Root Traits Determine Variation in Nonstructural Carbohydrates (NSCs) under Different Drought Intensities and Soil Substrates in Three Temperate Tree Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Sapling Preparation
2.2. Experimental Design
2.3. Sampling and Measurements
2.3.1. Plant Sample Collection
2.3.2. Soil Physical and Chemical Properties
2.3.3. Root Tip Morphological and Chemical Traits
2.3.4. NSC Concentration
2.4. Data Analysis
3. Results
3.1. Root Tip Morphology
3.2. Root Tip Carbon, Nitrogen, and Phosphorus
3.3. Root Tip NSC Concentration
3.4. Relationship between Root Tip Morphology and NSC
4. Discussion
4.1. Response of Root Morphological Traits to Drought and Soil Substrates
4.2. Effect of Drought and Soil Substrates on Fine Root NSC Concentration
4.3. Relationship between Root SRL, Diameter, and NSC Concentration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McDowell, N.; Pockman, W.; Allen, C. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; Mcdowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Berry, J.A.; Smith, D.D.; Sperry, J.S.; Anderegg, L.D.L.; Field, C.B. The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proc. Natl. Acad. Sci. USA 2012, 109, 233–237. [Google Scholar] [CrossRef]
- IPCC. Climate change 2007: Synthesis report. In Core Writing Team; Pachauri, R.K., Reisinger, A., Eds.; IPCC: Geneva, Switzerland, 2007; p. 104. [Google Scholar]
- Smith, A.M.; Stitt, M. Coordination of carbon supply and plant growth. Plant Cell Environ. 2007, 30, 1126–1149. [Google Scholar] [CrossRef]
- Wiley, E.; Huepenbecker, S.; Casper, B.B.; Helliker, B.R. The effects of defoliation on carbon allocation: Can carbon limitation reduce growth in favour of storage? Tree Physiol. 2013, 33, 1216–1228. [Google Scholar] [CrossRef]
- Myers, J.A.; Kitajima, K. Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest. J. Ecol. 2007, 95, 383–395. [Google Scholar] [CrossRef]
- Hartmann, H.; Ziegler, W.; Trumbore, S. Lethal drought leads to reduction in nonstructural carbohydrates in norway spruce tree roots but not in the canopy. Funct. Ecol. 2013, 27, 413–427. [Google Scholar] [CrossRef]
- Mitchell, P.J.; O’Grady, A.P.; Tissue, D.T.; White, D.A.; Ottenschlaeger, M.L.; Pinkard, E.A. Drought response strategies define the relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality. New Phytol. 2013, 197, 862–872. [Google Scholar] [CrossRef]
- Piper, F.I.; Fajardo, A. Carbon dynamics of Acer pseudoplatanus seedlings under drought and complete darkness. Tree Physiol. 2016, 36, 1400–1408. [Google Scholar]
- Christian, K.R.; Roman, A.; Olivier, B.; Stephan, H.T.; Keel, S.G.; Susanna, P.R.; Steeve, P.; Siegwolf, R.T.W.; Gerhard, Z. Carbon flux and growth in mature deciduous forest trees exposed to elevated CO2. Science 2005, 309, 1360–1362. [Google Scholar]
