative vegetative responses. However, an important caveat to our findings is that the thinning and prescribed burning applied in this study may have homogenized environmental conditions and fire effects, and this homogenization may have overridden season and interval effects. Mechanical thinning with spatially variable tree retention (sensu North et al. [88]) and prescribed burning with more natural fire behavior are increasingly applied on fire adapted landscapes. An unanswered question is how understory vegetation will respond to different seasons and intervals of prescribed burning in more spatially complex forests with more active fire behavior. This may be of particular importance with respect to invasive species control, as variable tree retention and more active fire behavior may exacerbate exotic invasive species such as Bromus tectorum, which has continued to increase throughout the study area with burning. Better integration of weed management into prescribed fire programs could mitigate such undesirable outcomes.
Author Contributions
Conceptualization, H.S.J.Z., B.K.K., and M.A.D.; methodology, H.S.J.Z., B.K.K., and M.A.D.; software, H.S.J.Z., and M.A.D.; validation, H.S.J.Z.; formal analysis, H.S.J.Z.; investigation, H.S.J.Z., B.K.K., and M.A.D.; resources, B.K.K.; data curation, M.A.D.; writing—original draft preparation, H.S.J.Z.; writing—review and editing, H.S.J.Z., B.K.K., and M.A.D.; visualization, H.S.J.Z.; supervision, B.K.K.; project administration, B.K.K.; funding acquisition, B.K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by USDI Joint Fire Sciences Program (Project 12-01-01-10), The APC was funded by the USDA Forest Service.
Acknowledgments
Special thanks to Douglas Westlind for project oversight, Walter Thies and Christine Niwa for their initial efforts implementing the study, and the Malheur National Forest, Emigrant Creek Ranger District, for conducting periodic prescribed burns and other operational support. We also thank the numerous field crews that worked on this study. Lastly, we thank the two anonymous reviewers for their insightful and constructive comments on the original manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Species codes, scientific names, and general groups for all understory plant species used in analyses. * after species codes denotes species groups or complexes
Table A1.
Species codes, scientific names, and general groups for all understory plant species used in analyses. * after species codes denotes species groups or complexes
| Code | Scientific Name | Family | Group |
|---|---|---|---|
| ACMI | Achillea millefolium ssp. lanulosa | Asteraceae | native perennial forb |
| AGOS * | Agoseris spp. | Asteraceae | native perennial forb |
| AGSP | Agropyron spicatum | Poaceae | native perennial grass |
| AMAL | Amelanchier alnifolia | Rosaceae | native perennial shrub |
| ANMI | Antennaria microphylla | Asteraceae | native perennial forb |
| ARCO | Arnica cordifolia | Asteraceae | native perennial forb |
| BERE | Berberis repens | Berberidaceae | native perennial shrub |
| BRCA | Bromus carinatus | Poaceae | native perennial grass |
| BRTE | Bromus tectorum | Poaceae | exotic annual grass |
| CAGE | Carex geyeri | Cyperaceae | native perennial sedge |
| CARO | Carex rossii | Cyperaceae | native perennial sedge |
| CARU | Calamagrostis rubescens | Poaceae | native perennial grass |
| CEVE | Ceanothus velutinus | Rhamnaceae | native perennial shrub |
| CIVU | Cirsium vulgare | Asteraceae | exotic biennial forb |
| CLRH | Clarkia rhomboidea | Onagraceae | native annual forb |
