Aboveground Carbon Storage and Cycling of Flooded and Upland Forests of the Brazilian Pantanal
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the Study Area
2.2. Collection of Litter Fall and Litter Pool
2.3. Measurement of Wood C Increment
2.4. Data Analysis
3. Results
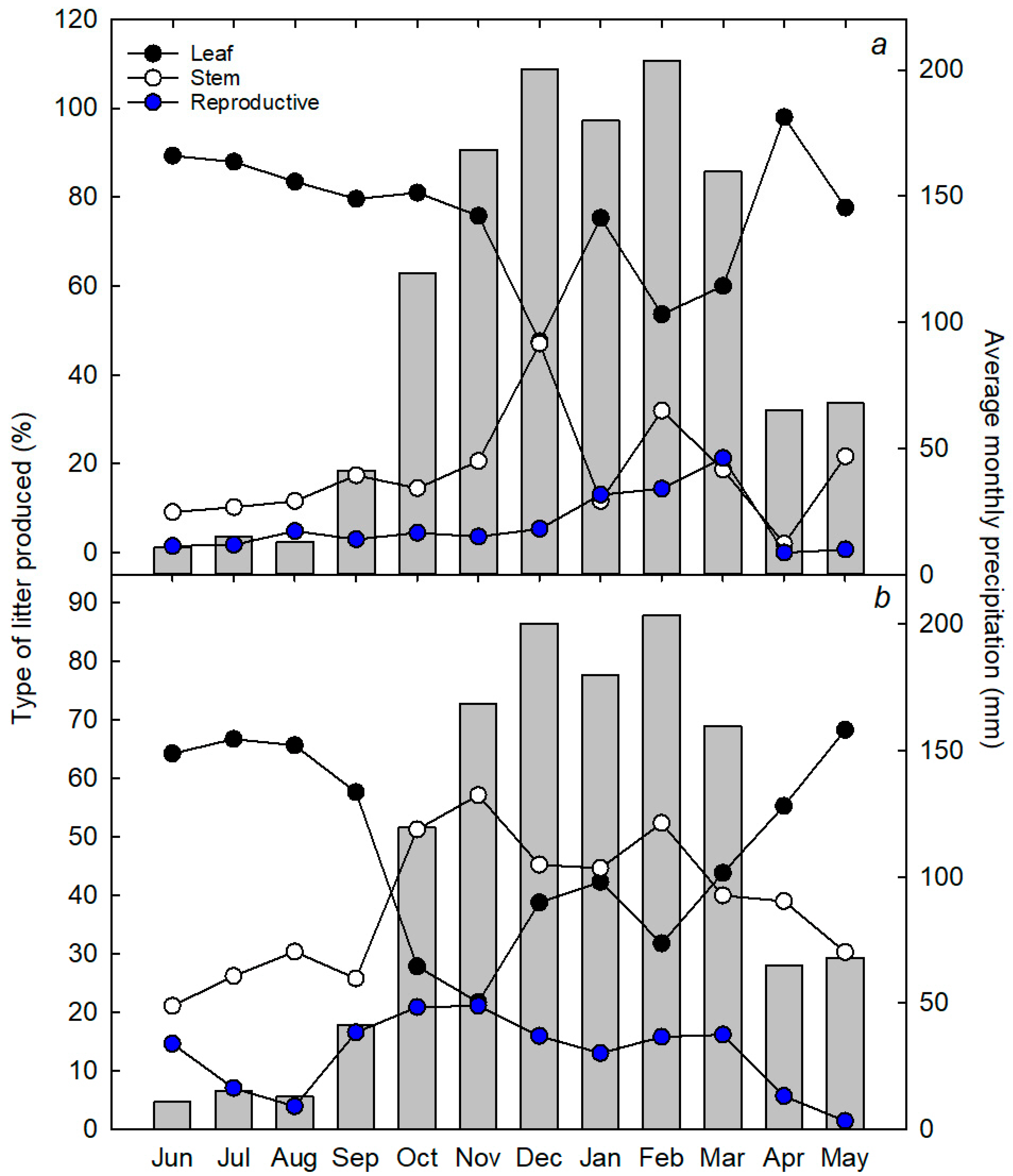
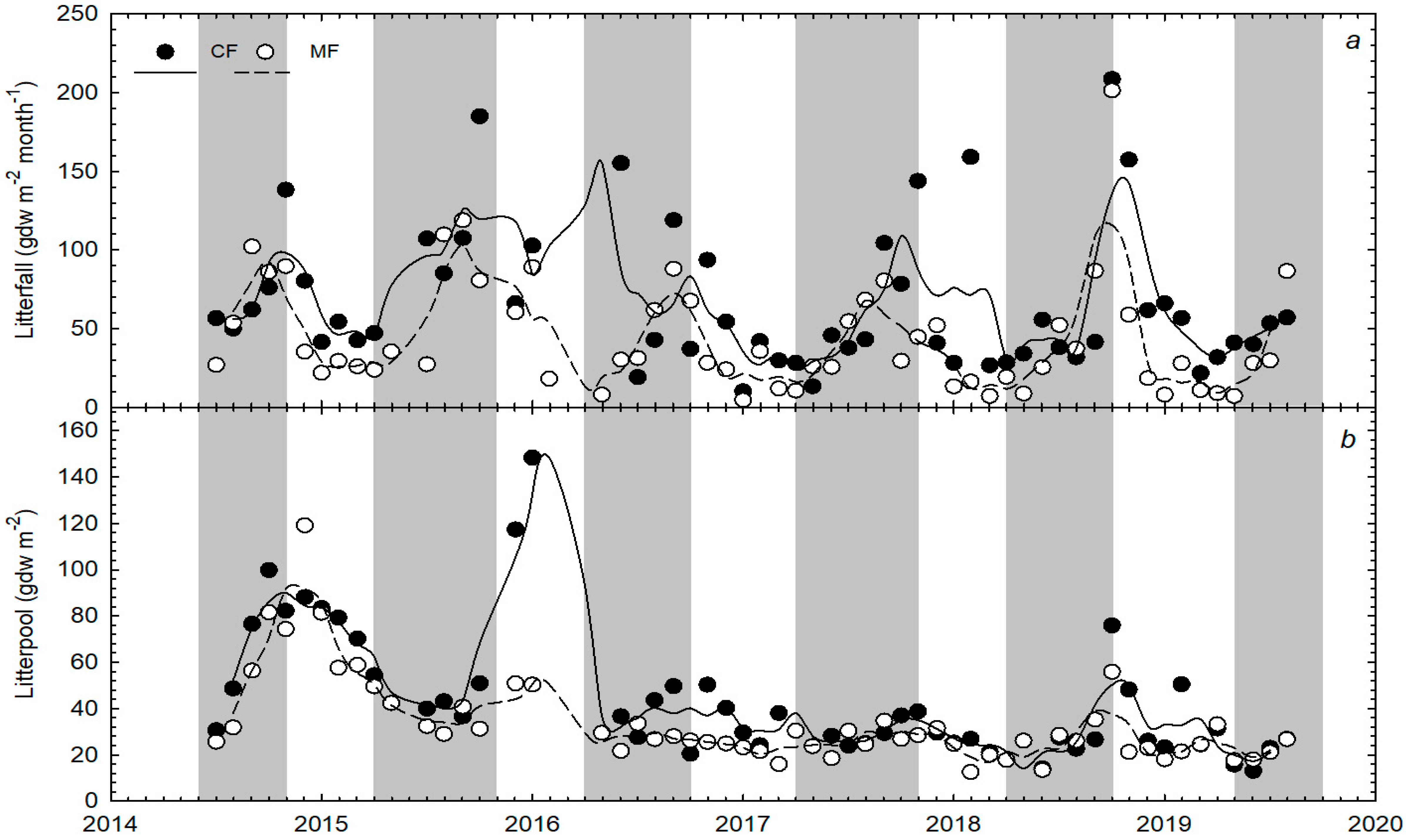
3.1. Litter Dynamics
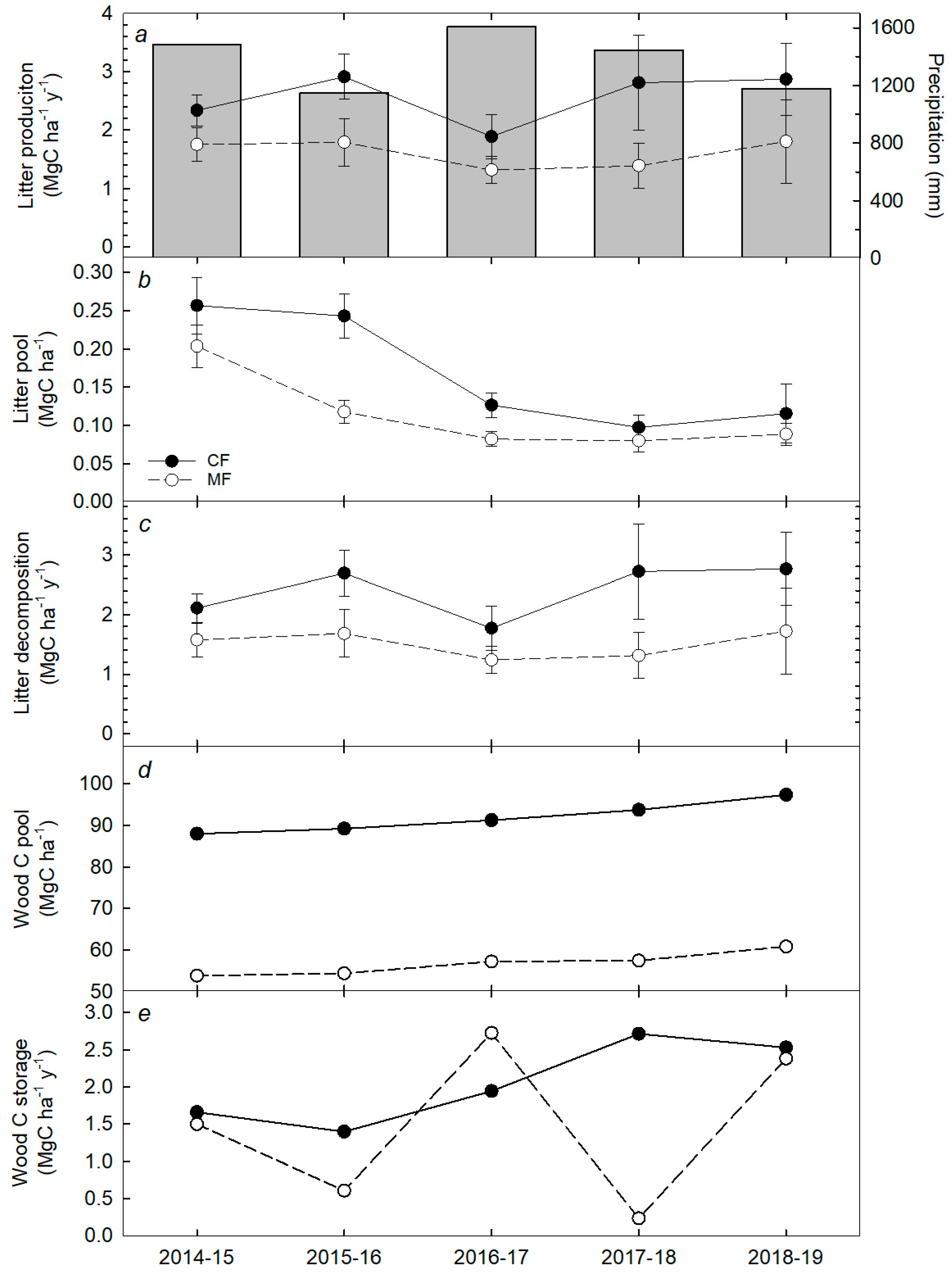
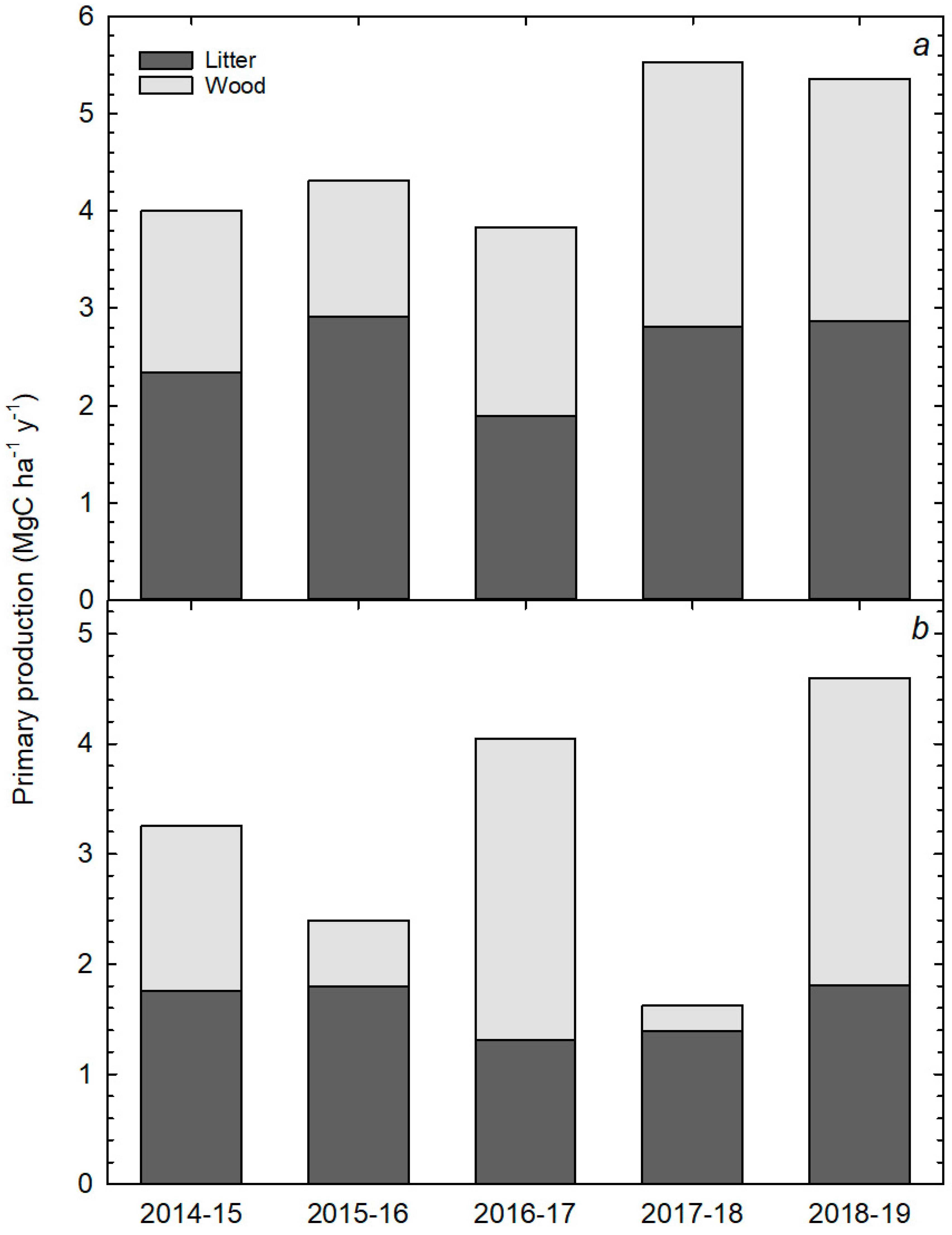
3.2. Annual Rates of Aboveground C Cycling Components
4. Discussion
4.1. Stand Differences in Aboveground C Cycling and Storage
4.2. Seasonal Variations in Litter Production
4.3. Annual Variations in Aboveground C Cycling and Storage Components
4.4. Comparisons to Other Studies
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schlesinger, W.H.; Bernhardt, E.S. Biogeochemistry: An Analysis of Global Change, 3rd ed.; Academic Press: Waltham, MA, USA, 2013; p. 668. [Google Scholar]
- Clark, D.A.; Clark, D.B.; Oberbauer, S.F. Field-quantified responses of tropical rainforest aboveground productivity to increasing CO2 and climatic stress, 1997–2009. J. Geophys. R.-Biogeosci. 2013, 118, 783–794. