Abstract
Research highlights: To understand differences in the establishment of balsam fir regeneration observed in the boreal forest, we examined how soil layer and microorganisms explained differences in growth and mycorrhization in three different stand types. Our experiment revealed positive and negative effects on growth of seedlings, and highlights the importance of biotic interactions in balsam fir establishment. Background and Objectives: In a context of climate change, understanding tree migration can be examined through changes in tree regeneration. At the ecotone between mixed and conifer boreal forest, regeneration of balsam fir northward is of particular interest because it thrives better under aspen-dominated stands as compared to adjacent spruce-dominated stands. As the understorey differs between these stands, with more Ericaceae under spruce and different ectomycorrhizal fungal communities in organic and mineral horizons, we hypothesized that biotic factors could explain differences in balsam fir establishment. Materials and Methods: Using a growth chamber experiment, we tested if differences in soil layers and modification of soil fungal communities would affect germination, mycorrhization, and growth of balsam fir seedlings in three different stand vegetation. We compared 12 treatments and followed 120 seedlings over three growth seasons. Results: We found similar survival in soils from aspen- and spruce-dominated stands, and a greater biomass on organic layers. In addition to this, a greater mycorrhization rate was found in aspen soils but improved germination in spruce soils. The presence of Ericaceae in spruce soils was associated with lower mycorrhization but did not affect other traits. Sterilization and therefore microorganisms affected mainly the number of ectomycorrhizae and the investment in root biomass. Finally, mycorrhization and biomass were correlated, but independent from N nutrition measured in needles. Conclusions: Our results highlighted the positive effects of organic soil layers and of mycorrhization on biomass, and showed that mycorrhization was increased under aspen as compared to other stand types. Our experiment also revealed positive effects of spruce soil on fir germination and showed that fir was able to grow and survive in all conditions. Our study suggests that fir establishment is affected by belowground multi-species interactions, and therefore highlights that biotic interactions shall be taken into account to understand and predict future tree migrations in the boreal forest.
1. Introduction
In a context of climate change, species migration and changes in tree species distribution are expected to occur. Migrations to the North or in elevation have already been observed for birds, butterflies, and grasses [1]. Tree responses to increasing temperatures seem more heterogeneous and Zhu et al. [2] concluded that only northern tree species would migrate north, these representing about 20% of tree species in North America. Tree migration models may include more than climatic parameters [3], and take into account local processes and heterogeneity [4]. Indeed, tree establishment into a new area depends not only on latitude or climatic conditions, but also on abiotic and biotic conditions, as well as on the available space [5]; all these factors delineate the realized niche of a tree species. New models try to integrate the dependence toward symbionts [6] or competition with other tree species [7], but may miss local heterogeneity of biotic interactions that could buffer the effect of stressors on population dynamics and maintenance of biotic interactions. Understanding mechanisms controlling tree regeneration would then greatly improve migration models and give guidelines for assisted migration programs [3].
The boreal forest represents a key ecosystem to study tree migration today, as fast changes are already observed [8] and threaten the largest forest biome on Earth [9]. In Quebec (Canada), boreal forests are mainly dominated by black spruce (Picea mariana (Miller) B.S.P.; [10]) and feather-mosses. Local changes in tree dominance are increasingly observed, such as patches of trembling aspen (Populus tremuloides Michaux), possibly favored by climate change and forest management practices [11]. In this heterogeneous landscape, tree species are extending their distribution northward, such as balsam fir (Abies balsamea (L.) Miller; [12]). Interestingly, balsam fir establishes and grows better under trembling aspen- than under black spruce-dominated stands of the boreal forest [13], suggesting a positive interaction between aspen and fir. Both stands occur in similar climatic and edaphic conditions but host different understorey vegetation [14], soil fungal communities, and more specifically saprotrophic and symbiotic fungi communities [15]. Investigations of these micro-organisms communities have also revealed strong differences within stands between organic and mineral layers [15], which could increase local heterogeneity and complexify interactions around balsam fir seedlings.
Among interactions, ectomycorrhizal symbioses might be of peculiar importance for tree establishment. These interactions between Asco and Basidiomycetes and tree roots are particularly rich and diverse in north temperate and boreal forests [16] and provide nutritional benefits to most tree species in these biomes [17], including balsam fir, trembling aspen, and black spruce [18]. In a previous study, Nagati et al. [19] observed ectomycorrhizae on balsam fir seedlings growing under trembling aspen or black spruce, showing that associations were possible for this migrating species. The colonization by ectomycorrhizal fungi, estimated by ramification indices and molecular markers, was greater under trembling aspen than under black spruce, especially when black spruce stands contained ericaceous shrubs [19]. Overall, this study showed that facilitation of fir under trembling aspen was most likely mediated by ectomycorrhizal fungi. On the other hand, ericaceous shrubs are known for their negative effects on coniferous regeneration [20,21,22,23] in conifer-ericaceous communities in boreal and temperate forests. Indeed, poor tree recruitment and slow seedling growth is often observed in nutrient-stressed environments dominated by ericaceous plants [20]. In a first study [19], we found that the presence of Ericaceae affected mycorrhizal communities on the surrounding fir roots, which may lead to a decrease of mycorrhization as observed in other ecosystems [22,23,24]. In addition to this, different fungal species with ectomycorrhizal or ericoid status can coexist in this particular habitat, and potentially compete in plant root systems [23], leading to differences in plant nutrient uptake or functioning.
To further disentangle these complex interactions and test the relative importance of soil microbial communities, soil layers and dominant vegetation (aspen or spruce) on balsam fir establishment, we launched an ex-situ experiment in a growth chamber. We did not isolate ectomycorrhizal fungi, but rather tested the effect of fungi occurrence by comparing sterile and non-sterile soils. Controlled conditions are particularly useful as ecological factors shape species traits and the outcome of biotic interactions, and experimental tests have already allowed to show the link between mycorrhizae and facilitation in other biomes (e.g., [25]). Moreover, our objective was also to monitor the early-stage steps of tree establishment, that are germination, seedling survival, and growth, under different conditions. We hypothesized that ectomycorrhizal fungi would play a role on seedling growth but not on seed germination, and tested that germination and survival was dependent mostly on the seedbed [26] and therefore soil layer (organic or mineral) and soil origin (from stands dominated either by aspen or spruce, with or without ericaceous shrubs). In line with our field observations [19], we expected that mycorrhization, growth and survival would be greater in non-sterile soils and organic layers, and greater on soils collected from aspen-dominated stands than from spruce-dominated stands, particularly with Ericaceae. Finally, we compared our results with in situ interactions drawn from sapling growth and mycorrhization, to sketch a synthesis of direct and indirect interactions leading to balsam fir establishment in the boreal forest, and conclude on the importance of soil fungi and mycorrhiza for balsam fir colonization.