| COLL * | Collomia spp. | Polemoniaceae | native annual forb |
| COPMIG * | Collinsia/Microsteris Group | Scroph.\Polem. | native annual forb |
| CREP * | Crepis spp. | Asteraceae | native perennial forb |
| CRTO | Cryptantha torreyana | Boraginaceae | native annual forb |
| ELCI | Elymus cinereus | Poaceae | native perennial grass |
| ELGL | Elymus glaucus | Poaceae | native perennial grass |
| ERCO | Erigeron corymbosus | Asteraceae | native perennial forb |
| ERHE | Eriogonum heracleoides | Polygonaceae | native perennial forb |
| ERIC | Ericameria spp. | Asteraceae | native perennial shrub |
| FEID | Festuca idahoensis | Poaceae | native perennial grass |
| FRVI | Fragaria virginiana | Rosaceae | native perennial forb |
| GAAP | Galium aparine | Rubiaceae | native annual forb |
| GEVI | Geranium viscosissimum var. nervosum | Geraniaceae | native perennial forb |
| HIAL2 | Hieracium albertinum | Asteraceae | native perennial forb |
| HYCA | Hydrophyllum capitatum | Hydrophyllaceae | native perennial forb |
| KEGA | Kelloggia galioides | Rubiaceae | native perennial forb |
| KOCR | Koeleria cristata | Poaceae | native perennial grass |
| LOTR | Lomatium triternatum | Apiaceae | native perennial forb |
| LUCA | Lupinus caudatus | Fabaceae | native perennial forb |
| MEFU | Melica fugax | Poaceae | native perennial grass |
| MOPE | Montia perfoliata | Portulacaceae | native annual forb |
| ONAG * | Onagraceae spp. | Onagraceae | native annual forb |
| PHAC * | Phacelia spp. | Hydrophyllaceae | native perennial forb |
| PHLO | Phlox longifolia | Polemoniaceae | native perennial forb |
Appendix B

Table A2.
Continued: Species codes, scientific names, and general groups for all understory plant species used in analyses.
Table A2.
Continued: Species codes, scientific names, and general groups for all understory plant species used in analyses.
| Code | Scientific Name | Family | Group |
|---|---|---|---|
| PODO | Polygonum douglasii | Polygonaceae | native annual forb |
| PONE | Poa nervosa var. wheeleri | Poaceae | native perennial grass |
| POSE | Poa secunda | Poaceae | native perennial grass |
| PRUN * | Prunus spp. | Rosaceae | native perennial shrub |
| RICE | Ribes cereum | Grossulariaceae | native perennial shrub |
| SEIN | Senecio integerrimus | Asteraceae | native perennial forb |
| SIHY | Sitanion hystrix | Poaceae | native perennial grass |
| SIME | Silene menziesii | Caryophyllaceae | native perennial forb |
| SIOR | Sidalcea oregana | Malvaceae | native perennial forb |
| SOMU | Solidago multiradiata var. scopulorum | Asteraceae | native perennial forb |
| STIP * | Stipa spp. | Poaceae | native perennial grass |
| SYMP * | Symphoricarpos spp. | Caprifoliaceae | native perennial shrub |
| TAOF | Taraxacum officinale | Asteraceae | exotic perennial forb |
| THFE | Thalictrum fendleri | Ranunculaceae | native perennial forb |
| VIPU | Viola purpurea | Violaceae | native perennial forb |
Notes: * after species codes denotes species groups or complexes. Agoseris spp = Agoseris aurantiaca, Agoseris glauca, Microseris nutans, and Microseris troximoides. Collomia spp. = Collomia grandiflora and Collomia linearis. Collinsia/Microsteris Group = Collinsia parviflora and Microsteris gracilis. Crepis spp. = Crepis acuminate and Crepis occidentalis. Onagraceae spp. = Epilobium minutum and Gayophytum heterozygum. Phacelia spp. = Phacelia hastate and Phacelia heterophylla. Prunus spp. = Prunus emarginata and Prunus virginiana var. melanocarpa. Stipa spp. = Stipa lemmonii and Stipa occidentalis. Symphoricarpos spp. = Symphoricarpos albus and Symphoricarpos oreophilus.