- Wiley, E.; Hoch, G.; Landhausser, S.M. Dying piece by piece: Carbohydrate dynamics in aspen (populus tremuloides) seedlings under severe carbon stress. J. Exp. Bot. 2017, 68, 5221–5232. [Google Scholar] [CrossRef] [PubMed]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Annu. Rev. Plant. Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef]
- Mei, L.; Xiong, Y.; Gu, J.; Wang, Z.; Guo, D. Whole-tree dynamics of non-structural carbohydrate and nitrogen pools across different seasons and in response to girdling in two temperate trees. Oecologia 2015, 177, 333–344. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees from what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef]
- Mei, L.; Wang, Z.Q.; Cheng, Y.H.; Guo, D.L. A review:factors influencing fine root longevity in forest ecosystems. Acta Phytoecol. Sin. 2004, 28, 704–710. (In Chinese) [Google Scholar]
- Pregitzer, K.S.; DeForest, J.L.; Burton, A.J. Fine root architecture of nine North American trees. Ecol. Monogr. 2002, 72, 293–309. [Google Scholar] [CrossRef]
- Guo, D.; Xia, M.; Wei, X.; Chang, W.; Liu, Y.; Wang, Z. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three chinese temperate tree species. New Phytol. 2008, 180, 673–683. [Google Scholar] [CrossRef]
- Guo, D.; Li, H.; Mitchell, R.J.; Han, W.; Hendricks, J.J.; Fahey, T.J.; Hendrick, R.L. Fine root heterogeneity by branch order: Exploring the discrepancy in root turnover estimates between minirhizotron and carbon isotopic methods. New Phytol. 2010, 177, 443–456. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, D.; Wang, X.; Gu, J.; Mei, L. Fine root architecture, morphology, and biomass of different branch orders of two chinese temperate tree species. Plant Soil 2006, 288, 155–171. [Google Scholar] [CrossRef]
- Guo, D.; Mitchell, R.J.; Withington, J.M.; Fan, P.P.; Hendricks, J.J. Endogenous and exogenous controls of root life span, mortality and nitrogen flux in a longleaf pine forest: Root branch order predominates. J. Ecol. 2008, 96, 737–745. [Google Scholar] [CrossRef]
- Gu, J.; Xu, Y.; Dong, X.; Wang, H.; Wang, Z. Root diameter variations explained by anatomy and phylogeny of 50 tropical and temperate tree species. Tree Physiol. 2014, 34, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.J.; Guo, D.L. Variation in root diameter among 45 common tree species in temperate, subtropical and tropical forests in china. J. Plant Ecol. 2008, 32, 1248–1257. [Google Scholar]
- Withington, J.M.; Reich, P.B.; Oleksyn, J.; Eissenstat, D.M. Comparisons of structure and life span in roots and leaves among temperate trees. Ecol. Monogr. 2006, 76, 381–397. [Google Scholar] [CrossRef]
- Valladares, F.; Sanchez-Gomez, D.; Zavala, M.A. Quantitative estimation of phenotypic plasticity: Bridging the gap between the evolutionary concept and its ecological applications. J. Ecol. 2006, 94, 1103–1116. [Google Scholar] [CrossRef]
