Abstract
To explore the possible relationship between diseased trees and wildfires, we assessed the flammability of canker-resistant and susceptible common cypress clones that were artificially infected with Seiridium cardinale compared to healthy trees. This study explored the effect of terpenoids produced by the host plant in response to infection and the presence of dead plant portions on flammability. Terpenoids were extracted and quantified in foliage and bark samples by gas chromatography–mass spectrometry (GC–MS). A Mass Loss Calorimeter was used to determine the main flammability descriptors. The concentration of terpenoids in bark and leaf samples and the flammability parameters were compared using a generalized linear mixed models (GLMM) model. A partial least square (PLS) model was generated to predict flammability based on the content of terpenoid, clone response to bark canker and the disease status of the plants. The total terpenoid content drastically increased in the bark of both cypress clones after infection, with a greater (7-fold) increase observed in the resistant clone. On the contrary, levels of terpenoids in leaves did not alter after infection. The GLMM model showed that after infection, plants of the susceptible clone appeared to be much more flammable in comparison to those of resistant clones, showing higher ignitability, combustibility, sustainability and consumability. This was mainly due to the presence of dried crown parts in the susceptible clone. The resistant clone showed a slightly higher ignitability after infection, while the other flammability parameters did not change. The PLS model (R2Y = 56%) supported these findings, indicating that dead crown parts and fuel moisture content accounted for most of the variation in flammability parameters and greatly prevailed on terpenoid accumulation after infection. The results of this study suggest that a disease can increase the flammability of trees. The deployment of canker-resistant cypress clones can reduce the flammability of cypress plantations in Mediterranean areas affected by bark canker. Epidemiological data of diseased tree distribution can be an important factor in the prediction of fire risk.
1. Introduction
The relationship between non-native plant disease and the frequency of wildfires (the effect on fire regimes), and the implications for fire management has become an increasing focus of research in recent years [1,2]. Both wildfires and disease caused by invasive pathogens (and insects) are key factors in determining tree mortality in forests worldwide and are linked to the global change context [3,4,5,6,7,8,9,10]. The relationship between wildfire and forest disease depends on the host–pathogen species involved and their mutual interaction, knowledge in this field is still lacking detailed information.
Current global change scenarios in terms of the combination of climate, shift in land use, and the expansion of trade networks and volume of goods, exacerbate the seasonal drought and warming stress periods that in turn influence plant physiology, biochemical defences and disease severity, in terms of pest and disease movement and outbreaks [9,11,12]. At the stand level, the interaction between wildfire and an emerging fungal forest disease was studied in Californian and Oregon forests affected by sudden oak death (SOD) (caused by Phytophthora ramorum) [1,4,13]. This new disease altered the physical and biochemical characteristics of the ecosystem e.g., fuel load, increasing the surface fuel, restructuring the forest canopy, decreasing canopy continuity and increasing tree mortality. This altered the species composition and in turn affected wildfire dynamics (severity, risk of crown ignition, etc.) [4,14,15,16,17]. In Californian forests affected by SOD, the rate of standing dead trees was higher, the tanoak (Notholithocarpus densiflorus) mass of woody debris on the soil was tens of times greater and the depth of the fuel bed in diseased stands was four times that in disease-free forest [18]. Simulation modelling with the BehavePlus fire model system, indicated that flame length, fire spread rate and fireline intensity, increased several times in infected Douglas fir and Redwood stands compared to their healthy counterparts [18].
At the tree level, both climatic and biotic stress factors affect the health of trees. These stresses decrease the water content of plant organs, increase the ratio of dead to alive crown portions, and especially in conifers, influence the qualitative and quantitative amount of several plant defensive compounds, such as terpenoids [19,20,21,22]. Terpenoids are considered to be one of the most important molecules affecting forest fuel flammability [23,24,25,26,27,28]. Terpenoids are constitutive induced lines of defence in conifers; an increase in absolute amounts, changes in their proportions and de novo production of molecules (phytoalexins) have been observed after infection depending on the pathosystem [29].
Cypress canker disease (CCD) is a non-native lethal disease affecting many Cupressaceae (above all Cupressus sempervirens L., in the Mediterranean area). It is caused by the invasive fungal pathogen Seiridium cardinale (Wagener) Sutton et Gibson introduced in Europe (and spread across the globe) from California, USA [30,31,32]. This destructive disease causes the dieback of crown portions and the desiccation of twigs and branches, due to the girdling of the woody organs by the necrotrophic fungal agent [33]. An additional effect of CCD is also the de novo genesis of traumatic resin ducts (TRD) in bark tissues affected by canker [34,35,36], and the consequent abundant exudation of resin that flows down from the infected organs [33]. Both of these effects supposedly affect the flammability of the infected trees or their portions. A long-term genetic research program developed since the 1970s in Italy, France and Greece led to the selection of several C. sempervirens genotypes resistant to CCD (some of which were patented and made commercially available).
The CCD resistant clones are able to block the growth of the fungus in the infected bark within a few weeks and completely heal the lesion within a few years [37,38,39,40,41]. The efforts undertaken to control the CCD are justified by the high ecological, symbolic, historical and cultural value of this tree in Mediterranean countries. Cupressus sempervirens is used in forestry, landscaping in peri-urban and urban contexts and also as a windbreak and hedge [32]. The induction of terpenoids as part of the defensive reaction of common cypress plants (both CCD-resistant and susceptible clones and not selected for CCD resistance plants) to Seiridium cardinale infection is characterized by the production and accumulation of several de novo specific compounds [29].
The flammability of the live crown of plants of C. sempervirens has already been studied extensively [42,43,44,45,46,47]; nevertheless, the flammability descriptors (ignitability, sustainability, combustibility and consumability) of healthy and diseased cypress clones selected for CCD resistance have not yet been assessed. This work explores the links between diseased trees and wildfire, comparing the flammability of canker-resistant and susceptible common cypress clones, artificially infected by S. cardinale, in comparison to healthy ramets of both clones. We set out to address the following questions: (i) Is a diseased plant more flammable than a healthy one, and if so, to what extent? (ii) Is a CCD-resistant cypress clone less flammable than the CCD-non-resistant equivalent? (iii) How do terpenoids produced by the host in response to infection, and dead plant portions killed by the fungal pathogen, affect flammability?
2. Materials and Methods
2.1. Experimental Set-Up: Plant Selection, Growth Conditions and Artificial Inoculation
Twenty 3-year-old grafted ramets of Cupressus sempervirens of the canker-resistant (PM-322; patented cypress clone ‘Bolgheri’) and CCD-susceptible (10 ramets each) clones were used for this study. The plants were grown under natural field conditions in 4 litre pots (15 × 15 × 20 cm) containing a mixture of peat, compost and perlite (3:1:1, v/v/v) in the experimental area of the Institute for Sustainable Plant Protection (IPSP) of the Italian National Research Council (CNR) in Sesto Fiorentino, Italy (43°49′05″ N; 11°12′07″ E). During the experiment, the potted cypress plants were irrigated 2 times per week and were fertilized every 20 days with half-strength Hoagland solution.