2. Materials and Methods
2.1. Soil Origin and Sterilization
Soils were sampled in August 2016 in three adjacent stands distant 20–100 m, either dominated by trembling aspen (TA) or black spruce (BS), the latter with or without the presence of ericaceous shrubs (Ledum groenlandicum; BSE (BS with Ericaceae) stand). Stands were previously described in Nagati et al. [15] (site 4, see Table S1 for soil characteristics). For each soil origin (stand) we collected 10 L of organic soil and 10 L of mineral soil independently. As texture of mineral soil was heavy clay the transition between organic and mineral horizons was clear in the field, at circa 20–30 cm depth and allowed to separate the two horizons (Figure 1a,b). To test the effect of biotic factors aside from the dominant vegetation, we sterilized our wet soils by microwaving them for 5 min at maximum power (1000 watts), three times: A first time, three hours later, and 24 h later (recommended by M. Bidartondo based on [27]). This sterilization method was supposed to decrease all microbial life [27] but we did not test the sterilization on plate. We therefore had 12 different soil treatments (Figure 1c), allowing to test for soil layer (organic vs. mineral), and sterilization (sterile, non-sterile) effects, and comparing stand type (BS, BSE, and TA).
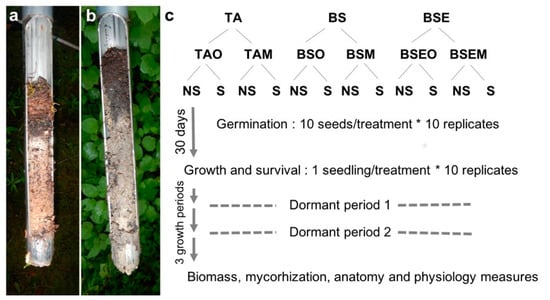
Figure 1.
Soil cores from black spruce (BS) (a) and trembling aspen (TA) (b) stands showing the difference between organic and mineral soil layers, and (c) experimental scheme showing the 12 different treatments and three growth seasons: TAO (Trembling aspen organic layer), TAM (Trembling aspen mineral layer), BSO (Black spruce organic layer), BSM (Black spruce mineral layer), BSEO (Black spruce and Ericaceae, organic layer), BSEM (Black spruce and Ericaceae, mineral layer), NS (non sterile treatment, and S (sterile treatment)).
2.2. Germination Process
Balsam fir seeds were provided by the Centre des semences forestières de Berthier, Quebec Ministry of Forests, Wildlife, and Parks. Seeds were stratified for germination by immersing bags containing circa 2000 seeds in running cold fresh water for 24 h (tap was let opened to ensure a water flow of 0.5 L/min), air dried for 48 h before putting them at 3 °C in a polyethylene bag for 28 days and mixed each week to ensure oxygenation. For each treatment, we put 10 seeds per pot and replicated it 10 times (100 seeds per treatment). Seeds were planted at 0.5 cm depth for two months in a growth chamber (Conviron model CG-108, Winnipeg, MB, Canada). The following conditions were set for germination period: 16h photoperiod with a photosynthetically active radiation at pot level of 450 μmol m−2 s−1, day/night temperature 20/10 °C and relative humidity 80%. After two months, we counted the number of germinated seeds per pot and calculated the germination rate for each pot and treatment (Table S2). One randomly selected seedling per pot was kept for the next part of the experiment. The selected seedlings were grown under these conditions for one supplementary month corresponding to the first growth phase.
2.3. Growing Period
In addition to the first growth phase, two additional growing seasons were simulated, each preceded by a dormancy period. First, a three-month period corresponded to the first growth season. Two additional growth seasons were carried out, and each growing season lasted 3 months under the same conditions as for the germination phase. Each dormancy phase lasted 2 months with a 4 h photoperiod with photosynthetically active radiation at pot level of 450 μmol m−2 s−1, temperature at 4 °C and humidity at 80%. At the beginning of the second growth phase, soils were fertilized with 20/20/20 (N/P/K) fertilizer at a 2 g/L concentration. Each pot received around 0.5 L of fertilized water.
2.4. Seedlings Mycorrhizal Status, Biomass, Anatomy, and Physiology
At the end of the experiment, we counted the number of ectomycorrhizal (EM) and non-EM apices under a magnifying glass for each seedling. The total number of EM apices and the percent of EM apices were reported (Table S2). Height and root collar diameter were measured before stems (with needles) and roots were separated and separately weighed fresh. Stem and roots were then oven-dried at 40 °C for 48 h for dry weight measurements. Needles were finely ground with a ball mill (Pulverisette 0, Fritsch, Idar-Oberstein, Germany). Nitrogen and carbon contents in needles were determined by combustion elemental analysis using a CNS-2000 Elemental Analyzer (Leco Instruments Ltd., St-Josephs, MI, USA). Element concentrations were calculated as the sum of the absolute amounts, divided by the total dry weight of the sample. Isotope analyses were performed with a PDZ Europa ANCA-GSL elemental analyzer, interfaced with a PDZ Europa 20–20 isotope-ratio mass spectrometer (Sercon Ltd., Crewe, Cheshire, UK) at the Stable Isotope Facility of University of California Davis (Davis, CA, USA). The δ13C was determined as:
where Rsample and Rstandard are, respectively, the 13C/12C ratios of the needle sample and the international standard V-PDB (Vienna PeeDee Belemnite). The δ15N followed the same formula, and Rsample and Rstandard were respectively the 15N/14N ratios of the needle sample and the atmospheric N2. This analysis was possible only for the larger seedlings with at least 1 mg of dried needles (i.e., from 3 to 10 individuals per treatment, see Table S2).
δ13 C = ((Rsample − Rstandard)/Rstandard) × 1000
2.5. Statistical Analyses
Statistical analyses were done with R software (version 3.5.2, [28]). To take our experimental design into account, we analyzed all our data with general linear mixed effect models (GLMM) and linear mixed effect models (LMM). These models are robust to assumptions on data distribution [29], and assume that our treatment are not completely independent. As soils were collected independently in each stand, the layer was considered as a fixed effect per se, and so was the sterilization nested in the soil layer. The stand was not replicated, as only one site was studied and could not be included as a fixed effect. However, to differentiate the variability due to stands from the variability within treatment (and between the ten pseudoreplicates), we included a random effect in models, where treatment was nested into the stand. We did not nest the presence of Ericaceae within spruce stands, as soils were collected independently. The final formula of our model was: response variable ~Layer + Layer/Steril + (1|Stand/Treatment).
To choose the family of models to use for each variable, we first plotted the distribution of our data through histograms and tested the normality with a Shapiro-Wilcoxon test. Height, Root collar diameter, total, shoot and root dry weight, δ13C, δ15N, and N quantity followed a Gaussian distribution (p-value > 0.05 for all Shapiro-Wilcoxon tests) and a LMM was used. For count variables (survival, germination, number of mycorrhizal apices), we plotted the variance and means for each treatment. If variance and means were correlated, we used a Poisson law in the GLMM. If no correlation was detected, we used a different law, notable a Poisson negative binomial law for the GLMM on germination. For survival, i.e., the presence or absence of seedling after three growth seasons, we used a binomial law. For the percent of mycorrhizal apices, percent of C and N, and root/shoot ratio, we used a quasi-binomial law, recommended for percent analysis. Finally for GLMM, to check if we used the right law, we used the TestDispersion test (DHARMA package, [30]) and checked that the p-value was non-significant, and therefore that residuals were neither over or under-dispersed.
