The Staining Effect of Iron (II) Sulfate on Nine Different Wooden Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Sample Preparation
2.2. Exposure and Evaluation of Color Differences
3. Results
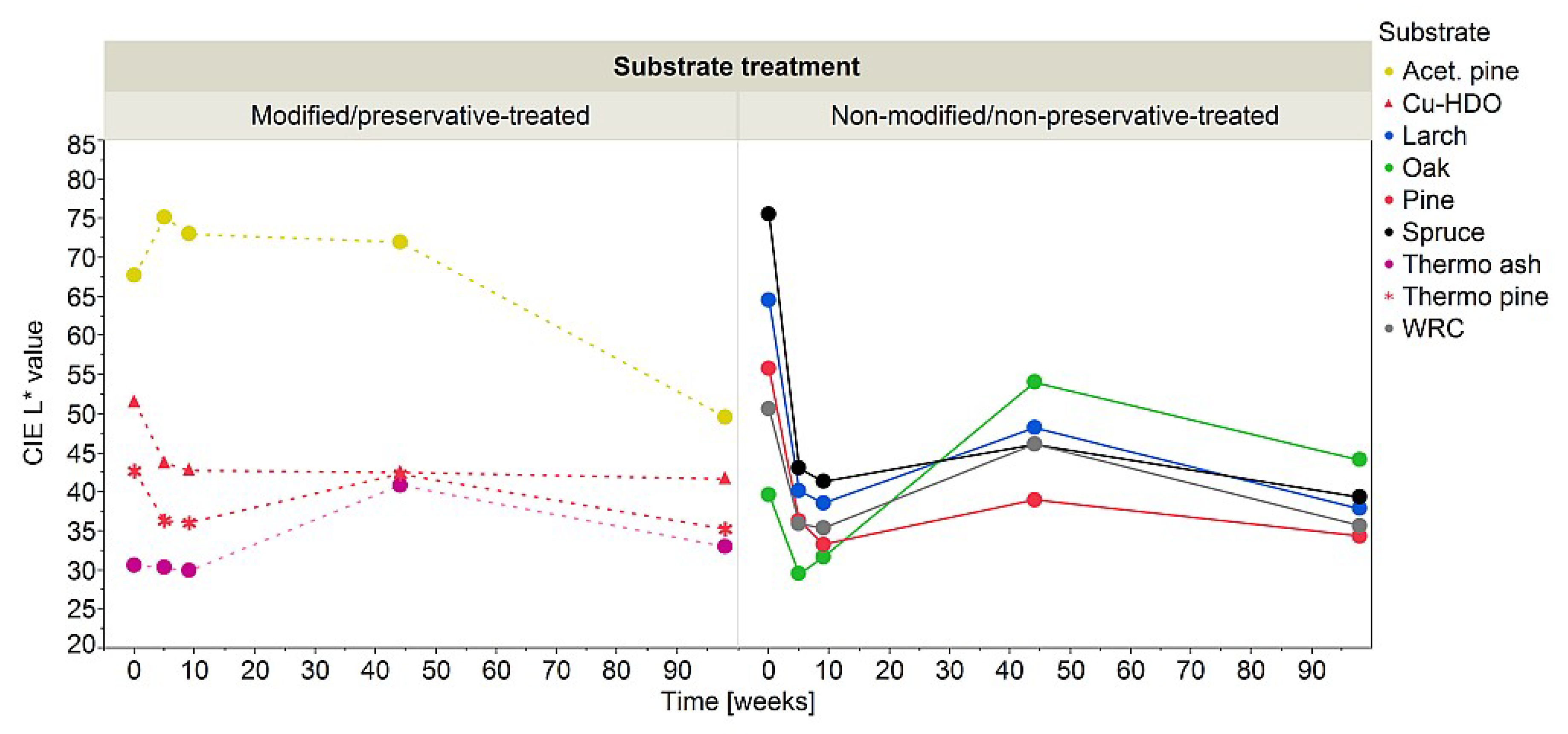
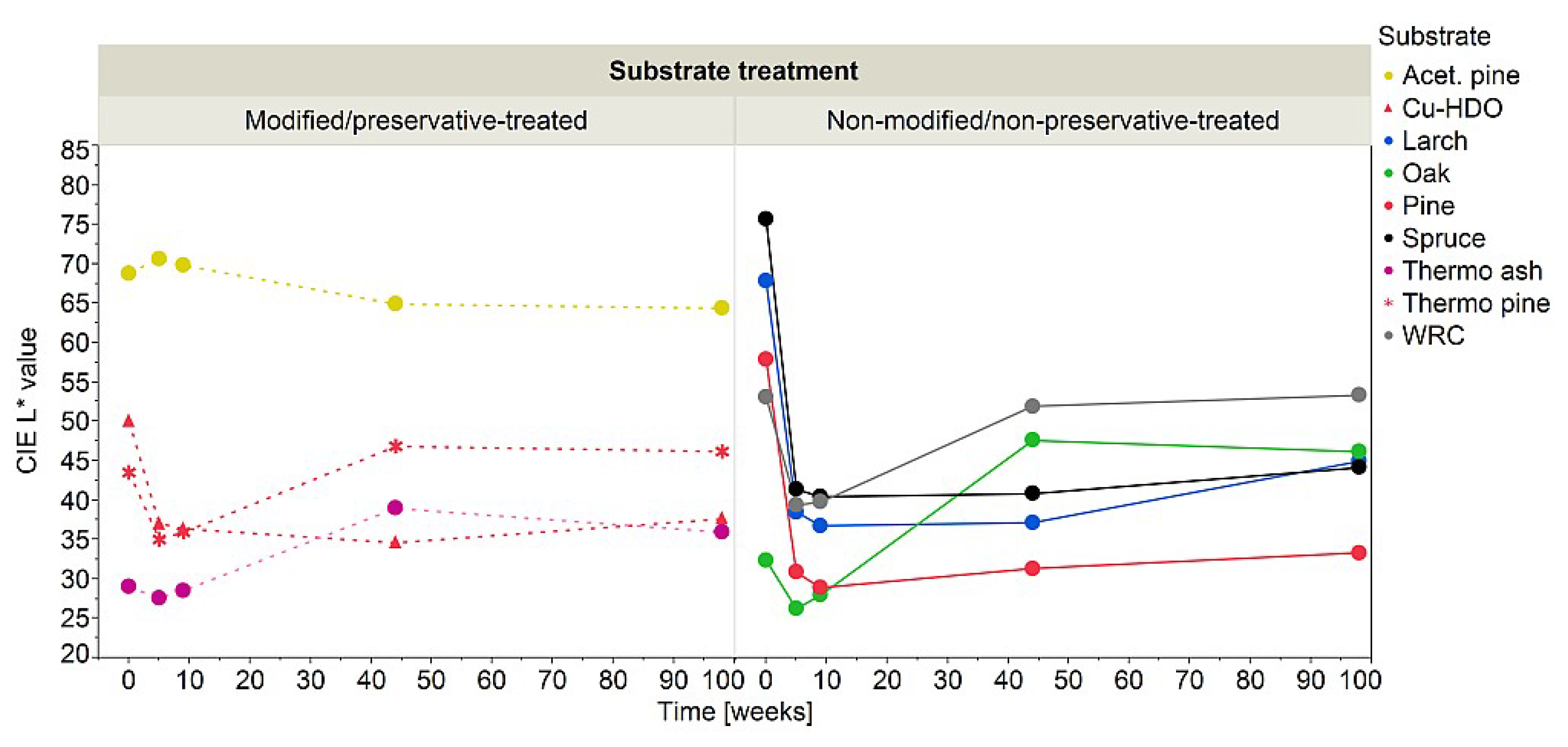
3.1. Indoor Exposure
3.2. Outdoor Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lin, S.Y.; Kringstad, K.P. Mechanisms in the yellowing of high-yield pulps by light: Structure and reactivity of free-radical intermediates in the photodegradation of lignin. Tappi J. 1970, 53, 2296–2300. [Google Scholar]
- Li, C.; Ragauskas, A.J. Brightness reversion of mechanical pulps. Part XVII: Diffuse reflectance study on brightness stabilization by additives under various atmospheres. Cellulose 2000, 7, 369–385. [Google Scholar] [CrossRef]
- Norrström, H. Light absorbing properties of pulp and pulp components. Sven. Papp. 1969, 72, 25–31. [Google Scholar]
- Carter, H.A. The chemistry of paper preservation: Part 2. The yellowing of paper and conservation bleaching. J. Chem. Educ. 1996, 73, 1068–1073. [Google Scholar] [CrossRef]
- Hon, D.N.-S.; Shiraishi, N. Wood and Cellulosic Chemistry, 2nd ed.; Hon, D.N.-S., Ed.; CRC Press: Boca Raton, FL, USA, 2000; p. 928. [Google Scholar]
- Kataoka, Y.; Kiguchi, M.; Williams, R.S.; Evans, P.D. Violet light causes photodegradation of wood beyond the zone affected by ultraviolet radiation. Holzforschung 2007, 61, 23–27. [Google Scholar] [CrossRef]
- Feist, W.; Hon, D. Chemistry of weathering and protection. In The Chemistry of Solid Wood, 1st ed.; Rowell, R.M., Ed.; American Chemical Soc.: Washington, DC, USA, 1984; pp. 401–451. [Google Scholar]