- Weemstra, M.; Sterck, F.J.; Visser, E.J.W.; Kuyper, T.W.; Goudzwaard, L.; Mommer, L. Fine-root trait plasticity of beech (Fagus sylvatica) and spruce (Picea abies) forests on two contrasting soils. Plant Soil 2016, 415, 175–188. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Sterck, F.J.; Weemstra, M. Plasticity influencing the light compensation point offsets the specialization for light niches across shrub species in a tropical forest understorey. J. Ecol. 2013, 101, 971–980. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Yu, D.P.; Zhou, W.M.; Bao, Y.; Lin, Q.I.; Zhou, L.; Dai, L.M. Forest management of korean pine and broadleaf mixed forest in northeast china since the implementation of natural forest protection project. Acta Ecol. Sin. 2015, 35, 10–17. [Google Scholar]
- Dai, L.; Gao, C.; Deng, H.; Lanzhu, J.I.; Hao, Z.; Wang, Q. Structure characteristics and health distance assessment of various disturbed communities of korean pine and broadleaved mixed forest in Changbai mountains. Chin. J. Appl. Ecol. 2004, 15, 1750. (In Chinese) [Google Scholar]
- Zhu, J.J. A review on fundamental studies of secondary forest management. Chin. J. Appl. Ecol. 2002, 13, 1689–1694. (In Chinese) [Google Scholar]
- Bharathan, G.; Goliber, T.E.; Moore, C.; Kessler, S.; Pham, T.; Sinha, N.R. Homologies in leaf form inferred from knoxi gene expression during development. Science 2002, 296, 1858–1860. [Google Scholar] [CrossRef]
- Klingenberg, C.P.; Duttke, S.; Whelan, S.; Kim, M. Developmental plasticity, morphological variation and evolvability: A multilevel analysis of morphometric integration in the shape of compound leaves. J. Evol. Biol. 2015, 25, 115–129. [Google Scholar] [CrossRef]
- Turner, I.M. The Ecology of Trees in the Tropical Rain Forest; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Mokany, K.; Mcmurtrie, R.E.; Atwell, B.J.; Keith, H. Interaction between sapwood and foliage area in alpine ash (Eucalyptus delegatensis) trees of different heights. Tree Physiol. 2003, 23, 949. [Google Scholar] [CrossRef]
- Tulik, M.; Marciszewska, K.; Adamczyk, J. Diminished vessel diameter as a possible factor in the decline of european ash (Fraxinus excelsior L.). Ann. For. Sci. 2009, 67, 103. [Google Scholar] [CrossRef]
- Niinemets, Ü. Are compound-leaved woody species inherently shade-intolerant? An analysis of species ecological requirements and foliar support costs. Plant Ecol. 1998, 134, 1–11. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Song, J.; Wang, M.; Li, N.; Niu, C.Y.; Hao, G.Y. Coordination of xylem hydraulics and stomatal regulation in keeping the integrity of xylem water transport in shoots of two compound-leaved tree species. Tree Physiol. 2015, 35, 1333. [Google Scholar] [CrossRef]
- Merine, A.K.; Rodríguez-García, E.; Alía, R.; Pando, V.; Bravo, F. Effects of water stress and substrate fertility on the early growth of acacia senegal and acacia seyal from ethiopian savanna woodlands. Trees 2015, 29, 593–604. [Google Scholar] [CrossRef]
- Yang, N.; Ji, L.; Yang, Y.; Yang, L. The influence of tree species on soil properties and microbial communities following afforestation of abandoned land in northeast china. Eur. J. Soil. Biol. 2018, 85, 73–78. [Google Scholar] [CrossRef]