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Townsend, A.R.; Taylor, P.; Alvarez-Clare, S.; Bustamante, M.M.C.; Chuyong, G.; Dobrowski, S.Z.; Grierson, P.; Harms, K.E.; Houlton, B.Z.; et al. Relationships among net primary productivity, nutrients and climate in tropical rain forest: A pan-tropical analysis. Ecol. Lett. 2011, 14, 1313–1317. [Google Scholar] [CrossRef]
- Quesada, C.A.; Phillips, O.L.; Schwarz, M.; Czimczik, C.I.; Baker, T.R.; Patino, S.; Fyllas, N.M.; Hodnett, M.G.; Herrera, R.; Almeida, S.; et al. Basin-wide variations in Amazon forest structure and function are mediated by both soils and climate. Biogeosciences 2012, 9, 2203–2246. [Google Scholar] [CrossRef]
- Vourlitis, G.L.; Zappia, A.; Pinto, O.B., Jr.; Arruda, P.H.Z.; Santanna, F.B.; Dalmagro, H.J.; Lobo, F.A.; Nogueira, J.S. Spatial and temporal variations in aboveground woody carbon storage for cerrado forests and woodlands of Mato Grosso, Brazil. J. Geophys. R.-Biogeosci. 2019, 124. [Google Scholar] [CrossRef]
- Miranda, S.C.; Bustamante, M.; Palace, M.; Hagen, S.; Keller, M.; Ferreira, L.G. Regional variations in biomass distribution in Brazilian savanna woodland. Biotropica 2014, 46, 125–138. [Google Scholar] [CrossRef]
- Vourlitis, G.L.; Lobo, F.A.; Pinto, O.B., Jr.; Dalmagro, H.J.; Arruda, P.H.Z.; Nogueira, J.S. Variations in aboveground vegetation structure along a nutrient availability gradient in the Brazilian Pantanal. Plant Soil 2015, 389, 307–321. [Google Scholar] [CrossRef]