For LMM, an ANOVA was performed on the model to detect the effect of stand, soil layer within stand and sterilization within soil layer. For factors showing significantly different estimates in models, differences between pairs of treatments were compared and represented based on contrasts between marginal means of the models (using emmeans package, [31]).
To better describe differences between stand, we finally extracted the random effect from our models (ranef function in nlme package, [32]). No statistical test was performed, as we could not differentiate our stand effect form a site effect, but the difference of variance between stand and treatments in LMM and GLMM allowed to identify traits more variable between stands than within treatments.
Finally, to examine the correlations between variables, we performed pairwise Spearman correlations, and corrected our p-value using a Bonferroni correction for the number of comparisons.
3. Results
3.1. Germination
Germination was successful for all treatment (Figure 2, Table S2). As no clear correlation was detected between germination variance and mean, we analyzed germination with a GLMM following a Poisson negative binomial distribution. No significant overdispersion was detected on residuals of this model (p-value = 0.448). Only seedlings growing under TA showed a slightly lower germination rate (Figure 2a), while soil layer and sterilization did not significantly influence germination (Table S3).
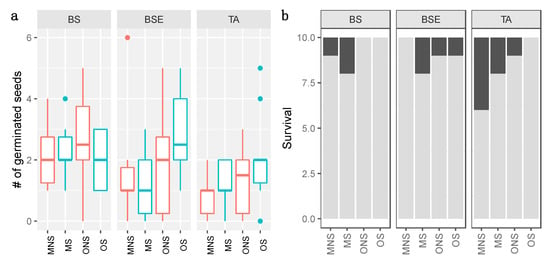
Figure 2.
Number (#) of germinated seeds per pot and per treatment over 10 seeds (a) and Survival after three growth seasons (b). BS: Black spruce stand, BSE: BS with Ericaceae, TA: Trembling Aspen stand, M: Mineral, O: Organic, S, Sterile (blue), NS: non sterile (red). For the Survival (b), the proportion of dead seedlings is represented in black, while live seedlings at the end of the experiment are in grey.
3.2. Survival
Over the 120 seedlings that remained for the growth experiment, 16 did not survive after 3 growing seasons. The number of seedlings at the end of the experiment varied between 6 and 10 per treatment (over 10 seedlings; Figure 2b; Table S2). For these binomial data, we analyzed the effect of stand, layer and sterilization through a GLMM, assuming a binomial distribution of the data. The GLMM did not detect any significant effect of soil layer and sterilization (Table S3) and the variance linked with stand was null in our model. These results reject the idea that survival after three growing seasons would be affected by stand, soil layer, or sterilization.
3.3. Mycorrhization
Ectomycorrhizal apices were observed on seedlings in all treatments after the three growing seasons. Seedlings growing on sterile soils also developed EM apices after three growth seasons but in lower proportion, with 1.8 to 7.2 times more ectomycorrhizae on non-sterile treatments as compared to sterile treatments (mean = 4.32).
These number and percent of ectomycorrhizal apices were analyzed with GLMM assuming, respectively, a Poisson and a quasibinomial distribution, based on the relationship between variance and means. No overdispersion was detected for these models (p-value > 0.05). The number and percent of EM apices was lower under BSE than under BS and TA, and the higher variance between stands than within treatment supported this difference (Table 1, Figure 3a,b). Across stands, the number of EM apices was always higher on organic than on mineral soil (Table 1, Figure 3c) and always higher on non-sterile soils as compared with sterile soils (Table 1, Figure 3c). All these differences were supported by pairwise comparisons of marginal means estimates (Figure 3c). These differences between soil layers were not observed on the percent of mycorrhizal apices (Table 1).

Table 1.
General linear mixed effect models (GLMM) and linear mixed effect models (LMM) estimates for the number (#) and percent (%) of ectomycorrhizal (EM) apices. Std: standard error, p: p-value. *: p-value < 0.01, **: p-value < 0.001, ***: p-value < 0.0001. Model formula: ~Layer + Layer/Steril + (1|Stand/Treatment).
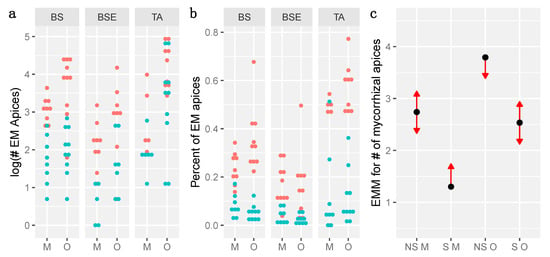
Figure 3.
Number (#) of ectomycorrhizal (EM) apices (a, log transformed), Percent of ectomycorrhizal apices (b) and comparison of estimated marginal means (EMM, c) between stands, layers and sterilization treatments. BS: Black spruce stand, BSE: BS with Ericaceae, TA: Trembling Aspen stand, NS: non sterile, S: sterile, M: Mineral, O: Organic. In (a,b), blue dots refer to sterile treatments, and red dots to non-sterile treatments. In (c), blue bars indicate confidence intervals of estimated marginal means, arrows refer to comparisons among marginal means. If two arrows overlap, Tukey post-hoc tests are non-significant and p-value for pairwise comparisons are > 0.05.
3.4. Growth and Biomass
For height, total, shoot and root dry weight and root collar diameter, we tested the effect of soil layer and sterilization through LMM, considering the Gaussian distribution of the data. Across stand types, soil layer explained most differences of anatomy and biomass (Table 2 and Table S3 for model estimates, and Table S4 for ANOVA). Indeed biomass (total, shoot and root dry weight), height and root collar diameter were always significantly greater for seedlings growing on organic than on mineral layers (Table 2, Table S3, Figure 4). ANOVA on LMM also supported an effect of sterilization only on root dry weight (Table S4, Figure 4d). The variance attributed to stands within the random effects was null or lower than the variance attributed to treatments, suggesting a lack of effect of the stand on these variables (Table 2 and Table S3).

Table 2.
LMM estimates for height, root collar (RC) diameter, total dry weight (DW). Std: standard error, p: p-value. *: p-value < 0.01, **: p-value < 0.001, ***: p-value < 0.0001. LMM formula: ~Layer + Layer/Steril + (1|Stand/Treatment).
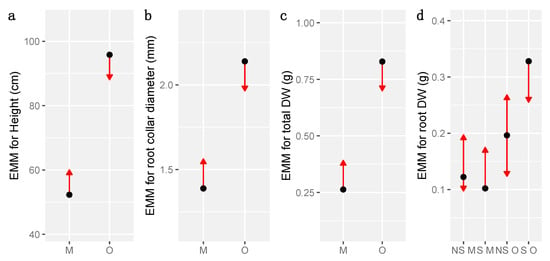
Figure 4.