- Schoeman, M.; Dickinson, D. Growth of Aureobasidium pullulans on lignin breakdown products at weathered wood surfaces. Mycologist 1997, 11, 168–172. [Google Scholar] [CrossRef]
- Evans, P.D.; Haase, J.G.; Seman, A.S.; Kiguchi, M. The search for durable exterior clear coatings for wood. Coatings 2015, 5, 830–864. [Google Scholar] [CrossRef]
- Nejad, M.; Cooper, P. Exterior wood coatings. In Wood in Civil Engineering, 1st ed.; Concu, G., Ed.; InTech: Rijeka, Croatia, 2017; pp. 111–129. [Google Scholar]
- Petric, M. Surface modification of wood: A critical review. Rev. Adhes. Adhes. 2013, 1, 216–247. [Google Scholar] [CrossRef]
- Hirche, M. Wood Weathering as Design Option. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2014. [Google Scholar]
- Gobakken, L.R.; Alfredsen, G.; Høibø, O.; Vestøl, G. Aesthetical aspects of discolouring fungi on wood in-service. In Proceedings of the 1st COST FP1303 International Conference—Performance and Maintenance of Bio-Based Building Materials Influencing the Life Cycle and LCA, Kranjska Gora, Slovenia, 23–24 October 2014; pp. 17–18. [Google Scholar]
- Podgorski, L.; Georges, V.; Izaskun Garmendia, I.; Sarachu, B.S. A fast and economic method to produce grey wooden surfaces for decking and cladding: Preliminary results. In Proceedings of the IRG/WP 40th IRG Annual Meeting, Beijing, China, 24–28 May 2009. [Google Scholar]
