Abstract
Absorptive and transport fine roots (diameter ≤ 2 mm) differ greatly in anatomy, morphology, and physiology, as well as their responses to environmental changes. However, it is still not well understood how their functional traits and biomass repartition respond to resource variability associated with increasing soil depth. Herein, we sampled the first five order roots of three hardwoods, i.e., Juglans mandshurica Maxim., Fraxinus mandshurica Rupr., and Phellodendron amurense Rupr. at surface (0–10 cm) and subsurface (20–30 cm) soil layers, respectively, and measured root biomass, anatomy, morphology, chemistry, and physiology at the branch-order level. Based on the anatomical characteristics, absorptive and transport fine roots were identified within each order, and their amounts and functional trait plasticity to soil depth were examined. The results showed that across soil layers, the first three order roots were mainly absorptive roots, while the fourth- and fifth-order roots were transport ones. From surface to subsurface soil layers, both the number and biomass proportion of absorptive fine roots decreased but those of transport fine roots increased. Transport fine root traits were more plastic to soil depth than absorptive ones, especially for the conduit-related traits. Absorptive fine roots in surface soil generally had stronger potential for resource acquisition than those in deeper soil, as indicated by their longer specific root length and greater root branching density. In comparison, transport fine roots in deeper soil were generally enhanced in their transportation function, with wider stele and higher hydraulic conductivity. Our findings suggest that functional specialization via multi-trait plasticity and coordination in both absorptive and transport fine roots along the soil depth would benefit the efficient soil resource exploitation of trees in forest ecosystems.
1. Introduction
Within the fine root system (diameter ≤ 2 mm), the individual roots vary greatly in anatomy, morphology, physiology, and mycorrhizal colonization, as well as in their inherent functions [1,2]. Functionally, fine roots can be categorized into two components, that is, absorptive and transport fine roots [3]. Any potential shift driven by environmental changes in root abundance and functional traits in both fine root types would result in a switch in dominant function (i.e., absorption or transportation) of a fine root system, and the related ecophysiological processes [4]. Generally, the huge differences in soil physical and chemical properties among different soil layers can induce distinct variations of root functional traits in forest ecosystems [5,6,7]. The root trait variations along the soil profile could indicate changes in tree resource acquisition strategies and belowground interspecific competition [8]. A previous study has reported that root tips (i.e., the first-order roots), the typical absorptive roots, show apparent changes in anatomy, morphology, and respiration associated with soil depths across three hardwood species [9]. To date, little is known about the responses of high-order roots that primarily function in transportation. Some studies have suggested that absorptive and transport fine roots respond differently to changed environmental conditions. For example, significant changes in root hydraulic conductance and root length were found for the low-order roots of Pinus tabulaeformis Carr. under nitrogen addition, while changes in in anatomy were observed for the high-order roots [10]. However, most previous studies focusing on fine root responses to soil depth generally included only several root traits [11] or concentrated on limited root orders such as the first-order roots [9]. Therefore, to achieve a better understanding of root trait variations associated with soil depth, the comprehensive assessments of multiple traits (e.g., morphology, anatomy, chemistry, and physiology) in both absorptive and transportive fine roots are needed.
The relative share of root biomass between absorptive and transportive roots within a fine-root system is crucial to resource uptake in woody plants [12]. If the first-order roots are considered as typical absorptive roots, the higher-order roots (from the second- to the fifth-order) are transport roots, and their biomass ratio ranges from 15:85 to 47:53 among seven temperate woody species, indicating substantial inter-specific variation [13]. Additionally, biomass repartition between absorptive and transport fine roots also exhibits great intra-specific variations along environmental gradients. For instance, in northeastern China, Wang et al. [14] reported that the biomass ratio of the first-order roots to the sum of second- to fifth-order roots decreased from 0–10 cm to 10–20 cm soil layers in both Fraxinus mandshurica and Larix gmelinii (Rupr.) Kuzen. plantations, mainly due to the lower nutrient availability and higher soil bulk density of deep soil. On a large scale, based on anatomical assessments, Zadworny et al. [15] found that both the number and biomass proportion of absorptive roots (i.e., roots absent in phellem) in Pinus sylvestris Linn. increased with the increase of latitude along a 2000 km gradient, showing cold-adapted adjustments for resource uptake. Overall, it is necessary to quantify the biomass repartition between absorptive and transport fine roots precisely based on their anatomical structure (e.g., disappearance of cortex or presence of cork) and to reveal how their ratio varies with soil depth.
In the past decades, phenotypic plasticity was extensively applied to understand the magnitude of plant functional traits responses to environmental changes [16,17,18,19]. Previous studies suggested that root trait plasticity was closely related to soil nutrients and root trait categories. For example, under nutrient limitation, root biomass allocation was more plastic than root morphology in both herbaceous [20] and woody [16] seedlings. However, under water plus phosphorus shortage conditions, the plasticity of Fabaceae species was largest for root physiology, followed by chemistry, morphology, and anatomy, and finally in biomass allocation [21]. Compared with those short-term experimental treatments, the resource variability among different soil layers is a result of long-term physical and biogeochemical processes. Thus, it would be interesting to know which root traits are more plastic to soil depth.