At the beginning of June 2018, four ramets of each cypress clone were artificially inoculated with a standard isolate of S. cardinale (ATCC 38654) following the procedure described in Danti et al. [48], while the other ramets were left intact. A 3 mm plug of stem bark was removed with a cork borer and replaced with a plug the same size of S. cardinale mycelium grown on PDA in Petri dishes for 15 days at 25 °C in the dark. The inoculum was then covered with wet cotton wrapped with parafilm around the trunk for one week.
We performed 5 stem inoculations for each plant to simulate a severe CCD attack and induce severe infection symptoms. The sites of inoculation started approximately 10 cm below the top of the plant and were spaced 5 cm apart from one another, where the stem was between 0.5 and 1 cm in width, determined using a stem calliper. The duration of the study was 3 months.
Three months after the inoculation (September 2018), when the typical CCD symptoms were fully evident (development of necrotic lesions around the inoculation points, a little resin exudation from the inoculation points, with apical twigs and shoot desiccation), the 4 diseased plants and 4 unaffected plants of each clone were sampled for the flammability tests and the determination of terpenoid content. At the same time, two more intact ramets of each clone were used to determine the moisture content (FMC) and dry mass (see below).
2.2. Fuel Moisture and Biomass Determination
To determine the moisture content and biomass, two intact ramets of each clone were subdivided into three parts: upper, middle and lower third, and each portion was in turn divided into green leaves and twigs, bark tissues and xylem. The cutting and splitting of the ramets was carried out in a cold room (5 °C) as fast as possible (taking a few minutes). Each type of sample was then immediately weighed (fresh weight) with a precision balance and placed in an oven at 70 °C until a constant weight was achieved and considered to represent the dry weight. The fresh and dry mass and the moisture content (FMC) of the leaves–-twigs, bark and xylem were separately determined for the three parts (upper third, middle third, lower third) of the ramets. These measures allowed us to determine the real amount of terpenoids contained in the leaves and bark of each sample used for flammability tests (see below).
2.3. Sample Splitting for the Determination of Terpenoids and Flammability Tests
For the extraction of terpenoids and to conduct the flammability tests, 16 ramets (8 per clone, 4 inoculated and 4 intact) were separately cut in 10 cm long stem portions (in a cold room as before) from the upper, lower and medium third of the ramets. From each portion, a 4 cm long stem segment (including the inoculation point, for the infected plants) and 5 g in fresh weight of leaves, randomly chosen, were sampled for the determination of terpenoids. From the 4 cm stem segments, bark tissues (from the cambium to the outer periderm) were removed and separately stored (while the xylem was discarded). For the determination of terpenoids, the leaves and bark samples were stored in falcon tubes at −20 °C until the extraction of the terpenoids. The remaining material from each of the stem portions, that were initially 10 cm in length, was placed in hermetically sealed plastic bags and immediately stored at −20 °C and shipped in dry ice (the day after with a 12 h courier) to the Forest Fire Laboratory of the INIA–CIFOR in Madrid, Spain, for the flammability tests (see below).
2.4. Terpenoids Extraction, Identification and Quantification
For the determination of terpenoids, 500 mg (fresh weight) of each foliage and bark tissues were quickly fragmented into small pieces, of about 0.5–1 cm in length, with a scalpel (in a cold room) and placed separately in sealed 230 mL vials with 1 mL of heptane as the solvent and tridecane (20 ppm) as an internal standard. The vials were then submitted to 3 sonication cycles of 10 min each at room temperature (25 °C) at a frequency of 38–40 KHz (Ultrasonic cleaner Sonica, S3 EP, Soltec, Milano, Italy) and subsequently stirred overnight (for 12 h) at 35 °C in a rotating incubator shaker (Thermoshake THO 500/1, Gerhardt, Königswinter, Germany) at 90 rpm. The vials were then centrifuged for 10 min at 20 °C at 5000 rpm (Centrifuge 5810 R, Eppendorf, Hamburg, Germany), and the supernatant pipetted in 2 mL vials sealed with a Teflon septum and crimped with an aluminum cap for the detection of the terpenoids via gas chromatography–mass spectrometry (GC–MS).
The terpenoids were analysed using a Gas Chromatograph Agilent 7820 GC-Cromatograph equipped with a 5975C MSD with EI ionisation (Agilent Tech., Palo Alto, AC, USA) as described in [27]. A 1 µL sample of the aforementioned supernatant was injected in a split/splitless injector operating in split mode with 1:10 split ratio. A Gerstel MPS2 XL autosampler equipped with liquid option was used. The analysis was carried out under the following conditions: H2 (carrier gas) at 1.2 mL min−1; the injector in splitless mode set at 260 °C, J&W innovax column (30 m, 0.25 mm i.d., 0.5 µm df); oven temperature program: initial temperature 40 °C for 1 min, then 5 °C/min until 200 °C, then 10 °C/min until 220 °C, then 30 °C/min until 260 °C, with a hold time of 3 min. The mass spectrometer was operating with an electron ionisation of 70 eV, in scan mode in the m/z range 29–330 at three scans/sec.
The deconvoluted peak spectra, obtained by Agilent MassHunter Workstation software, were matched against the NIST 11 spectral library for tentative identification. Kovats’ retention indices were calculated for further compound confirmation and compared with those reported in the literature for the chromatographic column used. In addition, terpenoids (mono- and sesquiterpenoids) were identified by the comparison of the retention times with those of authentic standards (high-purity components were obtained from Fluka, Aldrich and Acros) injected under the same conditions, and also by comparison with the tridecane internal standard for those compounds for which standards were not available. The identified terpenoids (TOTterp) were grouped into four categories: monoterpenoids (MT), oxygenated monoterpenoids (MTox), sesquiterpenoids (ST) and oxygenated sesquiterpenoids (STox), as outlined in Della Rocca et al. [27]. The amount of terpenoids was expressed as µg/g dry weight (DW) of the samples.
2.5. Flammability Test at MLC
An adapted Mass Loss Calorimeter (MLC) device was used [47,49,50,51]. The tests were performed using the MLC arranged in the standard horizontal configuration, to determine the main flammability descriptors [52]: ignitability (time to ignition, TTI), combustibility (peak of heat release rate, PHRR), sustainability (average effective heat of combustion, AEHC) and consumability (percentage mass lost, PML). A porous holder (10 × 10 × 5 cm) was used to allow the natural diffusion of air through the samples during the MLC tests. The MLC tests were conducted at 50 kW/m2, simulating severe fire conditions [53]. The fuel moisture content (FMC) of the live foliage was promptly determined on 8 g subsamples using a Computrac MAX R 2000XL moisture analyser (Arizona Instrument LLC). Based on their FMC values, the dry mass of the fresh samples was fixed at 10 g (to balance the variability in weight due to the differences in water content among the samples [50]). At the end of each test, the residual mass fraction was determined with a precision balance (Mettler AB104-S). All samples were stored in a refrigerated chamber (at 4 °C) and processed within 5 days.