- Evans, P.D.; Thay, P.; Schmalzl, K. Degradation of wood surfaces during natural weathering. Effects on lignin and cellulose and on the adhesion of acrylic latex primers. Wood Sci. Technol. 1996, 30, 411–422. [Google Scholar] [CrossRef]
- Schnabel, T.; Petutschnigg, A. Modelling colour changes of wood for architectural CAD simulations. Comput. Aided Des. 2011, 43, 1849–1853. [Google Scholar] [CrossRef]
- Zimmer, K.; Gobakken, L.R.; Flindall, O.; Nygaard, M. Colour changes in unpainted wooden façades—Fifty Shades of Grey. In Proceedings of the IRG/WP 49th IRG Annual Meeting, Johannesburg, South Africa, 29 April–3 May 2018. [Google Scholar]
- Evans, P.D. Weathering of wood and wood composites. In Handbook of Wood Chemistry and Wood Composites, 2nd ed.; Rowel, R.M., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 166–231. [Google Scholar]
- Høibø, O.; Nyrud, A.Q. Consumer perception of wood surfaces: The relationship between stated preferences and visual homogeneity. J. Wood Sci. 2010, 56, 276–283. [Google Scholar] [CrossRef]
- Nyrud, A.Q.; Roos, A.; Rødbotten, M. Product attributes affecting consumer preference for residential deck materials. Can. J. For. Res. 2008, 38, 1385–1396. [Google Scholar] [CrossRef]
- Gellerich, A.; Brischke, C.; Emmerich, L.; Meyer-Veltrup, L.; Kaudewitz, P. Evaluation of surface cracks on wood–physical assessment versus subjective sensation. In Proceedings of the IRG/WP 48th IRG Annual Meeting, Ghent, Belgium, 4–8 June 2017. [Google Scholar]
- Cassens, D.L.; Feist, W.C. Exterior Wood in the South: Selection, Application and Finishes; GTR-69; USDA Forest Service, Forest Products Laboratory: Madison, WA, USA, 1991; p. 60.
