Leachability and Decay Resistance of Wood Polyesterified with Sorbitol and Citric Acid
Abstract
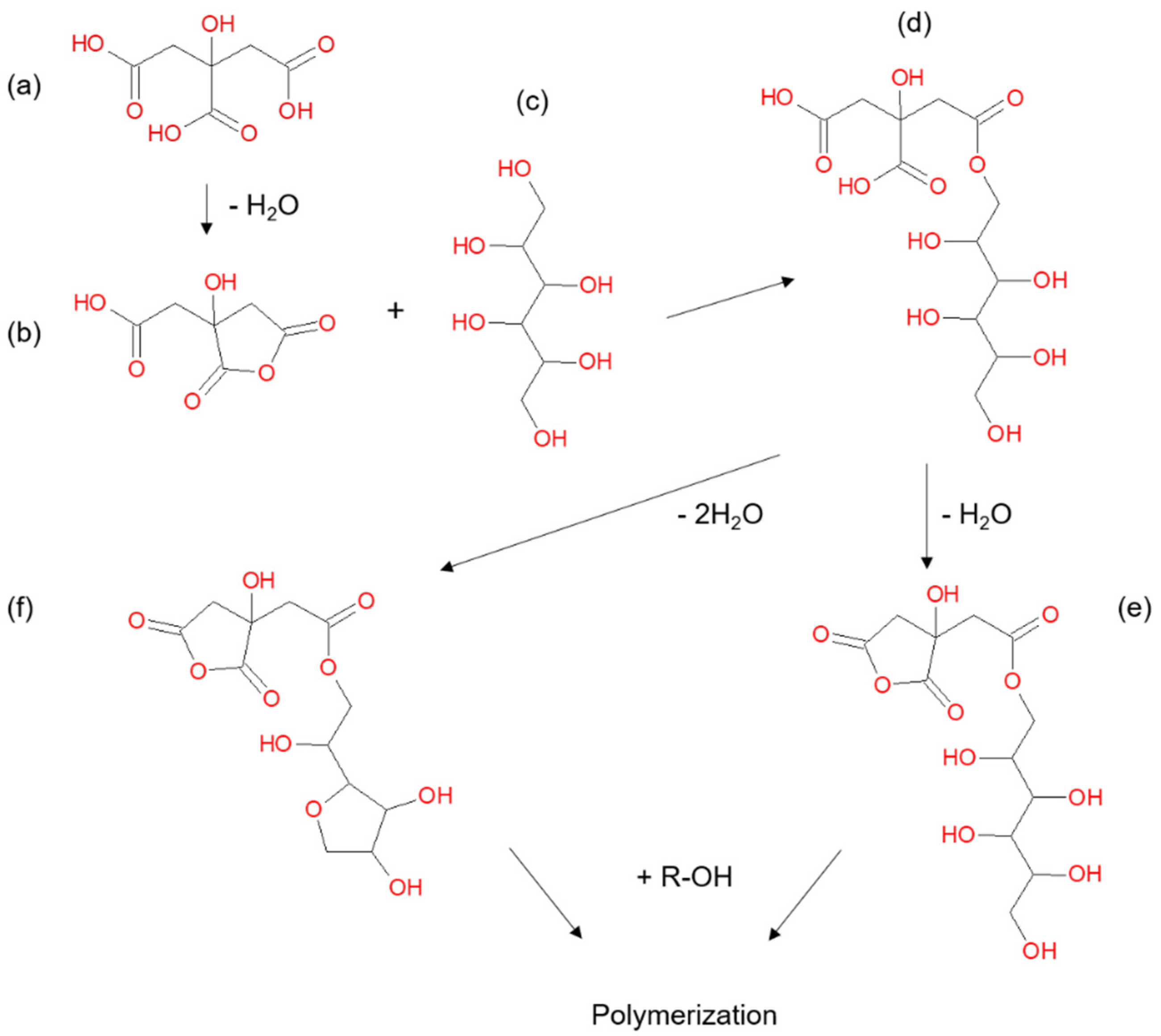
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Treatment
2.2. Dimensions Analysis
2.3. Analysis of Leachate
2.4. Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.5. Low-Field