Additionally, root trait plasticity to soil depth appears to be root-size-dependent. At the species level, the specific root length (SRL) of very fine roots (diameter < 0.5 mm) in both Quercus serrata Thunb. [22] and Chamaecyparis obtusa (Sieb. et Zucc.) Endl. [23] were lower at deep soil layers in comparison to shallow layers, but not for the fine roots (diameter of 0.5–1 mm and 1–2 mm). Similarly, at the community level, fine roots (diameter < 2 mm) rather than coarse roots (diameter 2–5 mm) had lower lignin concentrations at deep soil layers than at shallow layers [8]. Although the diameter size classification approach fails to differentiate the absorptive and transport fine roots, the studies mentioned above suggest that the former root group is possibly more plastic in responding to soil depth. Nonetheless, as both the magnitude and direction of the plasticity are also related to specific root traits, it is difficult to infer how trait plasticity varies between absorptive and transport fine roots.
In this study, we selected three broad-leaved hardwood species, Juglans mandshurica, Fraxinus mandshurica, and Phellodendron amurense, and measured 17 functional traits of the first five orders of roots at the surface (0–10 cm) and subsurface (20–30 cm) layers, including root biomass, morphology, physiology, chemistry, and anatomy, and then evaluated their plasticity in response to soil depth. Based on anatomical structure, roots with primary growth having intact cortex and mycorrhizal colonization were defined as absorptive roots, and roots with secondary growth including disrupted cortex, thickened secondary xylem, and continuous cork layer were defined as transport roots [1,15]. The potential for absorption and transportation among the first five orders was assessed. Here, our overall aims were to reveal how the biomass repartition between absorptive and transport fine roots changes with soil depth and to explore how trait plasticity to soil depth varies between two root functional groups. Specifically, we tested the hypotheses that: (1) the biomass proportion of absorptive fine roots decreases but that of transport ones increases with soil depth; (2) both absorptive and transport fine roots show marked responses to soil depth, but the former is more plastic.
2. Materials and Methods
2.1. Study Site
The research was conducted at the Maoershan Forest Research Station (45°21′–45°25′ N, 127°30′–127°34′ E, with an average elevation of 300 m) of the Northeast Forestry University, in Heilongjiang Province, China. The site has a continental temperate monsoon climate with mean January, July, and annual temperatures of −19.6, 20.9, and 2.8 °C, respectively, and annual precipitation ranging from 600 to 800 mm, of which 80% falls in June, July, and August [14]. The growing season ranges from 120 to 140 days. Soils are Hap-Boric Luvisols with well-developed horizons and are well drained. Soil bulk density increases from 0.7 to 1.2 g·cm−3, organic matter content declines from 235.2 to 77.1 mg·g−1, and total nitrogen concentration decreases from 8.7 to 3.5 mg·g−1; other details can be found in Wang et al. [9]. Pure plantations of three species were established in 1986 by planting nursery-raised 2 year-old bare root seedlings using a 1.5 × 2.0 m planting grid on a common flat slope. The area of each plantation was over 5 ha.
2.2. Root Sample Collection
At the end of April 2012, three plots (20 × 30 m) were randomly established in each plantation. In mid-July 2012, within each plot, root branches of three soil blocks at the surface (0–10 cm) and subsurface (20–30 cm) were sampled by using a specially designed 20 × 20 cm rectangular soil core with sharp edges [24]. All root branches of the target tree species were collected carefully by hand and gently washed in deionized water. Roots from shrubs or herbs were removed based on root form, color, and elasticity [1,25]. Root samples from the same soil layer from three plots were mixed into a composite sample then randomly divided into two subsamples: one was immediately fixed in formalin–aceto-alcohol solution (FAA, 90 ml of 50% ethanol, 5 mL of 100% glacial acetic acid, and 5 mL of 37% methanal) for subsequent anatomy analysis, and the other one was immediately put in a cooler with ice and transported to the laboratory within 4 h and frozen for subsequent morphology and biomass analysis.
2.3. Root Anatomy, Morphology, and Biomass
In the laboratory, at least five root branches fixed in FAA solution at each soil layer for each species were selected randomly. These were dissected into five branch orders following the procedure described in Pregitzer et al. [2], and the distal un-branched root tips were numbered as first-order roots. Most roots adhering to the branch were alive; dead roots were rare and were identified by their form, color, and elasticity then excluded from the samples. For each species, 20–30 root segments for the first and second order and 5–20 root segments for the third to fifth order at each soil layer were randomly chosen for final anatomy measurements. All root segments were less than 2 mm in diameter. Within each order, root segments were similar in both root age and distance from root tips, avoiding potential effects of these on the anatomical traits [26,27]. Slides 8 μm thick were prepared by dehydration in alcohol and embedded in paraffin and stained with safranin-fast green [1,25]. Root cross-sections were photographed under a biological microscope (Olympus Electronics Inc, Tsukuba, Japan) equipped with a Motic 3000 CCD camera (Motic Corporation, Xiamen, China). Root anatomy traits were measured to the nearest 1 μm using Motic Images Advanced 3.2 software, including root and stele diameter, cortex thickness, conduit diameter (e.g., mean, maximum, and hydraulic weighted conduit diameter). The ratio of stele diameter to root diameter was then calculated. Absorptive and transportive fine roots were classified with the following criteria: roots with primary growth having an intact cortex and mycorrhizal colonization were defined as absorptive roots, and roots with secondary growth due to vascular development were defined as transport roots, showing disrupted cortex, thickened secondary xylem, and a continuous cork layer [1,15]. In each order of roots, the number of absorptive and transport fine roots was recorded and their proportions (%) were calculated.