- Wei, X.; Li, G.Y.; Lv, L. Water and nutrient preservation of agri-forest residues used as nursery matrix. Sci. Silvae Sin. 2015, 51, 26–34. (In Chinese) [Google Scholar]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Osaki, M.; Shinano, T.; Tadano, T. Redistribution of carbon and nitrogen compounds from the shoot to the harvesting organs during maturation in field crops. Soil. Sci. Plant Nutr. 1991, 37, 117–128. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Ostonen, I.; Püttsepp, Ü.; Biel, C.; Alberton, O.; Bakker, M.R.; Lõhmus, K.; Majdi, H.; Metcalfe, D.; Olsthoorn, A.F.M.; Pronk, A.; et al. Specific root length as an indicator of environmental change. Plant Biosyst. 2007, 141, 426–442. [Google Scholar] [CrossRef]
- Olmo, M.; Lopez-Iglesias, B.; Villar, R. Drought changes the structure and elemental composition of very fine roots in seedlings of ten woody tree species. Implications for a drier climate. Plant Soil 2014, 384, 113–129. [Google Scholar] [CrossRef]
- Eissenstat, D.; Yanai, R. The ecology of root lifespan. Adv. Ecol. Res. 1997, 27, 1–60. [Google Scholar]
- Aerts, R.; Chapin, F. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv. Ecol. Res. 1999, 30, 1–67. [Google Scholar]
- Reich, P.B. The world-wide ‘fast-slow’: Plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Eissenstat, D. Costs and benefits of constructing roots of small diameter. J. Plant Nutr. 1992, 15, 763–782. [Google Scholar] [CrossRef]
- Ostonen, I.; Lõhmus, K.; Helmisaari, H.; Truu, J.; Meel, S. Fine root morphological adaptations in scots pine, norway spruce and silver birch along a latitudinal gradient in boreal forests. Tree Physiol. 2007, 27, 1627–1634. [Google Scholar] [CrossRef]
- Prieto, I.; Roumet, C.; Cardinael, R.; Dupraz, C.; Jourdan, C.; Kim, J.H.; Maeght, J.L.; Mao, Z.; Pierret, A.; Portillo, N.; et al. Root functional parameters along a land-use gradient: Evidence of a community-level economics spectrum. J. Ecol. 2015, 103, 361–373. [Google Scholar] [CrossRef]
- Comas, L.; Becker, S.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [PubMed]
- Henke, M.; Sarlikioti, V.; Kurth, W.; Buck-Sorlin, G.H.; Pagès, L. Exploring root developmental plasticity to nitrogen with a three-dimensional architectural model. Plant Soil 2014, 385, 49–62. [Google Scholar] [CrossRef]
- Linkohr, B.I.; Williamson, L.C.; Fitter, A.H.; Leyser, H.O. Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. Plant J. 2002, 29, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Krouk, G.; Lacombe, B.; Bielach, A.; Perrine-Walker, F.; Malinska, K.; Mounier, E.; Hoyerova, K.; Tillard, P.; Leon, S.; Ljung, K. Nitrate-regulated auxin transport by nrt1.1 defines a mechanism for nutrient sensing in plants. Dev. Cell 2010, 18, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Birouste, M.; Zamora-Ledezma, E.; Bossard, C.; Pérez-Ramos, I.M.; Roumet, C. Measurement of fine root tissue density: A comparison of three methods reveals the potential of root dry matter content. Plant Soil 2014, 374, 299–313. [Google Scholar] [CrossRef]