- Hundhausen, U. Holzfassaden in Norwegen—Tradition und moderne Architektur. In Proceedings of the Wiener Holzschutztage, Vienna, Austria, 28–29 November 2013; p. 12. [Google Scholar]
- Evensen, F. Reading in the Park: A New Context for the Library of Kungsholmen. Independent Thesis Advanced Level (Professional Degree), KTH Royal Institute of Technology, Stockholm, Sweden, 2014. [Google Scholar]
- Nilsson, K. Träskyddsbehandlingar: Jämförande Provningar Av Ett Urval Traditionella Och Moderna Medel; Meddelande 168; Svenska träskyddsinstitutet: Stockholm, Sweden, 1993; p. 64. [Google Scholar]
- Larsen, K.E.; Hakonsen, F. Kledd I Tre: Tre Som Fasademateriale, 1st ed.; Larsen, K.E., Hakonsen, F., Eds.; Gaidaros forlag AS: Oslo, Norway, 2008; p. 222. [Google Scholar]
- Krekel, C. The chemistry of historical iron gall inks. Int. J. Forensic Doc. Exam. 1999, 5, 54–58. [Google Scholar]
- Klöckl, I. Chemie der Farbmittel. In der Malerei, 1st ed.; Klöckl, I., Ed.; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2015; p. 691. [Google Scholar]
- Karpenko, V.; Norris, J.A. Vitriol in the history of chemistry. Chem. Listy 2002, 96, 997–1005. [Google Scholar]
- Canevari, C.; Delorenzi, M.; Invernizzi, C.; Licchelli, M.; Malagodi, M.; Rovetta, T.; Weththimuni, M. Chemical characterization of wood samples colored with iron inks: Insights into the ancient techniques of wood coloring. Wood Sci. Technol. 2016, 50, 1057–1070. [Google Scholar] [CrossRef]