A series of tests was carried out using the experimental design described for the extraction of terpenoids (see above). The portions cut from the 16 ramets (8 per clone), were divided into the three groups previously identified (upper, middle and lower thirds), and a total of 48 samples were obtained. From each portion, one 8 g subsample of leaves was used to obtain the FMC (see above), while the remaining samples of woody stem and foliage (10 g of dry weight) were subdivided in 3–5 subsamples to carry out the flammability tests. The MLC protocol for ‘alive’ samples generated a high level of variability, and non-repetitive tests must be removed from analysis to obtain at least two replicates complying with the repeatability criteria (errors less than 15% [53]). Therefore, the original set of 48 samples was reduced to 36 samples (8 replicates per condition: CCD-resistant clone inoculated (RI) or non-inoculated (RC), CCD-susceptible clone inoculated (SI) and non-inoculated (SC)). This data set (n = 36) was considered representative (upper, middle and lower third portion of trees), and was randomly extracted from 16 ramets (replicates), avoiding pseudo-replication. For each plant portion, the FMC and dry mass were measured and the real amount of terpenoids contained in each sample used for the flammability tests was determined, starting from the concentration of terpenoids per µg found in the leaves and the bark tissues.
2.6. Statistical Analysis
The total concentrations of terpenoids (TOTterp), as well as the subcategories of MT, MTox, ST, and STox extracted from both the bark and leaves and the flammability parameters (TTI, PHRR, AEHC, PML) were used as the response variables. Considering the hierarchical nature of the data (multiple observations on single ramets of a same clone), multilevel generalized linear mixed models (GLMM) with both fixed and random effects acting at ramet and portion (upper, middle and lower third) levels were fitted. The distribution of variables was checked for parametric requisites (skewness, kurtosis, influence points) and to select the suitable link function (Gaussian or Gamma distribution). Once the model was fitted, the assumptions of normality and homoscedasticity of the residuals were evaluated. Potential autocorrelation was controlled by residual analysis. Finally, the goodness-of-fit statistics, referring to both the marginal (not considering random effects) and conditional (including random effects) predictions were determined. A GLMM model was generated to predict variables for each clone (susceptible “S” and resistant “R”) using the factor ‘infection’ (infected “I” vs. healthy control “C”) and the covariable FMC as the fixed predictor variables. As some of the upper part of susceptible inoculated (SI) ramets died as consequence of the inoculation with the pathogen, to prevent the strong effect of dead parts in the prediction of terpenoid content and flammability, models were replicated removing those samples. An additional model was generated to detect the effect of the clone (R vs. S) in determining the terpenoid concentrations before (C) and after the treatment (I).
A partial least square (PLS) model was generated to predict the flammability parameters (TTI, PHRR, AEHC, PML) using terpenoid contents per sample (MT_tot, ST_tot, MTox_tot, STox_tot, Tot_Terp) expressed in µg and FMC as predictors, including a dummy variable “alive vs. dead samples”. An additional factor predictor with 4 levels (resistant infected “RI”, resistant control “RC”, susceptible infected “SI” and susceptible control “SC”) was included in the model. The technique prevented the problems associated with multicollinearity among the multiple initial explanatory variables (e.g., terpenoid content and FMC), and the main advantage was that a linear combination of the explanatory variables can be determined. For the determination of the optimal number of components, the cross-validation method was used by applying the Stone-Geiser Q2 statistic. To assess the relative contribution of each independent variable in the model, the value and physical sense of the scaled coefficients were checked. The final output was a multiple linear model with a fit estimated by the R2Y statistic, which was equivalent to the adjusted R2 of a multiple linear model obtained by generalized least squares, and the R2X statistic, which evaluated the collinearity between the independent variables. Statistica 10® and SPSS 20® packages were used to analyse the data.
3. Results
After three months, the inoculation with S. cardinale on the young stems of the S clone induced the dieback of the crowns which were partially desiccated, due to the girdling of the stem by the necrotic lesion (Figure 1).
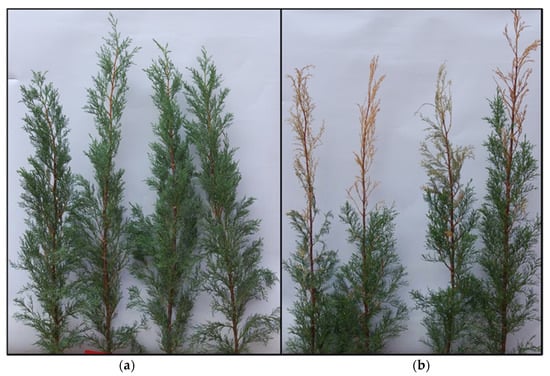
Figure 1.
Cupressus sempervirens L. clones 3 months after multiple artificial stem inoculation with the cypress canker disease fungal agent S. cardinale. (a) Resistant (R); (b) Susceptible (S). The susceptible ramets displayed the dieback of crown portions due to the effect of the bark pathogen that completely girdled the inoculated axes.
3.1. Clone and Infection Effects on Terpenoids Concentration
The differences in the concentration of terpenoids (µg/g) in the leaves and the bark samples were evaluated between the inoculated and the intact ramets of the two clones (Table 1 and Table 2), as well as within the same clone, between the intact (control) and the inoculated ramets (Table 3 and Table 4).

Table 1.
Generalized linear mixed models (GLMM) on the concentration of terpenoids (µg/g) in common cypress leaf samples from the control (C) and infected (I) treatments comparing the resistant (R) vs. susceptible (S) clones for the following variables: MT (monoterpenoids concentration), MTox (oxygenated monoterpenoids concentration), ST (sesquiterpenoids concentration), STox (oxygenated sesquiterpenoids concentration), TOTterp (total terpenoids concentration). The fuel moisture content (FMC) was used as the covariable. The model for the S clone was repeated removing dead samples (live fuels only)*. The average and standard deviation (in brackets) for each variable are shown (N = 12). The p-value shows the significance of the fixed variables (clone, R vs. S) and covariable (FMC) predictors. Significant differences (>95%) are highlighted in bold.

Table 2.
GLMM on the concentration of terpenoids (µg/g) in the common cypress bark samples from the control (C) and infected (I) treatments, comparing the resistant (R) vs. susceptible (S) clones for the following variables: MT (monoterpenoids concentration), MTox (oxygenated monoterpenoids concentration), ST (sesquiterpenoids concentration), STox (oxygenated sesquiterpenoids concentration), TOTterp (total terpenoids concentration). The average and standard deviation (sd) for each variable are shown (N = 12). The p-value shows the significance of the fixed variables (clone, R vs. S) and covariable (FMC) predictors. Significant differences (>95%) are highlighted in bold.

Table 3.
GLMM on the concentration of terpenoids (µg/g) in the common cypress leaf samples of the resistant (R) and susceptible (S) cypress clones comparing the control (C) vs. infected (I) treatments for the following variables: MT (monoterpenoids concentration), MTox (oxygenated monoterpenoids concentration), ST (sesquiterpenoids concentration), STox (oxygenated sesquiterpenoids concentration), TOTterp (total terpenoids concentration). Fuel moisture content (FMC) is used as a covariable. Model for the SI treatment was repeated removing the dead samples *. The average and standard deviation (in brackets) for each variable are shown (N = 12). The p-value shows the significance of the fixed variables (treatment, C vs. I) and covariable (FMC) predictors. Significant differences (>95%) are highlighted in bold.

Table 4.