- Rewald, B.; Rechenmacher, A.; Godbold, D.L. It’s complicated: Intraroot system variability of respiration and morphological traits in four deciduous tree species. Plant Physiol. 2014, 166, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Irène, H.; Denis, V.; Cyrille, V.; Jeremy, D.; Beno?T, R.; Alain, B.; Eric, G.; Catherine, R. Relating root structure and anatomy to whole-plant functioning in 14 herbaceous mediterranean species. New Phytol. 2010, 173, 313–321. [Google Scholar]
- Mccormack, M.L.; Guo, D. Impacts of environmental factors on fine root lifespan. Front. Plant Sci. 2014, 5, 205. [Google Scholar] [CrossRef]
- Mooney, K.A.; Rayko, H.; Andre, K.; Agrawal, A.A. Evolutionary trade-offs in plants mediate the strength of trophic cascades. Science 2010, 327, 1642–1644. [Google Scholar] [CrossRef]
- Hodge, A. The plastic plant: Root responses to heterogeneous supplies of nutrients. New Phytol. 2004, 162, 9–24. [Google Scholar] [CrossRef]
- Hoch, G.; Richter, A.; Körner, C. Non-structural carbon compounds in temperate forest trees. Plant Cell Environ. 2003, 26, 1067–1081. [Google Scholar] [CrossRef]
- Gaul, D.; Hertel, D.; Borken, W.; Matzner, E.; Leuschner, C. Effects of experimental drought on the fine root system of mature norway spruce. For. Ecol. Manag. 2008, 256, 1151–1159. [Google Scholar] [CrossRef]
- Mcdowell, N.G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef]
- Zang, U.; Goisser, M.; Häberle, K.; Matyssek, R.; Borken, W. Effects of drought stress on photosynthesis, rhizosphere respiration, and fine-root characteristics of beech saplings: A rhizotron field study. J. Plant Nutr. Soil Sci. 2014, 177, 168–177. [Google Scholar] [CrossRef]
- Galvez, D.A.; Landhäusser, S.M.; Tyree, M.T. Root carbon reserve dynamics in aspen seedlings: Does simulated drought induce reserve limitation? Tree Physiol. 2011, 31, 250–257. [Google Scholar] [CrossRef]
- Henrik, H.; Waldemar, Z.; Olaf, K.; Susan, T. Thirst beats hunger—Declining hydration during drought prevents carbon starvation in norway spruce saplings. New Phytol. 2013, 200, 340–349. [Google Scholar]
- Gu, J.; Yu, S.; Sun, Y.; Wang, Z.; Guo, D. Influence of root structure on root survivorship: An analysis of 18 tree species using a minirhizotron method. Ecol. Res. 2011, 26, 755–762. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Achor, D.S. Anatomical characteristics of roots of citrus rootstocks that vary in specific root lenght. New Phytol. 1999, 141, 309–321. [Google Scholar] [CrossRef]
- Wahl, S.; Ryser, P. Root tissue structure is linked to ecological strategies of grasses. New Phytol. 2010, 148, 459–471. [Google Scholar] [CrossRef]
- Fort, F.; Cruz, P.; Catrice, O.; Delbrut, A.; Luzarreta, M.; Stroia, C.; Jouany, C. Root functional trait syndromes and plasticity drive the ability of grassland fabaceae to tolerate water and phosphorus shortage. Environ. Exp. Bot. 2015, 110, 62–72. [Google Scholar] [CrossRef]