Nuclear Magnetic Resonance Relaxometry
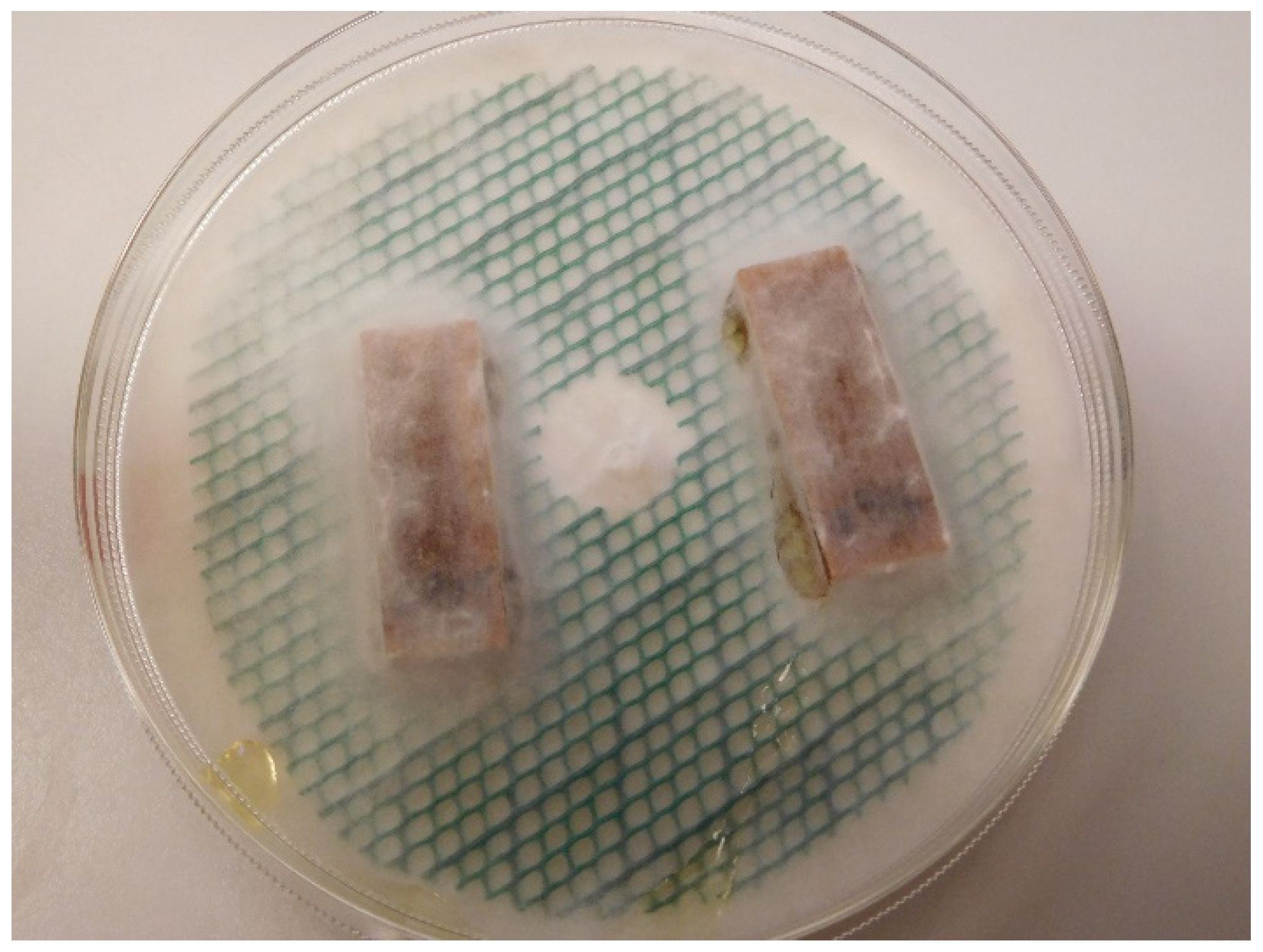
2.6. Decay Test
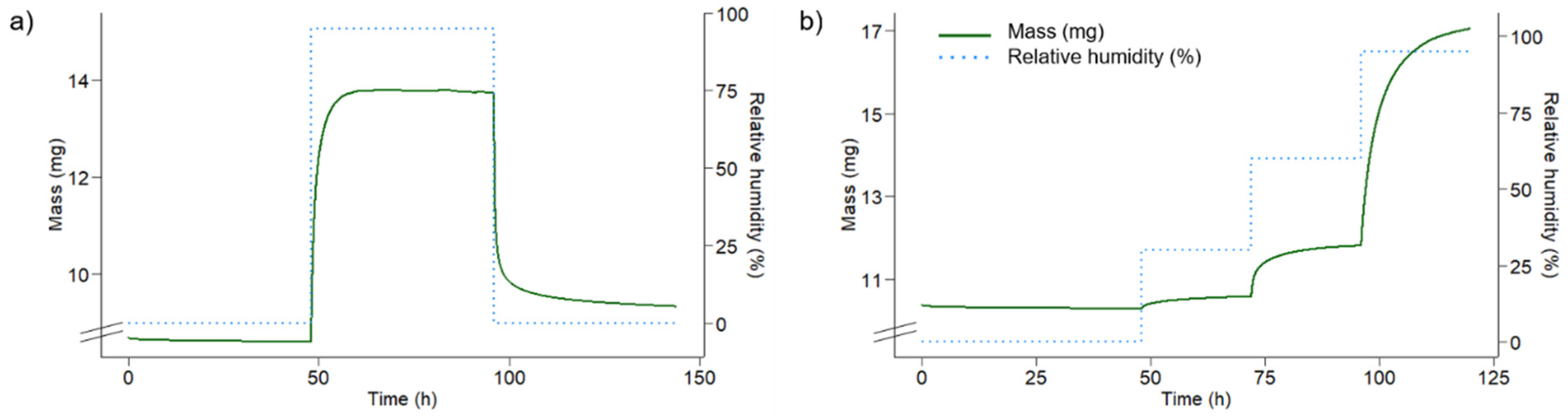
2.7. Preliminary Sorption Experiments
3. Results
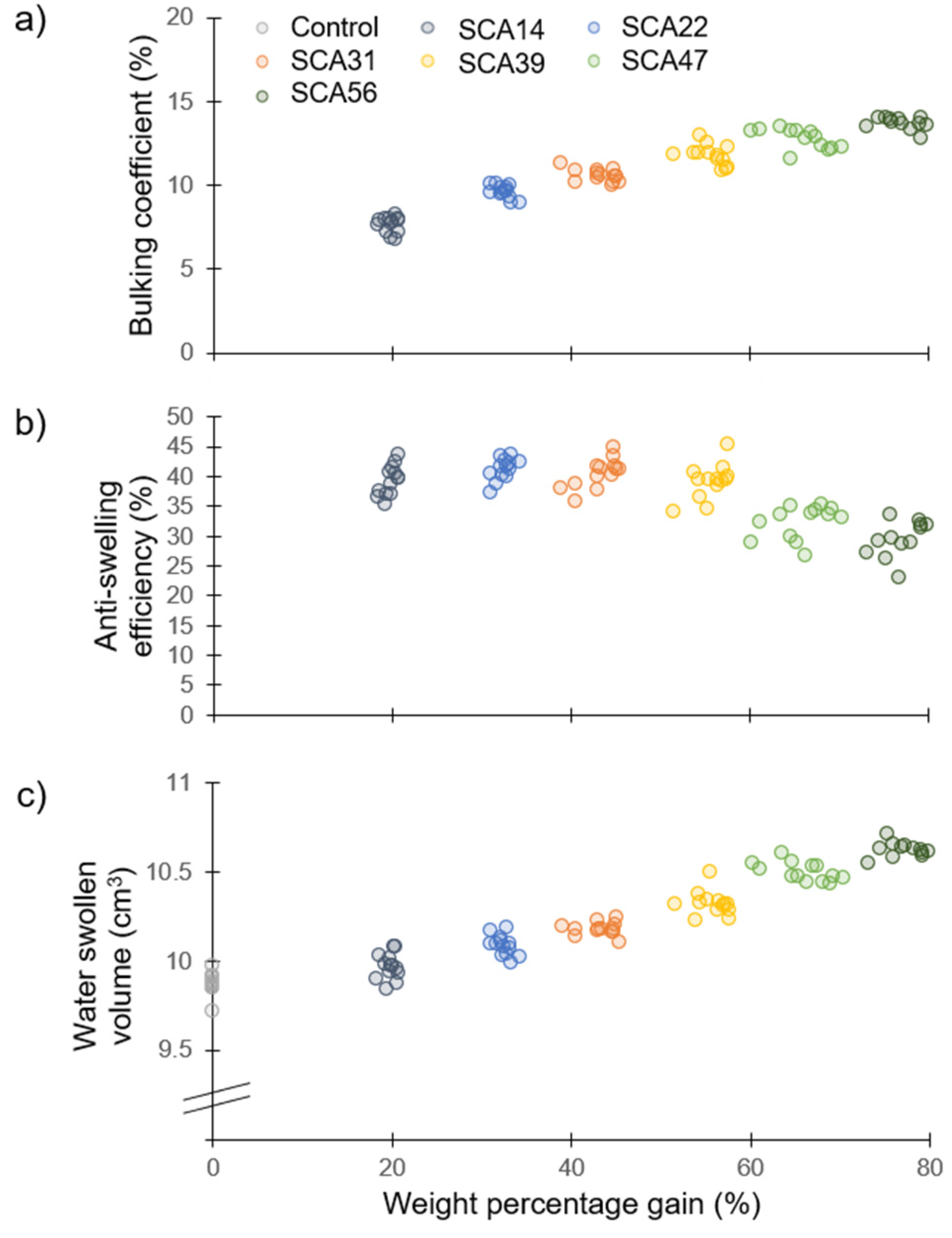
3.1. Dimensions Analysis
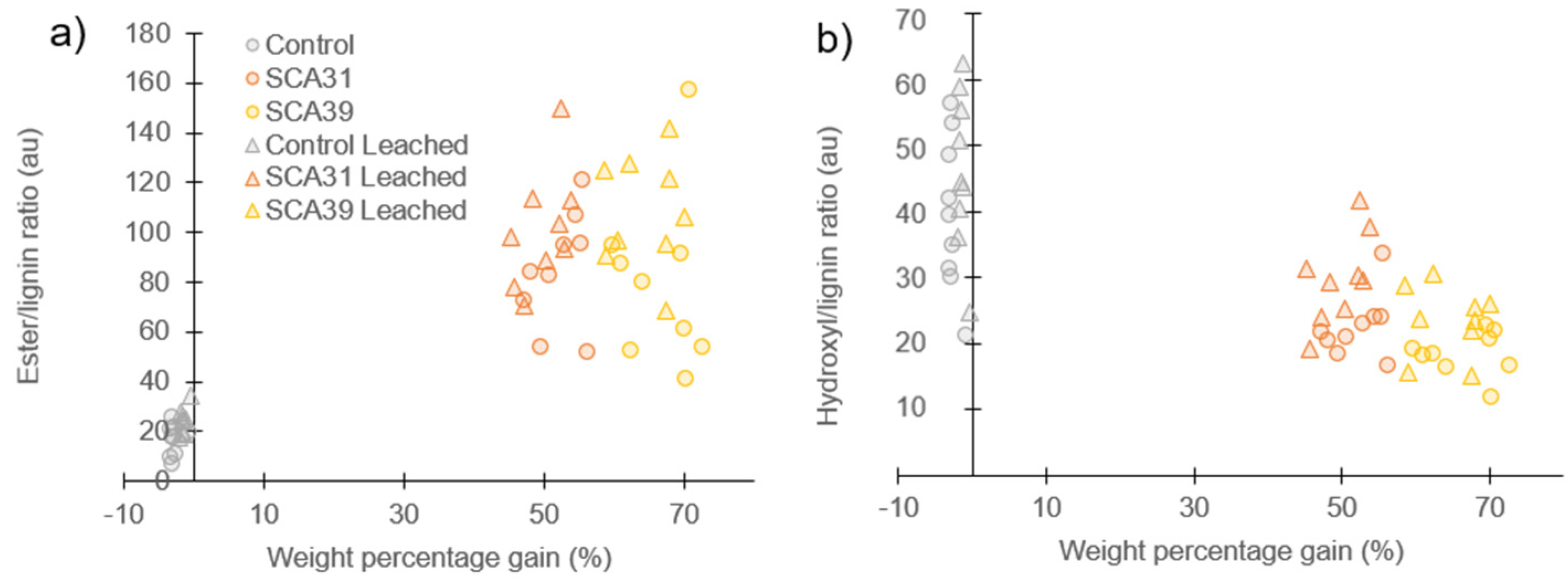