- Hoffmann, W.A.; Geiger, E.L.; Gotsch, S.G.; Rossatto, D.R.; Silva, L.C.R.; Lau, O.L.; Haridasan, M.; Franco, A.C. Ecological thresholds at the savanna-forest boundary: How plant traits, resources and fire govern the distribution of tropical biomes. Ecol. Lett. 2012, 15, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.C.R.; Hoffmann, W.A.; Rossatto, D.R.; Haridasan, M.; Franco, A.C.; Horwath, W.R. Can savannas become forests? A coupled analysis of nutrient stocks and fire thresholds in central Brazil. Plant Soil 2013, 373, 829–842. [Google Scholar] [CrossRef]
- Lopes, A.S.; Cox, F.R. Cerrado vegetation in Brazil: An edaphic gradient. Agron. J. 1977, 69, 828–831. [Google Scholar] [CrossRef]
- Junk, W.J.; Nunes da Cunha, C.; Wantzen, K.M.; Petermann, P.; Strussmann, C.; Marques, M.I.; Adis, J. Biodiversity and its conservation in the Pantanal of Mato Grosso, Brazil. Aquat. Sci. 2006, 68, 278–309. [Google Scholar] [CrossRef]
- Nunes da Cunha, C.; Junk, W.; Leitão-Filho, H. Woody vegetation in the Pantanal of Mato Grosso, Brazil: A preliminary typology. Amazoniana 2007, 19, 159–184. [Google Scholar]