For morphological traits and biomass proportion measurements, five to seven intact root branches (i.e., including at least the first five orders) in the same soil layer were selected for each species. For each root branch, root dissection was conducted with the same procedure as the root anatomical analysis mentioned above. The number of roots in each order within a root branch was recorded manually. Then, each order of roots was scanned using a digital scanner (Epson Expression 10000XL, Epson Telford Ltd., Suwa Nagano, Japan). The mean diameter, total length, and volume of each order of roots was analyzed using the root system analyzer software (WinRHIZO 2004b, Regent Instruments Inc., Québec, QC, Canada). Then, all the root samples were oven-dried at 60 °C for 48 hours to constant weight (0.0001 g). SRL (m·g−1) was calculated as total root length divided by dry weight. Root tissue density (RTD, g·cm−3) was calculated as root dry weight divided by volume. Root branching density (RBD, No.·cm−1) was calculated as the total number of daughter roots divided by the total length of the mother roots.
2.4. Root Respiration and Chemistry
In mid-July 2013, three soil blocks were randomly sampled at the plot level with the same procedure as mentioned above. Root samples from three blocks within the same plot were mixed into a composite sample, yielding a total of three replicates for each order of roots at each soil layer. Root respiration measurements of the first five orders were made as soon as possible after sampling within 4 h. Dissected root samples (0.5 g fresh weight) were immersed in a constant-temperature circulating water bath at 18 ℃ (the mean soil temperature at 0–10 cm depth in July at the study site) and allowed to equilibrate for 30 min. Root respiration was then measured by measuring O2 consumption using gas-phase O2 electrodes (Model LD 2/2, Hansatech Instruments Ltd, King’s Lynn, UK) connected to the circulating water bath [9,24,28]. Two complete O2 electrode systems and water baths were used, allowing simultaneous respiration measurements to be performed on separate root samples. Once respiration measurements were done, the same root samples were oven-dried at 60 ℃ to determine constant weight (0.0001 g). The root respiration rate was calculated as nmol O2 g−1·s−1 (dry weight). Then, dried root samples were ground and homogenized for chemical analysis. Total nitrogen (N) and carbon (C) concentrations and their ratio were determined using a Macro Elemental Analyzer (vario MACRO, Elementar Co., Hanau, Germany). Root respiration rates and N concentrations can be found in Wang et al. [9], while root C concentration and C/N ratio are shown in this study.
2.5. Data Analysis
The dry weight of root subsamples scanned for the morphological analysis of each root order was also used for the biomass proportion measurements. Within each root branch, the proportion of biomass allocated to absorptive fine roots (PRBa) (%) was calculated as
where Bi is the proportion (%) of biomass of the ith-order roots to total biomass of the first five orders of roots and Ai is the proportion of ith-order roots that are absorptive based on anatomical measurements. The proportion of biomass allocated to transport fine roots (PRBt) (%) was 100 − PRBa.
The theoretical hydraulic conductivity (Ks) (Kg·m−1·MPa−1·s−1) was calculated with the Hagen–Poiseu equation [29]:
where ρ and η are the water density and viscosity coefficient (the temperature was set at 18 °C, consistent with root respiration measurements), respectively. Vd is conduit density in stele area. Dh is the hydraulic weighted conduit diameter, which is calculated according to the following equation [30,31]:
where di is the diameter of the ith conduit, n is the number of conduits in xylem (n is the total number of conduits in the first- to third-order roots, and about one-third of the total number of conduits in the fourth- and fifth-order roots, respectively).
The relative distance of plasticity index (RDPI) was calculated at the species level to quantify root functional trait plasticity to soil depth through the following equation [19,32]:
where and are the mean root trait values at the surface and subsurface soil layers, respectively. The averaged RDPI across the three species was used to compare the differences among root functional traits.
For traits expressed as percentages (i.e., the proportion of biomass in each root order and functional group, respectively), the Bliss angular transformation was used before analysis. The effects of species, root order, and soil depth on the root traits were tested using a three-way factorial analysis of variance (ANOVA). Fisher’s least significant difference (LSD) test (p = 0.05) was used to identify the difference in biomass proportions of each order or functional group (absorptive or transport) as well as the differences in other traits of each order between the two soil layers. A t-test (p = 0.05) was used to verify the difference between the RDPI and zero (no plasticity) for each order of roots across the three species [33]. A principal component analysis (PCA) was applied to log-transformed data across the three species to determine major sources of variation across multiple traits and identify whether there were concerted trait adjustments to soil depth. All statistical analyses were performed using SPSS software (2010, V. 19.0; SPSS Inc., Chicago, IL, USA). Data visualizations were made using SigmaPlot 10.0 (Systat Software Inc., San Jose, CA, USA) and ggplot2 [34].
3. Results
3.1. Assessment of Absorptive and Transport Fine Roots at Different Soil Layers
Root anatomical traits differed widely among species, soil depth, and root orders (Figure 1, Table 1). Across the three species, nearly all of the first- and second-order roots were absorptive, having an intact cortex and mycorrhizal colonization, while the fourth- and fifth-order roots were transportive, with a disrupted cortex, well-developed secondary xylem, and continuous cork layer, which was consistent at the two soil layers (Figure 1, Table 1). However, some third-order roots in all species showed structural transition from primary to secondary development, with cortex disruption and cork layer development, representing a functional shift from absorption to transport, which was more remarkable in deep soil (Figure 1, Table 1). The diameter cutoff of 0.5 mm, or the third order, could better discriminate the absorptive and transport fine roots across the three hardwoods.
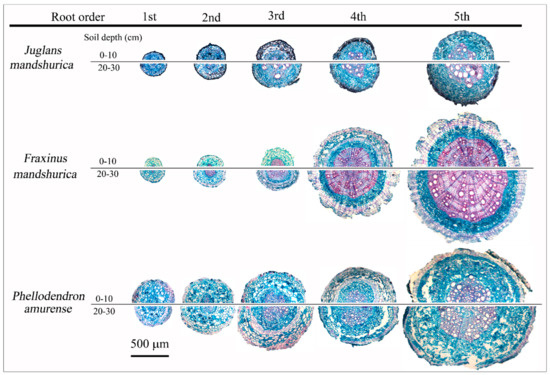
Figure 1.