- Edlund, M.-L.; Nilsson, K.; Henningsson, B. Jämförande Provning Av Ett Ett Uravl Traditionella Och Moderna medel; Svenska Träskyddsinstitutet: Stockholm, Sverige, 1997; p. 9. [Google Scholar]
- Sandermann, W.; Lüthgens, M. Untersuchungen über Verfärbungen von Hölzern. Holz als Roh-Und Werkst. 1953, 11, 435–440. [Google Scholar] [CrossRef]
- NWPC. Nordic Wood Protection Classes and Product Requirements for Industrially Protected Wood. Part 1: Scots Pine and Other Permeable Softwoods; Nordic Wood Preservation Council: Stockholm, Sweden, 2017; p. 8. [Google Scholar]
- CEN. EN 927-3:2012: Paints and Varnishes—Coating Materials and Coating Systems for Exterior Wood—Part 3: Natural Weathering. Test European Committee for Standardization, 2012. Available online: https://standards.iteh.ai/catalog/standards/cen/71371f20-5bfb-4d7b-8e3c-3925e7d7c92f/en-,927-3-2012 (accessed on 23 April 2020).
- Hosseinpourpia, R.; Mai, C. Mode of action of brown rot decay resistance of acetylated wood: Resistance to Fenton’s reagent. Wood Sci. Technol. 2016, 50, 413–426. [Google Scholar] [CrossRef]
- Hosseinpourpia, R.; Mai, C. Mode of action of brown rot decay resistance in phenol-formaldehyde-modified wood: Resistance to Fenton’s reagent. Holzforschung 2016, 70, 253–259. [Google Scholar] [CrossRef]