References
- Naveh, Z. Effects of fire in the Mediterranean region. In Fire and Ecosystems; Academic Press: New York, NY, USA, 1974; pp. 401–434. [Google Scholar]
- Wein, R.W.; Maclean, D.A. The Role of Fire in Northern Circumpolar Ecosystems; John Wiley & Sons: New York, NY, USA, 1983. [Google Scholar]
- Agee, J.K. Ecology of Pacific Northwest Forests; Island Press: Washington, DC, USA, 1993. [Google Scholar]
- Bond, W.J.; Keeley, J.E. Fire as a global ‘herbivore’: The ecology and evolution of flammable ecosystems. Trends Ecol. Evol. 2005, 20, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.F. Changes in vegetation, structure, and growth of south western pine forests since white settlement. Ecol. Monogr. 1960, 30, 29–164. [Google Scholar] [CrossRef]
- Parsons, D.J.; DeBenedetti, S.H. Impact of fire suppression on a mixed-conifer forest. For. Ecol. Manag. 1979, 2, 21–33. [Google Scholar] [CrossRef]
- Johnston, J.D.; Bailey, J.D.; Dunn, C.J. Influence of fire disturbance and biophysical heterogeneity on pre-settlement ponderosa pine and mixed conifer forests. Ecosphere 2016, 7, e01581. [Google Scholar] [CrossRef]
- Stephens, S.; Fry, D.; Franco-Vizcaíno, E. Wildfire and spatial patterns in forests in northwestern Mexico: The United States wishes it had similar fire problems. Ecol. Soc. 2008, 13, 10. [Google Scholar] [CrossRef]
- Collins, B.M.; Lydersen, J.M.; Fry, D.L.; Wilkin, K.; Moody, T.; Stephens, S.L. Variability in vegetation and surface fuels across mixed-conifer-dominated landscapes with over 40 years of natural fire. For. Ecol. Manag. 2016, 381, 74–83. [Google Scholar] [CrossRef]
- Koontz, M.J.; North, M.P.; Werner, C.M.; Fick, S.E.; Latimer, A.M. Local forest structure variability increases resilience to wildfire in dry western U.S. coniferous forests. Ecol. Lett. 2020, 23, 483–494. [Google Scholar] [CrossRef]
- Agee, J.K.; Skinner, C.N. Basic principles of forest fuel reduction treatments. For. Ecol. Manag. 2005, 211, 83–96. [Google Scholar] [CrossRef]
- Ryan, K.C.; Knapp, E.E.; Varner, J.M. Prescribed fire in North American forests and woodlands: History, current practice, and challenges. Front. Ecol. Environ. 2013, 11, e15–e24. [Google Scholar] [CrossRef]
- Jewell, S.; Vilsack, T.J. The National Strategy: The Final Phase in the Development of the National Cohesive Wildland Fire Management Strategy. United States Department of Agriculture-United States Department of Interior; 2014. Available online: https://www.forestsandrangelands.gov/documents/strategy/strategy/CSPhaseIIINationalStrategyApr2014.pdf (accessed on 29 July 2020).
- North, M.; Collins, B.M.; Stephens, S. Using fire to increase the scale, benefits, and future maintenance of fuels treatments. J. For. 2012, 110, 392–401. [Google Scholar] [CrossRef]
- North, M.; Brough, A.; Long, J.; Collins, B.; Bowden, P.; Yasuda, D.; Miller, J.; Sugihara, N. Constraints on mechanized treatment significantly limit mechanical fuels reduction extent in the Sierra Nevada. J. For. 2015, 113, 40–48. [Google Scholar] [CrossRef]
- North, M.P.; Stephens, S.L.; Collins, B.M.; Agee, J.K.; Aplet, G.; Franklin, J.F.; Fulé, P.Z. Reform forest fire management. Science 2015, 349, 1280–1281. [Google Scholar] [CrossRef]
- Thompson, M.; Dunn, C.; Calkin, D. Wildfires: Systemic changes required. Science 2015, 350, 920–921. [Google Scholar] [CrossRef] [PubMed]
- Covington, W.W.; Fule, P.Z.; Moore, M.M.; Hart, S.C.; Kolb, T.E.; Mast, J.N.; Sackett, S.S.; Wagner, M.R. Restoring ecosystem health in ponderosa pine forests of the Southwest. J. For. 1997, 95, 23–29. [Google Scholar]