Comparison of estimated marginal means (EMM) showing significant differences of height (a), root collar diameter (b), total dry weight (c, DW), and root DW (d) of seedlings per soil layer (Mineral: M, Organic: O) and sterilization treatment (S: sterile, NS: non sterile). Blue bars indicate confidence intervals of estimated marginal means, arrows refer to comparisons among marginal means. If two arrows overlap, Tukey post-hoc tests are non-significant and p-value for pairwise comparisons are > 0.05.
3.5. Anatomy and Physiology
All measurements of root/shoot ratio, percent of C and N content were analyzed with GLMM assuming a quasibinomial distribution of the variables. Measurements of δ13C and δ15N followed a Gaussian distribution and were analyzed with a LMM. No overdispersion was detected for these models (p-value > 0.05).
Among these variables, only the root/shoot ratio was significantly different between soil layers (Table S4), with more investment in roots than in shoots in the mineral layer (Table S3, Figure 5a). Moreover, the root/shoot ratio was less variable between stands than within treatments (Table S3), suggesting that seedlings growing on soil from TA stand would develop more roots than shoots, as compared with soils from other stands.
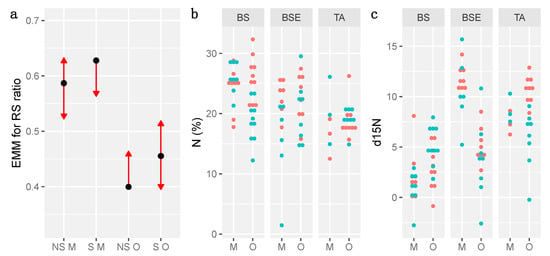
Figure 5.
Comparison of estimated marginal means (EMM) showing significant differences of Root/shoot ratio between soil layers and sterilization treatments (a), and distribution of percent of N (b) and δ15N (c). BS: Black spruce stand, BSE: BS with Ericaceae, TA: Trembling Aspen stand, NS: non sterile, S: sterile, M: Mineral, O: Organic. In (a), blue bars indicate confidence intervals of estimated marginal means, arrows refer to comparisons among marginal means. If two arrows overlap, Tukey post-hoc tests are non-significant and p-value for pairwise comparisons are > 0.05. In (b,c), blue dots refer to sterile treatments, and red dots to non-sterile treatments.
Variations of percent of C, δ13C, N and δ15N in needles were not explained by layer and sterilization (Table S3). Measures of δ15N and percent of N were more variable within stand than between treatments, but showed that seedlings growing on soils from TA stand had a lower N and higher δ15N than those growing on soil from BS stand (Figure 5b,c).
3.6. The Effects of Ericaceous Shrubs
We expected that the presence of Ericaceae would result in a negative effect on seedling growth as compared to BS soil. According to different models, based on the comparison between variability within stand and between treatments, only few traits were different between BS and BSE stands (Table 3), and the main difference was observed on mycorrhization, as seedlings growing in BSE soil had less mycorrhizal apices (in number and percent) than those growing in BS soil (Figure 3a,b, Table 3). We also observed that seedling growing under BSE developed less roots than shoots, as compared to seedlings growing under other stands.
3.7. Correlations between Variables
In order to have a global overview of seedlings response, and notably of the effect of mycorhization, we finally investigated the correlations between variables. The use of Bonferroni corrections decreased the number of significant correlations, and we observed different groups of correlations (Figure 6). The number of EM apices was significantly and positively correlated with the percent of EM apices, height and total dry weight of seedlings (Figure 6). Interestingly, δ15N, N content and N concentration were correlated (or anti-correlated) with each other, but never significantly correlated with mycorrhizal indices (Figure 6). These results show that growth was globally responding to mycorrhization, through a positive interaction, but not through N nutrition.
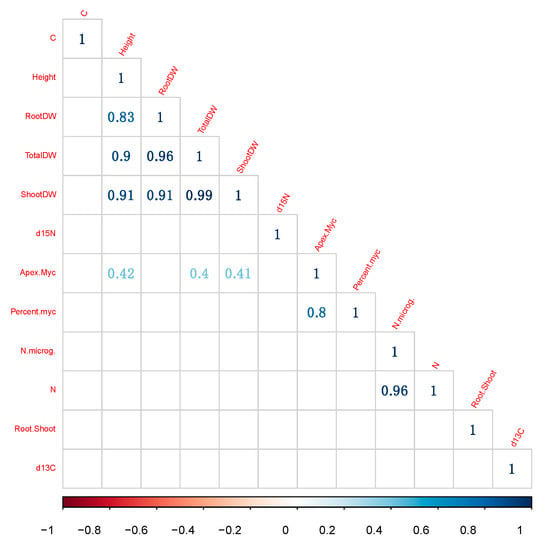
Figure 6.
Significant correlations between variables, after Bonferroni corrections for multiple comparisons. N. microg: N content (µg).
4. Discussion
Our experiment on the germination, growth and survival of balsam fir on boreal soils has revealed complex interactions between soil layers, dominant vegetation, and microorganisms. Soil layer was the main structuring factor affecting seedling biomass and anatomy, and soil sterilization only affected the mycorrhization and the root/shoot ratio. Differences between stand were rather observed than tested, but revealed differences of mycorrhization, germination, and root/shoot ratio. Interestingly, results were different on germination, survival, and growth, allowing to disentangle interactions between soil, fungi and stand along the first steps of balsam fir establishment into the boreal forest.
Following the experiment of McLaren and Janke [26] in Michigan, boreal forest soils can be favorable to seed germination, especially the organic layer. In our experiment, seed germination was similar between organic horizons (14 to 28%) and mineral soil (9 to 22%). Our germination rate was relatively high, confirming that boreal soils are favorable to balsam fir establishment and that this species is relatively well adapted to these soil conditions. Interestingly, soils from spruce stands (BS and BSE) were more favorable to fir germination that those from TA stands (Table 3), while we expected the opposite. It is possible that the presence of mosses and particularly Pleurozium spp. in the organic layer of BS stands represents a good seedbed for balsam fir seeds contrary to the broadleaf litter [26,33]. However, beside this initial difference in germination rate, final survival after three growth seasons was similar between treatments.