GLMM of the concentration of terpenoids (µg/g) in the common cypress bark samples of the resistant (R) and susceptible (S) cypress clones comparing the control (C) vs. infected (I) treatments for the following variables: MT (monoterpenoids concentration), MTox (oxygenated monoterpenoids concentration), ST (sesquiterpenoids concentration), STox (oxygenated sesquiterpenoids concentration), TOTterp (total terpenoids concentration). The average and standard deviation (sd) for each variable are shown (N = 12). The p-value shows the significance of the fixed variables (treatment, C vs. I). Significant differences (>95%) are highlighted in bold.
The results showed strong differences between the clones for both the intact (uninoculated) (C) and the inoculated plants (I) (Table 1). In the leaves of intact plants, clone (S) had a higher concentration (p < 0.001) of total terpenoids compared to clone (R) (7514 vs. 6730 µg/g) due to the higher MT and MTox (4319 vs. 3093 µg/g and 221 vs. 156 µg/g, respectively; p < 0.001). In contrast, a higher concentration of ST was shown by the R clone. In the bark tissues (Table 2), the total concentration of terpenoids was higher (p < 0.001) in the R clone than in the S (1727 vs. 1295 µg/g), despite the concentration of monoterpenoids being slightly higher (MT) or higher (MTox) in the S clone (604 vs. 549 µg/g and 42 vs. 32 µg/g, respectively); a similar pattern was also observed in the leaves. Both classes of sesquiterpenoids (ST and STox) were much higher in the R clone (p < 0.001).
The comparison of the inoculated plants of the two clones (I) showed that the clone R generated a higher total concentration of terpenoids (TOTterp) (p < 0.001) in both leaves (6487 vs. 6003 µg/g) (Table 1) and bark tissues (more than twice the concentration of the S one, that was 12,542 vs. 5760 µg/g) (Table 2). With regard to the intact plants C, the higher (p < 0.001) concentration of MT (3134 vs. 2894 µg/g) and MTox (239 vs. 158 µg/g) in the leaves was found in the S clone (Table 1), while the ST and STox were higher (p < 0.001) in the R clone (2231 vs. 1529 µg/g and 1204 vs. 1100 µg/g, respectively). After infection, the significant differences between the clones were maintained even when the dead leaf samples were removed: TOTterp, MT and MTox were slightly higher in the clone S (live) compared to S (live and dead) while the STs were even lower. After the fungal infection, the concentration of all terpenoid categories in the bark tissues were markedly higher (p < 0.001) in the R clone compared to the S clone (Table 2). The effect of the FMC on the concentration of terpenoids in the leaves was always significant, excluding for the concentration of MT in the C plants (comparing R and S clones) (Table 1). The results suggested that the effect of the infection should be analysed separately within the two clones R and S (Table 3 and Table 4).
The results showed little differences between the control (C) and the infected (I) plants of the clone R (Table 3), with only slight decreases in the STox concentration in the leaves after infection (from 1352 to 1204 µg/g; p = 0.026) (Table 3). The susceptible (S) clone reacted to the infection in a different way, the TOTterp significantly decreased in the inoculated plants (from 7514 to 6003 µg/g, p = 0.025), mainly due to the strong reduction in the concentration of MT (from 4319 to 3134 µg/g; p = 0.017) (Table 3). Nevertheless, when the dead samples were removed from the analysis, this difference between the clones was not significant with only a difference in ST concentration occurring (decreasing to 1239 µg/g) (p = 0.045). The relationship between the FMC and terpenoids was always positive: the higher the FMC, the higher the concentration of terpenoids (Table 3). The effect of infection in both clones was strongly evident in the bark tissues (Table 4). A strong increase in TOTterp was detected in the clone S (with an almost 4-fold increase: from 1295 to 5760 µg/g) but especially in clone R in which the value in inoculated plants was more than seven times higher than in the C plants (from 1727 to 12,542 µg/g; p < 0.001). All the classes of terpenoids increased after the infection (p < 0.001), and irrespective of their concentration in the bark tissues of the intact plants, the values reached in the R clone were always higher than those in the S clone. The levels of MT and MTox in the R clone showed the highest relative increase (from 549 to 5926 µg/g, almost 11 times, for MT; from 32 to 489 µg/g, more than 15 times, for MTox) (Table 4).
3.2. Clone and Infection Effects on Flammability
Significant differences between the clones were detected for all the flammability parameters in the intact plants (C) (Table 5). The R clone showed a significantly (p < 0.001) higher TTI compared to the S clone (140.78 vs. 121.67 s) due to the higher FMC, AEHC (p < 0.001) (5.54 vs. 5.07 MJ/Kg) and PML (22.90 vs. 19%). Susceptible infected (SI) clones showed significantly higher ignitability (lower TTI, 73 vs. 116 s, p < 0.001), combustibility (higher PHRR, 143 vs. 69 kW/m2, p < 0.001), sustainability (higher AEHC, 8.10 vs. 4.93 MJ/Kg, p < 0.038) and consumability (higher PML, 47.50 vs. 21.67%, p < 0.001) than the resistant infected (RI) clones. Excluding the dead samples of the S clone (when comparing the live R and the live S portions), many differences among the clones were markedly reduced and only the TTI remained lower in the S clone (109 vs. 116 s, p < 0.001) (Table 5). The effect of the FMC was always significant (p ranging from <0.001 to 0.003) except for the AEHC of the clones before the infection (Table 5).

Table 5.
GLMM of the flammability parameters from the control (C) and the infected (I) treatments of the resistant (R) vs. susceptible (S) common cypress clones for the following variables: TTI (time to ignition, s), PHRR (peak heat release rate, kW/m2), AEHC (average effective heat of combustion, MJ/Kg), PML (percentage of mass lost, %). The average and standard deviation (in brackets) for each variable and treatment are shown (N = 9). The fuel moisture content (FMC) is used as a covariable. The model for the SI treatment was repeated removing dead samples (*). The p-value indicates the significance of the fixed variables (clone, R vs. S). The factor ramet is included in the model as a random variable. Significant differences (>95%) are highlighted in bold.
The comparison of infection effects within the R and S clones showed that the main differences were related to ignitability (TTI) and combustibility (PHRR) (Table 6). In fact, in the R clone the TTI was the only parameter that changed (decreased) in the inoculated plants (from 141 to 116 s, p < 0.001) (Table 6) which showed a higher ignitability. The effect of the inoculation in the S clone was even stronger and involved all of the flammability parameters: ignitability (TTI dropped from 122 to 73 s, p = 0.002), combustibility (PHRR, p < 0.001), sustainability (AEHC, p = 0.005) and consumability (PML, p < 0.001) all increased. Nevertheless, when the dead samples were removed, only the TTI remained significantly lower than in the control (Table 6). The effect on the FMC was significant for all of the flammability parameters in the S clone, whereas in the R clone, it was only the TTI and PHRR that were significantly affected (p < 0.001). In the S clone, when the dead parts were removed from the computation, the effect of the infection on the FMC was still significant (p < 0.001) (Table 6).

Table 6.
GLMM of the flammability parameters of the resistant (R) and susceptible (S) common cypress clones comparing the control (C) and infected (I) treatments for the following variables: TTI (time to ignition, s, PHRR (peak heat release rate, kW/m2), AEHC (average effective heat of combustion, MJ/Kg), PML (percentage of mass lost, %). The average and standard deviation (in brackets) for each variable and treatment are shown (N = 9). The fuel moisture content (FMC) is used as a covariable. The model for the SI treatment was repeated removing the dead samples (*). The p-value indicates the significance of the fixed variables (treatment, C vs. I). Significant differences (>95%) are highlighted in bold.
