Abstract
Storms are the main abiotic disturbance in European forests, effects of which are expected to intensify in the future, hence the importance of forest stand stability is increasing. The predisposition of Norway spruce to wind damage appears to be enhanced by pathogens such as Heterobasidion spp., which reduce stability of individual trees. However, detailed information about the effects of the root rot on the stability of individual trees across diverse soil types is still lacking. The aim of the study was to assess the effect of root rot on the individual tree stability of Norway spruce growing on drained peat and mineral soils. In total, 77 Norway spruce trees (age 50–80 years) growing in four stands were tested under static loading. The presence of Heterobasidion spp. had a significant negative effect on the bending moment at primary and secondary failure of the tested trees irrespectively of soil type. This suggests increased legacy effects (e.g., susceptibility to pathogens and pests due to fractured roots and altered water uptake) of storms. Damaged trees act as weak spots increasing the susceptibility of stands to wind damage, thus forming a negative feedback loop and contributing to an ongoing decline in vitality of Norway spruce stands following storms in the study region in the future. Accordingly, the results support the importance of timely identification of the decayed trees, lowering stand density and/or shortening rotation period as the measures to counteract the increasing effects of storms on Norway spruce stands.
1. Introduction
The increasing frequency of storms causes growing losses of both economic and ecological (e.g., carbon sequestration) value in European forests [1], which is expected to intensify in the future [2,3]. The susceptibility of forests to wind damage is amplified by the presence of additional disturbance agents, such as pathogens (e.g., Heterobasidion spp.), thus increasing the vulnerability of a forest stand [4]. Moreover, in the future, northern forests are expected to become more susceptible to wind impact during extra-tropical cyclones in the autumn-winter period, as well as in summer thunderstorms [5,6]. Under such conditions, management measures improving the mechanical stability of forest stands are becoming crucial [7,8].
The collective stability of forest stands is largely attributed to individual tree stability because the collapse of weakened individual trees initiates further damage in the stand via the domino effect [2,9]. The stability of an individual tree is determined by the species, stand properties, and tree health conditions, as well as aerial tree characteristics and root anchorage [2,9]. The size of soil-root plate is considered as the factor determining individual stability of a tree, which might be reduced by root rot [10]. High stand density is known to affect individual tree stability because competing trees invest in stem growth rather than root development [11,12]. Furthermore, denser stands are more susceptible to the spread of pathogens, which affect roots and, consequently, also stems [13]. Accordingly, forest management measures aimed at increasing the individual stability of the trees have been considered a major measure to reduce the consequences of storms [14,15,16].
Norway spruce (Picea abies L.) is economically important in Northern Europe; hence, efforts are aimed at maintaining its productivity [17]. A rather large proportion of highly productive stands of Norway spruce are growing on fertile, drained peat soils in Northern Europe [18]. The species is prone to wind damage due to shallow rooting and its relatively dense crown [19,20,21]. Among the pathogens of Norway spruce, root rot caused by Heterobasidion spp. is the most common, causing severe economic losses due to a decrease in stem quality [22]. In stands growing on mineral soils, the effect of root rot on the mechanical stability of conifers, such as Norway spruce, has been investigated using winching tests [23,24,25,26], suggesting the reduction of root anchorage due to root rot by up to 33%. However, no studies on this aspect exist in stands on peat soils. Soil is a significant factor, affecting tree wind stability [23], thus, it is important to assess the impact of root rot on the stability of Norway spruce growing on diverse soils. The aim of the study is to assess the effect of root rot caused by Heterobasidion spp. on the individual tree stability of Norway spruce growing on peat and mineral soils. We hypothesise that the reduction of the soil-root anchorage by Heterobasidion spp. is stronger in peat soils than in mineral soils due to the reduction of soil-root plate.
2. Materials and Methods
2.1. Study Site and Sample Trees
The study was conducted in the summer seasons of 2018 to 2019 in Norway spruce stands situated on mineral and peat soils in the central part of Latvia (Table 1). Mature Norway spruce dominated forest stands with deep drained peat and fine dry sandy soils were selected for the study. Evenly distributed within stands, dominant trees without visual damage were selected for sampling. Prior information on the presence of Heterobasidion spp. in the stands was collected during previous research (unpublished data). Additionally, the presence of fungal pathogens in sample trees’ wood was tested in the laboratory from increment cores extracted below the root collar from the opposite sides of stem. The presence of Heterobasidion spp. in the samples was confirmed by observing its characteristic asexual sporulation (conidiophores). Based on these results, in each stand infected (root rot group) and not infected sample trees (control group) were selected and pulled to failure within the same season. Additionally, Armillaria spp. was found in 70% of trees of the root rot group.