Typical anatomical structures the first five orders of roots in surface (0–10 cm) and subsurface (20–30 cm) soil layers in Juglans mandshurica, Fraxinus mandshurica, and Phellodendron amurense.

Table 1.
The relative abundance (%) of absorptive and transportive fine roots in three hardwood species at surface (0–10 cm) and subface (20–30 cm) soil layers.
3.2. Relative Share of Biomass between Absorptive and Transport Fine Roots at Different Soil Layers
Within each root branch, the first three orders of roots generally had higher biomass proportions in the surface rather than the subsurface soil layer, but the opposite pattern was observed in the fourth- and fifth-order roots (Figure 2). When classifying the roots into absorptive or transport based on their anatomical traits (Table 1), the biomass proportion varied greatly between root functional groups and soil depths (Figure 2). With an increase in soil depth, the biomass proportion of absorptive fine roots decreased significantly, but that of transport fine roots increased in all three species (Figure 2, p < 0.05). The biomass proportion of absorptive fine roots decreased by 44% in J. mandshurica, 34% in F. mandshurica, and 33% in P. amurense from the surface to subsurface soil layer but correspondingly increased by 53%, 96%, and 100% in transport roots, respectively.
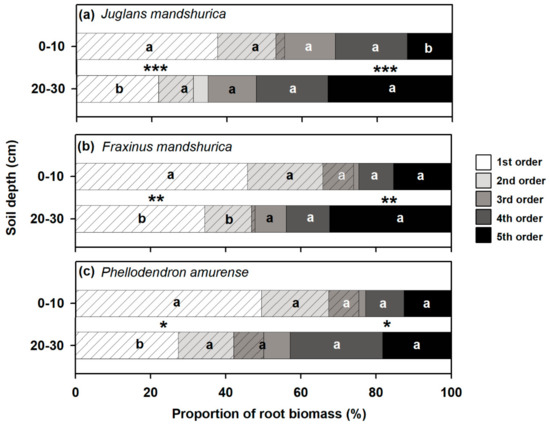
Figure 2.
Proportions of root biomass (PRB) in different root orders to total fine root biomass (first five root orders) at surface (0–10 cm) and subsurface (20–30 cm) soil layers in Juglans mandshurica (a), Fraxinus mandshurica (b), and Phellodendron amurense (c), respectively. Different lowercase letters indicate significant differences (p < 0.05) in PRB within a root order between soil layers according to Fisher’s least significant difference (LSD) test. Asterisks indicates significant differences (*** p < 0.001, ** p < 0.01, * p < 0.05) in the PRB of absorptive (with diagonal lines) or transport (without diagonal line) fine roots (based on Table 1) between soil layers according to Fisher’s LSD test, respectively.
3.3. Root Functional Trait Responses and Plasticity to Soil Depth
Most root functional traits varied greatly between the two soil depths in each order; however, the statistical significance depended on root order and tree species (Table 2). Generally, with the increase of soil depth, each order of roots tended to be thicker in root diameter (RD), smaller in SRL and RBD (Figure S1), and lower in root carbon concentration (RCC) (Figure S2). In addition, deep roots were wider in terms of the diameters of stele (SD) (Figure S3) and conduits (Figure S4), as well as higher in hydraulic conductivity (Ks) (Figure S5).

Table 2.
Results of a three-way (species × soil depth × root order) factorial ANOVA of root functional traits.
The plasticity of root traits to soil depth differed greatly between absorptive and transport fine root groups. Transport root traits generally had higher RDPIs than absorptive ones, except for the stele-to-root diameter ratio (SRR), RCC, RTD, and root respiration rate (RRR) (Figure 3). Among all root traits, the RDPI was much higher for anatomy, especially for the conduit-related traits, followed by root physiology and biomass, and lastly in root morphology and chemistry (Figure 3).
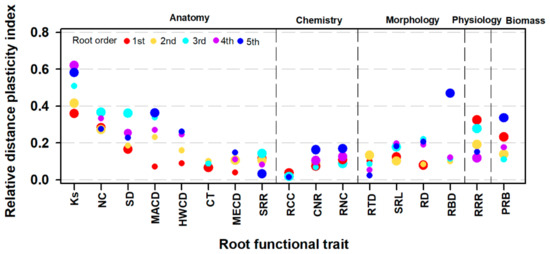
Figure 3.
The relative distance plasticity index (RDPI) of root functional traits in response to soil depth in the first five root orders. Large points indicate that the averaged RDPI across the three species is significantly different from zero (no plasticity) according to a t-test (p < 0.05), while small points indicate that the difference is not significant (p > 0.05). The trait abbreviations are as in Table 2.
3.4. Root Functional Trait Shift Coordinately along Soil Depth
Most root functional traits coordinately changed along soil depth. The PCA showed that the first two trait axes explained 68.3% and 12.9% of total variations, respectively (Figure 4), with SD scoring high on the first axis and PRB scoring high on the second axis (Table S1). Specifically, one vascular-trait-related dimension occupied the first axis, with the traits related to absorption (i.e., SRL and root nitrogen concentration (RNC)) and transportation (i.e., SD and NC) clustering at the two endpoints, respectively. Another biomass-allocation-related dimension, including root biomass allocation (PRB) and branching properties (RBD), occupied the second axis. Generally, for a given order of roots, increasing soil depth moved the roots from the left to the right side along the first axis, indicating that the surface roots were more absorption-centric and the subsurface roots were more transport-centric.