3.2. Analysis of Leachate
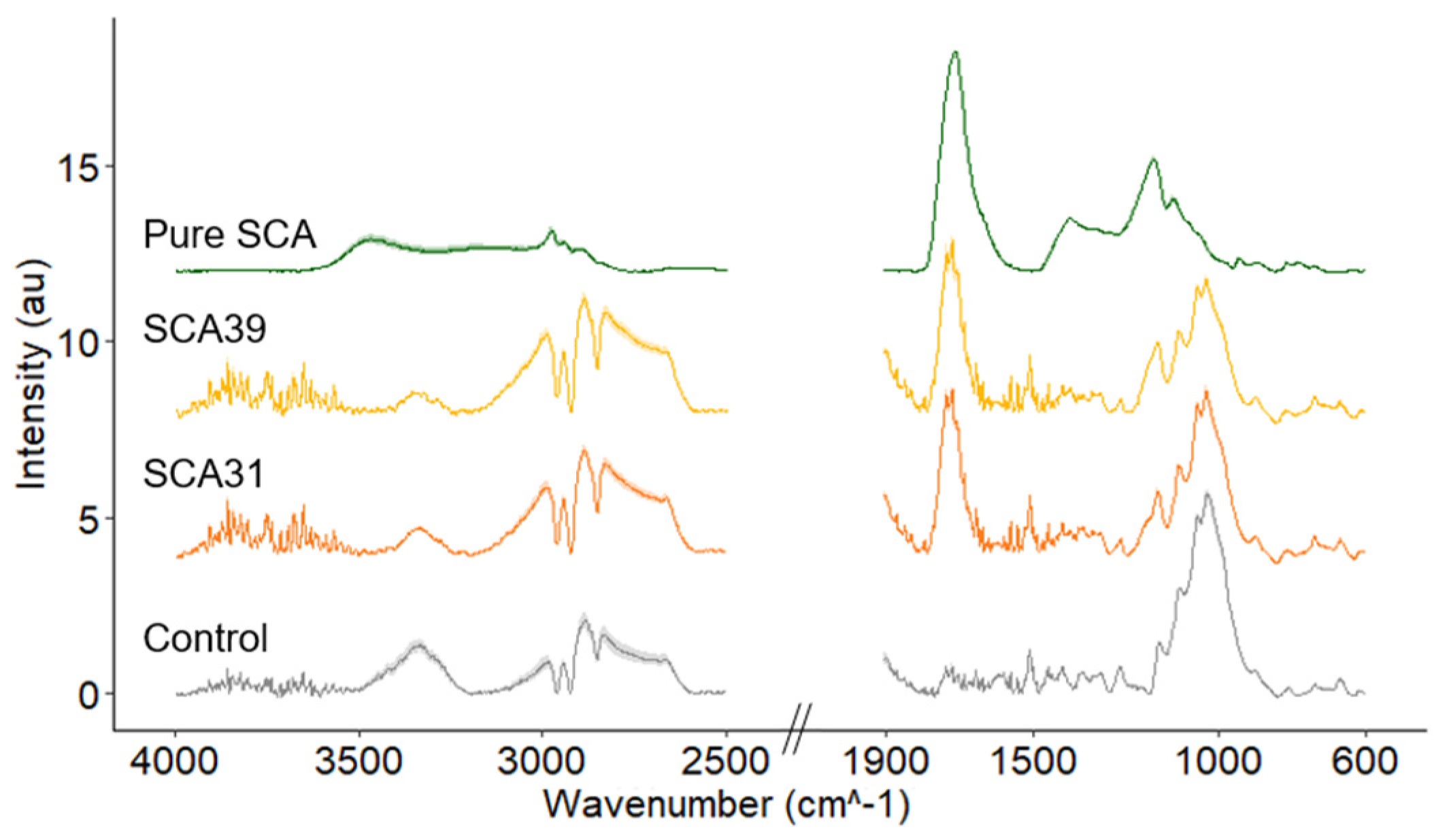
3.3. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy
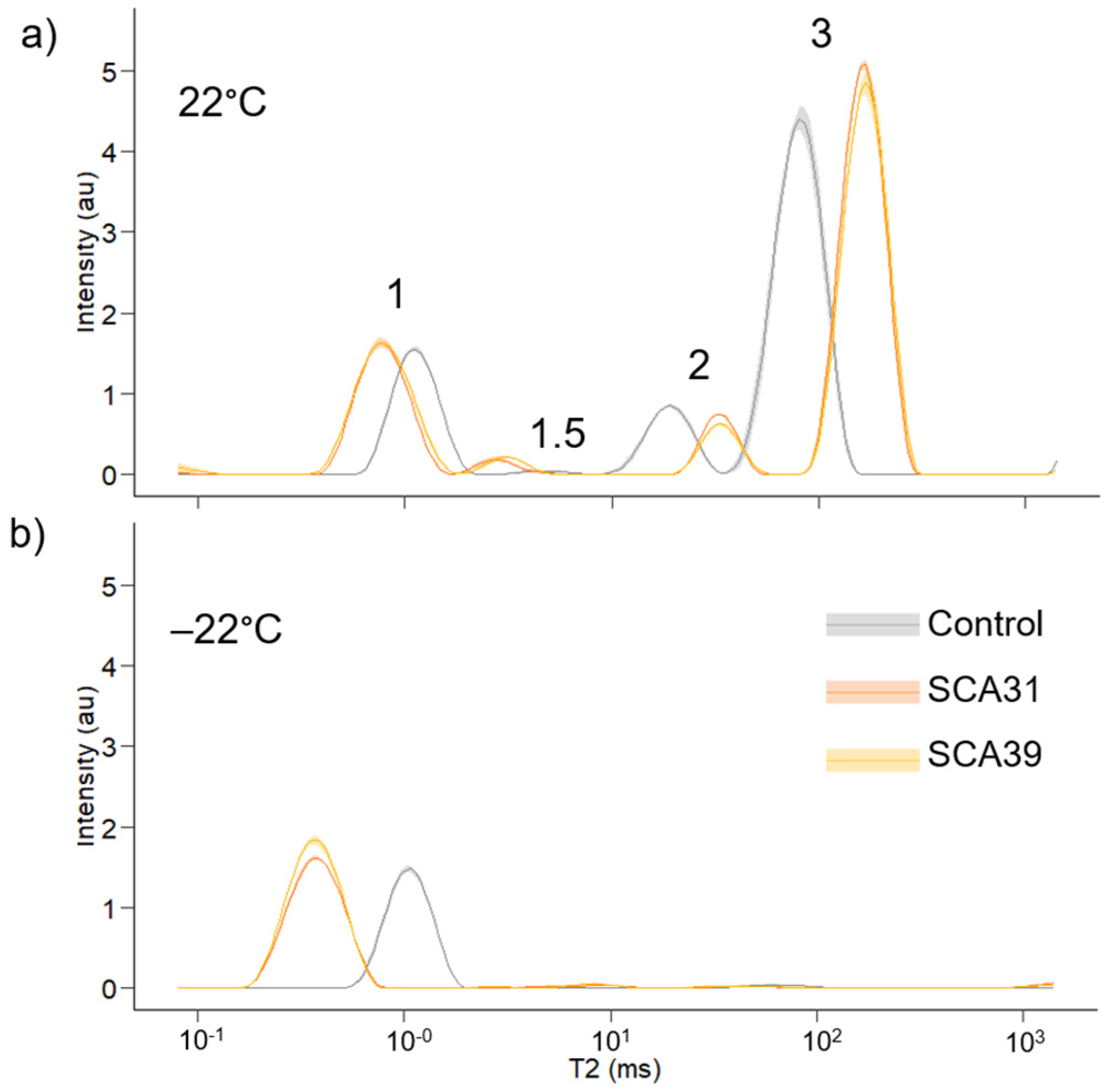
3.4. Low-Field Nuclear Magnetic Resonance Relaxometry
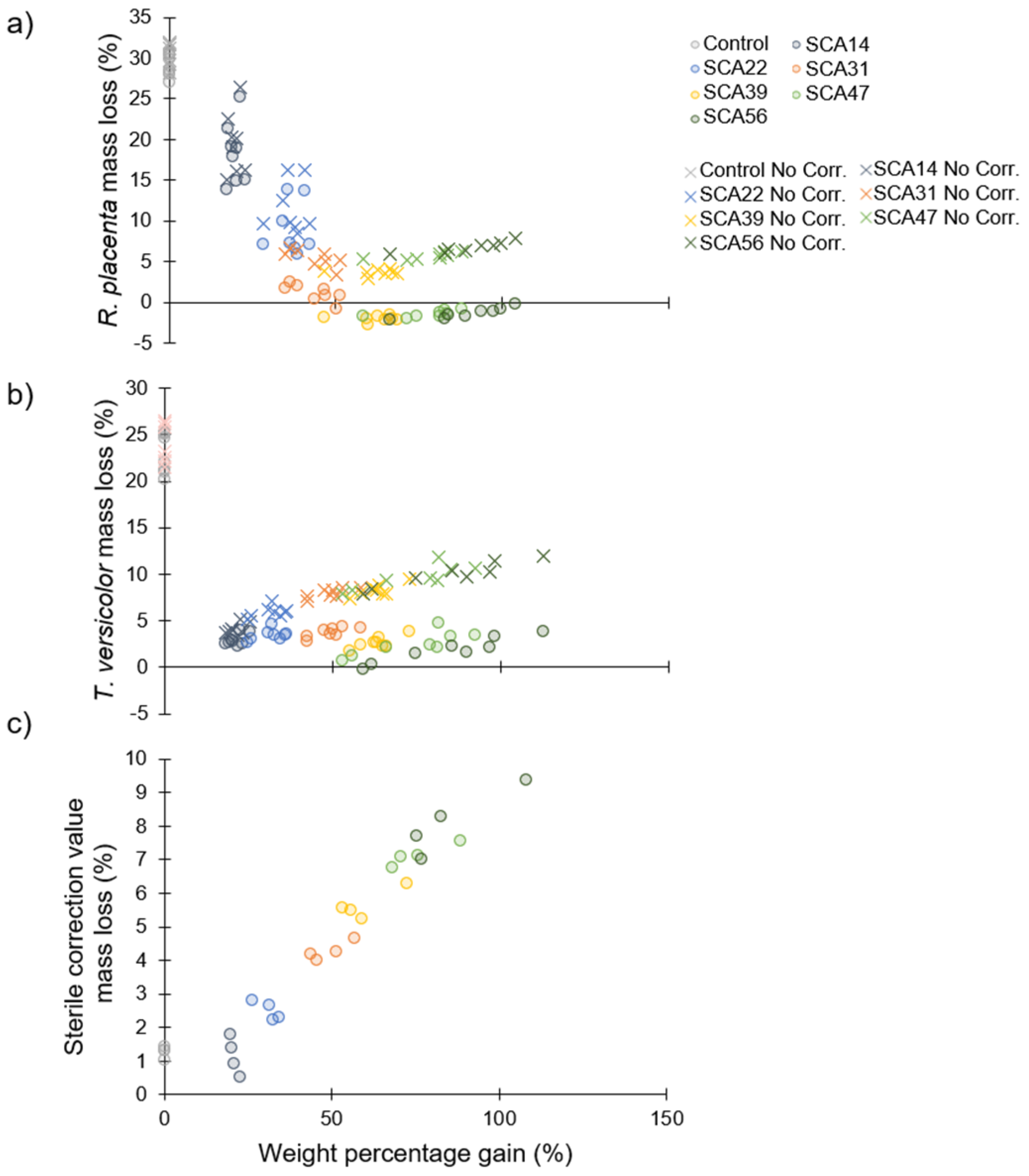
3.5. Decay Test
3.6. Preliminary Sorption Experiments
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 Concerning the Making Available on the Market and use of Biocidal Products Text with EEA Relevance. 2012, Volume 167. Available online: http://data.europa.eu/eli/reg/2012/528/oj/eng (accessed on 5 May 2020).