3.3. Linking Disease and Flammability
The PLS model indicated that 56% of the variation in the flammability can be explained by the following selected variables: clone x treatment (RC, RI, SC, SI), fuel moisture content (FMC), the dummy variable ‘alive vs. dead’ samples, and terpenoid concentration for each class. The fitted parameters (Table 7) show that the flammability components were explained by the predictive variables with different fits between 24% and 77%. Therefore, the model accounts for the combustibility (R2Y = 77% for PHRR) and consumability (R2Y = 66% for PML) more effectively than ignitability (R2Y = 24% for TTI) or sustainability (R2Y = 52% for AEHC). The PLS model showed that the FMC and ‘alive vs. dead’ (dummy variable) explain most of the variability in the data (higher scaled coefficients) (Figure 2). With regard to the amount of terpenoids, ST showed the highest scaled coefficients and a positive relationship with flammability (higher PHRR, AEHC and PML) (Figure 2). In terms of the treatment effects, the scaled coefficients in the model showed that the most important parameter related to flammability was the dead portions of plant instead of the changes in the content of terpenoids (Figure 2). When the dead samples were included (Figure 2), the susceptible infected clone (SI) showed the highest flammability.

Table 7.
Fitted parameters for the partial least square (PLS) model to predict the flammability (TTI, PHRR, AEHC, PML) using: the amount of terpenoids (MT_tot, ST_tot, MTox_tot, STox_tot, Tot_Terp) contained in each sample (µg), the FMC, the treatment x clone (4 levels: resistant control, RC, resistant infected, RI, susceptible control, SC, susceptible infected, SI) as predictors. The model selects two components and includes the dummy variable ‘alive vs. dead’ samples to highlight the importance of the dead samples (4 samples belonging to the SI treatment) in the prediction of flammability (N = 36). The total model fit (R2Y) and the partial model fit for the predicted flammability variables are highlighted in bold.
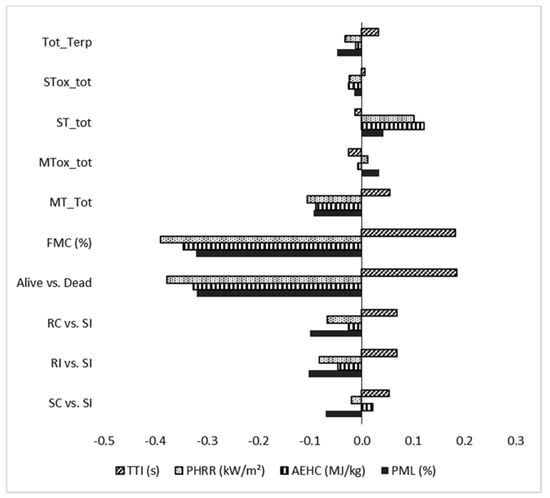
Figure 2.
Scaled coefficients for the predictive variables of the flammability parameters: TTI (ignitability), PHRR (combustibility), AEHC (sustainability) and PML (consumability). The scaled coefficients show the relative importance of each variable in the structure of the PLS model (N = 36).
4. Discussion
Volatile terpenoids are secondary plant metabolites that undertake many ecological functions and roles [54,55]. As part of resins, terpenoids play an essential role in the plant defence against microbes, especially in conifers [22,56,57,58,59]. The expected increase in the content of terpenoids after infection with S. cardinale was observed in both the cypress clones in the bark tissue (although it was much stronger in the resistant clone). The accumulation of terpenoids did not occur in the leaves, in accordance with the biology of the fungal pathogen acting at the cortical level [30,33]. This result was consistent with the known de novo production and the accumulation of all the classes of terpenoids as a reaction to the attack of fungal pathogens in many species of conifers [20,29,59,60,61,62,63]. In addition, it was recently reported that a S. cardinale inoculation on the cypress stems or branches induced a reaction in the host which consisted in the production of traumatic resin ducts in the phloem [36] and the consequent accumulation of terpenoids in the bark tissues near the site of infection. This defence reaction was observed to be stronger in canker resistant cypress genotypes [29], confirming that the production of resin terpenoids was an important and effective response of cypress to CCD. In unaffected plants (C), the differences among the clones mainly concerned STs that were higher than in the R clone, indicating a possible deployment of a constitutive chemical barrier of quantitatively ‘minor’ terpenoids with a higher biological efficacy, instead of MTs that have less antifungal activity against S. cardinale [29].
Low weight terpenoids (volatiles terpenoids), such as monoterpenoids (C10) and sesquiterpenoids (C15), possess relatively low boiling and flash points, and as a consequence high flammability [26,27]. The role played by the plant volatile terpenoids in driving the flammability of vegetation is now widely accepted [26,64], though the quantitative effect is still debated [27]. In other words, to what extent does the terpenoid content influence combustion, and the wildfires on the scale of a forest fire, is not yet well defined. Few laboratory studies have attempted to evaluate the effect of these compounds on the variability of flammability parameters in different tree species. This is particularly relevant given the amount of terpenoids contained in the leaf tissues or plant twigs [27].
Inoculation with S. cardinale on the young cypress plants induced the dieback of the upper part of the crowns of the S clone after three months (Figure 1). This has two implications: firstly, the presence of dried plant material, and secondly, the accumulation of terpenoids in bark tissues around the necrotic lesions. It is well known that cypress canker disease may cause large bark lesions, inducing serious diebacks of cypress crowns [33] and causing the copious exudation of resin which flows down the affected trunks in severely cankered stems. In this study, the tissues around the inoculated points on the stem did not show the exudation of resin outside the cankered lesion. This could be due to the relatively short time between the inoculations and the collection of samples (3 months), the young age of the plants or the relatively small diameters of the inoculated stems and their subsequent early death.
To our knowledge this is one of the first studies to evaluate the effect of the estimated amount of terpenoids contained in plant tissues (µg) on flammability tests. The GLMM model showed that the SI (susceptible–infected) clone samples appeared to be significantly more flammable in comparison to the RI (resistant–infected) samples, showing higher ignitability, combustibility, sustainability and consumability. This is mainly due to the presence of dead crown portions, as when the dead samples of SI were removed from the computation most of the differences between the treatments disappeared. This finding was also supported by the values of the R clone, which showed a slightly higher ignitability in infected plants, while the other parameters did not change as a consequence of the S. cardinale infection. In contrast, the ignitability of the S clone was higher than that of the R clone, when only the living tissues were considered.
The outcomes from the GLMM were supported by the PLS model results, which indicated that the FMC and the ‘alive vs. dead’ accounted for most of the data variability. Moreover, concerning the treatment, the model showed that the most important parameter related to flammability was the presence of dead plant portions rather than changes in the content of terpenoids (µg). As accounted for above, this could be partially explained by the absence of a copious amount of resin flowing on the stems, and by the relatively small proportion of bark tissues (where the highest increase in terpenoids was observed) when compared to the sample as a whole. In fact, the scaled coefficients showed a negative correlation between both ‘non-dead’ plant material and FMC vs. all of the tested flammability parameters. An interesting positive relationship was found between the total amount of ST and combustibility and sustainability, confirming the role of terpenoids in flammability, as already hypothesized in Della Rocca et al. [27]. In contrast, a surprising negative relationship between the total amount of MTs and all the flammability parameters was found. This might be explained by the observed strong reduction in MT concentration in the dead tissues of the susceptible clone, while in the live tissues of the same inoculated clone the MT concentration increased. These results suggested a possible MT leak as the diseased crown portions dry, which requires further investigation to fully account for the possible relationship between the tissues’ water content and the terpenoids loss.