- Ribeiro, J.F.; Walter, B.M.T. As Principais Fitofisionomias do Bioma Cerrado. In Cerrado Ecologia e Flora; Sano, S.M., Pedrosa de Almeida, S., Ribeiro, J.F., Eds.; Emprapa Informacao Technologica, Ministerio da Agricultura, Pecuaria e Abastecimento, Brasilia: Districto Federal, Brasil, 2008; Volume 1, pp. 151–212. [Google Scholar]
- Pott, A.; Oliveiraa, A.K.M.; Damasceno-Junior, G.A.; Silva, J.S.V. Plant diversity of the Pantanal wetland. Brazil J. Biol. 2011, 71, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, E.L.; Santos, S.A.; Urbanetz, C.; Filho, A.C.; Naime, U.J.; Silva, M.L.N.; Curi, N. Relação entre solos e unidades da paisagem no ecossistema Pantanal. Pesq. Agropec. Bras. 2016, 51, 1231–1240. [Google Scholar] [CrossRef]
- Boni, P.V.; Gradella, F.D.S.; Decco, H.F. Comparação granulométrica com vistas para o entendimento sedimentar em áreas úmidas no Pantanal da Nhecolândia-MS. Per. Elet. Fórum Amb. Alta Paulista 2016, 12, 2. [Google Scholar] [CrossRef]
- Dalmagro, H.J.; Lathuillière, M.J.; Vourlitis, G.L.; Campos, R.C.; Pinto, O.B., Jr.; Johnson, M.S.; Ortiz, C.E.R.; Couto, E.G. Soil oxidation-reduction potential and its impacts on plant physiology in seasonally inundated soils of the Brazilian Pantanal. J. Veg. Sci. 2016, 27, 568–577. [Google Scholar] [CrossRef]
- Fantin-Cruz, I.; Girard, P.; Zeilhofer, P.; Collischonn, W.; Nunes Da Cunha, C. Unidades fitofisionômicas em mesoescala no Pantanal Norte e suas relações com a geomorfologia. Biota Neotropica 2010, 10, 31–38. [Google Scholar] [CrossRef]