- Hill, C.A.S. Wood Modification: Chemical, Thermal and Other Processes; John Wiley & Sons: Chichester, UK, 2006; ISBN 978-0-470-02173-6. [Google Scholar]
- Sandberg, D.; Kutnar, A.; Mantanis, G. Wood modification technologies—A review. IForest Biogeosci. For. 2017, 10, 895. [Google Scholar] [CrossRef]
- Heräjärvi, H.; Kunttu, J.; Hurmekoski, E.; Hujala, T. Outlook for modified wood use and regulations in circular economy. Holzforschung 2020, 74, 334–343. [Google Scholar] [CrossRef]
- L’Hostis, C.; Fredon, E.; Thévenon, M.-F.; Santiago-Medina, F.-J.; Gérardin, P. Beech wood treated with polyglycerol succinate: A new effective method for its protection and stabilization. Holzforschung 2020, 74, 351–361. [Google Scholar] [CrossRef]
- L’Hostis, C.; Thévenon, M.-F.; Fredon, E.; Gérardin, P. Improvement of beech wood properties by in situ formation of polyesters of citric and tartaric acid in combination with glycerol. Holzforschung 2018, 72, 291–299. [Google Scholar] [CrossRef]
- Guo, W.; Xiao, Z.; Wentzel, M.; Emmerich, L.; Xie, Y.; Militz, H. Modification of Scots Pine with Activated Glucose and Citric Acid: Physical and Mechanical Properties. BioResources 2019, 14, 3445–3458. [Google Scholar]
- Essoua Essoua, G.G.; Blanchet, P.; Landry, V.; Beauregard, R. Pine wood treated with a citric acid and glycerol mixture: Biomaterial performance improved by a bio-byproduct. BioResources 2016, 11, 3049–3072. [Google Scholar] [CrossRef]
- Grosse, C.; Grigsby, W.J.; Noël, M.; Treu, A.; Thévenon, M.-F.; Gérardin, P. Optimizing Chemical Wood Modification with Oligomeric Lactic Acid by Screening of Processing Conditions. J. Wood Chem. Technol. 2019, 39, 385–398. [Google Scholar] [CrossRef]
- Mubarok, M.; Hadi, Y.S.; Suryana, J.; Darmawan, W.; Simon, F.; Dumarcay, S.; Gérardin, C.; Gérardin, P. Feasibility study of utilization of commercially available polyurethane resins to develop non-biocidal wood preservation treatments. Eur. J. Wood Wood Prod. 2017, 75, 877–884. [Google Scholar] [CrossRef]
- Noël, M.; Grigsby, W.; Vitkeviciute, I.; Volkmer, T. Modifying wood with bio-polyesters: Analysis and performance. Int. Wood Prod. J. 2015, 6, 14–20. [Google Scholar] [CrossRef]
- Noël, M.; Grigsby, W.J.; Ormondroyd, G.A.; Spear, M.J. Influence of water and humidity on chemically modifying wood with polybutylene succinate bio-polyester. Int. Wood Prod. J. 2016, 7, 80–88. [Google Scholar] [CrossRef]
- Berube, M.-A.; Schorr, D.; Ball, R.J.; Landry, V.; Blanchet, P. Determination of In Situ Esterification Parameters of Citric Acid-Glycerol Based Polymers for Wood Impregnation. J. Polym. Environ. 2018, 26, 970–979. [Google Scholar] [CrossRef]
- Larnøy, E.; Karaca, A.; Gobakken, L.R.; Hill, C.A.S. Polyesterification of wood using sorbitol and citric acid under aqueous conditions. Int. Wood Prod. J. 2018, 9, 66–73. [Google Scholar] [CrossRef]
- Mubarok, M.; Militz, H.; Dumarçay, S.; Gérardin, P. Beech wood modification based on in situ esterification with sorbitol and citric acid. Wood Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Klaushofer, H.; Berghofer, E.; Steyrer, W. Stärkecitrate—Produktion und anwendungs-technische Eigenschaften. Starch Stärke 1978, 30, 47–51. [Google Scholar] [CrossRef]
- Doll, K.M.; Shogren, R.L.; Willett, J.L.; Swift, G. Solvent-free polymerization of citric acid and D-sorbitol. J. Polym. Sci. Part Polym. Chem. 2006, 44, 4259–4267. [Google Scholar] [CrossRef]
- Centolella, A.P.; Razor, B.G. Polyesters of Citric Acid and Sorbitol. U.S. Patent US3661955A, 9 May 1972. [Google Scholar]
- Palencia, M.S.; Mora, M.A.; Palencia, S.L. Biodegradable Polymer Hydrogels Based in Sorbitol and Citric Acid for Controlled Release of Bioactive Substances from Plants (Polyphenols). Available online: https://www.ingentaconnect.com/content/ben/ccb/2017/00000011/00000001/art00008 (accessed on 6 May 2020).
- Barrett, D.G.; Yousaf, M.N. Design and Applications of Biodegradable Polyester Tissue Scaffolds Based on Endogenous Monomers Found in Human Metabolism. Molecules 2009, 14, 4022–4050. [Google Scholar] [CrossRef]
- Shogren, R.; Gonzalez, S.; Willett, J.L.; Graiver, D.; Swift, G. Preparation of Sorbitol Citrate Polyesters by Reactive Extrusion and Application as Inhibitors of Calcium Carbonate Precipitation. Available online: https://www.ingentaconnect.com/content/asp/jbmb/2007/00000001/00000002/art00007 (accessed on 6 May 2020).