Table 1.
Sample size, soil type, species composition, and DBH (diameter at breast height) of the pulled Norway spruce (Picea abies L.) trees in tested stands.
2.2. Pulling Tests
Trees were pulled with a hand winch (working load limit 32 kN) to determine the maximum force needed for failure, either uprooting or stem fracture. The winch was anchored at the base of a second tree with a polyester roundsling (working load limit of 40 kN) at a distance that exceeded the sample tree height. The pulling line consisted of a 20-m-long (16 mm diameter) steel cable, which was extended using a static polyester rope (Tenex Tec 16; diameter 16 mm; working load limit 77 kN; Samson Rope Technologies Inc, Ferndale, USA). On the sample tree, the pulling line was anchored at the half of the height. To minimise the potential underestimation of the pulling force caused by the effects of wind and the canopy weight above the anchoring point of the sample tree, the sample trees were topped 1 m above the anchor point and pruned prior the test.
TreeQinetic System instruments (Argus electronic GmbH, Rostock, Germany) were used for the simultaneous measurements of the pulling force, stem inclination, and wood fibre deformation. A dynamometer was used for measuring the pulling force, and the angle of the pulling line was placed between the winch and the polyester roundsling. The stem inclination was measured at two heights (at the root collar and at 5 m) using inclinometers placed on the same side of the stem perpendicularly to the pulling direction. The wood fiber deformation of the stem was measured using a strain gauge on the compression side (facing the winch) at the height of 1 m from the root collar. This measurement was done from the beginning of the pulling test until the root collar inclination of 0.25° as bending moment at this threshold correlates well with anchorage [27,28].
2.3. Soil and Root Measurements
The soil water content was measured for each tree after the pulling test using an ML3 ThetaKit (Delta-T Devices Ltd., United Kingdom). For the uprooted trees, the largest (half of the width) and smallest (height) radius of the soil-root plate were measured from the centre of the stem to point where roots were damaged. The soil-root plate depth was measured from ground surface to depth of roots with diameter larger than 1 cm. The cross-sections of stumps, as well as the decay, were transferred to transparent films, and measured using a planimeter (Planix 10S; Tamaya, Japan) in the laboratory. The cross-section area of wood decay ranged from 75.7 to 1512.8 cm2.
2.4. Data Analysis
The basal bending moment (BBM) was calculated as follows:
where F is the pulling force, hanchor is the height of the anchor point on the sample tree, and medianα rope is the median of the rope angle. The stem curvature was expressed as the difference in the stem inclination measured at different heights (at the height of 5 m and at the base) as follows:
BBM = F · hanchor · cos(medianα rope)
NΔ = N5m − Nbase
During the static pulling, the stem curvature (NΔ,) and BBM increase proportionally until the point of irreversible wood fibre kinking on the compression side of the stem [29,30]. This point characterises the primary failure (BBMprim) after which NΔ increases faster than BBM. Secondary failure (BBMsec) occurs at the maximum loading as tree collapses.
The modulus of elasticity (MOE) was calculated according to a previous study [31]:
where BBM is the bending moment at the height of 1 m above the root collar, y is the radius of the stem section to the centre of the strain gauge, I is the area moment of inertia of the section, and e is the strain. The volume of the soil-root plate was calculated as the volume of an elliptical paraboloid as follows:
where a and b are the largest and smallest radii of the soil-root plate and h is the depth of the soil-root plate.
Considering the split-site study design, the effects of soil type (nested within site), root rot, and their interaction on force (BBM) necessary for the primary and secondary failures to occur were tested using the fractional analysis of variance (ANOVA) [32]. As trees of different size were analysed, BBM was expressed per tree size; tree height, DBH, stem volume, root rot cross-sectional area, soil-root plate volume, and tree height multiplied by the second power of DBH were tested as proxies for the tree size; stem volume was identified as the best performing one. Presence and cross-section area of decay on stump were tested as proxies for the root rot, among which presence (binomial variable) showed the best performance. The best performing proxies were selected according to the arbitrary principle considering residual variance as a criterion. Due to limited scope of the study, the total number of factors analysed was kept to a minimum. The statistical analysis was conducted in R software (v. 3.5.3) [33], using packages “tidyverse” [34] and “ez” [35]. The stem wood volume was calculated according to the local equation [36].