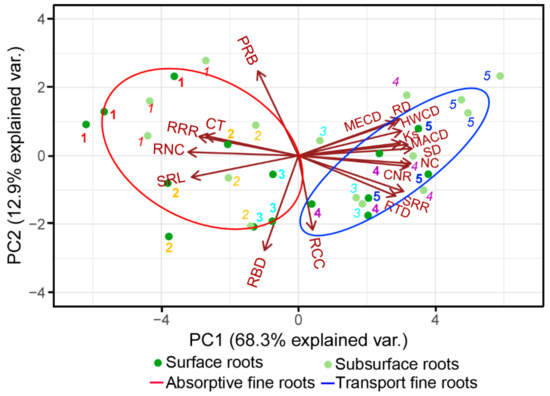
Figure 4.
Principal component analysis (PCA) of the functional traits of the first five orders of roots in response to soil depth. The numbers in bold non-italics and in non-bold italics indicate the order of roots at surface (0–10 cm) and subsurface (20–30 cm) soil layers, respectively. The absorptive and transportive fine roots are classified based on their anatomical traits: when the abundance of absorptive roots was more than 50% in a given order (according to the Table 1), then roots in this order were defined as absorptive fine roots, otherwise they were transport fine ones. The trait abbreviations are as in Table 2.
4. Discussion
Here, we assessed the direction, magnitude, and coordination of multi-traits shifts in absorptive and transport fine roots along soil depth across three tree species in term of their biomass repartition, anatomy, morphology, physiology, and chemistry. To the best of our knowledge, this is the first report of an anatomical approach to quantifying the changes in biomass repartition between absorptive and transport fine roots along the soil profile. We found that the plasticity of functional traits in response to soil depth differed in absorptive and transport fine roots, but not their coordination. Root functional specialization along soil depth occurred in both root groups, i.e., the shallower absorptive fine roots are stronger in uptake capacity, while the deeper transport fine roots are stronger in transport capacity. Undoubtedly, these shifts in root biomass and functional traits are important adjustments to soil stratification for root resources acquisition in woody species.
4.1. Variations in Biomass Repartition between Absorptive and Transport Fine Roots along Soil Depth
Our results support the first hypothesis that the proportion of root biomass decreases in absorptive fine roots with increasing soil depth but increases in transport ones. Although our previous study also found that the biomass proportion of the lower-order roots declined in deeper soils in two temperate tree species, the classification of absorptive and transport fine roots was not done concurrently [14]. Based on anatomical measurements, Trocha et al. [11] further revealed that the ratio of absorptive roots decreased significantly at deep soil layers in P. sylvestris, but biomass data on each order were not reported. Many studies have confirmed that fine root standing biomass decreases with increasing soil depth [35,36,37]; to date, if and how the relative share of biomass between absorptive and transport fine roots responds to soil depth still remains unclear. Herein, all three examined hardwood species showed a consistent decline of biomass proportion in absorptive fine roots at deep soil, similar to the fine root standing biomass found in previous studies.
Why does the proportion of absorptive fine root biomass decrease in deeper soil? There are two possible reasons. Firstly, this is mainly related to the inherent preference of absorptive fine roots for nutrients. Numerous studies have confirmed that nutrient-rich patches can stimulate root proliferation [4,38,39,40]. In our study, compared with deeper soil, greater water and nutrient availability in surface soil [9] could promote the growth of absorptive fine roots, manifested by the larger number (Figure S1) and biomass proportion (Figure 1). Thus, more absorptive fine roots located in surface soil layers could yield a high benefit-to-cost ratio (resource acquisition vs. C investment).
Secondly, the decrease of root biomass proportion in absorptive fine roots at subsurface soil layer was possibly related to the lower root branching density (Figure S1). Less daughter roots per mother root length (Figure S1) could result in a dramatic decrease in the total number of distal first- and second-order roots at the subsurface soil layer, and consequently a lower share of biomass. Wang et al. [14] also found a smaller root branching ratio and lower biomass proportion of lower root orders at the deeper soil layer in both F. mandshurica and L. gmelinii. Overall, further study of whether the systematic decline in the proportion of absorptive fine roots associated with soil depth is common in diverse tree species and forests is warranted.
4.2. Variations of Functional Traits of Absorptive and Transport Fine Roots along Soil Depth
The functional traits of both absorptive and transport fine roots varied remarkably along soil depth, confirming our second hypothesis. However, no distinct discrepancies in their responses were found in the two functional groups, manifested by the interaction effect of soil depth and species with branch order (Table 2). The dramatic changes in root functional traits across soil depths have been found in very fine root tips [9] and coarse roots (diameter > 2 mm [41,42] and > 4 mm [7]). Nonetheless, these studies only focused on one single pool, i.e., absorptive fine roots or coarse transport roots, leaving the transport fine roots less understood. Thus, our results, together with other studies, suggest that different root functional groups, i.e., absorptive fine and transport fine roots (diameter ≤ 2 mm) and coarse transport roots (diameter > 2 mm) can all alter their functional traits to adapt to soil depth changes, which seems to be general across forest ecosystems.