- Nunes da Cunha, C.; Junk, W.J. Distribution of woody plant communities along the flood gradient in the Pantanal of Pocone, Mato Grosso, Brazil. Int. J. Ecol. Environ. Sci. 2001, 27, 63–70. [Google Scholar]
- Pott, A.; Pott, V.J. Plantas do Pantanal; Empresa Brasileira de Pesquisa, Agropecuaria, Centro de Pesquisa Agropecuaria do Pantanal: Corumba, MS, Brasil, 1994; p. 319. [Google Scholar]
- Vourlitis, G.L.; Hentz, C.S.; Pinto, O.B., Jr.; Carneiro, E.; Nogueira, J.S. Soil N, P, and C dynamics of upland and seasonally flooded forests of the Brazilian Pantanal. Glob. Ecol. Conserv. 2017, 12, 227–240. [Google Scholar] [CrossRef]
- Pinto Junior, O.B.; Vourlitis, G.L.; Carneiro, E.M.; Dias, M.D.F.; Hentz, C.; Nogueira, J.D.S. Interactions between Vegetation, hydrology, and litter inputs on decomposition and soil CO2 efflux of tropical forests in the Brazilian Pantanal. Forests 2018, 9, 281. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Responses of woody plants to flooding and salinity. Tree Phys. 1997, 17, 490. [Google Scholar] [CrossRef]
- Nunes da Cunha, C.; Junk, W. Year-to-year changes in water level drive the invasion of Vochysia divergens in Pantanal grasslands. Appl. Veg. Sci. 2004, 7, 103–110. [Google Scholar]