- Covington, W.W. Helping western forests heal. Nature 2000, 408, 135–136. [Google Scholar] [CrossRef]
- Allen, C.D.; Savage, M.; Falk, D.A.; Suckling, K.F.; Swetnam, T.W.; Schulke, T.; Stacey, P.B.; Morgan, P.; Hoffman, M.; Klingel, J.T. Ecological restoration of southwestern ponderosa pine ecosystems: A broad perspective. Ecol. Appl. 2002, 12, 1418–1433. [Google Scholar] [CrossRef]
- Whisenant, S.; Shevock, J.R.; Palik, B.J.; Engstrom, R.T.; Egan, D.; Volland, L.A.; Dell, J.D.; Parker, V.T. Can native flora survive prescribed burns? In Maintaining Biodiversity in Forest Ecosystems; Hunter, M.J., Ed.; Cambridge University Press: New York, NY, USA, 1999; pp. 691–709. [Google Scholar]
- Shevock, J.R. Status of Are and Endemic Plants. Sierra Nevada Ecosystem Project: Final Report to Congress; Report No. 37; Assessments and Scientific Basis for Management Options University of California Press, Centers for Water and Wildland Resources: Davis, CA, USA, 1996; Volume 2. [Google Scholar]
- Palik, B.J.; Engstrom, R.T. Species composition. In Maintaining Biodiversity in Forest Ecosystems; Hunter, M.J., Ed.; Cambridge University Press: New York, NY, USA, 1999; pp. 65–94. [Google Scholar]
- Brown, J.K. Ecological principles, shifting fire regimes and management consideration. In Wildland Fire in Ecosystems: Effects of Fire on Flora; Brown, J.K., Smith, J.K., Eds.; USDA Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 2000; pp. 185–203. [Google Scholar]
- Keeley, J.E.; Pausas, J.G.; Rundel, P.W.; Bond, W.J.; Bradstock, R.A. Fire as an evolutionary pressure shaping plant traits. Trends Plant Sci. 2011, 16, 406–411. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E. Evolutionary ecology of resprouting and seeding in fire-prone ecosystems. New Phytol. 2014, 204, 55–65. [Google Scholar] [CrossRef]
- Battles, J.J.; Shlisky, A.J.; Barrett, R.H.; Heald, R.C.; Allen-Diaz, B.H. The effects of forest management on plant species diversity in a Sierran conifer forest. For. Ecol. Manag. 2001, 146, 211–222. [Google Scholar] [CrossRef]
- Ryan, K.C.; Frandsen, W.H. Basal injury from smoldering fires in mature Pinus ponderosa Laws. Int. J. Wildland Fire 1991, 1, 107–118. [Google Scholar] [CrossRef]
- Varner, J.M.; Gordon, D.R.; Putz, E.; Hiers, J.K. Restoring fire to long-unburned Pinus palustris ecosystems: Novel fire effects and consequences for long-unburned ecosystems. Restor. Ecol. 2005, 13, 536–544. [Google Scholar] [CrossRef]
- Hood, S.M. Mitigating Old Tree Mortality in Long-Unburned, Fire-Dependent Forests: A Synthesis; Report No.: RMRS-GTR-283; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2010. [Google Scholar]
- Egan, D.; Howell, E.A. (Eds.) The Historical Ecology Handbook: A Restorationist’s Guide to Reference Ecosystems; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Baker, W.L.; Ehle, D. Uncertainty in surface-fire history: The case of ponderosa pine forests in the western United States. Can. J. For. Res. 2001, 31, 1205–1226. [Google Scholar] [CrossRef]
- Falk, D.A.; Heyerdahl, E.K.; Brown, P.M.; Farris, C.; Fulé, P.Z.; McKenzie, D.; Swetnam, T.W.; Taylor, A.H.; Van Horne, M.L. Multi-scale controls of historical forest-fire regimes: New insights from fire-scar networks. Front. Ecol. Environ. 2011, 9, 446–454. [Google Scholar] [CrossRef]
- Westerling, A.L.; Bryant, B.P. Climate change and wildfire in California. Clim. Change 2007, 87, 231–249. [Google Scholar] [CrossRef]
- Rogers, B.M.; Neilson, R.P.; Drapek, R.; Lenihan, J.M.; Wells, J.R.; Bachelet, D.; Law, B.E. Impacts of climate change on fire regimes and carbon stocks of the U.S. Pacific Northwest. J. Geophys. Res. 2011, 116, 303037. [Google Scholar] [CrossRef]