3. Results and Discussion
Uprooting was the most common type of failure as only nine out of 77 had stem fracture. The stem fracture occurred similarly in all groups according to root rot and soil types. Fractured trees tended to be smaller and were in dryer soil conditions (Table 2). However, a statistical analysis to describe the influencing factors for the failure type was not possible due to the limited number of the fractured trees.

Table 2.
Mean values and standard deviations of diameter at breast height (DBH), soil-root plate volume (Vsrp), soil moisture (SM), bending moment at primary (BBMprim) and secondary (BBMsec) failure, and modulus of elasticity (MOE) for all tested trees.
The presence of root rot had explicit negative effect on mechanical stability of Norway spruce, significantly reducing BBM both at the primary and secondary failures (p < 0.05; Table 3, Figure 1) on peat, as well as mineral soils. This indicated explicit increase in vulnerability to wind damage of root rot affected Norway spruce stands. The mean BBM (Table 3) in our study were lower than observed in an earlier study [37] implying regional differences in Norway spruce wind stability. The other terms tested, i.e., soil type and plot were not significant for BBMprim and BBMsec (Table 3).

Table 3.
Results of the fractional analysis of variance between bending moment at primary (BBMprim) and secondary (BBMsec) failure, soil type, the presence of root rot, and the interaction between soil type and the presence of root rot.
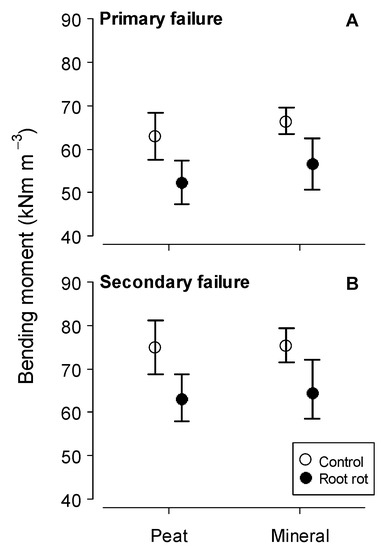
Figure 1.
Basal bending moment (BBM) of the Norway spruce at the primary failure (A) and at secondary failure (B) according to stem wood volume and root rot.
The hypothesis of the study was confirmed partly, as the soil-root anchorage was reduced by the presence of Heterobasidion spp. while the volume of the soil-root plate did not appear as the primary factor affecting changes in tree stability in relation to a slowly decomposing pathogen. This pathogen develops wood decay in roots and stem base [13]. This suggests that the uprooting of the affected trees was facilitated by the reduction of the mechanical strength of the lateral roots regardless of soil type.
The presence of root rot appeared to be a better proxy for the effect of root rot on tree stability than the area of rot on stem (according to the arbitrary selection principle), thus implying facilitated detection of the “weak” trees within a stand. As the trees affected and unaffected by root rot were tested simultaneously within each stand, the effect of the seasonal differences in the soil moisture [38] on our results was minimal. This is important as the linkage between increased soil moisture and tree stability is explained by reduced bearing capacity of soil [39,40,41]. Alternatively, considering Norway spruce as a water demanding species, the effect of soil moisture on root anchorage might be indirect via faster growth under moister soil conditions, hence wood strength has been decreased due to lower wood density [42].
Primary failure is the irreversible deformation of wood fibres as tangential sideways kinking occurs in the compression zone under loading [29]. Such damage affects tree hydraulics [43], triggering physiological drought stress [29,30], consequently subjecting storm-surviving trees to consecutive disturbances, such as pests or pathogens [2,3]. Thus, the reduced force needed to cause primary failure in moister soils could cause a potential underestimation of the negative legacy effect of storms. Reduced hydraulic conductivity caused by primary failure in stems might also contribute to pervasive growth reduction observed in Norway spruce forests after storms [44]. An additional storm legacy effect is caused by fracturing the roots, as it reduces the water uptake and facilitates the spread of root rot. A faster spread of root rot reduces the tree wind stability; thus, a negative feedback loop progressively decreases the sustainability of spruce stands in Northern Europe [45].
The cumulative probability of wind effects on Norway spruce stands could be reduced by the application of silvicultural measures, such as early identification and removal of infected trees, gradual lowering of the stand density, or shortening the length of the rotation period [2]. Also, the spread of Heterobasidion spp. could be slowed by reduced root contact in stands with lower density [13]. However, the decrease of stand density might facilitate effects of other disturbances, such as bark-stripping [46] and pests [3] that might increase the susceptibility to wind damage. Accordingly, a regional evaluation of silvicultural measures is necessary.