- Padilla, F.M.; Aarts, B.H.J.; Roijendijk, Y.O.A.; Caluwe, H.D.; Mommer, L.; Visser, E.J.W.; Kroon, H.D. Root plasticity maintains growth of temperate grassland species under pulsed water supply. Plant Soil 2013, 369, 377–386. [Google Scholar] [CrossRef]
- Hernández, E.I.; Vilagrosa, A.; Pausas, J.G.; Bellot, J. Morphological traits and water use strategies in seedlings of mediterranean coexisting species. Plant Ecol. 2010, 207, 233–244. [Google Scholar] [CrossRef]
- Mccormack, M.; Adams, T.S.; Smithwick, E.A.H.; Eissenstat, D.M. Predicting fine root lifespan from plant functional traits in temperate trees. New Phytol. 2012, 195, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Markesteijn, L.; Poorter, L. Seedling root morphology and biomass allocation of 62 tropical tree species in relation to drought- and shade-tolerance. J. Ecol. 2009, 97, 311–325. [Google Scholar] [CrossRef]
- Sun, Y.; Gu, J.; Zhuang, H.; Guo, D.; Wang, Z. Lower order roots more palatable to herbivores: A case study with two temperate tree species. Plant Soil 2011, 347, 351–361. [Google Scholar] [CrossRef]
- Guo, D.L.; Mitchell, R.J.; Hendricks, J.J. Fine root branch orders respond differentially to carbon source-sink manipulations in a longleaf pine forest. Oecologia 2004, 140, 450–457. [Google Scholar] [CrossRef]
- Comas, L.H.; Eissenstat, D.M. Patterns in root trait variation among 25 co-existing north american forest species. New Phytol. 2010, 182, 919–928. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Kucharski, J.M.; Zadworny, M.; Adams, T.S.; Koide, R.T. Linking root traits to nutrient foraging in arbuscular mycorrhizal trees in a temperate forest. New Phytol. 2015, 208, 114–124. [Google Scholar] [CrossRef]
- Holdaway, R.J.; Coomes, D.A. Species- and community-level patterns in fine root traits along a 120 000-year soil chronosequence in temperate rain forest. J Ecol. 2011, 99, 954–963. [Google Scholar] [CrossRef]
- Chen, W.; Zeng, H.; Eissenstat, D.M.; Guo, D.L. Variation of first-order root traits across climatic gradients and evolutionary trends in geological time. Glob. Ecol. Biogeogr. 2013, 22, 846–856. [Google Scholar] [CrossRef]
- Kong, D.L.; Ma, C.E.; Zhang, Q.; Li, L.; Chen, X.Y.; Zeng, H.; Guo, D.L. Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytol. 2014, 203, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.T.; Li, H.B.; Zhu, B.; Koide, R.T.; Eissenstat, D.M.; Guo, D.L. Complementarity in nutrient foraging strategies of absorptive fine roots and arbuscular mycorrhizal fungi across 14 coexisting subtropical tree species. New Phytol. 2015, 208, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Makita, N.; Hirano, Y.; Dannoura, M.; Kominami, Y.; Mizoguchi, T.; Ishii, H.; Kanazawa, Y. Fine root morphological traits determine variation in root respiration of quercus serrata. Tree Physiol. 2009, 29, 579–585. [Google Scholar] [CrossRef]