- Keeley, J.E.; Stephenson, N.L. Restoring natural fire regimes to the Sierra Nevada in an era of global change. In Wilderness Science in a Time of Change Conference-Volume 5: Wilderness Ecosystem, Threats and Management, Proceedings of the RMRS-P-15-VOL-5, Missoula, MT, USA, 23–27 May 1999; Cole, D.N., McCool, S.F., Borrie, W.T., O’Loughlin, J., Eds.; USDA Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 2000; pp. 255–265. [Google Scholar]
- Wright, H.A. A method to determine heat-caused mortality in bunchgrasses. Ecology 1970, 51, 582–587. [Google Scholar] [CrossRef]
- Volland, L.A.; Dell, J.D. Fire Effects on Pacific Northwest Forest and Range Vegetation; USDA Forest Service: Portland, OR, USA, 1981. [Google Scholar]
- Wright, H.A.; Klemmedson, J.O. Effect of fire on bunchgrasses of the sagebrush-grass region in southern Idaho. Ecology 1965, 46, 680–688. [Google Scholar] [CrossRef]
- Brockway, D.G.; Gatewood, R.G.; Paris, R.B. Restoring fire as an ecological process in shortgrass prairie ecosystems: Initial effects of prescribed burning during the dormant and growing seasons. J. Environ. Manag. 2002, 65, 135–152. [Google Scholar] [CrossRef]
- Parker, V.T. Can native flora survive prescribed burns? Fremontia 1987, 15, 3–6. [Google Scholar]
- Kauffman, J.B.; Martin, R.E. Factors influencing the scarification and germination of three montane Sierra Nevada shrubs. Northwest Sci. 1991, 65, 180–187. [Google Scholar]
- Kauffman, J.B.; Martin, R. Fire behavior, fuel consumption, and forest-floor changes following prescribed understory fires in Sierra Nevada mixed conifer forests. Can. J. For. Res. 1989, 19, 455–462. [Google Scholar] [CrossRef]
- Knapp, E.E.; Keeley, J.E.; Ballenger, E.A.; Brennan, T.J. Fuel reduction and coarse woody debris dynamics with early season and late season prescribed fire in a Sierra Nevada mixed conifer forest. For. Ecol. Manag. 2005, 208, 383–397. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Martin, R.E. Sprouting shrub response to different seasons and fuel consumption levels of prescribed fire in Sierra Nevada mixed conifer ecosystems. For. Sci. 1990, 36, 748–764. [Google Scholar]
- Thies, W.G.; Westlind, D.J.; Loewen, M. Season of prescribed burn in ponderosa pine forests in eastern Oregon: Impact of pine mortality. Int. J. Wildland Fire 2005, 14, 223–231. [Google Scholar] [CrossRef]
- Knapp, E.E.; Keeley, J.E. Heterogeneity in fire severity within early season and late season prescribed burns in a mixed-conifer forest. Int. J. Wildland Fire 2006, 15, 37–45. [Google Scholar] [CrossRef]
- Billings, W.D. Cheatgrass and resultant fire on ecosystems in the western Great Basin. In Proceedings-Ecology and Management of Annual Rangelands; Monsen, S.B., Kitchen, S.G., Eds.; General Technical Reports INT-GTR-313; USDA Forest Service, Intermountain Research Station: Ogden, UT, USA, 1994; pp. 22–30. [Google Scholar]
- Kerns, B.K.; Tortorelli, C.; Day, M.A.; Nietupski, T.; Barros, A.M.G.; Kim, J.B.; Krawchuk, M.A. Invasive grasses: A new perfect storm for forested ecosystems? For. Ecol. Manag. 2020, 463, 117985. [Google Scholar] [CrossRef]
- Bradley, B.A.; Curtis, C.A.; Fusco, E.J.; Abatzoglou, J.T.; Balch, J.K.; Dadashi, S.; Tuanmu, M.-N. Cheatgrass (Bromus tectorum) distribution in the intermountain Western United States and its relationship to fire frequency, seasonality, and ignitions. Biol. Invasions 2018, 20, 1493–1506. [Google Scholar] [CrossRef]
- Keeley, J.E.; McGinnis, T.W. Impact of prescribed fire and other factors on cheatgrass persistence in a Sierra Nevada ponderosa pine forest. Int. J. Wildland Fire 2007, 16, 96–106. [Google Scholar] [CrossRef]