4. Conclusions
Under the increasing frequency of storms and related disturbances in the future, the reduction of mechanical stability will increase the cumulative probability of wind damage in Norway spruce stands across different soil types. Considering the importance of Norway spruce in Northern Europe, silvicultural measures, such as gradual lowering of the stand density or shortening the length of the rotation period, will become essential to ensure the long-term vitality and to decrease the vulnerability of Norway spruce stands to wind damage. Considering regional differences in mechanical strength, local evaluations of tree stability are necessary.
Author Contributions
Conceptualization, O.K. and A.J.; methodology, O.K., R.M., S.R.; formal analysis, D.E., data curation, L.K., N.B., L.B.; writing—original draft preparation, O.K.; writing—review and editing, R.M., S.R., A.J.; project administration, A.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by European Regional Development Fund grant number 1.1.1.1/16/A/260.
Acknowledgments
This study was funded by the European Regional Development Fund project Development of decision support tool for prognosis of storm damages in forest stands on peat soils (No. 1.1.1.1/16/A/260).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nabuurs, G.J.; Lindner, M.; Verkerk, P.J.; Gunia, K.; Deda, P.; Michalak, R.; Grassi, G. First sign of carbon sink saturation in European forest biomass. Nat. Clim. Change 2013, 3, 792–796. [Google Scholar] [CrossRef]
- Gardiner, B.; Schuck, A.R.T.; Schelhaas, M.J.; Orazio, C.; Blennow, K.; Nicoll, B. Living with Storm Damage to Forests; European Forest Institute: Joensuu, Finland, 2013; pp. 1–132. [Google Scholar]
- Seidl, R.; Rammer, W. Climate change amplifies the interactions between wind and bark beetle disturbances in forest landscapes. Landsc. Ecol. 2017, 32, 1485–1498. [Google Scholar] [CrossRef] [PubMed]
- Schelhaas, M.J.; Nabuurs, G.J.; Schuck, A. Natural disturbances in the European forests in the 19th and 20th centuries. Glob. Change Biol. 2003, 9, 1620–1633. [Google Scholar] [CrossRef]
- Laapas, M.; Lehtonen, I.; Venäläinen, A.; Peltola, H.M. The 10-Year Return Levels of Maximum Wind Speeds under Frozen and Unfrozen Soil Forest Conditions in Finland. Climate 2019, 7, 62. [Google Scholar] [CrossRef]
- Suvanto, S.; Henttonen, H.M.; Nöjd, P.; Mäkinen, H. Forest susceptibility to storm damage is affected by similar factors regardless of storm type: Comparison of thunder storms and autumn extra-tropical cyclones in Finland. For. Ecol. Manag. 2016, 381, 17–28. [Google Scholar] [CrossRef]
- Lindner, M.; Fitzgerald, J.B.; Zimmermann, N.E.; Reyer, C.; Delzon, S.; van der Maaten, E.; Schelhaas, M.J.; Lasch, P.; Eggers, J.; van der Maaten-Theunissen, M.; et al. Climate change and European forests: What do we know, what are the uncertainties, and what are the implications for forest management? J. Environ. Manag. 2014, 146, 69–83. [Google Scholar] [CrossRef]
- Suvanto, S.; Peltoniemi, M.; Tuominen, S.; Strandström, M.; Lehtonen, A. High-resolution mapping of forest vulnerability to wind for disturbance-aware forestry. For. Ecol. Manag. 2019, 453, 117619. [Google Scholar] [CrossRef]
- Díaz-Yáñez, O.; Mola-Yudego, B.; González-Olabarria, J.R.; Pukkala, T. How does forest composition and structure affect the stability against wind and snow? For. Ecol. Manag. 2017, 401, 215–222. [Google Scholar] [CrossRef]
- Honkaniemi, J.; Lehtonen, M.; Väisänen, H.; Peltola, H. Effects of wood decay by Heterobasidion annosum on the vulnerability of Norway spruce stands to wind damage: A mechanistic modelling approach. Can. J. For. Res. 2017, 47, 777–787. [Google Scholar] [CrossRef]
- Gardiner, B.A.; Stacey, G.R.; Belcher, R.E.; Wood, C.J. Field and wind tunnel assessments of the implications of respacing and thinning for tree stability. Forestry 1997, 70, 233–252. [Google Scholar] [CrossRef]