- Geeti, D.K.; Niranjan, K. Environmentally benign bio-based waterborne polyesters: Synthesis, thermal- and bio-degradation studies. Prog. Org. Coat. 2019, 127, 419–428. [Google Scholar] [CrossRef]
- Kiljunen, S.; Koski, A.; Kunttu, M.; Valkonen, T. Impregnation of Chemicals into Wood. U.S. Patent EP2485880A1, 15 August 2012. [Google Scholar]
- Dix, N.J. Fungal Ecology; Springer Science & Business Media: Berlin, Germany, 2012; ISBN 978-94-011-0693-1. [Google Scholar]
- Rayner, A.D.M.; Boddy, L. Fungal Decomposition of Wood. Its Biology and Ecology; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1988. [Google Scholar]
- Thybring, E.E. The decay resistance of modified wood influenced by moisture exclusion and swelling reduction. Int. Biodeterior. Biodegrad. 2013, 82, 87–95. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Ringman, R.; Pilgård, A.; Thybring, E.E.; Jakes, J.E.; Richter, K. The role of chemical transport in the brown-rot decay resistance of modified wood. Int. Wood Prod. J. 2016, 7, 66–70. [Google Scholar] [CrossRef]
- Ringman, R.; Beck, G.; Pilgård, A. The Importance of Moisture for Brown Rot Degradation of Modified Wood: A Critical Discussion. Forests 2019, 10, 522. [Google Scholar] [CrossRef]
- Thybring, E.; Kymäläinen, M.; Rautkari, L. Moisture in modified wood and its relevance for fungal decay. IForest Biogeosciences For. 2018, 11, 418–422. [Google Scholar] [CrossRef]
- Phuong, L.X.; Takayama, M.; Shida, S.; Matsumoto, Y.; Aoyagi, T. Determination of the accessible hydroxyl groups in heat-treated Styrax tonkinensis (Pierre) Craib ex Hartwich wood by hydrogen-deuterium exchange and 2H NMR spectroscopy. Holzforschung 2007, 61, 488–491. [Google Scholar] [CrossRef]
- Altgen, M.; Willems, W.; Hosseinpourpia, R.; Rautkari, L. Hydroxyl accessibility and dimensional changes of Scots pine sapwood affected by alterations in the cell wall ultrastructure during heat-treatment. Polym. Degrad. Stab. 2018, 152, 244–252. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Hill, C.A.S.; Curling, S.; Ormondroyd, G.; Xie, Y. The water vapour sorption behaviour of acetylated birch wood: How acetylation affects the sorption isotherm and accessible hydroxyl content. J. Mater. Sci. 2014, 49, 2362–2371. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Hill, C.A.S.; Popescu, M.-C. Water adsorption in acetylated birch wood evaluated through near infrared spectroscopy. Int. Wood Prod. J. 2016, 7, 61–65. [Google Scholar] [CrossRef]
- Hill, C.; Popescu, C.M.; Rautkari, L.; Curling, S.; Ormondroyd, G.; Xie, Y.; Jalaludin, Z. The role of hydroxyl groups in determining the sorption properties of modified wood. In Proceedings of the 7th European Conference on Wood Modification, Lisbon, Portugal, 1 January 2014. [Google Scholar] [CrossRef]
- Beck, G.; Strohbusch, S.; Larnøy, E.; Militz, H.; Hill, C. Accessibility of hydroxyl groups in anhydride modified wood as measured by deuterium exchange and saponification. Holzforschung 2017, 72. [Google Scholar] [CrossRef]
- Thybring, E.E.; Piqueras, S.; Tarmian, A.; Burgert, I. Water accessibility to hydroxyls confined in solid wood cell walls. Cellulose 2020. [Google Scholar] [CrossRef]
- Epmeier, H.; Westin, M.; Rapp, A. Differently modified wood: Comparison of some selected properties. Scand. J. For. Res. 2004, 19, 31–37. [Google Scholar] [CrossRef]
- Beck, G.; Hill, C.; Cocher, P.M.; Alfredsen, G. Accessibility of hydroxyl groups in furfurylated wood at different weight percent gains and during Rhodonia placenta decay. Eur. J. Wood Wood Prod. 2019, 77, 953–955. [Google Scholar] [CrossRef]
- Dieste, A.; Krause, A.; Mai, C.; Militz, H. The calculation of EMC for the analysis of wood/water relations in Fagus sylvatica L. modified with 1,3-dimethylol-4,5-dihydroxyethyleneurea. Wood Sci. Technol. 2010, 44, 597–606. [Google Scholar] [CrossRef][Green Version]
- Dieste, A.; Krause, A.; Mai, C.; Sèbe, G.; Grelier, S.; Militz, H. Modification of Fagus sylvatica L. with 1,3-dimethylol-4,5-dihydroxy ethylene urea (DMDHEU). Part 2: Pore size distribution determined by differential scanning calorimetry. Holzforschung 2009, 63, 89–93. [Google Scholar] [CrossRef]
- Dieste, A.; Krause, A.; Militz, H. Modification of Fagus sylvatica (L.) with 1,3-dimethylol-4,5-dihydroxyethylene urea (DMDHEU): Part 1. Estimation of heat adsorption by the isosteric method (Hailwood-Horrobin model) and by solution calorimetry. Holzforschung 2008, 62, 577–583. [Google Scholar] [CrossRef]
- CEN European Committee for Standardization. Wood Preservatives—Accelerated Ageing of Treated Wood Prior to Biological Testing—Leaching Procedure; CEN (European Committee for Standardization): Brussels, Belgium, 1996. [Google Scholar]
- Faix, O. Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 1991, 45, 21–28. [Google Scholar] [CrossRef]
- Fackler, K.; Stevanic, J.S.; Ters, T.; Hinterstoisser, B.; Schwanninger, M.; Salmén, L. Localisation and characterisation of incipient brown-rot decay within spruce wood cell walls using FT-IR imaging microscopy. Enzyme Microb. Technol. 2010, 47, 257–267. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Mitchell, J.; Stark, S.C.; Strange, J.H. Probing surface interactions by combining NMR cryoporometry and NMR relaxometry. J. Phys. Appl. Phys. 2005, 38, 1950–1958. [Google Scholar] [CrossRef]
- Araujo, C.D.; MacKay, A.L.; Hailey, J.R.T.; Whittall, K.P.; Le, H. Proton magnetic resonance techniques for characterization of water in wood: Application to white spruce. Wood Sci. Technol. 1992, 26, 101–113. [Google Scholar] [CrossRef]
- Telkki, V.-V.; Yliniemi, M.; Jokisaari, J. Moisture in softwoods: Fiber saturation point, hydroxyl site content, and the amount of micropores as determined from NMR relaxation time distributions. Holzforschung 2013, 67, 291–300. [Google Scholar] [CrossRef]