However, partly contradicting our second hypothesis, the phenotypic plasticity was generally higher in transport fine roots than in absorptive ones, especially for the hydraulic and vascular traits (Figure 4). Wang et al. [10] also found that the vascular traits of high-order transport roots were more plastic to fertilization in P. tabulaeformis. The higher plasticity of transport fine roots may be related to their higher priority for C utilization. Compared with the distal lower-order absorptive fine roots, the basal higher-order transport roots generally contained more and wider phloem elements (Figure 1), consequently indicating a priority for C allocation [43]. Therefore, for roots in deeper soils, C availability could be reduced due to long-distance transload, but relatively greater C is invested to transport fine roots for constructing cells and tissues (e.g., having greater conduit and stele, respectively, Figures S3 and S4) and individual roots (showing thicker root diameters, Figure S1). But the deep roots within each order generally had a greater CNR than the shallow ones across species (Figure S2), which indicated their lower maintenance costs compared with the higher construction investments [2], implying an efficient C allocation strategy for building roots in different soil layers. It is also worth noting that the RDPI of Ks was higher than other traits across all root orders (Figure 3), which was mainly caused by the substantial increase of Ks in deeper transport fine roots (Figure S5). Some previous studies also confirmed that both absorptive fine roots [9] and coarse transport roots [7,41] had higher Ks in deeper soil. Therefore, all deeper roots possessing greater Ks is likely to be a common phenomenon across diverse root functional types and tree species and may enable them to minimize flow resistance and maximize water transportation efficiency [44], despite empirical validation being needed.
In addition, we found that root functional traits coordinately responded to soil depth, which was closely related to the inherent functions of root groups (Figure 4, Table S1). For instance, for the absorptive fine roots, those traits associated with resource absorption, such as SRL, RNC, and RRR, were correlated with each other and had greater values in surface soil, indicating an enhanced absorption ability [9,28,45]. In comparison, in the transport fine roots, traits associated with resource transportation, such as SD, NC, and Ks, were correlated with each other and had higher values in deeper soil, indicating a strengthened transportation capacity. Moreover, considering the fact that absorptive fine roots at the surface soil layer had a greater biomass proportion while transport fine roots showed an inverse trend, our findings confirm that both root functional traits and biomass repartition changed in a similar manner across soil depths. Collectively, the functional specialization of absorptive and transport fine roots via multi-trait plasticity and coordination could provide a benefit to trees for the efficient exploitation of soil resources.
5. Conclusions
With an increase in soil depth, a portion of the second- and third-order roots transformed from absorptive to transport fine roots, as manifested by their anatomical traits in all three species, resulting in a decreased biomass proportion of absorptive fine roots to the total fine roots. It is suggested that roots with a diameter ≤ 0.5 mm, or the first three orders, could well represent the absorptive fine roots across these hardwood species. In addition, all root functional traits showed marked responses to soil depth, and transport fine roots were more plastic than absorptive ones, especially in terms of root hydraulic conductivity. Furthermore, root functional traits generally responded to soil depth in a coordinated way, which enhanced the uptake capacity of absorptive fine roots at the surface soil layer and the transportation ability of transport fine roots in deep soil. These findings highlight that both biomass repartition and functional specialization occur in absorptive and transport fine roots with increasing soil depth.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4907/11/1/42/s1, Table S1. The loadings scores of root functional traits of the first two PCA axes; Figure S1. Mean diameter, specific root length, root tissue density, and root branching density of the first five orders of roots in surface (0–10 cm) and subsurface (20–30 cm) soil layers for Juglans mandshurica (a, d, g, j), Fraxinus mandshurica (b, e, h, k), and Phellodendron amurense (c, f, i, l). The error bars represent 1 standard error of mean (SEM). Different lowercase letters within the clusters of bars indicate significant differences (p < 0.05) in root traits between soil layers according to Fisher’s LSD test. Figure S2. Root carbon concentration and the nitrogen and carbon concentration ratio in the first five orders of roots in surface (0–10 cm) and subsurface (20–30 cm) soil layers for J. mandshurica (a, d), F. mandshurica (b, e), and P. amurense (c, f). The error bars represent 1 SEM. Different lowercase letters within the clusters of bars indicate significant differences (p < 0.05) in root traits between soil layers according to Fisher’s LSD test. Figure S3. Root cortex thickness, stele diameter, and stele:root diameter ratio of the first five orders of roots in surface (0–10 cm) and subsurface (20–30 cm) soil layers for J. mandshurica (a, d, g), F. mandshurica (b, e, h), and P. amurense (c, f, i). The error bars represent 1 SEM. Different lowercase letters within the clusters of bars indicate significant differences (p < 0.05) in root traits between soil layers according to Fisher’s LSD test. Figure S4. Mean, maximum, and hydraulic weight conduit diameter and number of conduits per stele area in the first five orders of roots in surface (0-10 cm) and subsurface (20–30 cm) soil layers for J. mandshurica (a, d, g, j), F. mandshurica (b, e, h, k), and P. amurense (c, f, i, l). The error bars represent 1 SEM. Different lowercase letters within the clusters of bars indicate significant differences (p < 0.05) in root traits between soil layers according to Fisher’s LSD test. Figure S5. Theoretical hydraulic conductivity (Ks) of the first five orders of roots in surface (0–10 cm) and subsurface (20–30 cm) soil layers for J. mandshurica (a), F. mandshurica (b), and P. amurense (c). The error bars represent 1 SEM. Different lowercase letters within the clusters of bars indicate significant differences (p < 0.05) in root traits between soil layers according to Fisher’s LSD test.
Author Contributions
Conceptualization, J.G. and Z.W.; Data curation, Y.W. and Z.L.; Formal analysis, Y.W. and Z.L.; Funding acquisition, J.G.; Investigation, Y.W. and Z.L.; Methodology, Y.W. and Z.L.; Project administration, J.G. and Z.W.; Software, Y.W. and Z.L.; Writing—original draft, Y.W. and Z.L.; Writing—review & editing, Y.W., J.G. and Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was jointly supported by the Fundamental Research Funds for the Central Universities (2572018BA11) and the National Natural Science Foundation of China (31870608 and 31100470).