- Hosseinpourpia, R.; Mai, C. Mode of action of brown rot decay resistance of thermally modified wood: Resistance to Fenton’s reagent. Holzforschung 2016, 70, 691–697. [Google Scholar] [CrossRef]
- Wikberg, H.; Maunu, S.L. Characterisation of thermally modified hard-and softwoods by 13C CPMAS NMR. Carbohydr. Polym. 2004, 58, 461–466. [Google Scholar] [CrossRef]
- Esteves, B.; Pereira, H. Wood modification by heat treatment: A review. BioResources 2009, 4, 370–404. [Google Scholar]
- Kielmann, B.C.; Butter, K.; Mai, C. Modification of wood with formulations of phenolic resin and iron-tannin-complexes to improve material properties and expand colour variety. Eur. J. Wood Wood Prod. 2018, 76, 259–267. [Google Scholar] [CrossRef]
- Willför, S.; Hemming, J.; Reunanen, M.; Holmbom, B. Phenolic and lipophilic extractives in Scots pine knots and stemwood. Holzforschung 2003, 57, 359–372. [Google Scholar] [CrossRef]
- Willför, S.; Reunanen, M.; Eklund, P.; Sjöholm, R.; Kronberg, L.; Fardim, P.; Pietarinen, S.; Holmbom, B. Oligolignans in Norway spruce and Scots pine knots and Norway spruce stemwood. Holzforschung 2004, 58, 345–354. [Google Scholar] [CrossRef]
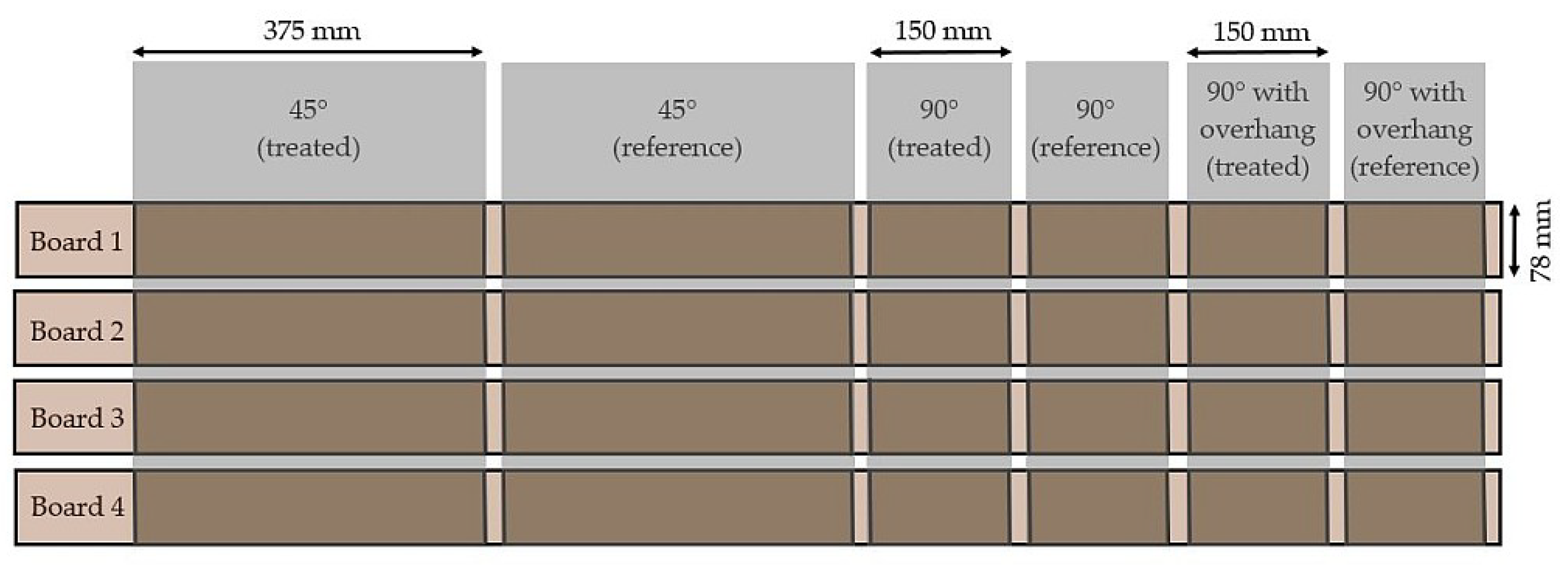
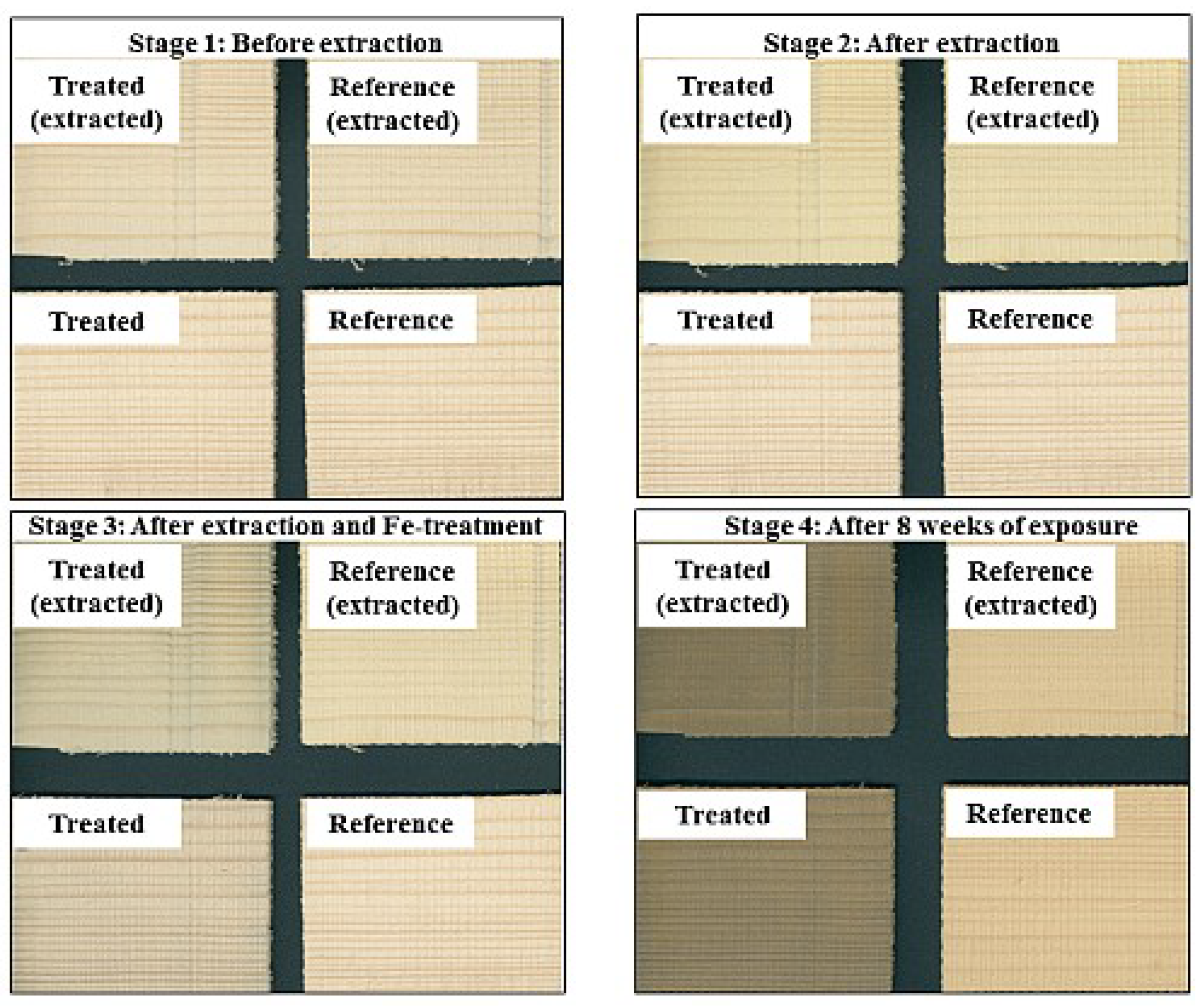




| Sample Set | Substrate | Extracted (y/n) | Iron (II) Treatment (y/n) | Exposure | n/Exposure Condition |
|---|---|---|---|---|---|
| 1 | Norway spruce | y | y | Indoor, sunlight | 1 |
| n | y | Indoor, sunlight | 1 | ||