- Thies, W.G.; Westlind, D.J.; Loewen, M. Field Note: Impact of spring or fall repeated prescribed fire on growth of ponderosa pine in eastern Oregon, USA. West. J. Appl. For. 2013, 28, 128–132. [Google Scholar] [CrossRef]
- Westlind, D.J.; Kerns, B.K. Repeated fall prescribed fire in previously thinned ponderosa pine (Pinus ponderosa) increases growth and resistance to other disturbances. For. Ecol. Manag. 2020. in revision. [Google Scholar]
- Kerns, B.K.; Westlind, D.J. Effect of season and interval of prescribed burn on ponderosa pine butterfly defoliation patterns. Can. J. For. Res. 2013, 43, 979–983. [Google Scholar] [CrossRef]
- Westlind, D.; Kerns, B. Long-term effects of burn season and frequency on ponderosa pine forest fuels and seedlings. Fire Ecol. 2017, 13, 42–61. [Google Scholar] [CrossRef]
- Kerns, B.K.; Buonopane, M.; Thies, W.G.; Niwa, C. Reintroducing fire into a ponderosa pine forest with and without cattle grazing: Understory vegetation response. Ecosphere 2011, 2, 1–23. [Google Scholar] [CrossRef]
- Kerns, B.K.; Day, M.A. Prescribed fire regimes subtly alter ponderosa pine forest plant community structure. Ecosphere 2018, 9, e02529. [Google Scholar] [CrossRef]
- Kerns, B.K.; Thies, W.G.; Niwa, C.G. Season and severity of prescribed burn in ponderosa pine forests: Implications for understory native and exotic plants. Ecoscience 2006, 13, 44–55. [Google Scholar] [CrossRef]
- Hatten, J.; Zabowski, D.; Ogden, A.; Thies, W.; Choi, B. Role of season and interval of prescribed burning on ponderosa pine growth in relation to soil inorganic N and P and moisture. For. Ecol. Manag. 2012, 269, 106–115. [Google Scholar] [CrossRef]
- Johnston, J.D. Influence of Fire Disturbance and Biophysical Heterogeneity on Pre-Settlement Ponderosa Pine and Mixed Conifer Forests; Oregon State University: Corvallis, OR, USA, 2016. [Google Scholar]
- Marchant, R. How important are rare species in aquatic community ecology and bioassessment? A comment on the conclusions of Cao et al. Author’s reply. Limnol. Oceanogr. 1999, 44, 1840–1841. [Google Scholar]
- Gauch, H.G. Multivariate Analysis in Community Ecology; Cambridge University Press: Cambridge, UK, 1982. [Google Scholar]
- McCune, B.; Grace, J.B.; Urban, D.L. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Clarke, K.R.; Green, R.H. Statistical Design and Analysis for a “Biological Effects” Study. Mar. Ecol. Prog. Ser. 1988, 46, 213–226. [Google Scholar] [CrossRef]
- Bailey, R.C.; Linke, S.; Yates, A.G. Bioassessment of Freshwater Ecosystems using the Reference Condition Approach: Comparing Established and New Methods with Common Data Sets. Freshw. Sci. 2014, 33, 1204–1211. [Google Scholar] [CrossRef]
- Sgarbi, L.F.; Bini, L.M.; Heino, J.; Jyrkänkallio-Mikkola, J.; Landeiro, V.L.; Santos, E.P.; Schneck, F.; Siqueira, T.; Soininen, J.; Tolonen, K.T.; et al. Sampling effort and information quality provided by rare and common species in estimating assemblage structure. Ecol. Indic. 2020, 110, 105937. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research; Freeman and Company: New York, NY, USA, 1995. [Google Scholar]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef] [PubMed]
- De Cáceres, M.; Coll, L.; Legendre, P.; Allen, R.B.; Wiser, S.K.; Fortin, M.J.; Condit, R.; Hubbell, S. Trajectory analysis in community ecology. Ecol. Monogr. 2019, 89, e01350. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.5-6. 2019. Available online: https://cran.rproject.org/package=vegan (accessed on 18 May 2020).