- Kekkonen, P.M.; Ylisassi, A.; Telkki, V.-V. Absorption of Water in Thermally Modified Pine Wood As Studied by Nuclear Magnetic Resonance. J. Phys. Chem. C 2014, 118, 2146–2153. [Google Scholar] [CrossRef]
- Beck, G.; Thybring, E.E.; Thygesen, L.G.; Hill, C. Characterization of moisture in acetylated and propionylated radiata pine using low-field nuclear magnetic resonance (LFNMR) relaxometry. Holzforschung 2018, 72, 225–233. [Google Scholar] [CrossRef]
- Fredriksson, M.; Thygesen, L.G. The states of water in Norway spruce (Picea abies (L.) Karst.) studied by low-field nuclear magnetic resonance (LFNMR) relaxometry: Assignment of free-water populations based on quantitative wood anatomy. Holzforschung 2016, 71, 77–90. [Google Scholar] [CrossRef]
- Cai, C.; Javed, M.A.; Komulainen, S.; Telkki, V.-V.; Haapala, A.; Heräjärvi, H. Effect of natural weathering on water absorption and pore size distribution in thermally modified wood determined by nuclear magnetic resonance. Cellulose 2020, 27, 4235–4247. [Google Scholar] [CrossRef]
- Carr, H.Y.; Purcell, E.M. Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1954, 94, 630–638. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef]
- Claridge, T. High-Resolution NMR Techniques in Organic Chemistry; Elsevier: Amsterdam, The Netherlands, 1999; ISBN 978-0-08-042798-0. [Google Scholar]
- Whittall, K.P.; Bronskill, M.J.; Henkelman, R.M. Investigation of analysis techniques for complicated NMR relaxation data. J. Magn. Reson. 1991, 95, 221–234. [Google Scholar] [CrossRef]
- Lawson, C.L.; Hanson, R.J. Linear Least Squares with Linear Inequality Constraints; Solving Least Squares Problems; Prentice-Hall: Englewood Cliffs, NJ, USA, 1974. [Google Scholar]
- CEN European Committee for Standardization. Wood Preservatives—Test Method for Determining the Protective Effectiveness against Wood Destroying Basidiomycetes—Determination of the Toxic Values; CEN (European Committee for Standardization): Brussels, Belgium, 1996. [Google Scholar]
- Bravery, A.F. A Miniaturised Wood-Block Test for the Rapid Evaluation of Wood Preservative Fungicides; Swedish Wood Preservation Institute: Stockholm, Sweden, 1979; pp. 58–65. [Google Scholar]
- Sepall, O.; Mason, S.G. Hydrogen exchange between cellulose and water: I. measurement of accessibility. Can. J. Chem. 1961, 39, 1934–1943. [Google Scholar] [CrossRef]
- Morrison, J. Deuterium-hydrogen exchange between water and macromolecules: Accessibility of cellulose. Nature 1960, 185, 160–161. [Google Scholar] [CrossRef]
- Uimonen, T.; Hautamäki, S.; Altgen, M.; Kymäläinen, M.; Rautkari, L. Dynamic vapour sorption protocols for the quantification of accessible hydroxyl groups in wood. Holzforschung 2020, 74, 412–419. [Google Scholar] [CrossRef]
- Pönni, R.; Rautkari, L.; Hill, C.A.S.; Vuorinen, T. Accessibility of hydroxyl groups in birch kraft pulps quantified by deuterium exchange in D2O vapor. Cellulose 2014, 21, 1217–1226. [Google Scholar] [CrossRef]
- Mantanis, G.; Young, R.A.; Rowell, R. Swelling of Wood. Part II. Swelling in Organic Liquids. Holzforschung 1994, 48. [Google Scholar] [CrossRef]
- Sessa, D.J.; Wing, R.E. Thermochemically-modified soybean and corn protein products with enhanced metal-binding properties. Food Nahr. 1998, 42, 266–268. [Google Scholar] [CrossRef]
- Chang, T.-C.; Chang, H.-T.; Wu, C.-L.; Chang, S.-T. Influences of extractives on the photodegradation of wood. Polym. Degrad. Stab. 2010, 95, 516–521. [Google Scholar] [CrossRef]
- Pandey, K.K.; Pitman, A.J. FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Pandey, K.K.; Nagveni, H.C. Rapid characterisation of brown and white rot degraded chir pine and rubberwood by FTIR spectroscopy. Holz als Roh-und Werkstoff 2007, 65, 477–481. [Google Scholar] [CrossRef]
- Pandey, K.K. A note on the influence of extractives on the photo-discoloration and photo-degradation of wood. Polym. Degrad. Stab. 2005, 87, 375–379. [Google Scholar] [CrossRef]
- Beck, G.; Thybring, E.E.; Thygesen, L.G. Brown-rot fungal degradation and de-acetylation of acetylated wood. Int. Biodeterior. Biodegrad. 2018, 135, 62–70. [Google Scholar] [CrossRef]
- Mao, J.; Holtman, K.M.; Scott, J.T.; Kadla, J.F.; Schmidt-Rohr, K. Differences between Lignin in Unprocessed Wood, Milled Wood, Mutant Wood, and Extracted Lignin Detected by 13C Solid-State NMR. J. Agric. Food Chem. 2006, 54, 9677–9686. [Google Scholar] [CrossRef]
- Cai, C.; Antikainen, J.; Luostarinen, K.; Mononen, K.; Heräjärvi, H. Wetting-induced changes on the surface of thermally modified Scots pine and Norway spruce wood. Wood Sci. Technol. 2018, 52, 1181–1193. [Google Scholar] [CrossRef]
- Andersson, S.; Serimaa, R.; Väänänen, T.; Paakkari, T.; Jämsä, S.; Viitaniemi, P. X-ray scattering studies of thermally modified Scots pine (Pinus sylvestris L.). Holzforschung 2005, 59, 422–427. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Cannella, D.; Jørgensen, H.; Felby, C.; Thygesen, L.G. Cellulase Inhibition by High Concentrations of Monosaccharides. J. Agric. Food Chem. 2014, 62, 3800–3805. [Google Scholar] [CrossRef]
- Thygesen, L.G.; Elder, T. Moisture in Untreated, Acetylated, and Furfurylated Norway Spruce Monitored During Drying Below Fiber Saturation Using Time Domain NMR. Wood Fiber Sci. 2009, 41, 194–200. [Google Scholar]
- Thybring, E.E.; Kymäläinen, M.; Rautkari, L. Experimental techniques for characterising water in wood covering the range from dry to fully water-saturated. Wood Sci. Technol. 2018, 52, 297–329. [Google Scholar] [CrossRef]