- Biudes, M.S.; Machado, N.G.; Danelichen, V.H.M.; Souza, M.C.; Vourlitis, G.L.; Nogueira, J.S. Ground and remote sensing-based measurements of leaf area index in a transitional forest and seasonal flooded forest in Brazil. Int. J. Biomet. 2013. [Google Scholar] [CrossRef] [PubMed]
- Lathuillière, M.J.; Pinto, O.B., Jr.; Johnson, M.S.; Jassal, R.S.; Dalmagro, H.J.; Leite, N.K.; Speratti, A.B.; Krampe, D.; Couto, E.G. Soil CO2 concentrations and efflux dynamics of a tree island in the Pantanal wetland. J. Geophys. Res. Biogeosci. 2017, 122, 2154–2169. [Google Scholar] [CrossRef]
- Couto, E.G.; Oliveira, V.A. The Soil Diversity of the Pantanal. In The Pantanal: Ecology, Biodiversity, and Sustainable Management of a Large Neotropical Seasonal Wetland; Junk, W.J., da Silva, C.J., Nunes da Cunha, C., Wantzen, K.M., Eds.; Pensoft Publishers: Sofia-Moscow, Bulgaria, 2011; pp. 71–102. [Google Scholar]
- Marthews, T.R.; Metcalfe, D.; Malhi, Y.; Phillips, O.; Huaraca Huasco, W.; Riutta, T.; Ruiz Jaén, M.; Girardin, C.; Urrutia, R.; Butt, N.; et al. Measuring Tropical Forest Carbon Allocation and Cycling: A RAINFOR-GEM Field Manual for Intensive Census Plots (v2.2); Global Ecosystems Monitoring Network. 2012. Available online: http://gem.tropicalforests.ox.ac.uk/ (accessed on 29 September 2014).