- De Caceres, M.; De Caceres, M.M. Vegclust: Fuzzy Clustering of Vegetation Data. R Package Version 1.7.7. 2019. Available online: https://cran.r-project.org/package=vegclust (accessed on 18 May 2020).
- Gower, J.C. Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika 1966, 53, 325–338. [Google Scholar] [CrossRef]
- Clarke, K.R.; Somerfield, P.J.; Airoldi, L.; Warwick, R.M. Exploring interactions by second-stage community analyses. J. Exp. Mar. Biol. Ecol. 2006, 338, 179–192. [Google Scholar] [CrossRef]
- Roberts, D.W. Labdsv: Ordination and Multivariate Analysis for Ecology. R Package 2.0-1. 2019. Available online: https://cran.r-project.org/package=labdsv (accessed on 18 May 2020).
- Gratkowski, H. Heat as a factor in germination of seeds of Ceanothus velutinus var. laevigatus T. & G. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 1962. [Google Scholar]
- Keeley, J.E. Seed germination and life history syndromes in the California chaparral. Bot. Rev. 1991, 57, 81–116. [Google Scholar] [CrossRef]
- Kondoh, M. Unifying the relationships of species richness to productivity and disturbance. Proc. R. Soc. B Biol. Sci. 2001, 268, 269–271. [Google Scholar] [CrossRef]
- Laughlin, D.C.; Bakker, J.D.; Daniels, M.L.; Moore, M.M.; Casey, C.A.; Springer, J.D. Restoring plant species diversity and community composition in a ponderosa pine-bunchgrass ecosystem. Plant Ecol. 2008, 197, 139–151. [Google Scholar] [CrossRef]
- Webster, K.M.; Halpern, C.B. Long-term vegetation responses to reintroduction and repeated use of fire in mixed-conifer forests of the Sierra Nevada. Ecosphere 2010, 1, 1–27. [Google Scholar] [CrossRef]
- Wayman, R.B.; North, M. Initial response of a mixed-conifer understory plant community to burning and thinning restoration treatments. For. Ecol. Manag. 2007, 239, 32–44. [Google Scholar] [CrossRef]
- Goodwin, M.J.; North, M.P.; Zald, H.S.J.; Hurteau, M.D. The 15-year post-treatment response of a mixed-conifer understory plant community to thinning and burning treatments. For. Ecol. Manag. 2018, 429, 617–624. [Google Scholar] [CrossRef]
- Zald, H.S.J.; Gray, A.N.; North, M.; Kern, R.A. Initial tree regeneration responses to fire and thinning treatments in a Sierra Nevada mixed-conifer forest, USA. For. Ecol. Manag. 2008, 256, 168–179. [Google Scholar] [CrossRef]
- Johnston, J.D.; Dunn, C.J.; Vernon, M.J.; Bailey, J.D.; Morrissette, B.A.; Morici, K.E. Restoring historical forest conditions in a diverse inland Pacific Northwest landscape. Ecosphere 2018, 9, e02400. [Google Scholar] [CrossRef]
- Kinucan, R.J.; Smeins, F.E. Soil Seed Bank of a Semiarid Texas Grassland Under Three Long-Term (36-Years) Grazing Regimes. Am. Midl. Nat. 1992, 128, 11–21. [Google Scholar] [CrossRef]
- Carr, C.A.; Krueger, W.C. The Role of the Seed Bank in Recovery of Understory Species in an Eastern Oregon Ponderosa Pine Forest. Northwest Sci. 2012, 86, 168–178. [Google Scholar] [CrossRef]
- North, M.P.; Stine, P.; O’Hara, K.; Zielinksi, W.; Stephens, S.L. An Ecosystem Management Strategy for Sierran Mixed-Conifer Forests; Report No.: PSW-GTR-220; USDA Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2009. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).