While germination was variable but successful in all soil types, highlighting the ability of fir seedlings to grow on contrasted substrates, we observed more variability in seedling growth, in terms of biomass, anatomy and physiology. Based on field observations [19], we expected growth to be better in TA soils than in BS soils. The responses were more complex, and for example only root/shoot ratio differed between stands, with seedlings growing on soil from TA stand producing more roots than shoots as compared to other stands. As the effect of stand relied only on differences within a site, more sites shall be sampled to confirm both the lack of effect of stands on growth, and the peculiar difference of root/shoot ratio. Considering that previous studies including more sites detected consistent differences between soils of closely located stands [14,15,34], we expect to observe similar results with soils from other local sites. Across stands, most differences were observed between soil layers, and indeed seedlings growing in organic soils were taller, and developed more dry weight than seedlings growing in mineral soils. This result was consistent across soils from different stands, showing the importance of soil characteristics and organic layer on fir seedling growth. Interestingly, for other trees of the boreal forests, such as black spruce and trembling aspen, their germination and establishment are on the contrary favored by bare mineral soil, a situation encountered after severe fire [35,36,37,38,39,40]. Black spruce are also able to grow on thick layer of organic soil [35], a situation encountered later along post-fire succession in the boreal fire [41]. The response of fir to organic layer is therefore more typical of a late colonizer [41], and indeed the forest at our site resulted from a post-fire recolonization dated around 1916 [42].
Beside the contrasted soil layers and differences between stands, seedlings managed to form ectomycorrhizae, but developed up to 7.2 times more ectomycorrhizae in non-sterile compared to sterile soils, after three growth seasons. The difference in mycorrhization between sterile and non-sterile soils confirms that our sterilization targeted micro-organisms, but the growth chamber did not exclude a possible post-colonization by airborne spores (a frequent phenomenon at least in greenhouses, [43]). We assume that these spores would come from local forests around the lab (campus of Amos), located 100 km apart from our field site. Beside the colonization of sterile soils, our results showed that mycorrhization was greater in TA soils, and significantly higher in organic layers (in number, not in percent). Moreover, the mycorrhization was correlated with seedlings biomass and height (whatever the treatment, Figure 6). The difference in mycorrhization observed between stand types shall be confirmed on other sites of course, but these results are consistent with our hypothesis and the results obtained on root ramification indices of naturally-growing and older saplings [19]. Interestingly, soil analyses did not detect differences of abundance and diversity of ectomycorrhizal fungi on the very same site and other neighboring sites [15], suggesting that young fir encounter not more EM fungi under TA, but more compatible mycorrhizal fungi. In this experiment, we did not sequence the mycorrhizal community in soils or on roots, but such data might be useful to identify fungi possibly linked with the better in-situ growth of young balsam fir in boreal forest soils dominated by trembling aspen, as responses to mycorrhization are also sensitive to fungi identity (e.g., [44,45]).
Although we expected N nutrition to be better in TA than in BS soils, seedlings physiology did not reveal such a trend. Indeed, N concentration was more variable within treatments than between stand, soil layer or sterilization treatments. Moreover, N concentration in needles was uncorrelated with mycorrhizal parameters, while in general, mycorrhizal symbioses are expected to provide N and P benefits to their partners [17]. We shall confirm this trend with a larger sampling, but the lack of correlation between N parameters, and even growth, also invite to consider the different N forms (ammonium and nitrate) accessible to young balsam fir. The greater mycorrhization and slightly higher δ15N of seedlings growing on soils from TA stand suggests that balsam fir rather relies on mycorrhizae for N acquisition in these conditions, perhaps also linked with increased P availability in soils under aspen [46]. Indeed, δ15N variation may be related to the use of different N sources [47] and notably from ectomycorrhizal fungi. For example Mayor et al. [48] revealed that higher δ15N of black spruce needles reflected a greater dependency on ectomycorrhizal fungi. On the contrary, the lower mycorrhization and slightly lower δ15N in black spruce soil suggests that balsam fir rather relied on other N sources in these treatments, notably ammonium, directly acquired from soil. In pots containing soil from BS stand, we indeed noticed the development of Pleurozium species, mosses able to fix N [49]. These mosses could have released ammonium in BS treatments. Interestingly, in Northern Quebec, black spruce δ15N values are generally low [50], suggesting a minor dependence toward mycorrhizal fungi. More generally, conifers and early-succession species are known for their greater preference for ammonium than for nitrate [51,52]. A more precise analysis on balsam fir preferences would be complementary, but our data already suggest the ability of young balsam fir to acquire N, whatever their mycorrhization rate and possibly from different sources.
Our results differed from previous field observations where we had detected a negative effect of the presence of ericaceous shrubs on growth of fir seedlings, possibly mediated by mycorrhizal fungi [19]. This growth chamber study did not confirm any negative effect from microorganisms present in soils from ericaceous shrubs, except that mycorrhization was lower in these soils. Interestingly, soils from BSE did not decrease needle N concentrations, contrary to the field [19]. We also found that germination, survival and growth were all relatively high in black spruce soils in our experiment as compared to field observations. Several explanations could be formulated to explain these differences between field and growth chamber conditions (Figure 7). Among them, root competition for nutrient acquisition with BS roots [53] and water logging constrain in BS stands [54] have both been demonstrated in these very same forest stands. The presence of a common mycorrhizal network between young and older balsam fir or TA in TA stands could also explain these differences.
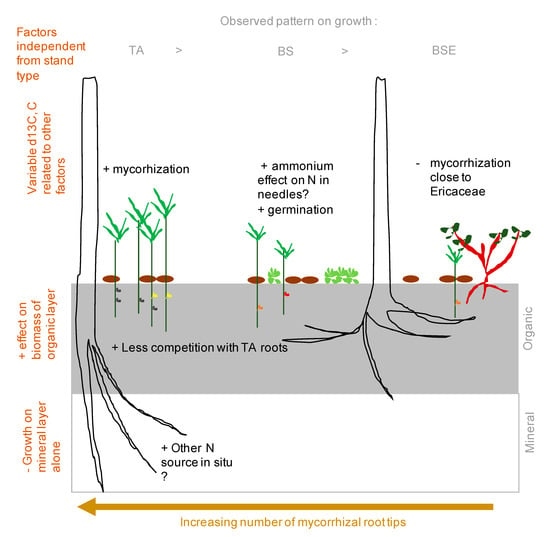
Figure 7.
Synthetic summary of positive and negative effect of soil layer, stand, trees, and fungi based on ex-situ and in-situ observations.
Except for mycorrhization, we did not detect such a positive effect of trembling aspen soils on balsam fir germination and growth in the growth chamber. We had expected a more direct effect of trembling aspen soils or other co-occurring species, since balsam fir thrived better under aspen and fewer adult trees occurred on our black spruce adjacent sites [19]. As mycorrhizae are essential for balsam fir growth, more direct interactions with neighboring tree roots suggest that balsam fir could depend on a common mycorrhizal network, linking them to trembling aspen roots and organic C (e.g., [55,56,57,58]). To go further with this hypothesis, a field experiment would be required to exclude mycorrhizal networks by regularly cutting soils around seedlings or setting a fine mesh around the young seedlings [58]. Moreover, such experiment would allow testing for root competition, from both ericaceous shrubs and black spruce trees. Such an experiment, handled in a Pinus radiata forest, revealed that canopy tree roots reduced growth of seedlings but that common mycorrhizal networks counteracted this competition [25]. Tracing of marked N injected to adult balsam fir trees has also revealed a possible transfer of nitrogen to balsam fir seedlings [59]. Such transfers could occur on our study sites, as balsam fir adults were scattered but more abundant under trembling aspen than under black spruce [19]. Bent et al. [60] also suggested common mycorrhizal network involving trembling aspen in boreal forests of Alaska, but did not observe any balsam fir seedlings in these forests. Setting an experiment, excluding root competition and common mycorrhizal networks, and tracing N from adult aspen and balsam fir would definitely allow us to understand how boreal forests favor balsam fir establishment today.