Acknowledgments
The authors thank Na Wang, Xueyun Dong, and Hongfeng Wang for their help with field and laboratory work, as well as the anonymous reviewers and the editor for comments that improved an earlier draft of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guo, D.; Xia, M.; Wei, X.; Chang, W.; Liu, Y.; Wang, Z. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol. 2008, 180, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Pregitzer, K.S.; Deforest, J.L.; Burton, A.J.; Allen, M.F.; Ruess, R.W.; Hendrick, R.L. Fine root architecture of nine North American trees. Ecol. Monogr. 2002, 72, 293–309. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A. The plastic plant: Root responses to heterogeneous supplies of nutrients. New Phytol. 2004, 162, 9–24. [Google Scholar] [CrossRef]
- Maeght, J.; Rewald, B.; Pierret, A. How to study deep roots-and why it matters. Front Plant Sci. 2013, 4, 299. [Google Scholar] [CrossRef]
- Majdi, H.; Truus, L.; Johansson, U.; Nylund, J.; Wallander, H. Effects of slash retention and wood ash addition on fine root biomass and production and fungal mycelium in a Norway spruce stand in SW Sweden. Forest Ecol. Manag. 2008, 255, 2109–2117. [Google Scholar] [CrossRef]
- McElrone, A.J.; Pockman, W.T.; Vilalta, J.M.; Jackson, R.B. Variation in xylem structure and function in stems and roots of trees to 20 m depth. New Phytol. 2004, 163, 507–517. [Google Scholar] [CrossRef]
- Prieto, I.; Roumet, C.; Cardinael, R.; Dupraz, C.; Jourdan, C.; Kim, J.H.; Maeght, J.L.; Mao, Z.; Pierret, A.; Portillo, N.; et al. Root functional parameters along a land-use gradient: Evidence of a community-level economics spectrum. J. Ecol. 2015, 103, 361–373. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, X.; Wang, H.; Wang, Z.; Gu, J. Root tip morphology, anatomy, chemistry and potential hydraulic conductivity vary with soil depth in three temperate hardwood species. Tree Physiol. 2016, 36, 99–108. [Google Scholar] [CrossRef]
- Wang, G.; Liu, F.; Xue, S. Nitrogen addition enhanced water uptake by affecting fine root morphology and coarse root anatomy of Chinese pine seedlings. Plant Soil 2017, 418, 177–189. [Google Scholar] [CrossRef]
- Trocha, L.K.; Bułaj, B.; Kutczyńska, P.; Mucha, J.; Rutkowski, P.; Zadworny, M. The interactive impact of root branch order and soil genetic horizon on root respiration and nitrogen concentration. Tree Physiol. 2017, 37, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, G.; Wang, N.; Wang, Z.; Gu, J. Effects of morphology and stand structure on root biomass and length differed between absorptive and transport roots in temperate trees. Plant Soil 2019, 442, 355–367. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, D.; Wang, X.; Gu, J.; Mei, L. Fine root architecture, morphology, and biomass of different branch orders of two Chinese temperate tree species. Plant Soil 2006, 288, 155–171. [Google Scholar] [CrossRef]
- Zadworny, M.; McCormack, M.L.; Mucha, J.; Reich, P.B.; Oleksyn, J. Scots pine fine roots adjust along a 2000-km latitudinal climatic gradient. New Phytol. 2016, 212, 389–399. [Google Scholar] [CrossRef]
- Kramer-Walter, K.R.; Laughlin, D.C. Root nutrient concentration and biomass allocation are more plastic than morphological traits in response to nutrient limitation. Plant Soil 2017, 416, 539–550. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef]
- Ryser, P.; Eek, L. Consequences of phenotypic plasticity vs. interspecific differences in leaf and root traits for acquisition of aboveground and belowground resources. Am. J. Bot. 2000, 3, 402–411. [Google Scholar] [CrossRef]
- Valladares, F.; Sanchez-Gomez, D.; Zavala, M.A. Quantitative estimation of phenotypic plasticity: Bridging the gap between the evolutionary concept and its ecological applications. J. Ecol. 2006, 94, 1103–1116. [Google Scholar] [CrossRef]
- Freschet, G.T.; Swart, E.M.; Cornelissen, J.H.C. Integrated plant phenotypic responses to contrasting above- and below-ground resources: Key roles of specific leaf area and root mass fraction. New Phytol. 2015, 206, 1247–1260. [Google Scholar] [CrossRef]
- Fort, F.; Cruz, P.; Catrice, O.; Delbrut, A.; Luzarreta, M.; Stroia, C.; Jouany, C. Root functional trait syndromes and plasticity drive the ability of grassland Fabaceae to tolerate water and phosphorus shortage. Environ. Exp. Bot. 2015, 110, 62–72. [Google Scholar] [CrossRef]
- Makita, N.; Hirano, Y.; Mizoguchi, T.; Kominami, Y.; Dannoura, M.; Ishii, H.; Finér, L.; Kanazawa, Y. Very fine roots respond to soil depth: Biomass allocation, morphology, and physiology in a broad-leaved temperate forest. Ecol. Res. 2011, 26, 95–104. [Google Scholar] [CrossRef]
- Miyatani, K.; Mizusawa, Y.; Okada, K.; Tanikawa, T.; Makita, N.; Hirano, Y. Fine root traits in Chamaecyparis obtusa forest soils with different acid buffering capacities. Trees 2016, 30, 415–429. [Google Scholar] [CrossRef]