| y | n | Indoor, sunlight | 1 | ||
| n | n | Indoor, sunlight | 1 | ||
| 2 | Norway spruce | n | y | Indoor, sunlight | 3 |
| n | y | Indoor, no light | 3 | ||
| 3 | Norway spruce | n | y | Outdoor, 45°/90°/90° with roof | 3(1) |
| Norway spruce | n | n | Outdoor, 45°/90°/90° with roof | 3(1) | |
| Siberian larch | n | y | Outdoor, 45°/90°/90° with roof | 3(1) | |
| Siberian larch | n | n | Outdoor, 45°/90°/90° with roof | 3(1) | |
| Oak | n | y | Outdoor, 45°/90°/90° with roof | 3(1) | |
| Oak | n | n | Outdoor, 45°/90°/90° with roof | 3(1) | |
| WRC | n | y | Outdoor, 45°/90°/90° with roof | 3(1) | |
| WRC | n | n | Outdoor, 45°/90°/90° with roof | 3(1) | |
| Scots pine heartwood | n | y | Outdoor, 45°/90°/90° with roof | 3(1) | |
| Scots pine heartwood | n | n | Outdoor, 45°/90°/90° with roof | 3(1) | |
| Cu-HDO pine | n | y | Outdoor, 45°/90°/90° with roof | 3(1) | |
| Cu-HDO pine | n | n | Outdoor, 45°/90°/90° with roof | 3(1) | |
| Thermo pine | n | y | Outdoor, 45°/90°/90° with roof | 3(1) | |
| Thermo pine | n | n | Outdoor, 45°/90°/90° with roof | 3(1) | |
| Thermo ash | n | y | Outdoor, 45°/90°/90° with roof | 3(1) | |
| Thermo pine | n | n | Outdoor, 45°/90°/90° with roof | 3(1) | |
| Acetylated pine | n | y | Outdoor, 45°/90°/90° with roof | 3(1) | |
| Acetylated pine | n | n | Outdoor, 45°/90°/90° with roof | 3(1) |
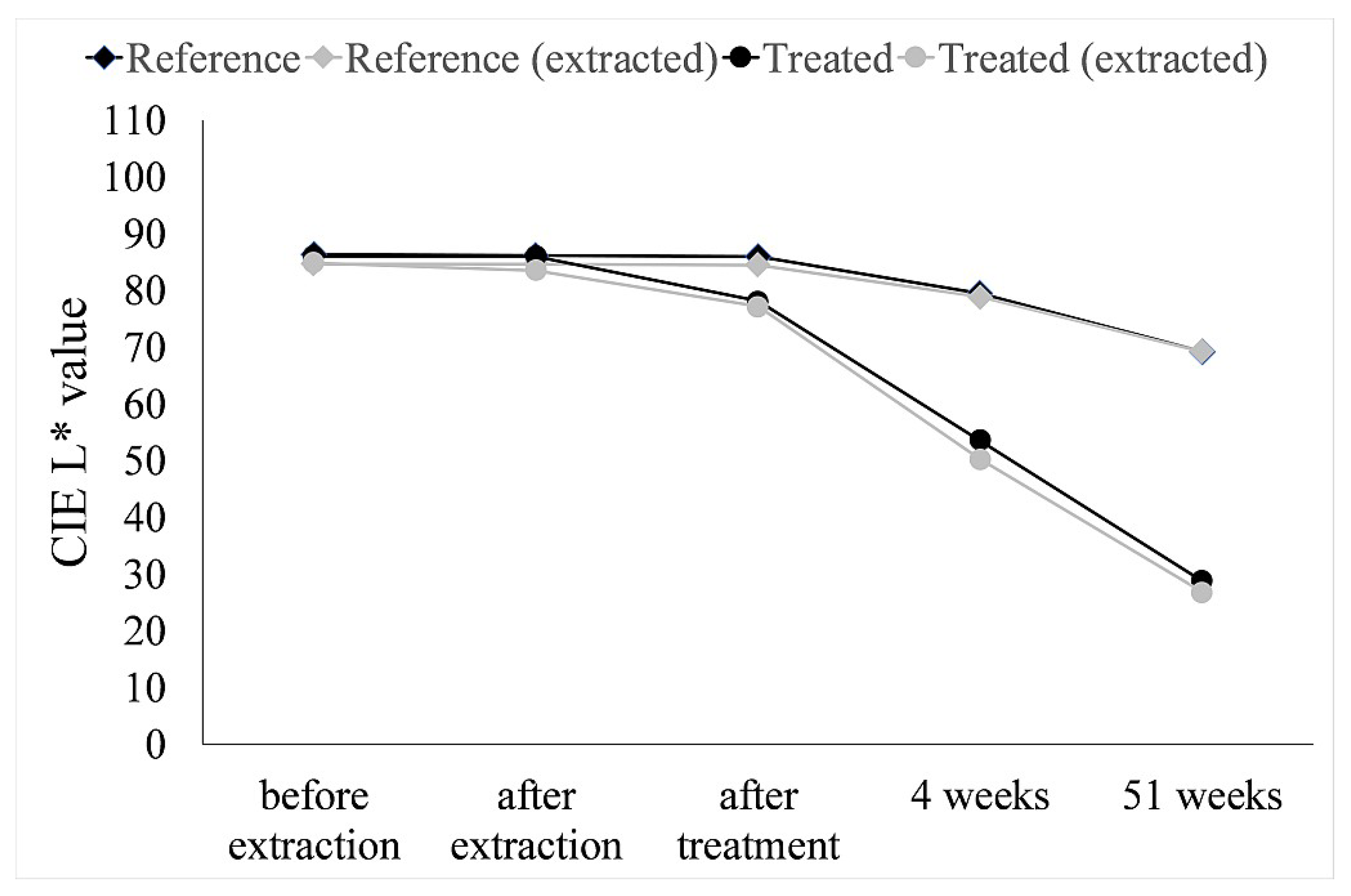