- George, K.; Norby, R.J.; Hamilton, J.G.; Delucia, E.H. Fine-root respiration in a loblolly pine and sweetgum forest growing in elevated co2. New Phytol. 2003, 160, 511–522. [Google Scholar] [CrossRef]
- Xu, X.; Kuzyakov, Y.; Wanek, W.; Richter, A. Root-derived respiration and non-structural carbon of rice seedlings. Eur. J. Soil. Biol. 2008, 44, 22–29. [Google Scholar] [CrossRef]
- Desrochers, A.; Landhäusser, S.M.; Lieffers, V.J. Coarse and fine root respiration in aspen (Populus tremuloides). Tree Physiol. 2002, 22, 725. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhou, L.; Rao, X.; Lin, Y.; Fu, S. Effects of root diameter and root nitrogen concentration on in situ root respiration among different seasons and tree species. Ecol. Res. 2010, 25, 983–993. [Google Scholar] [CrossRef]
- Burton, A.J.; Jarvey, J.C.; Jarvi, M.P.; Zak, D.R.; Pregitzer, K.S. Chronic n deposition alters root respiration-tissue n relationship in northern hardwood forests. Glob. Chang. Biol. 2015, 18, 258–266. [Google Scholar] [CrossRef]
- Wells, C.E.; Eissenstat, D.M. Beyond the roots of young seedlings: The influence of age and order on fine root physiology. J. Plant Growth Regul. 2002, 21, 324–334. [Google Scholar] [CrossRef]
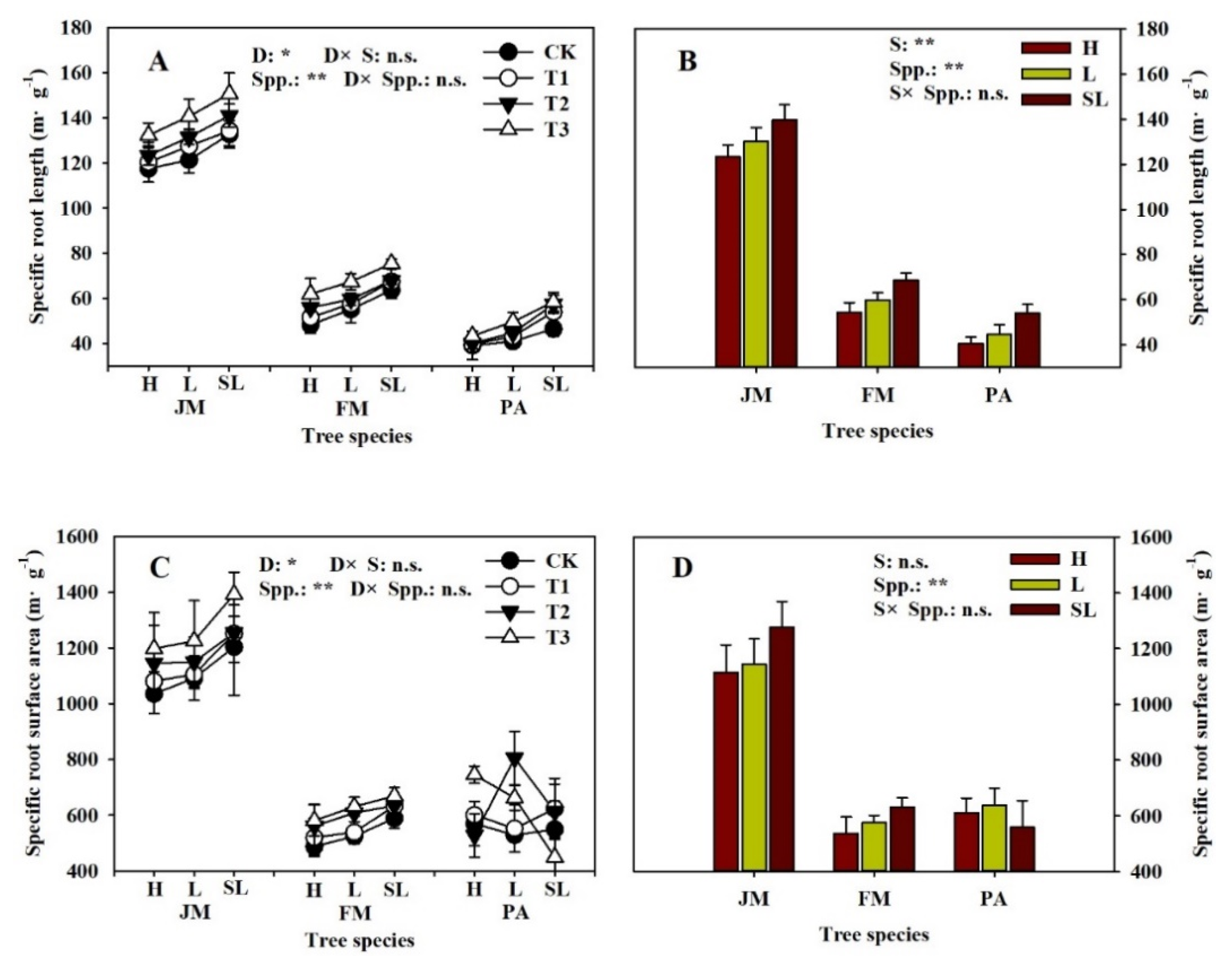

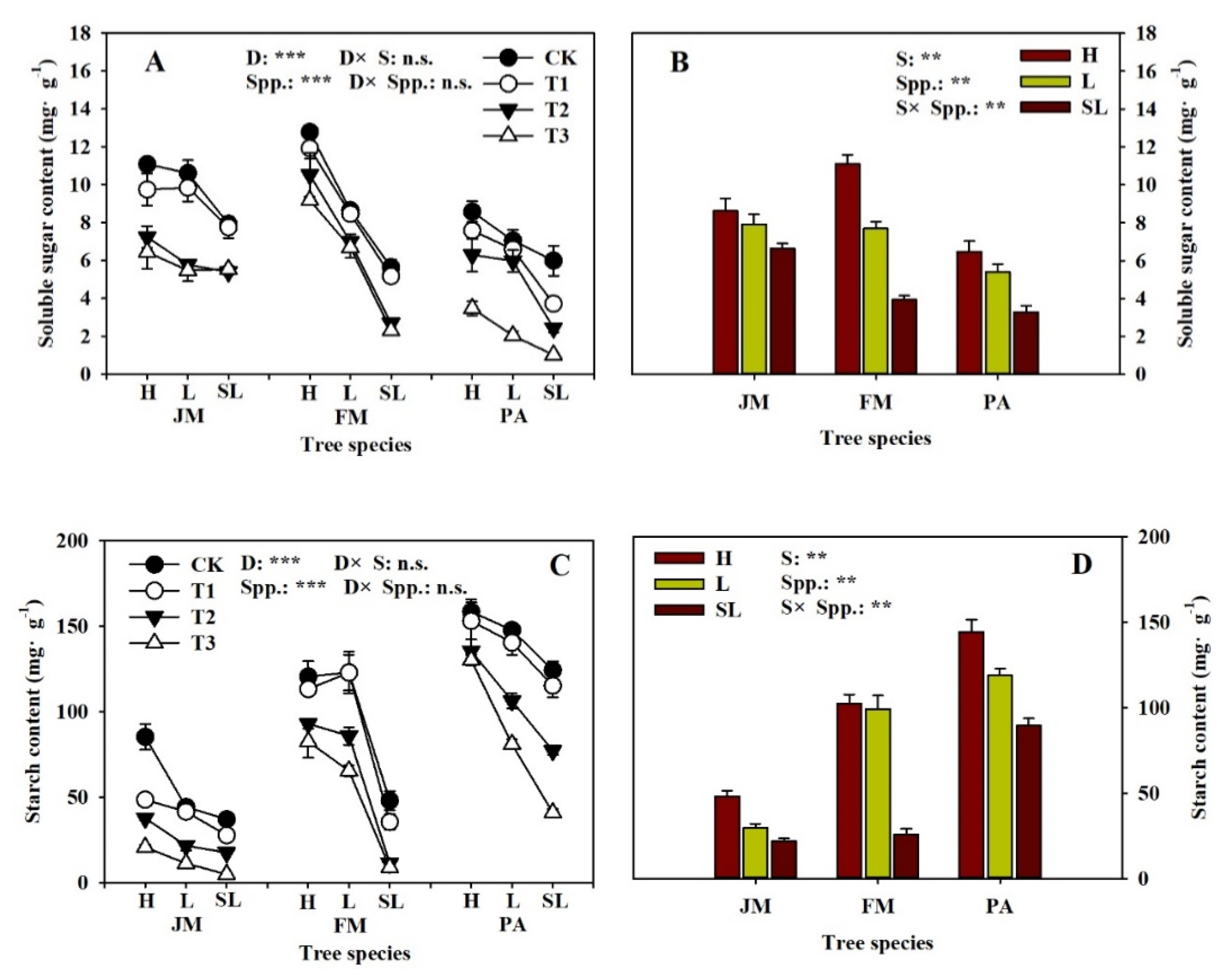
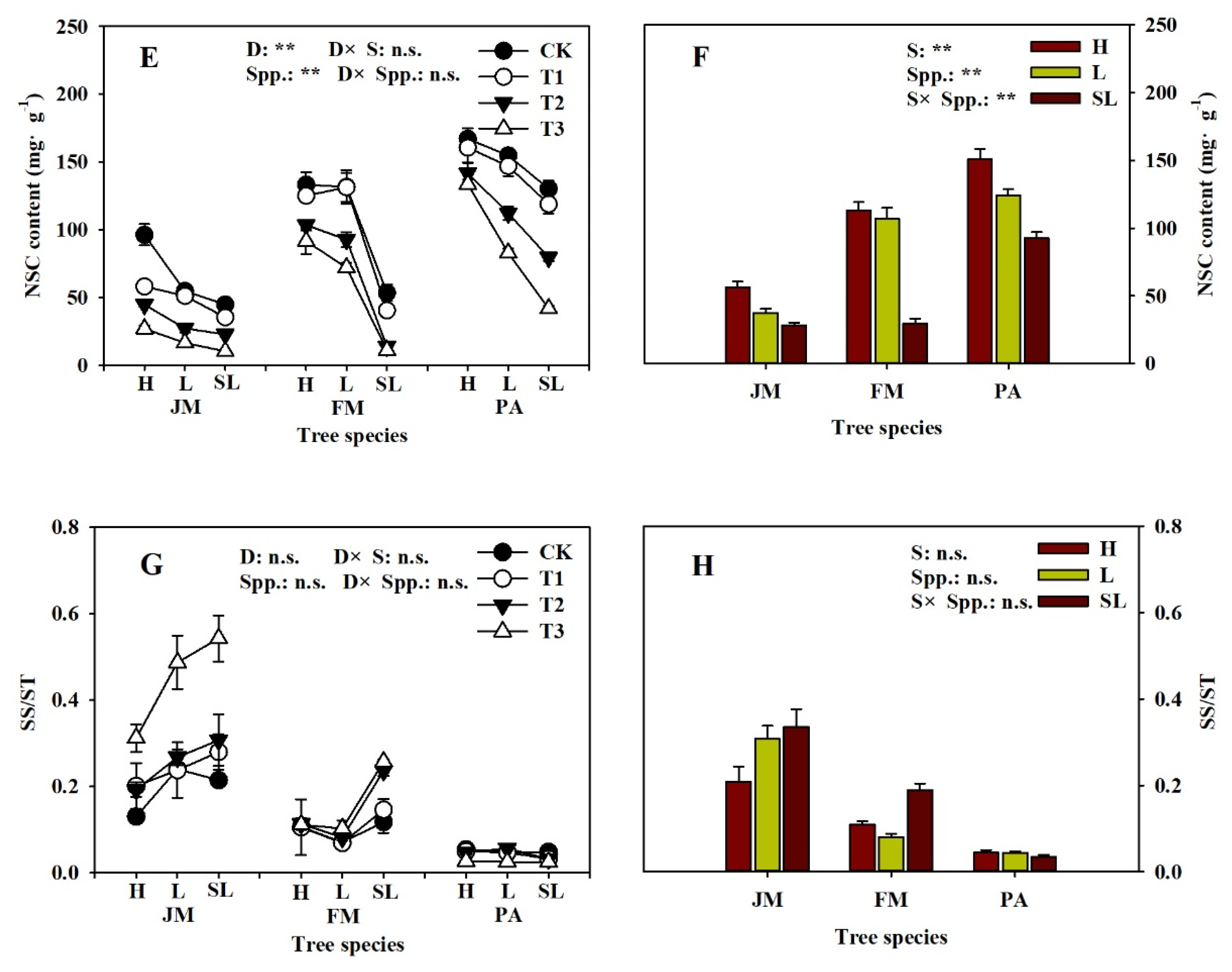
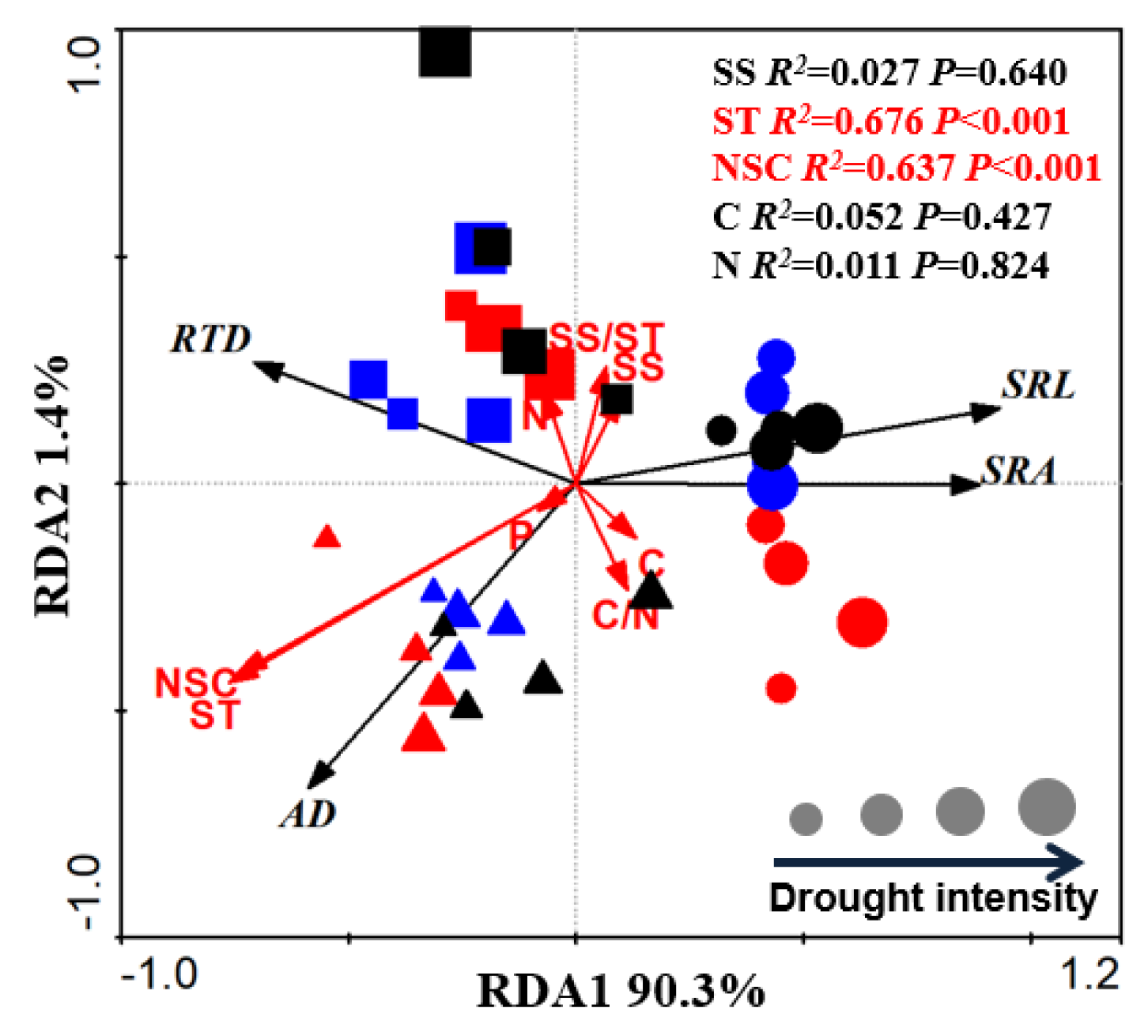

| Soil Substrate Physicochemical Property | Humus | Loam | Sandy-Loam |
|---|---|---|---|
| Bulk density (g·cm−3) | 1.18 ± 0.02b | 1.35 ± 0.02a | 1.32 ± 0.02a |
| Total porosity (%) | 53.19 ± 1.12b | 58.90 ± 0.91a | 36.72 ± 0.95c |
| Aeration porosity (%) | 25.61 ± 0.48a | 23.52 ± 0.53a | 13.01 ± 0.99b |
| Water absorption capacity | 0.23 ± 0.01b | 0.26 ± 0.01a | 0.18 ± 0.01c |
| Penetrate rate (g·min−1) | 4.49 ± 0.04a | 4.01 ± 0.12b | 3.04 ± 0.18c |
| Evaporation rate (g·h−1) | 0.59 ± 0.01a | 0.50 ± 0.01b | 0.37 ± 0.01c |
| Total nitrogen (mg·g−1) | 7.09 ± 0.78a | 3.11 ± 0.05b | 1.20 ± 0.03c |
| Total phosphorus (mg·g−1) | 0.71 ± 0.04a | 0.34 ± 0.09b | 0.32 ± 0.01b |
| Available phosphorus (mg·kg−1) | 13.10 ± 0.82a | 5.04 ± 0.21b | 12.75 ± 0.69a |
| Source of Variation | df | C | N | P | |||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| D | 3 | 0.399 | 0.754 | 0.188 | 0.904 | 3.001 | 0.036 |
| S | 2 | 22.109 | <0.001 | 11.669 | <0.001 | 6.191 | 0.003 |
| Spp. | 2 | 2.461 | 0.092 | 5.369 | 0.007 | 2.03 | 0.139 |
| D × S | 6 | 1.745 | 0.123 | 0.895 | 0.503 | 2.405 | 0.036 |
| D × Spp. | 6 | 1.897 | 0.093 | 2.226 | 0.050 | 4.567 | 0.001 |
| S × Spp. | 4 | 19.142 | <0.001 | 16.257 | <0.001 | 13.802 | <0.001 |
| D × S × Spp. | 12 | 3.801 | <0.001 | 2.871 | 0.003 | 2.245 | 0.018 |
| Soil Substrates | Drought Stress | JM | FM | PA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C (%) | N (%) | p (%) | C (%) | N (%) | p (%) | C (%) | N (%) | p (%) | ||