- Chave, J.; Rejou-Mechain, M.; Burquez, A.; Chidumayo, E.; Colgan, M.S.; Deltiti, W.B.C.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Chang. Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef] [PubMed]
- Schöngart, J.; Arieira, J.; Felfili Fortes, C.; de Arruda, E.C.; Nunes da Cunha, C.N. Age-related and stand-wise estimates of carbon stocks and sequestration in the aboveground coarse wood biomass of wetland forests in the northern Pantanal, Brazil. Biogeoscinces 2011, 8, 3407–3421. [Google Scholar] [CrossRef]
- del Valle, J.I.; Guarin, J.R.; Sierra, C.A. Unambiguous and low-cost determination of growth rates and ages of tropical trees and palms. Radiocarbon 2014, 56, 39–52. [Google Scholar] [CrossRef]
- Olsen, J.S. Energy Storage and the Balance of Producers and Decomposers in Ecological Systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Clark, D.A.; Brown, S.B.; Kicklighter, D.W.; Chambers, J.Q.; Thomlinson, J.R.; Ni, J. Measuring net primary production in forests: Concepts and field methods. Ecol. Appl. 2001, 11, 356–370. [Google Scholar] [CrossRef]
- Dalmolin, Â.C.; Lobo, F.A.; Vourlitis, G.; Silva, P.R.; Dalmagro, P.R.; Antunes, M.Z., Jr.; Ortíz, C.E.R. Is the dry season an important driver of phenology and growth for two Brazilian savanna tree species with contrasting leaf habits? Plant Ecol. 2015, 216, 407–417. [Google Scholar] [CrossRef]
- Keller, M.; Alencar, A.; Asner, G.P.; Braswell, B.; Bustamante, M.; Davidson, E.; Feldpausch, T.; Fernandes, E.; Goulden, M.; Kabat, P.; et al. Ecological Research in the Large Scale Biosphere Atmosphere Experiment in Amazonia (LBA): Early Results. Ecol. Appl. 2004, 14, S3–S16. [Google Scholar] [CrossRef]
- Sanches, L.; Valentini, C.M.A.; Pinto, O.B., Jr.; Nogueira, J.S.; Vourlitis, G.L.; Biudes, M.S.; da Silva, C.J.; Bambi, P.; Lobo, F.A. Seasonal and interannual litter dynamics of a tropical semideciduous forest of the southern Amazon Basin, Brazil. J. Geophys. Res.-Biogeosci. 2008, 113, G04007. [Google Scholar] [CrossRef]
- Chave, J.; Navarrete, D.; Almeida, S.; lvarez, E.A.; Aragao, L.E.; Bonal, D.; Chatelet, P.; Silva Espejo, J.; Goret, J.-Y.; von Hildebrand, P.; et al. Regional and temporal patterns of litterfall in tropical South America. Biogeosci. Discuss. 2009, 6, 7565–7597. [Google Scholar] [CrossRef]
- Wieder, R.K.; Wright, S.J. Tropical forest litter dynamics and dry season irrigation on Barro Colorado Island, Panama. Ecology 1995, 76, 1971–1979. [Google Scholar] [CrossRef]
- Valentini, C.M.A.; Sanches, L.; de Paula, S.R.; Vourlitis, G.L.; Nogueira, J.S.; Pinto, O.B., Jr.; Lobo, F.A. Soil respiration and aboveground litter dynamics of a tropical transitional forest in northwest Mato Grosso, Brazil. J. Geophys. Res. Biogeosci. 2008, 113, G00B10. [Google Scholar] [CrossRef]
- Powers, J.S.; Montgomery, R.A.; Adair, E.C.; Brearley, F.Q.; DeWalt, S.J.; Castanho, C.T.; Chave, J.; Deinert, E.; Ganzhorn, J.U.; Gilbert, M.E.; et al. Decomposition in tropical forests: A pan-tropical study of the effects of litter type, litter placement and mesofaunal exclusion across a precipitation gradient. J. Ecol. 2009, 97, 801–811. [Google Scholar] [CrossRef]
- Dalmagro, H.J.; de Arruda, P.H.Z.; Vourlitis, G.L.; Lathuillière, M.J.; Nogueira, J.S.; Couto, E.G.; Johnson, M.S. Radiative forcing of methane fluxes offsets net carbon dioxide uptake for a tropical flooded forest. Glob. Chang. Biol. 2019, 25, 1967–1981. [Google Scholar] [CrossRef]
- Johnson, M.S.; Couto, E.G.; Pinto, O.B., Jr.; Milesi, J.; Amorim, R.S.S.; Messias, I.A.M.; Biudes, M.S. Soil CO2 dynamics in a tree island soil of the Pantanal: The role of soil water potential. PLoS ONE 2013, 8, e64874. [Google Scholar] [CrossRef]