- Hale, S.E.; Gardiner, B.A.; Wellpott, A.; Nicoll, B.C.; Achim, A. Wind loading of trees: Influence of tree size and competition. Eur. J. For. Res. 2012, 131, 203–217. [Google Scholar] [CrossRef]
- Stenlid, J.; Redfern, D.B. Spread within the Tree and Stand. In Heterobasidion Annosum. Biology, Ecology, Impact and Control; Woodward, S., Stenlid, J., Karjalainen, R., Hüttemann, A., Eds.; CAB International: Wallingford, CT, USA, 1998; pp. 125–143. [Google Scholar]
- Zeng, H.; Pukkala, T.; Peltola, H. The use of heuristic optimisation in risk management of wind damage in forest planning. For. Ecol. Manag. 2007, 241, 189–199. [Google Scholar] [CrossRef]
- Heinonen, T.; Pukkala, T.; Ikonen, V.P.; Peltola, H.; Venäläinen, A.; Dupont, S. Integrating the risk of wind damage into forest planning. For. Ecol. Manag. 2009, 258, 1567–1577. [Google Scholar] [CrossRef]
- Heinonen, T.; Pukkala, T.; Ikonen, V.P.; Peltola, H.; Gregow, H.; Venäläinen, A. Consideration of strong winds, their directional distribution and snow loading in wind risk assessment related to landscape level forest planning. For. Ecol. Manag. 2011, 261, 710–719. [Google Scholar] [CrossRef]
- Pretzsch, H.; Biber, P.; Schütze, G.; Uhl, E.; Rötzer, T. Forest stand growth dynamics in Central Europe have accelerated since 1870. Nat. Commun. 2014, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Päivänen, J.; Hånell, B. Peatland Ecology and Forestry—A Sound Approach; Department of Forest Ecology, University of Helsinki: Helsinki, Finland, 2012; pp. 1–267. [Google Scholar]
- Albrecht, A.; Hanewinkel, M.; Bauhus, J.; Kohnle, U. How does silviculture affect storm damage in forests of south-western Germany? Results from empirical modeling based on long-term observations. Eur. J. For. Res. 2012, 131, 229–247. [Google Scholar] [CrossRef]
- Richter, C. Wood Characteristics: Description, Causes, Prevention, Impact on Use and Technological Adaptation. Springer Internationale Publishing: Basel, Switzerland, 2015; p. 222. [Google Scholar]
- Lodin, I. Choice of Tree Species in the Aftermath of Two Major Storms—A Qualitative Study of Private Forest Owners in Southern Sweden. Master’s Thesis, Swedish University of Agricultural Sciences, Alnarp, Sweden, January 2016. [Google Scholar]
- Woodward, S.; Stenlid, J.; Karjalainen, R.; Hüttemann, A. Heterobasidion Annosum. Biology, Ecology, Impact and Control; CAB International: Wallingford, CT, USA, 1998; p. 589. [Google Scholar]
- Nicoll, B.C.; Gardiner, B.A.; Rayner, B.; Peace, A.J. Anchorage of coniferous trees in relation to species, soil type, and rooting depth. Can. J. For. Res. 2006, 36, 1871–1883. [Google Scholar] [CrossRef]
- Lundström, T.; Jonas, T.; Stöckli, S.; Ammann, W. Anchorage of mature conifers: Resistive turning moment, root–soil plate geometry and root growth orientation. Tree Physiol. 2007, 27, 1217–1227. [Google Scholar]
- Bergeron, C.; Ruel, J.C.; Èlie, J.G.; Mitchell, S.J. Root anchorage and stem strength of black spruce (Picea mariana) trees in regular and irregular stands. Forestry 2009, 82, 29–41. [Google Scholar] [CrossRef]
- Giordano, L.; Lione, G.; Nicolotti, G.; Gonthier, P. Effect of Heterobasidion annosum s.l. root and butt rots on the stability of Norway spruce: An. uprooting test. In Proceedings of the XIII International Conference on Root and Butt Root of Forest Trees, Firenze (FI), S. Martino di Castrozza (TN), Italy, 4–10 September 2012; Capretti, P., Comparini, P., Garbelotto, M., La Porta, N., Santini, A., Eds.; University Press: Firenze, Italy, 2012; pp. 247–250. [Google Scholar]
- Göcke, L.; Rust, S.; Ruhl, F. Assessing the Anchorage and Critical Wind Speed of Urban Trees using Root Plate Inclination in high Winds. Arboric. Urban. For. 2018, 44, 1–11. [Google Scholar]
- Detter, A.; van Wassenaer, P.; Rust, S. Stability recovery in London Plane trees 8 years after primary anchorage failure. Arboric. Urban. For. 2019, 45, 279–288. [Google Scholar]