5. Conclusions
In response to the experimental questions outlined above: (i) in the common cypress—cypress bark canker pathosystem (at least for young plants such as those considered in this study), the diseased plants were more ignitable and showed increased combustibility and enhanced sustainability; (ii) the CCD-resistant cypress clone appeared less flammable than the susceptible clone when infected; and (iii) the proportion of the dried crown parts (as a consequence of the disease) was a stronger factor in determining the overall flammability than the terpenoid accumulation.
The selection of CCD-resistant cypress genotypes [65,66] for their use in plantations to replace trees killed or compromised by the disease, and sanitation to remove the heavily infected portions of trees [67] not only improves the health, aesthetic and recreational attributes of plantations [32,48] but also reduces their flammability. The results of this work suggest some general considerations for the phytosanitary management of woodlands, plantations and hedges. First, a disease can strongly increase the flammability of a tree, especially conifers, because it can cause the dehydration or desiccation of crown portions, the retention of dead material in the crowns and the accumulation of flammable compounds, such as terpenoids, in living bark tissues as a reaction to the disease. Secondly, the flammability of a tree species can be highly genotype dependent (i.e., genotypes resistant to a disease or more tolerant to drought stress can be less flammable than their susceptible or less tolerant counterparts). Finally, the spread of disease at the tree and stand level can be an important factor in the prediction of fire risk and should be actively monitored.
Author Contributions
Conceptualization, G.D.R. and J.M.; methodology, G.D.R., R.D. and J.M.; formal analysis, G.D.R., M.M., C.C. and J.M.; investigation, G.D.R. and J.M.; data curation, G.D.R. and J.M.; writing—original draft, G.D.R., R.D. and J.M.; writing—review & editing, C.H., M.G. and C.C.; funding acquisition, C.H. and J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the VIS4FIRE (Integrated Vulnerability of Forest Systems to Wildfire: Implications on Forest Management Tools), Spanish R&D project (RTA2017-00042-C05-01). VIS4FIRE is co-funded by the EU through the FEDER program. This study was also co-financed by INIA (FPI-SGIT 2018) and a European Social Fund grant awarded to Cristina Carrillo.
Acknowledgments
We thank Carmen Díez from INIA–CIFOR for the assistance during the flammability experiment, Vincenzo Di Lonardo for growing the cypress plants and Matthew Haworth for the revision of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, G.; He, Y.; De Santis, A.; Li, G.; Cobb, R.; Meentemeyer, R.K. Assessing the impact of emerging forest disease on wildfire using Landsat and KOMPSAT-2 data. Remote. Sens. Environ. 2017, 195, 218–229. [Google Scholar] [CrossRef]
- Metz, M.; Varner, J.M.; Frangioso, K.M.; Meentemeyer, R.K.; Rizzo, D.M. Unexpected redwood mortality from synergies between wildfire and an emerging infectious disease. Ecology 2013, 94, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Killi, D.; Bussotti, F.; Gottardini, E.; Pollastrini, M.; Mori, J.; Tani, C.; Papini, A.; Ferrini, F.; Fini, A. Photosynthetic and morphological responses of oak species to temperature and [CO2] increased to levels predicted for 2050. Urban For. Urban Green. 2018, 31, 26–37. [Google Scholar] [CrossRef]
- Metz, M.; Varner, J.M.; Simler-Williamson, A.; Frangioso, K.M.; Rizzo, D.M. Implications of sudden oak death for wildland fire management. For. Phytophthoras 2017, 7, 30–44. [Google Scholar] [CrossRef]
- Boyd, I.L.; Freer-Smith, P.H.; Gilligan, C.A.; Godfray, H.C.J. The Consequence of Tree Pests and Diseases for Ecosystem Services. Science 2013, 342, 1235773. [Google Scholar] [CrossRef] [PubMed]
- Stephens, S.L.; Agee, J.K.; Fulé, P.Z.; North, M.P.; Romme, W.H.; Swetnam, T.W.; Turner, M.G. Managing Forests and Fire in Changing Climates. Science 2013, 342, 41–42. [Google Scholar] [CrossRef]
- Ruffault, J.; Curt, T.; Martin-StPaul, N.K.; Moron, V.; Trigo, R.M. Extreme wildfire events are linked to global-change-type droughts in the northern Mediterranean. Nat. Hazards Earth Syst. Sci. 2018, 18, 847–856. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, S.; Kaplan, J. Sensitivity of global wildfire occurrences to various factors in the context of global change. Atmos. Environ. 2015, 121, 86–92. [Google Scholar] [CrossRef]
- Ramsfield, T.; Bentz, B.; Faccoli, M.; Jactel, H.; Brockerhoff, E.G. Forest health in a changing world: Effects of globalization and climate change on forest insect and pathogen impacts. Forestry 2016, 89, 245–252. [Google Scholar] [CrossRef]
- Loehman, R.; Keane, R.; Holsinger, L.M.; Wu, Z. Interactions of landscape disturbances and climate change dictate ecological pattern and process: Spatial modeling of wildfire, insect, and disease dynamics under future climates. Landsc. Ecol. 2016, 32, 1447–1459. [Google Scholar] [CrossRef]
- Pautasso, M.; Schlegel, M.; Holdenrieder, O. Forest Health in a Changing World. Microb. Ecol. 2014, 69, 826–842. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Veneklaas, E.J.; Hardy, G.E.S.J.; Poot, P. Tree host–pathogen interactions as influenced by drought timing: Linking physiological performance, biochemical defence and disease severity. Tree Physiol. 2018, 39, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.; Frangioso, K.M.; Meentemeyer, R.K.; Rizzo, D.M. Interacting disturbances: Wildfire severity affected by stage of forest disease invasion. Ecol. Appl. 2011, 21, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Meentemeyer, R.K.; Rank, N.E.; Shoemaker, D.A.; Oneal, C.B.; Wickland, A.C.; Frangioso, K.M.; Rizzo, D.M. Impact of sudden oak death on tree mortality in the Big Sur ecoregion of California. Boil. Invasions 2007, 10, 1243–1255. [Google Scholar] [CrossRef]
- Cobb, R.C.; Filipe, J.A.N.; Meentemeyer, R.K.; Gilligan, C.A.; Rizzo, D.M. Ecosystem transformation by emerging infectious disease: Loss of large tanoak from California forests. J. Ecol. 2012, 100, 712–722. [Google Scholar] [CrossRef]