5. Conclusions
Our results show that young fir were able to germinate and survive in all conditions, and that their growth were mainly favored by the organic layer. Aspen stands appear more favorable to mycorrhization of balsam fir compared to other stands, and across stands, mycorrhization, seedlings height, and biomass were positively correlated. Therefore, the increase in aspen due to climate change, natural or anthropogenic disturbances [61,62] offers an opportunity to increase balsam fir abundance in the black spruce dominated forests to the north. For future predictions in a context of climate change, our results highlight the dependency of balsam fir toward biotic interactions, involving mycorrhizal fungi, but also interactions with trees. These interactions are essential for species migration [63,64], and taking them into account could definitely improve our models of tree distribution as illustrated by several recent studies, notably on the boreal forest [65,66]. The case of balsam fir is not isolated, and for example Acer saccharum establishment in boreal forest also depends on soil characteristics and is limited by the access to mycorrhizal fungi [67]. Our study shows that setting controlled experiments can greatly improve our understanding of tree adaptability to new conditions, migration, and possible climate change effect on boreal forest.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4907/11/8/815/s1. Table S1: Soil characteristics measured in situ from [15]. Table S2: Means (and standard deviation) of seedlings measurements during germination and the three growth seasons. Diameter: root collar diameter, R/S: root/shoot ratio and DW: dry weight. Germination refers to the number of germinated seedlings over 100 (10*10 replicates, survival to the number of saplings at the end of the three growth season (over 10) and EM to ectomycorrhizal apices. For physiological measurements, only seedlings producing >1 mg of needles were used (n: number of individuals used for these measures). Table S3: Estimates of LMM and GLMM on Germination, survival rate, shoot and root dry weigh (DW), d13C, percent of C, d15N, percent of N, N content (µg) and Root/Shoot (RS) ratio. Table S4: Statistics from ANOVA on seedling height, root collar diameter, δ13 C, δ15N, total C and N content, total dry weight, shoot and root dry weight.
Author Contributions
Conceptualization, M.N., M.G., M.R., A.D. and Y.B.; methodology, M.N., M.G., A.D. and M.R.; statistical analysis, M.N. and M.R.; writing—original draft preparation, M.N. and M.R.; writing—review and editing, M.N., M.R., M.G., A.D. and Y.B.; visualization, M.N. and M.R.; supervision, M.G., A.D., Y.B. and M.R.; project administration, Y.B., A.D. and M.R.; funding acquisition, Y.B. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by a Mitacs acceleration fellowship to MN in collaboration with Norbord Inc. and from The UQAT-UQAM NSERC industrial chair in sustainable forest management to Y.B. We benefitted from the support of an “Investissement d’Avenir” grants also managed by Agence Nationale de la Recherche (CEBA, ref. ANR-10-LABX-25-01; ANAEE-France: ANR-11-INBS-0001, TULIP: ANR-10-LABX-0041), and from a Champlain grant from the CFQCU.
Acknowledgments
Authors sincerely thank Lyne Blackburn for field assistance, Aurélie Suzanne for laboratory assistance and Martin Bidartondo for giving helpful advice for soil sterilization. We deeply thank Philippe Marchand for his help on statistics.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Woodall, C.W.; Clark, J.S. Failure to migrate: Lack of tree range expansion in response to climate change. Glob. Chang. Biol. 2012, 18, 1042–1052. [Google Scholar] [CrossRef]
- Iverson, L.R.; McKenzie, D. Tree-species range shifts in a changing climate: Detecting, modeling, assisting. Landsc. Ecol. 2013, 28, 879–889. [Google Scholar] [CrossRef]
- Neilson, R.P.; Pitelka, L.F.; Solomon, A.M.; Nathan, R.A.N.; Midgley, G.F.; Fragoso, J.M.; Lischke, H.; Thompson, K.E.N. Forecasting regional to global plant migration in response to climate change. Bioscience 2005, 55, 749–759. [Google Scholar] [CrossRef]
- Peay, K.G. The mutualistic niche: Mycorrhizal symbiosis and community dynamics. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 143–164. [Google Scholar] [CrossRef]
- Karst, J.; Burns, C.; Cale, J.A.; Antunes, P.M.; Woods, M.; Lamit, L.J.; Hoeksema, J.D.; Zabinski, C.; Gehring, C.A.; La Flèche, M. Tree species with limited geographical ranges show extreme responses to ectomycorrhizas. Glob. Ecol. Biogeogr. 2018, 27, 839–848. [Google Scholar] [CrossRef]
- Meier, E.S.; Lischke, H.; Schmatz, D.R.; Zimmermann, N.E. Climate, competition and connectivity affect future migration and ranges of European trees. Glob. Ecol. Biogeogr. 2012, 21, 164–178. [Google Scholar] [CrossRef]
- Goldblum, D.; Rigg, L.S. The deciduous forest–boreal forest ecotone. Geogr. Compass 2010, 4, 701–717. [Google Scholar] [CrossRef]
- Brandt, J.P.; Flannigan, M.D.; Maynard, D.G.; Thompson, I.D.; Volney, W.J.A. An introduction to Canada’s boreal zone: Ecosystem processes, health, sustainability, and environmental issues. Environ. Rev. 2013, 21, 207–226. [Google Scholar] [CrossRef]
- Grondin, P.; Ansseau, C.; Bélanger, L.; Bergeron, Y.; De Grandpré, L.; Gagnon, G.; Lavoie, C. Cadre Bioclimatique de Référence des Régions Ecologiques du Québec; Manuel de Foresterie; Les Presses de l’Université Laval: Québec City, QC, Canada, 1996; pp. 134–279. [Google Scholar]
- Laquerre, S.; Leduc, A.; Harvey, B.D. Augmentation du couvert en peuplier faux-tremble dans les pessières noires du nord-ouest du Québec après coupe totale. Ecoscience 2009, 16, 483–491. [Google Scholar] [CrossRef]
- Messaoud, Y.; Bergeron, Y.; Asselin, H. Reproductive potential of balsam fir (Abies balsamea), white spruce (Picea glauca), and black spruce (P. mariana) at the ecotone between mixedwood and coniferous forests in the boreal zone of western Quebec. Am. J. Bot. 2007, 94, 746–754. [Google Scholar] [CrossRef]