| H | CK | 41.35 ± 0.12a | 2.12 ± 0.01b | 0.23 ± 0.01a | 41.05 ± 0.03a | 2.06 ± 0.02a | 0.25 ± 0.01a | 41.17 ± 0.08a | 2.10 ± 0.03a | 0.25 ± 0.01a |
| T1 | 41.30 ± 0.12a | 2.12 ± 0.02a | 0.24 ± 0.01a | 41.05 ± 0.05a | 2.06 ± 0.03a | 0.25 ± 0.01a | 41.04 ± 0.01a | 2.03 ± 0.01b | 0.25 ± 0.01a | |
| T2 | 41.41 ± 0.16a | 2.16 ± 0.06ab | 0.23 ± 0.01a | 41.09 ± 0.05a | 2.08 ± 0.03a | 0.25 ± 0.01a | 41.16 ± 0.02a | 2.07 ± 0.01ab | 0.24 ± 0.01a | |
| T3 | 41.51 ± 0.11a | 2.20 ± 0.04a | 0.23 ± 0.01a | 41.01 ± 0.04a | 2.03 ±0.02a | 0.25 ± 0.01a | 41.04 ± 0.03a | 2.03 ± 0.01b | 0.25 ± 0.01a | |
| L | CK | 41.12 ± 0.08a | 2.06 ± 0.03a | 0.25 ± 0.01a | 41.02 ± 0.06b | 2.04 ± 0.03a | 0.25 ± 0.01a | 41.00 ± 0.15a | 2.01 ± 0.06a | 0.25 ± 0.01a |
| T1 | 40.98 ± 0.02ab | 2.00 ± 0.01bc | 0.25 ± 0.01bc | 41.05 ± 0.08b | 2.07 ± 0.04a | 0.25 ± 0.01a | 41.00 ± 0.04a | 2.01 ± 0.02a | 0.25 ± 0.01a | |
| T2 | 40.87 ± 0.03b | 1.96 ± 0.01c | 0.26 ± 0.01c | 41.01 ± 0.04b | 2.04 ± 0.02a | 0.25 ± 0.01a | 41.11 ± 0.06a | 2.06 ± 0.03a | 0.25 ± 0.01a | |
| T3 | 40.92 ± 0.03a | 2.02 ± 0.01ab | 0.25 ± 0.01ab | 41.44 ± 0.10a | 2.02 ± 0.04a | 0.22 ± 0.01b | 40.93 ± 0.10a | 2.05 ± 0.01a | 0.25 ± 0.01a | |
| SL | CK | 41.03 ± 0.01a | 2.02 ± 0.01a | 0.25 ± 0.01a | 41.00 ± 0.13a | 2.02 ± 0.06b | 0.25 ± 0.01a | 41.08 ± 0.04a | 2.05 ± 0.02a | 0.25 ± 0.01a |
| T1 | 40.97 ± 0.02ab | 2.00 ± 0.01b | 0.25 ± 0.01b | 41.24 ± 0.09a | 2.16 ± 0.05ab | 0.24 ± 0.01a | 41.05 ± 0.04a | 2.03 ± 0.02a | 0.25 ± 0.01a | |
| T2 | 40.93 ± 0.01b | 1.98 ± 0.01bc | 0.25 ± 0.01bc | 41.15 ± 0.07a | 2.11 ± 0.03ab | 0.25 ± 0.01a | 41.09 ± 0.07a | 2.05 ± 0.03a | 0.25 ± 0.01a | |
| T3 | 40.92 ± 0.03b | 1.98 ± 0.01c | 0.25 ± 0.01c | 41.44 ± 0.10a | 2.26 ± 0.05a | 0.24 ± 0.01a | 40.93 ± 0.10a | 1.98 ± 0.04a | 0.25 ± 0.01a | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, L.; Attaullah, K.; Wang, J.; Yu, D.; Yang, Y.; Yang, L.; Lu, Z. Root Traits Determine Variation in Nonstructural Carbohydrates (NSCs) under Different Drought Intensities and Soil Substrates in Three Temperate Tree Species. Forests 2020, 11, 415. https://doi.org/10.3390/f11040415
Ji L, Attaullah K, Wang J, Yu D, Yang Y, Yang L, Lu Z. Root Traits Determine Variation in Nonstructural Carbohydrates (NSCs) under Different Drought Intensities and Soil Substrates in Three Temperate Tree Species. Forests. 2020; 11(4):415. https://doi.org/10.3390/f11040415
Chicago/Turabian StyleJi, Li, Khan Attaullah, Jun Wang, Dapao Yu, Yuchun Yang, Lixue Yang, and Zhimin Lu. 2020. "Root Traits Determine Variation in Nonstructural Carbohydrates (NSCs) under Different Drought Intensities and Soil Substrates in Three Temperate Tree Species" Forests 11, no. 4: 415. https://doi.org/10.3390/f11040415
APA StyleJi, L., Attaullah, K., Wang, J., Yu, D., Yang, Y., Yang, L., & Lu, Z. (2020). Root Traits Determine Variation in Nonstructural Carbohydrates (NSCs) under Different Drought Intensities and Soil Substrates in Three Temperate Tree Species. Forests, 11(4), 415. https://doi.org/10.3390/f11040415