- Javed, M.A.; Kekkonen, P.M.; Ahola, S.; Telkki, V.-V. Magnetic resonance imaging study of water absorption in thermally modified pine wood. Holzforschung 2015, 69, 899–907. [Google Scholar] [CrossRef]
- Hiltunen, S.; Mankinen, A.; Javed, M.A.; Ahola, S.; Venäläinen, M.; Telkki, V.-V. Characterization of the decay process of Scots pine caused by Coniophora puteana using NMR and MRI. Holzforschung 2020, 1. [Google Scholar] [CrossRef]
- Berthold, J.; Rinaudo, M.; Salmeń, L. Association of water to polar groups; estimations by an adsorption model for ligno-cellulosic materials. Colloids Surf. Physicochem. Eng. Asp. 1996, 112, 117–129. [Google Scholar] [CrossRef]
- Ostrofsky, A.; Jellison, J.; Smith, K.T.; Shortle, W.C. Changes in cation concentrations in red spruce wood decayed by brown rot and white rot fungi. Can. J. For. Res. 1997, 27, 567–571. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Jakes, J.E.; Tang, J.; Ohno, K.; Bishell, A.; Finney, L.; Maxey, E.R.; Vogt, S.; Kirker, G.T. Fungal–copper interactions in wood examined with large field of view synchrotron-based X-ray fluorescence microscopy. Wood Mater. Sci. Eng. 2019, 14, 174–184. [Google Scholar] [CrossRef]
- Hassan, R.; El-Kadi, S.; Sand, M. Effect of some organic acids on some fungal growth and their toxins produciton. Int. J. Adv. Biol. 2015, 2, 11. [Google Scholar]
- Lawrence, C.L.; Botting, C.H.; Antrobus, R.; Coote, P.J. Evidence of a New Role for the High-Osmolarity Glycerol Mitogen-Activated Protein Kinase Pathway in Yeast: Regulating Adaptation to Citric Acid Stress. Mol. Cell. Biol. 2004, 24, 3307–3323. [Google Scholar] [CrossRef]
- Nielsen, M.K.; Arneborg, N. The effect of citric acid and pH on growth and metabolism of anaerobic Saccharomyces cerevisiae and Zygosaccharomyces bailii cultures. Food Microbiol. 2007, 24, 101–105. [Google Scholar] [CrossRef]
- Brul, S.; Coote, P. Preservative agents in foods: Mode of action and microbial resistance mechanisms. Int. J. Food Microbiol. 1999, 50, 1–17. [Google Scholar] [CrossRef]
- Vom Stein, T.; Grande, P.; Sibilla, F.; Commandeur, U.; Fischer, R.; Leitner, W.; María, P.D. de Salt-assisted organic-acid-catalyzed depolymerization of cellulose. Green Chem. 2010, 12, 1844–1849. [Google Scholar] [CrossRef]
- Jakes, J.E.; Hunt, C.G.; Zelinka, S.L.; Ciesielski, P.N.; Plaza, N.Z. Effects of Moisture on Diffusion in Unmodified Wood Cell Walls: A Phenomenological Polymer Science Approach. Forests 2019, 10, 1084. [Google Scholar] [CrossRef]
| Analysis | Concentration of Treatment Solution (%) | Weight Percentage Gain (%) * | Dimensions L × T × R (mm) | Number of Replicates |
|---|---|---|---|---|
| Dimensions analysis | 0 | −1.0 (0.1) | 15 × 25 × 25 | 12 |
| Dimensions analysis | 14 | 16.4 (0.7) | 15 × 25 × 25 | 12 |
| Dimensions analysis | 22 | 28.2 (1.0) | 15 × 25 × 25 | 12 |
| Dimensions analysis | 31 | 38.7 (1.9) | 15 × 25 × 25 | 12 |
| Dimensions analysis | 39 | 50.6 (1.8) | 15 × 25 × 25 | 12 |
| Dimensions analysis | 47 | 60.2 (2.9) | 15 × 25 × 25 | 12 |
| Dimensions analysis | 56 | 72.0 (2.3) | 15 × 25 × 25 | 12 |
| ATR-FTIR | 0 | −1.49 (0.44) | 10 × 5 × 5 | 9 |
| ATR-FTIR | 31 | 49.8 (3.2) | 10 × 5 × 5 | 9 |
| ATR-FTIR | 39 | 64.5 (4.5) | 10 × 5 × 5 | 9 |
| LFNMR/HPLC leachate | 0 | −2.6 (0.2) | 10 × 5 × 5 | 4 |
| LFNMR/HPLC leachate | 31 | 47.6 (3.5) | 10 × 5 × 5 | 4 |
| LFNMR/HPLC leachate | 39 | 62.5 (4.7) | 10 × 5 × 5 | 4 |
| Decay test | 0 | 0 † | 30 × 5 × 10 | 8 ‡ |
| Decay test | 14 | 20.4 (2.2) | 30 × 5 × 10 | 8 ‡ |
| Decay test | 22 | 34.1 (5.0) | 30 × 5 × 10 | 8 ‡ |
| Decay test | 31 | 46.4 (6.3) | 30 × 5 × 10 | 8 ‡ |
| Decay test | 39 | 62.5 (6.0) | 30 × 5 × 10 | 8 ‡ |
| Decay test | 47 | 76.0 (11.9) | 30 × 5 × 10 | 8 ‡ |
| Decay test | 56 | 87.2 (15.4) | 30 × 5 × 10 | 8 ‡ |
| Leachate Number | Time in Leachate (days) | Sorbitol Concentration SCA31 (g/L) | Sorbitol Concentration SCA39 (g/L) | Citric Acid Concentration SCA31 (g/L) | Citric Acid Concentration SCA39 (g/L) |
|---|---|---|---|---|---|
| 2 | 1 | 0.009 (0.006) | 0.011 (0.002) | 0.32 (0.11) | 0.32 (0.06) |
| 3 | 1 | 0 | 0 | 0.12 (0.05) | 0.15 (0.01) |
| 4 | 1 | 0 | 0 | 0.07 (0.02) | 0.08 (0.01) |
| 5 | 1 | 0 | 0 | 0.05 (0.00) | 0.06 (0.01) |
| 10 | 1 | 0 | 0 | 0.03 (0.00) | 0.04 (0.00) |
| 11 | 7 | 0 | 0 | 0.12 (0.01) | 0.15 (0.00) |
| 12 | 21 | 0 | 0 | 0.27 (0.02) | 0.28 (0.04) |
| Treatment | Peak 1 | Peak 1.5 | Peak 2 | Peak 3 | ||||
|---|---|---|---|---|---|---|---|---|
| Area (%) | T2 (ms) | Area (%) | T2 (ms) | Area (%) | T2 (ms) | Area (%) | T2 (ms) | |
| Control | 21.7 (1.8) | 1.1 (0.03) | 0.5 (0.4) | 5.1 (0.8) | 11.8 (0.4) | 19.0 (1.4) | 66.0 (2.1) | 82.6 (7.2) |
| SCA31 | 24.8 (1.6) | 0.8 (0.02) | 2.1 (0.6) | 3.0 (0.6) | 7.9 (0.4) | 33.1 (0.9) | 65.3 (1.5) | 166.5 (8.4) |
| SCA39 | 27.2 (0.8) | 0.8 (0.02) | 2.4 (0.6) | 3.2 (0.2) | 6.9 (0.5) | 33.7 (3.2) | 63.4 (1.7) | 169.0 (10.1) |
| Treatment | Peak 1 T2 (ms) | Non-Freezing Moisture Content (%) * |
|---|---|---|
| Control | 1.05 (0.03) | 28.0 (0.3) |
| SCA31 | 0.38 (0.02) | 31.6 (0.8) |
| SCA39 | 0.37 (0.01) | 35.1 (3.0) |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beck, G. Leachability and Decay Resistance of Wood Polyesterified with Sorbitol and Citric Acid. Forests 2020, 11, 650. https://doi.org/10.3390/f11060650
Beck G. Leachability and Decay Resistance of Wood Polyesterified with Sorbitol and Citric Acid. Forests. 2020; 11(6):650. https://doi.org/10.3390/f11060650
Chicago/Turabian StyleBeck, Greeley. 2020. "Leachability and Decay Resistance of Wood Polyesterified with Sorbitol and Citric Acid" Forests 11, no. 6: 650. https://doi.org/10.3390/f11060650
APA StyleBeck, G. (2020). Leachability and Decay Resistance of Wood Polyesterified with Sorbitol and Citric Acid. Forests, 11(6), 650. https://doi.org/10.3390/f11060650