- Jia, S.; Mclaughlin, N.B.; Gu, J.; Li, X.; Wang, Z. Relationships between root respiration rate and root morphology, chemistry and anatomy in Larix gmelinii and Fraxinus mandshurica. Tree Physiol. 2013, 33, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Xu, Y.; Dong, X.; Wang, H.; Wang, Z. Root diameter variations explained by anatomy and phylogeny of 50 tropical and temperate tree species. Tree Physiol. 2014, 34, 415–425. [Google Scholar] [CrossRef]
- Anfodillo, T.; Petit, G.; Crivellaro, A. Axial conduit widening in woody species: A still neglected anatomical pattern. IAWA J. 2013, 34, 352–364. [Google Scholar] [CrossRef]
- Olson, M.E.; Rosell, J.A. Vessel diameter–stem diameter scaling across woody angiosperms and the ecological causes of xylem vessel diameter variation. New Phytol. 2013, 197, 1204–1213. [Google Scholar] [CrossRef]
- Burton, A.J.; Pregitzer, K.S.; Ruess, R.W.; Hendrick, R.L.; Allen, M.F. Root respiration in North American forests: Effects of nitrogen concentration and temperature across biomes. Oecologia 2002, 131, 559–568. [Google Scholar] [CrossRef]
- Tyree, M.T.; Ewers, F.W. The hydraulic architecture of trees and other woody plants. New Phytol. 1991, 119, 345–360. [Google Scholar] [CrossRef]
- Sterck, F.J.; Zweifel, R.; Sass-Klaassen, U.; Qumruzzaman, C. Persisting soil drought reduces leaf specific conductivity in Scots pine (Pinus sylvestris) and pubescent oak (Quercus pubescens). Tree Physiol. 2008, 28, 529–536. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, K.; Fan, Z.; Zhang, J. Potential hydraulic efficiency in angiosperm trees increases with growth-site temperature but has no trade-off with mechanical strength. Glob. Ecol. Biogeogr. 2013, 22, 971–981. [Google Scholar] [CrossRef]
- Scoffoni, C.; Kunkle, J.; Pasquet-Kok, J.; Vuong, C.; Patel, A.J.; Montgomery, R.A.; Givnish, T.J.; Sack, L. Light-induced plasticity in leaf hydraulics, venation, anatomy, and gas exchange in ecologically diverse Hawaiian lobeliads. New Phytol. 2015, 207, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Tsakaldimi, M.; Tsitsoni, T.; Ganatsas, P.; Zagas, T. A comparison of root architecture and shoot morphology between naturally regenerated and container-grown seedlings of Quercus ilex. Plant Soil 2009, 324, 103–113. [Google Scholar] [CrossRef]
- Wickham, H.; Chang, W.; Henry, L.; Pederson, T.L.; Takahashi, K.; Wikle, C.; Woo, K.; Yutani, H.; RStudio. ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. Available online: http://lib.ugent.be/CRAN/web/packages/ggplot2/index.html (accessed on 7 November 2019).
- Borken, W.; Kossmann, G.; Matzner, E. Biomass, morphology and nutrient contents of fine roots in four Norway spruce stands. Plant Soil 2007, 292, 79–93. [Google Scholar] [CrossRef]
- Liu, R.; Huang, Z.; McCormack, M.L.; Zhou, X.; Wan, X.; Yu, Z.; Wang, M.; Zheng, L. Plasticity of fine-root functional traits in the litter layer in response to nitrogen addition in a subtropical forest plantation. Plant Soil 2017, 415, 317–330. [Google Scholar] [CrossRef]
- Wang, W.; Wu, X.; Hu, K.; Liu, J.; Tao, J. Understorey fine root mass and morphology in the litter and upper soil layers of three Chinese subtropical forests. Plant Soil 2016, 406, 219–230. [Google Scholar] [CrossRef]
- Arredondo, J.T.; Johnson, D.A. Root architecture and biomass allocation of three range grasses in response to nonuniform supply of nutrients and shoot defoliation. New Phytol. 1999, 143, 373–385. [Google Scholar] [CrossRef]
- Fransen, B.; De Kroon, H. Long-term disadvantages of selective root placement: Root proliferation and shoot biomass of two perennial grass species in a 2-year experiment. J. Ecol. 2001, 89, 711–722. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.; Zhu, B.; Koide, R.T.; Eissenstat, D.M.; Guo, D. Complementarity in nutrient foraging strategies of absorptive fine roots and arbuscular mycorrhizal fungi across 14 coexisting subtropical tree species. New Phytol. 2015, 208, 125–136. [Google Scholar] [CrossRef]
- Gebauer, R.; Volařík, D. Root hydraulic conductivity and vessel structure modification with increasing soil depth of two oak species: Quercus pubescens and Quercus robur. Trees 2013, 27, 523–531. [Google Scholar] [CrossRef]
- Pate, J.S.; Jeschke, W.D.; Aylward, M.J. Hydraulic architecture and xylem structure of the dimorphic root systems of South-West Australian species of Proteaceae. J. Exp. Bot. 1995, 289, 907–915. [Google Scholar] [CrossRef]
- Aguirrezabal, L.A.; Pellerin, S.; Rtradieu, F. Carbon nutrition, root branching and elongation: Can the present state of knowledge allow a predictive approach at a whole-plant level? Environ. Exp. Bot. 1993, 33, 121–130. [Google Scholar] [CrossRef]
- North, G.B. A long drink of water how xylem changes with depth. New Phytol. 2004, 163, 447–449. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Laskowski, M.J.; Burton, A.J.; Lessard, V.C.; Zak, D.R. Variation in sugar maple root respiration with root diameter and soil depth. Tree Physiol. 1998, 18, 665–670. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).