- Arbour, M.-L.; Bergeron, Y. Effect of increased Populus cover on Abies regeneration in the Picea–feathermoss boreal forest. J. Veg. Sci. 2011, 22, 1132–1142. [Google Scholar] [CrossRef]
- Cavard, X.; Bergeron, Y.; Chen, H.Y.; Paré, D. Effect of forest canopy composition on soil nutrients and dynamics of the understorey: Mixed canopies serve neither vascular nor bryophyte strata. J. Veg. Sci. 2011, 22, 1105–1119. [Google Scholar] [CrossRef]
- Nagati, M.; Roy, M.; Manzi, S.; Richard, F.; Desrochers, A.; Gardes, M.; Bergeron, Y. Impact of local forest composition on soil fungal communities in a mixed boreal forest. Plant Soil 2018, 432, 1–13. [Google Scholar] [CrossRef]
- Taylor, D.L.; Hollingsworth, T.N.; McFarland, J.W.; Lennon, N.J.; Nusbaum, C.; Ruess, R.W. A first comprehensive census of fungi in soil reveals both hyperdiversity and fine-scale niche partitioning. Ecol. Monogr. 2014, 84, 3–20. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2008; p. 605. ISBN 440026354. [Google Scholar]
- Wang, B.; Qiu, Y.-L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef]
- Nagati, M.; Roy, M.; Desrochers, A.; Manzi, S.; Bergeron, Y.; Gardes, M. Facilitation of balsam fir by trembling aspen in the boreal forest: Do ectomycorrhizal communities matter? Front. Plant Sci. 2019, 10, 932. [Google Scholar] [CrossRef]
- Mallik, A.U. Conifer Regeneration Problems in Boreal and Temperate Forests with Ericaceous Understory: Role of Disturbance, Seedbed Limitation, and Keytsone Species Change. Crit. Rev. Plant Sci. 2003, 22, 341–366. [Google Scholar] [CrossRef]
- Mallik, A.; Kravchenko, D. Recruitment and ontogenic patterns of stunting and growth release of black spruce (Picea mariana) in post-fire Kalmia heaths. For. Ecol. Manag. 2018, 407, 135–144. [Google Scholar] [CrossRef]
- Kennedy, P.G.; Smith, D.P.; Horton, T.R.; Molina, R.J. Arbutus menziesii (Ericaceae) facilitates regeneration dynamics in mixed evergreen forests by promoting mycorrhizal fungal diversity and host connectivity. Am. J. Bot. 2012, 99, 1691–1701. [Google Scholar] [CrossRef]
- Sietiö, O.-M.; Tuomivirta, T.; Santalahti, M.; Kiheri, H.; Timonen, S.; Sun, H.; Fritze, H.; Heinonsalo, J. Ericoid plant species and Pinus sylvestris shape fungal communities in their roots and surrounding soil. New Phytol. 2018, 218, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Richard, F.; Selosse, M.-A.; Gardes, M. Facilitated establishment of Quercus ilex in shrub-dominated communities within a Mediterranean ecosystem: Do mycorrhizal partners matter? FEMS Microbiol. Ecol. 2009, 68, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Booth, M.G.; Hoeksema, J.D. Mycorrhizal networks counteract competitive effects of canopy trees on seedling survival. Ecology 2010, 91, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- McLaren, B.E.; Janke, R.A. Seedbed and canopy cover effects on balsam fir seedling establishment in Isle Royale National Park. Can. J. For. Res. 1996, 26, 782–793. [Google Scholar] [CrossRef]
- Trevors, J.T. Sterilization and inhibition of microbial activity in soil. J. Microbiol. Methods 1996, 26, 53–59. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Schielzeth, H.; Dingemanse, N.J.; Nakagawa, S.; Westneat, D.F.; Allegue, H.; Teplitsky, C.; Réale, D.; Dochtermann, N.A.; Garamszegi, L.Z.; Araya-Ajoy, Y.G. Robustness of linear mixed-effects models to violations of distributional assumptions. Methods Ecol. Evol. 2020. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.1. 2017, Volume 5. Available online: https://cran.r-project.org/web/packages/DHARMa/vignettes/DHARMa.html (accessed on 30 June 2020).
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version. 2018, Volume 1, p. 3. Available online: https://rdrr.io/cran/emmeans/ (accessed on 30 June 2020).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Team, R.C. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version. 2013, Volume 3, p. 111. Available online: https://cran.r-project.org/web/packages/nlme/index.html (accessed on 30 June 2020).
- Parent, S.; Simard, M.-J.; Morin, H.; Messier, C. Establishment and dynamics of the balsam fir seedling bank in old forests of northeastern Quebec. Can. J. For. Res. 2003, 33, 597–603. [Google Scholar] [CrossRef]
- Laganière, J.; Angers, D.A.; Paré, D.; Bergeron, Y.; Chen, H.Y. Black spruce soils accumulate more uncomplexed organic matter than aspen soils. Soil Sci. Soc. Am. J. 2011, 75, 1125–1132. [Google Scholar] [CrossRef]
- Greene, D.F.; Noël, J.; Bergeron, Y.; Rousseau, M.; Gauthier, S. Recruitment of Picea mariana, Pinus banksiana, and Populus tremuloides across a burn severity gradient following wildfire in the southern boreal forest of Quebec. Can. J. For. Res. 2004, 34, 1845–1857. [Google Scholar] [CrossRef]
- Johnstone, J.F.; Chapin, F.S. Effects of soil burn severity on post-fire tree recruitment in boreal forest. Ecosystems 2006, 9, 14–31. [Google Scholar] [CrossRef]
- Johnstone, J.F.; Hollingsworth, T.N.; Chapin, F.S., III; Mack, M.C. Changes in fire regime break the legacy lock on successional trajectories in Alaskan boreal forest. Glob. Chang. Biol. 2010, 16, 1281–1295. [Google Scholar] [CrossRef]
- Lafleur, B.; Fenton, N.; Paré, D.; Simard, M.; Bergeron, Y. Contrasting effects of season and method of harvest on soil properties and the growth of black spruce regeneration in the boreal forested peatlands of eastern Canada. Silva Fenn. 2010, 44, 799–813. [Google Scholar] [CrossRef]
- Lafleur, B.; Cazal, A.; Leduc, A.; Bergeron, Y. Soil organic layer thickness influences the establishment and growth of trembling aspen (Populus tremuloides) in boreal forests. For. Ecol. Manag. 2015, 347, 209–216. [Google Scholar] [CrossRef]
- Trugman, A.T.; Fenton, N.J.; Bergeron, Y.; Xu, X.; Welp, L.R.; Medvigy, D. Climate, soil organic layer, and nitrogen jointly drive forest development after fire in the North American boreal zone. J. Adv. Model. Earth Syst. 2016, 8, 1180–1209. [Google Scholar] [CrossRef]
- De Grandpré, L.; Morissette, J.; Gauthier, S. Long-term post-fire changes in the northeastern boreal forest of Quebec. J. Veg. Sci. 2000, 11, 791–800. [Google Scholar] [CrossRef]