- Detter, A.; Richter, K.; Rust, C.; Rust, S. Aktuelle Untersuchungen zum Primärversagen von grünem Holz-Current studies on primary failure in green wood. In Proceedings of the Conference Deutsche Baumpflegetage, Augsburg, Germany, 5–7 May 2015; pp. 156–167. [Google Scholar]
- Detter, A.; Rust, S.; Rust, C.; Maybaum, G. Determining strength limits for standing tree stems from bending tests. In Proceedings of the 18th International Nondestructive Testing and Evaluation of Wood Symposium, Madison, WI, USA, 4–27 September 2013; Ross, R.J., Wang, X., Eds.; U.S. Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2013; p. 226. [Google Scholar]
- Rogers, M.; Casey, A.; McMenamin, C. An experimental investigation of the effects of dynamic loading on coniferous trees planted on wet mineral soils. In Wind and Trees; Coutts, M.P., Grace, J., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 204–219. [Google Scholar]
- Bakeman, R. Recommended effect size statistics for repeated measures designs. Behav. Res. Methods 2005, 37, 379–384. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 5 December 2019).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Lawrence, M.A. EZ: Easy analysis and visualization of factorial experiments [Software]. 2011. (R package version 3.0-0). Available online: https://CRAN.R-project.org/package=ez (accessed on 2 November 2016).
- Liepa, I. Pieauguma mācība; [Increment theory]; Latvia University of Agriculture: Jelgava, Latvia, 1996; p. 123. [Google Scholar]
- Peltola, H.; Kellomäki, S.; Hassinen, A.; Granander, M. Mechanical stability of Scots pine, Norway spruce and birch: An analysis of tree-pulling experiments in Finland. For. Ecol. Manag. 2000, 135, 143–153. [Google Scholar] [CrossRef]
- Orth, R.; Seneviratne, S.I. Analysis of soil moisture memory from observations in Europe. J. Geophys. Res. 2012, 117, D15115. [Google Scholar] [CrossRef]
- Ray, D.; Nicoll, B.C. The effect of soil water-table depth on root-plate development and stability of Sitka spruce. Forestry 1998, 71, 169–182. [Google Scholar] [CrossRef]
- Kamimura, K.; Kitagawa, K.; Saito, S.; Mizunaga, H. Root anchorage of hinoki (Chamaecyparis obtuse (Sieb. Et Zucc.) Endl.) under the combined loading of wind and rapidly supplied water on soil: Analyses based on tree-pulling experiments. Eur J. Forest Res. 2012, 131, 219–227. [Google Scholar] [CrossRef]
- Detter, A.; Rust, S.; Böttcher, J.; Bouillon, J. Ambient influences on the results of non-destructive pulling tests. In Proceedings of the 21st International Nondestructive Testing and Evaluation of Wood Symposium, Freiburg, Germany, 24–27 September 2019; Forest Products Laboratory, General Technical Report: Madison, WI, USA, 2019. [Google Scholar]
- Pollet, C.; Henin, J.M.; Hébert, J.; Jourez, B. Effect of growth rate on the physical and mechanical properties of Douglas-fir in western Europe. Can. J. For. Res. 2017, 47, 1056–1065. [Google Scholar] [CrossRef]
- Mayr, S.; Bertel, C.; Dämon, B.; Beikircher, B. Static and dynamic bending has minor effects on xylem hydraulics of conifer branches (Picea abies, Pinus sylvestris). Plant. Cell Environ. 2014, 37, 2151–2157. [Google Scholar] [CrossRef]
- Seidl, R.; Blennow, K. Pervasive growth reduction in Norway Spruce forests following wind disturbance. PLoS ONE 2012, 7, e33301. [Google Scholar] [CrossRef]
- Honkaniemi, J.; Ojansuu, R.; Kasanen, R.; Heliövaara, K. Interaction of disturbance agents on Norway spruce: A mechanistic model of bark beetle dynamics integrated in simulation framework WINDROT. Ecol. Model. 2018, 388, 45–60. [Google Scholar] [CrossRef]
- Krisans, O.; Saleniece, R.; Rust, S.; Elferts, D.; Kapostins, R.; Jansons, A.; Matisons, R. Effect of Bark-Stripping on Mechanical Stability of Norway Spruce. Forests 2020, 11, 357. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).