- Cobb, R.C.; Rizzo, D.M. Decomposition and N cycling changes in redwood forests caused by sudden oak death. In Proceedings of the Coast Redwood Forests in a Changing California: A Symposium for Scientists and Managers, Albany, CA, USA, 21–23 June 2011; 2012; pp. 357–362. [Google Scholar]
- Varner, J.M.; Kuljian, H.G.; Kreye, J.K. Fires without tanoak: The effects of a non-native disease on future community flammability. Boil. Invasions 2017, 19, 2307–2317. [Google Scholar] [CrossRef]
- Forrestel, A.B.; Ramage, B.; Moody, T.; Moritz, M.A.; Stephens, S.L. Disease, fuels and potential fire behavior: Impacts of Sudden Oak Death in two coastal California forest types. For. Ecol. Manag. 2015, 348, 23–30. [Google Scholar] [CrossRef]
- Trapp, S.; Croteau, R. Defensive resin biosynthesis in conifers. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 689–724. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Krokene, P.; Christiansen, E.; Krekling, T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 2005, 167, 353–376. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Boulogne, I.; Petit, P.; Ozier-Lafontaine, H.; Desfontaines, L.; Loranger-Merciris, G. Insecticidal and antifungal chemicals produced by plants: A review. Environ. Chem. Lett. 2012, 10, 325–347. [Google Scholar] [CrossRef]
- Chetehouna, K.; Barboni, T.; Zarguili, I.; Leoni, E.; Simeoni, A.; Fernandez-Pello, A.C. Investigation on the Emission of Volatile Organic Compounds from Heated Vegetation and Their Potential to Cause an Accelerating Forest Fire. Combust. Sci. Technol. 2009, 181, 1273–1288. [Google Scholar] [CrossRef]
- Chetehouna, K.; Courty, L.; Garo, J.P.; Viegas, D.; Fernandez-Pello, C. Flammability limits of biogenic volatile organic compounds emitted by fire-heated vegetation (Rosmarinus officinalis) and their potential link with accelerating forest fires in canyons: A Froude-scaling approach. J. Fire Sci. 2013, 32, 316–327. [Google Scholar] [CrossRef]
- Courty, L.; Chetehouna, K.; Lemee, L.; Mounaïm-Rousselle, C.; Halter, F.; Garo, J. Pinus pinea emissions and combustion characteristics of limonene potentially involved in accelerating forest fires. Int. J. Therm. Sci. 2012, 57, 92–97. [Google Scholar] [CrossRef]
- Pausas, J.G.; Alessio, G.A.; Moreira, B.; Segarra-Moragues, J.G. Secondary compounds enhance flammability in a Mediterranean plant. Oecologia 2015, 180, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Della Rocca, G.; Madrigal, J.; Marchi, E.; Michelozzi, M.; Moya, B.; Danti, R. Relevance of terpenoids on flammability of Mediterranean species: An experimental approach at a low radiant heat flux. iFor. Biogeosci. For. 2017, 10, 766–775. [Google Scholar] [CrossRef]
- Fares, S.; Bajocco, S.; Salvati, L.; Camarretta, N.; Dupuy, J.-L.; Xanthopoulos, G.; Guijarro, M.; Madrigal, J.; Hernando, C.; Corona, P. Characterizing potential wildland fire fuel in live vegetation in the Mediterranean region. Ann. For. Sci. 2017, 74, 1–14. [Google Scholar] [CrossRef]
- Achotegui-Castells, A.; Della Rocca, G.; Llusià, J.; Danti, R.; Barberini, S.; Bouneb, M.; Simoni, S.; Michelozzi, M.; Penuelas, J. Terpene arms race in the Seiridium cardinale—Cupressus sempervirens pathosystem. Sci. Rep. 2016, 6, 18954. [Google Scholar] [CrossRef]
- Graniti, A. CYPRESS CANKER: A Pandemic in Progress. Annu. Rev. Phytopathol. 1998, 36, 91–114. [Google Scholar] [CrossRef]
- Della Rocca, G.; Osmundson, T.; Danti, R.; Doulis, A.G.; Pecchioli, A.; Donnarumma, F.; Casalone, E.; Garbelotto, M. AFLP analyses of California and Mediterranean populations ofSeiridium cardinaleprovide insights on its origin, biology and spread pathways. For. Pathol. 2013, 43, 211–221. [Google Scholar] [CrossRef]
- Danti, R.; Della Rocca, G. Epidemiological History of Cypress Canker Disease in Source and Invasion Sites. Forests 2017, 8, 121. [Google Scholar] [CrossRef]
- Danti, R.; Della Rocca, G.; Panconesi, A. Chapter 17 Cypress canker. In Infectious Forest Disease; Nicolotti, G., Gonthier, P., Eds.; CAB International: Wallingford, UK; Boston, MA, USA, 2013; pp. 358–371. [Google Scholar]
- Ponchet, J.; Andreoli, C. Histopathologie du cancre du cyprès à Seiridium cardinale. Eur. J. For. Path. 1989, 19, 212–221. [Google Scholar]
- Spanos, B.K.A.; Pirrie, A.; Woodward, S.; Xenopoulos, S. Responses in the bark of Cupressus sempervirens clones artificially inoculated with Seiridium cardinale under field conditions. Eur. J. For. Pathol. 1999, 29, 135–142. [Google Scholar] [CrossRef]
- Papini, A.; Moricca, S.; Danti, R.; Tani, C.; Posarelli, I.; Falsini, S.; Della Rocca, G. Ultrastructure of traumatic resin duct formation in Cupressus sempervirens L. in response to the attack of the fungus Seiridium cardinale (Wag.) Sutton & Gibson. In Proceedings of the 14th Multinational Congress on Microscopy, Belgrade, Serbia, 15–20 September 2019; pp. 310–311. [Google Scholar]
- Raddi, P.; Panconesi, A. Pathogenicity of some isolates of Seiridium (Coryneum) cardinale, agent of cypress canker disease. For. Pathol. 1984, 14, 348–354. [Google Scholar] [CrossRef]
- Santini, A.; Donardo, V. Genetic variability of the ‘bark canker resistance’ character in several natural provenances of Cupressus sempervirens. For. Pathol. 2000, 30, 87–96. [Google Scholar] [CrossRef]
- Danti, R.; Panconesi, A.; Di Lonardo, V.; Della Rocca, G.; Raddi, P. Italico’and Mediterraneo’: Two Seiridium cardinale Canker-Resistant Cypress Cultivars of Cupressus sempervirens. Am. Soc. Hort. Sci. 2006, 41, 1357–1359. [Google Scholar] [CrossRef]
- Danti, R.; Di Lonardo, V.; Pecchioli, A.; Della Rocca, G. ‘Le Crete 1’ and ‘Le Crete 2’: Two newly patentedSeiridium cardinalecanker-resistant cultivars ofCupressus sempervirens. For. Pathol. 2012, 43, 204–210. [Google Scholar] [CrossRef]
- Danti, R.; Rotordam, M.G.; Emiliani, G.; Giovannelli, A.; Papini, A.; Tani, C.; Barberini, S.; Della Rocca, G. Different clonal responses to cypress canker disease based on transcription of suberin-related genes and bark carbohydrates’ content. Trees 2018, 32, 1707–1722. [Google Scholar] [CrossRef]