- Fanin, N.; Hättenschwiler, S.; Barantal, S.; Schimann, H.; Fromin, N. Does variability in litter quality determine soil microbial respiration in an Amazonian rainforest? Soil Biol. Biochem. 2011, 43, 1014–1022. [Google Scholar] [CrossRef]
- Fang, X.; Zhao, L.; Zhou, G.; Huang, W.; Liu, J. Increased litter input increases litter decomposition and soil respiration but has minor effects on soil organic carbon in subtropical forests. Plant Soil 2015, 392, 139–153. [Google Scholar] [CrossRef]
- Vourlitis, G.L.; Lobo, F.A.; Lawrence, S.; Holt, K.; Zappia, A.; Pinto, O.B., Jr.; Nogueira, J.S. Nutrient resorption in tropical savanna forests and woodlands of central Brazil. Plant Ecol. 2014, 215, 963–975. [Google Scholar] [CrossRef]
- Haase, R. Litterfall and nutrient return in seasonally flooded and non-flooded forest of the Pantanal, Mato Grosso, Brazil. For. Ecol. Manag. 1999, 117, 129–147. [Google Scholar] [CrossRef]
- Breitsprecher, A.; Bethel, J.S. Stem-growth periodicity of trees in a tropical wet forest of Costa Rica. Ecology 1990, 71, 1156–1164. [Google Scholar] [CrossRef]
- Girard, P. Hydrology of Surface and Ground Water in the Pantanal Floodplains. In The Pantanal: Ecology, Biodiversity and Sustainable Management of a Large Neotropical Seasonal Wetland; Junk, W.J., da Silva, C.J., Nunes da Cunha, C., Wantzen, K.M., Eds.; Pensoft Publishers: Sofia, Bulgaria, 2010; pp. 103–126. [Google Scholar]
- Condit, R.; Hubbell, S.P.; Foster, R.B. Mortality rates of 205 neotropical tree and shrub species and the impact of a severe drought. Ecol. Monogr. 1995, 65, 419–439. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, M.; Zhang, Y.; Zeng, X.; Xiao, X. Response of tropical terrestrial gross primary production to the super El Niño event in 2015. J. Geophys. R. Biogeosci. 2018, 123, 3193–3203. [Google Scholar] [CrossRef]
- Phillips, O.L.; Aragão, L.E.O.C.; Lewis, S.L.; Fisher, J.B.; Lloyd, J.; Lopez-Gonzalez, G.; Malhi, Y.; Monteagudo, A.; Peacock, J.; Quesada, C.A.; et al. Drought sensitivity of the Amazon rainforest. Science 2009, 323, 1344–1347. [Google Scholar] [CrossRef]
- Sjögersten, S.; Black, C.R.; Evers, S.; Hoyos-Santillan, J.; Wright, E.L.; Turner, B.L. Tropical wetlands: A missing link in the global carbon cycle? Glob. Biogeochem. Cycles 2014, 28, 1371–1386. [Google Scholar] [CrossRef]

| Variable | Units | Mixed Forest | Cerrado Forest | ||
|---|---|---|---|---|---|
| r | Lag (Months) | r | Lag (Months) | ||
| Litterfall | gdw/m2 | −0.87 | 1 | −0.76 | 2 |
| Litterpool | gdw/m2 | −0.80 | 3 | −0.75 | 3 |
| Leaf litter | % | −0.84 | 0 | −0.79 | 0 |
| Stem litter | % | 0.86 | 0 | 0.65 | 0 |
| Reproductive litter | % | NS | ─ | 0.63 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges Pinto, O., Jr.; Marques, A.C.A.; Vourlitis, G.L. Aboveground Carbon Storage and Cycling of Flooded and Upland Forests of the Brazilian Pantanal. Forests 2020, 11, 665. https://doi.org/10.3390/f11060665
Borges Pinto O Jr., Marques ACA, Vourlitis GL. Aboveground Carbon Storage and Cycling of Flooded and Upland Forests of the Brazilian Pantanal. Forests. 2020; 11(6):665. https://doi.org/10.3390/f11060665
Chicago/Turabian StyleBorges Pinto, Osvaldo, Jr., Ana Carolina Amorim Marques, and George L. Vourlitis. 2020. "Aboveground Carbon Storage and Cycling of Flooded and Upland Forests of the Brazilian Pantanal" Forests 11, no. 6: 665. https://doi.org/10.3390/f11060665
APA StyleBorges Pinto, O., Jr., Marques, A. C. A., & Vourlitis, G. L. (2020). Aboveground Carbon Storage and Cycling of Flooded and Upland Forests of the Brazilian Pantanal. Forests, 11(6), 665. https://doi.org/10.3390/f11060665