| Treatment | dE (After Extraction) | dE (After Treatment) | dE (After 4 Weeks) | dE (After 51 Weeks) |
|---|---|---|---|---|
| Reference | 0.09 | 0.23 | 9.93 | 22.05 |
| Reference (extracted) | 4.85 | 2.56 | 8.77 | 21.18 |
| Treated | 0.07 | 8.03 | 32.68 | 58.50 |
| Treated (extracted) | 5.58 | 8.01 | 34.68 | 59.56 |
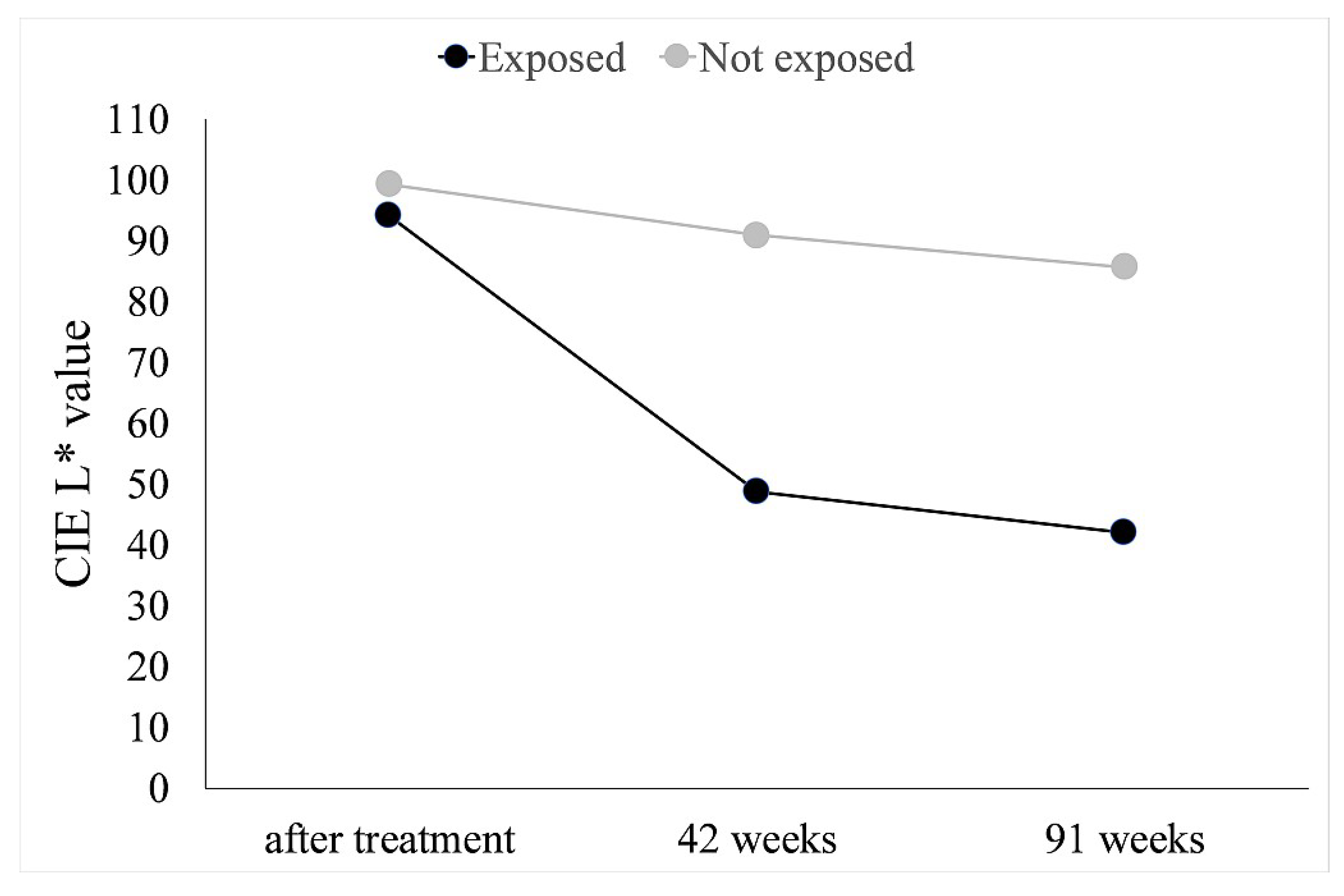
| Exposed to Light | dE (After 42 Weeks) | dE (After 91 Weeks) |
|---|---|---|
| Yes | 46.56 | 53.51 |
| No | 9.13 | 14.48 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hundhausen, U.; Mai, C.; Slabohm, M.; Gschweidl, F.; Schwarzenbrunner, R. The Staining Effect of Iron (II) Sulfate on Nine Different Wooden Substrates. Forests 2020, 11, 658. https://doi.org/10.3390/f11060658
Hundhausen U, Mai C, Slabohm M, Gschweidl F, Schwarzenbrunner R. The Staining Effect of Iron (II) Sulfate on Nine Different Wooden Substrates. Forests. 2020; 11(6):658. https://doi.org/10.3390/f11060658
Chicago/Turabian StyleHundhausen, Ulrich, Carsten Mai, Maik Slabohm, Florian Gschweidl, and Ronald Schwarzenbrunner. 2020. "The Staining Effect of Iron (II) Sulfate on Nine Different Wooden Substrates" Forests 11, no. 6: 658. https://doi.org/10.3390/f11060658
APA StyleHundhausen, U., Mai, C., Slabohm, M., Gschweidl, F., & Schwarzenbrunner, R. (2020). The Staining Effect of Iron (II) Sulfate on Nine Different Wooden Substrates. Forests, 11(6), 658. https://doi.org/10.3390/f11060658