- Légaré, S.; Paré, D.; Bergeron, Y. Influence of aspen on forest floor properties in black spruce-dominated stands. Plant Soil 2005, 275, 207–220. [Google Scholar] [CrossRef]
- Stottlemyer, A.D.; Wang, G.G.; Wells, C.E.; Stottlemyer, D.W.; Waldrop, T.A. Reducing airborne ectomycorrhizal fungi and growing non-mycorrhizal loblolly pine (Pinus taeda L.) seedlings in a greenhouse. Mycorrhiza 2008, 18, 269–275. [Google Scholar] [CrossRef]
- Hoeksema, J.D.; Chaudhary, V.B.; Gehring, C.A.; Johnson, N.C.; Karst, J.; Koide, R.T.; Pringle, A.; Zabinski, C.; Bever, J.D.; Moore, J.C. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 2010, 13, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Nara, K. Ectomycorrhizal networks and seedling establishment during early primary succession. New Phytol. 2006, 169, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Torres Aquino, M.; Plassard, C. Dynamics of ectomycorrhizal mycelial growth and P transfer to the host plant in response to low and high soil P availability. FEMS Microbiol. Ecol. 2004, 48, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Vallano, D.M.; Sparks, J.P. Foliar δ15N is affected by foliar nitrogen uptake, soil nitrogen, and mycorrhizae along a nitrogen deposition gradient. Oecologia 2013, 172, 47–58. [Google Scholar] [CrossRef]
- Mayor, J.R.; Schuur, E.A.G.; Mack, M.C.; Hollingsworth, T.N.; Bååth, E. Nitrogen Isotope Patterns in Alaskan Black Spruce Reflect Organic Nitrogen Sources and the Activity of Ectomycorrhizal Fungi. Ecosystems 2012, 15, 819–831. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Zackrisson, O.; Gentili, F.; Sellstedt, A.; Nilsson, M.-C. Ecosystem controls on nitrogen fixation in boreal feather moss communities. Oecologia 2007, 152, 121–130. [Google Scholar] [CrossRef]
- Houle, D.; Moore, J.-D.; Ouimet, R.; Marty, C. Tree species partition N uptake by soil depth in boreal forests. Ecology 2014, 95, 1127–1133. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Siddiqi, M.Y.; Glass, A.D.M. Conifer root discrimination against soil nitrate and the ecology of forest succession. Nature 1997, 385, 59–61. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Siddiqi, M.Y.; Glass, A.D.M.; Britto, D.T. Root ammonium transport efficiency as a determinant in forest colonization patterns: An hypothesis. Physiol. Plant. 2003, 117, 164–170. [Google Scholar] [CrossRef]
- Ghotsa Mekontchou, C.; Houle, D.; Bergeron, Y.; Drobyshev, I. Contrasting Root System Structure and Belowground Interactions between Black Spruce (Picea mariana (Mill.) B.S.P) and Trembling Aspen (Populus tremuloides Michx) in Boreal Mixedwoods of Eastern Canada. Forests 2020, 11, 127. [Google Scholar] [CrossRef]
- Lavoie, M.; Paré, D.; Bergeron, Y. Quality of growth substrates of post-disturbed lowland black spruce sites for black spruce (Picea mariana) seedling growth. New For. 2007, 33, 207–216. [Google Scholar] [CrossRef]
- Simard, S.W.; Perry, D.A.; Jones, M.D.; Myrold, D.D.; Durall, D.M.; Molina, R. Net transfer of carbon between ectomycorrhizal tree species in the field. Nature 1997, 388, 579–582. [Google Scholar] [CrossRef]
- Simard, S.W.; Jones, M.D.; Durall, D.M. Carbon and nutrient fluxes within and between mycorrhizal plants. In Mycorrhizal Ecology; Springer: Berlin, Germany, 2003; pp. 33–74. [Google Scholar]
- Simard, S.W.; Beiler, K.J.; Bingham, M.A.; Deslippe, J.R.; Philip, L.J.; Teste, F.P. Mycorrhizal networks: Mechanisms, ecology and modelling. Fungal Biol. Rev. 2012, 26, 39–60. [Google Scholar] [CrossRef]
- Hoeksema, J.D. Experimentally Testing Effects of Mycorrhizal Networks on Plant-Plant Interactions and Distinguishing Among Mechanisms. In Mycorrhizal Networks; Springer: Berlin, Germany, 2015; pp. 255–277. [Google Scholar]
- Gélinas-Pouliot, M. The Fate of 15N-Labeled Ammonium and Nitrate Applied on Trees Canopy in a Mature Balsam-Fir Stand. Ph.D. Thesis, Université du Québec ą Chicoutimi, Saguenay, QC, Canada, 2013. [Google Scholar]
- Bent, E.; Kiekel, P.; Brenton, R.; Taylor, D.L. Root-Associated Ectomycorrhizal Fungi Shared by Various Boreal Forest Seedlings Naturally Regenerating after a Fire in Interior Alaska and Correlation of Different Fungi with Host Growth Responses. Appl. Environ. Microbiol. 2011, 77, 3351–3359. [Google Scholar] [CrossRef]
- Marchais, M.; Arseneault, D.; Bergeron, Y. Composition changes in the boreal mixedwood forest of western Quebec since Euro-Canadian settlement. Front. Plant Sci. 2020. In Press. [Google Scholar] [CrossRef]
- Danneyrolles, V.; Dupuis, S.; Fortin, G.; Leroyer, M.; de Römer, A.; Terrail, R.; Vellend, M.; Boucher, Y.; Laflamme, J.; Bergeron, Y. Stronger influence of anthropogenic disturbance than climate change on century-scale compositional changes in northern forests. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Van der Putten, W.H.; Macel, M.; Visser, M.E. Predicting species distribution and abundance responses to climate change: Why it is essential to include biotic interactions across trophic levels. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2025–2034. [Google Scholar] [CrossRef]
- Urban, M.C.; Zarnetske, P.L.; Skelly, D.K. Moving forward: Dispersal and species interactions determine biotic responses to climate change: Dispersal and species interactions. Ann. N. Y. Acad. Sci. 2013, 1297, 44–60. [Google Scholar] [CrossRef]
- Pither, J.; Pickles, B.J.; Simard, S.W.; Ordonez, A.; Williams, J.W. Below-ground biotic interactions moderated the postglacial range dynamics of trees. New Phytol. 2018, 220, 1148–1160. [Google Scholar] [CrossRef]
- Hewitt, R.E.; Bennett, A.P.; Breen, A.L.; Hollingsworth, T.N.; Taylor, D.L.; Chapin, F.S.; Rupp, T.S. Getting to the root of the matter: Landscape implications of plant-fungal interactions for tree migration in Alaska. Landsc. Ecol. 2016, 31, 895–911. [Google Scholar] [CrossRef]
- Carteron, A.; Parasquive, V.; Blanchard, F.; Guilbeault-Mayers, X.; Turner, B.L.; Vellend, M.; Laliberté, E. Soil abiotic and biotic properties constrain the establishment of a dominant temperate tree into boreal forests. J. Ecol. 2020, 108, 931–944. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).