- Valette, J.-C. Inflammabilités des espèces forestières méditerranéennes. Conséquences sur la combustibilité des formations forestières. Rev. For. Française 1990, 42, 76–92. [Google Scholar] [CrossRef]
- Dimitrakopoulos, A.; Papaioannou, K.K. Flammability Assessment of Mediterranean Forest Fuels. Fire Technol. 2001, 37, 143–152. [Google Scholar] [CrossRef]
- Liodakis, S.; Bakirtzis, D.; Lois, E. TG and autoignition studies on forest fuels. J. Therm. Anal. Calorim 2002, 69, 519–528. [Google Scholar] [CrossRef]
- Neyisci, T.; Intini, M. The use of cypress barriers for limiting fires in Mediterranean countries. In Proceedings of the Il Cipresso e Gli Incendi, Valencia, Spain, 14–16 June 2006; Arsia Toscana: Firenze, Italy, 2006; pp. 3–18. [Google Scholar]
- Ganteaume, A.; Jappiot, M.; Lampin, C.; Guijarro, M.; Hernando, C. Flammability of Some Ornamental Species in Wildland–Urban Interfaces in Southeastern France: Laboratory Assessment at Particle Level. Environ. Manag. 2013, 52, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Della Rocca, G.; Hernando, C.; Madrigal, J.; Danti, R.; Moya, J.; Guijarro, M.; Pecchioli, A.; Moya, B. Possible land management uses of common cypress to reduce wildfire initiation risk: A laboratory study. J. Environ. Manag. 2015, 159, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Danti, R.; Barberini, S.; Pecchioli, A.; Di Lonardo, V.; Della Rocca, G. The Epidemic Spread of Seiridium cardinaleon Leyland Cypress Severely Limits Its Use in the Mediterranean. Plant Dis. 2014, 98, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, J.; Hernando, C.; Guijarro, M.; Díez, C.; Marino, E.; De Castro, A.J. Evaluation of Forest Fuel Flammability and Combustion Properties with an Adapted Mass Loss Calorimeter Device. J. Fire Sci. 2009, 27, 323–342. [Google Scholar] [CrossRef]
- Madrigal, J.; Hernando, C.; Guijarro, M. A new bench-scale methodology for evaluating the flammability of live forest fuels. J. Fire Sci. 2012, 31, 131–142. [Google Scholar] [CrossRef]
- Della Rocca, G.; Danti, R.; Hernando, C.; Guijarro, M.; Madrigal, J. Flammability of Two Mediterranean Mixed Forests: Study of the Non-additive Effect of Fuel Mixtures in Laboratory. Front. Plant Sci. 2018, 9, 825. [Google Scholar] [CrossRef]
- White, R.H.; Zipperer, W.C. Testing and classification of individual plants for fire behaviour: Plant selection for the wildland—urban interface. Int. J. Wildland Fire 2010, 19, 213–227. [Google Scholar] [CrossRef]
- Cruz, M.G.; Butler, B.W.; Viegas, D.; Palheiro, P. Characterization of flame radiosity in shrubland fires. Combust. Flame 2011, 158, 1970–1976. [Google Scholar] [CrossRef]
- Tholl, D. Biosynthesis and Biological Functions of Terpenoids in Plants. Plant Cells 2015, 148, 63–106. [Google Scholar] [CrossRef]
- Yazaki, K.; Arimura, G.; Ohnishi, T. ‘Hidden’ Terpenoids in Plants: Their Biosynthesis, Localization and Ecological Roles. Plant Cell Physiol. 2017, 58, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Freeman, K. An Overview of Plant Defenses against Pathogens and Herbivores. Plant Heal. Instr. 2008, 149. [Google Scholar] [CrossRef]
- Selim, S.A.; E Adam, M.E.; Hassan, S.M.; AlBalawi, A.R. Chemical composition, antimicrobial and antibiofilm activity of the essential oil and methanol extract of the Mediterranean cypress (Cupressus sempervirens L.). BMC Complement. Altern. Med. 2014, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Zulak, K.G.; Bohlmann, J. Terpenoid Biosynthesis and Specialized Vascular Cells of Conifer Defense. J. Integr. Plant Boil. 2010, 52, 86–97. [Google Scholar] [CrossRef]
- Celedon, J.M.; Bohlmann, J. Oleoresin defenses in conifers: Chemical diversity, terpene synthases and limitations of oleoresin defense under climate change. New Phytol. 2019, 224, 1444–1463. [Google Scholar] [CrossRef]
- Krokene, P.; Nagy, N.E.; Krekling, T. Chapter 7 Traumatic resin ducts and polyphenolic parenchyma cells in conifers. In Induced Plant Resistance to Herbivory; Springer: Berlin/Heidelberg, Germany, 2008; pp. 147–169. [Google Scholar]
- Bonello, P.; Capretti, P.; Luchi, N.; Martini, V.; Michelozzi, M. Systemic effects of Heterobasidion annosum s.s. infection on severity of Diplodia pinea tip blight and terpenoid metabolism in Italian stone pine (Pinus pinea). Tree Physiol. 2008, 28, 1653–1660. [Google Scholar] [CrossRef]
- Pepori, A.L.; Michelozzi, M.; Santini, A.; Cencetti, G.; Bonello, P.; Gonthier, P.; Sebastiani, F.; Luchi, N. Comparative transcriptional and metabolic responses of Pinus pinea to a native and a non-native Heterobasidion species. Tree Physiol. 2018, 39, 31–44. [Google Scholar] [CrossRef]
- Whitehill, J.; Yuen, M.M.S.; Henderson, H.; Madilao, L.L.; Kshatriya, K.; Bryan, J.; Jaquish, B.; Bohlmann, J. Functions of stone cells and oleoresin terpenes in the conifer defense syndrome. New Phytol. 2018, 221, 1503–1517. [Google Scholar] [CrossRef]
- Ormeño, E.; Céspedes, B.; Sánchez, I.A.; García, A.V.; Moreno, J.M.; Fernandez, C.; Baldy, V. The relationship between terpenes and flammability of leaf litter. For. Ecol. Manag. 2009, 257, 471–482. [Google Scholar] [CrossRef]
- Raddi, P.; Panconesi, A.; Xenopoulos, S.; Ferrandes, P.; Andreoli, C. Genetic improvement for resistance to cypress canker. In Agrimed Reserach Programme, Progress in EEC Research on Cypress Disease; Ponchet, J., Ed.; Report EU 12493 EN; Commission of the European Communities: Brussels, Belgium; Luxembourg, 1990; pp. 127–134. [Google Scholar]
- Danti, R.; Della Rocca, G.; Di Lonardo, V.; Pecchioli, A.; Raddi, P. Genetic improvement program of cypress: Results and outlook. In Status of the Experimental Network of Mediterranean Forest Genetic Resources; Besacier, C., Ducci, F., Malagnoux, M., Souvannavong, O., Eds.; CRA SAL, Arezzo and FAO Silva Mediterranea: Rome, Italy, 2011; pp. 88–96. [Google Scholar]
- Panconesi, A.; Danti, R. Esperienze técnico-scientifiche nella bonifica del cipresso. In Proceedings of the Il Recupero Del Cipresso Nel Paesaggio E Nel Giardino Storico’ Collodi, Pistoia, Italy, 15 March 1995; Regione Toscana Giunta Regionale Dipartimento Agricoltura e Foreste: Florence, Italy, 1995; pp. 9–21. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).