Small-Scale Abiotic Factors Influencing the Spatial Distribution of Phytophthora cinnamomi under Declining Quercus ilex Trees
Abstract
1. Introduction
2. Material and Methods
2.1. Study Zone
2.2. Soil Sampling Design
2.3. Processing and Analysis of Soil Samples
2.4. Quantification of Cfu
2.5. Statistical Analysis
3. Results
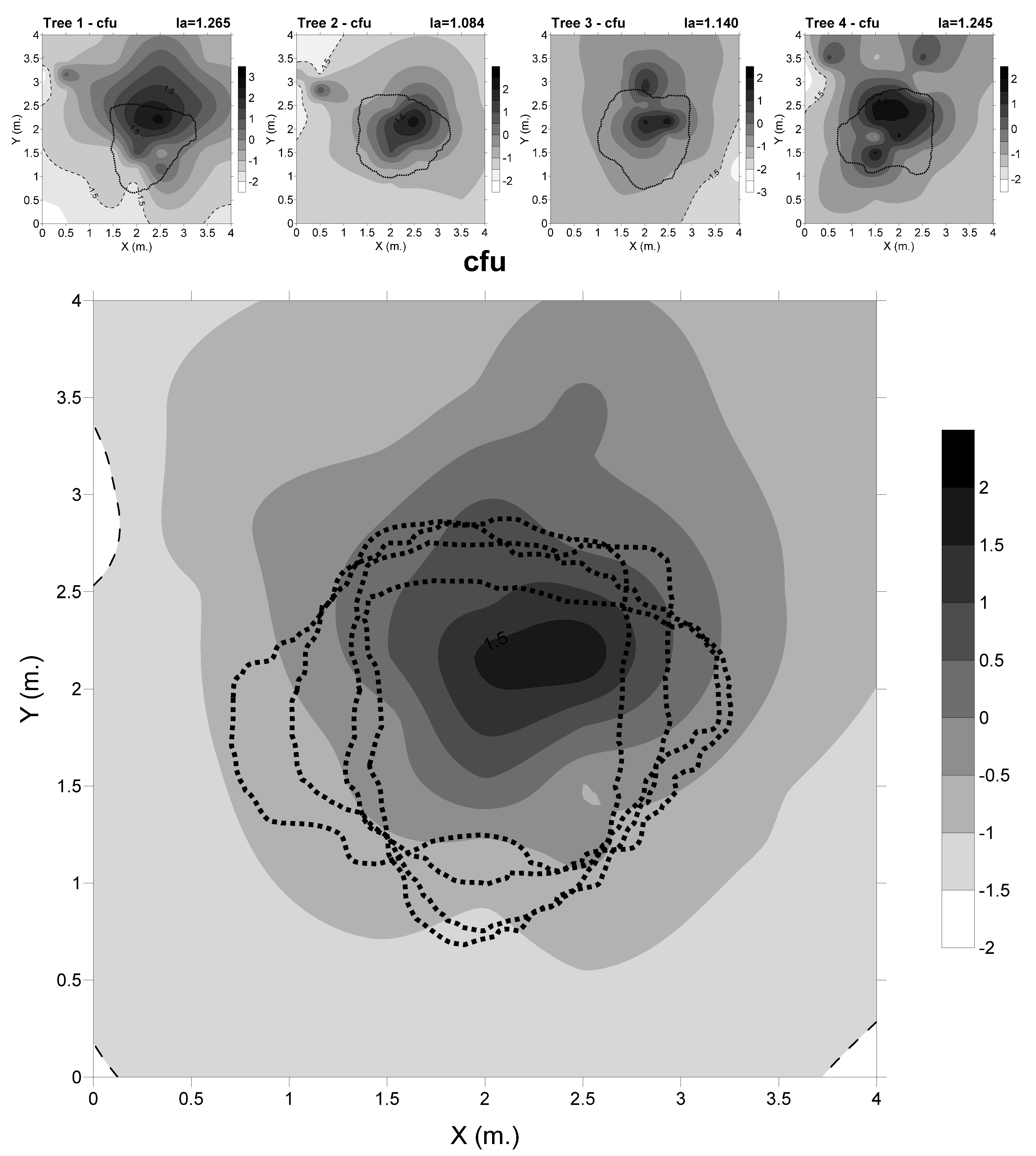
3.1. Spatial Distribution of the Cfu
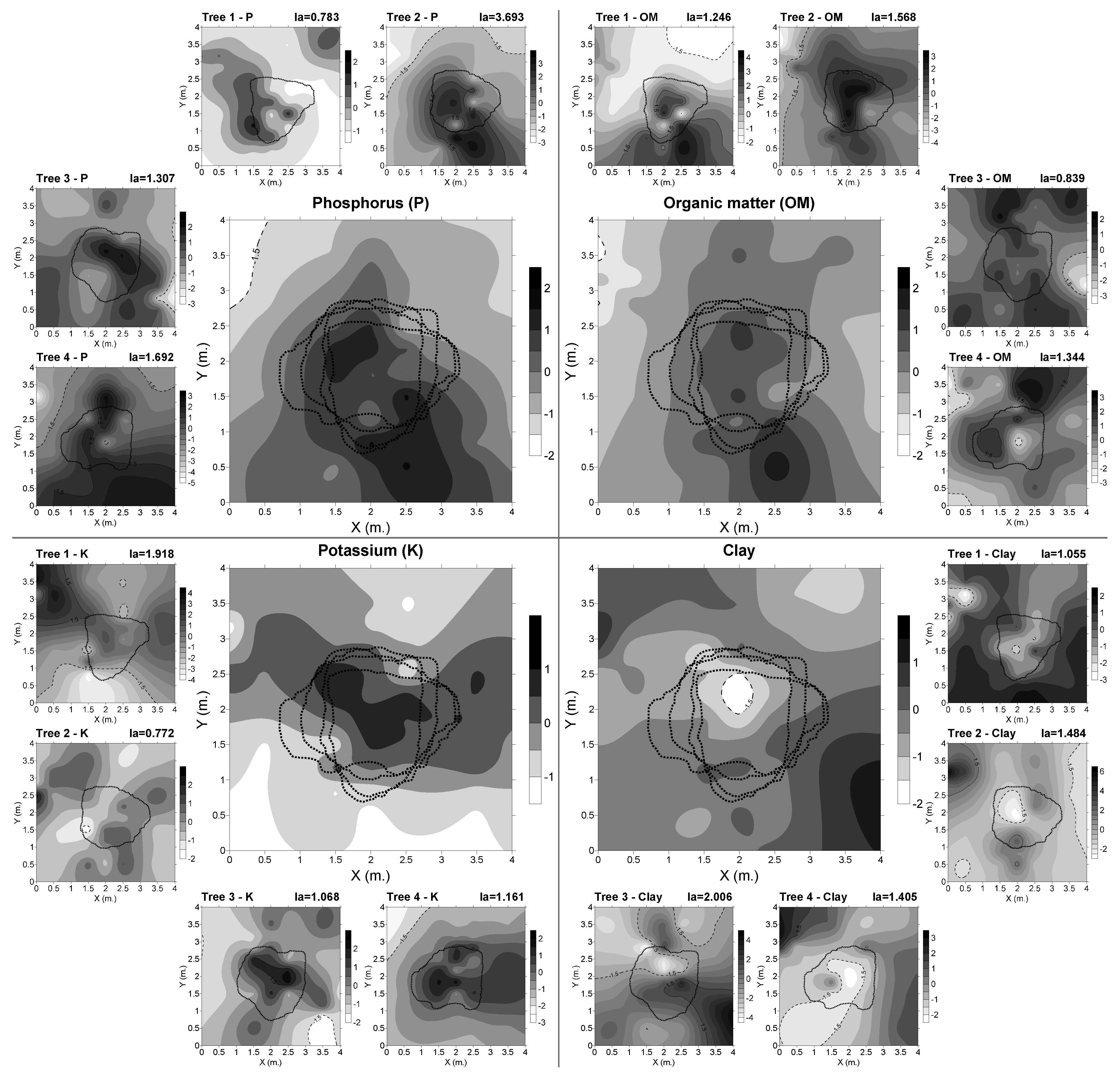
3.2. Spatial Distribution of Edaphic Variables
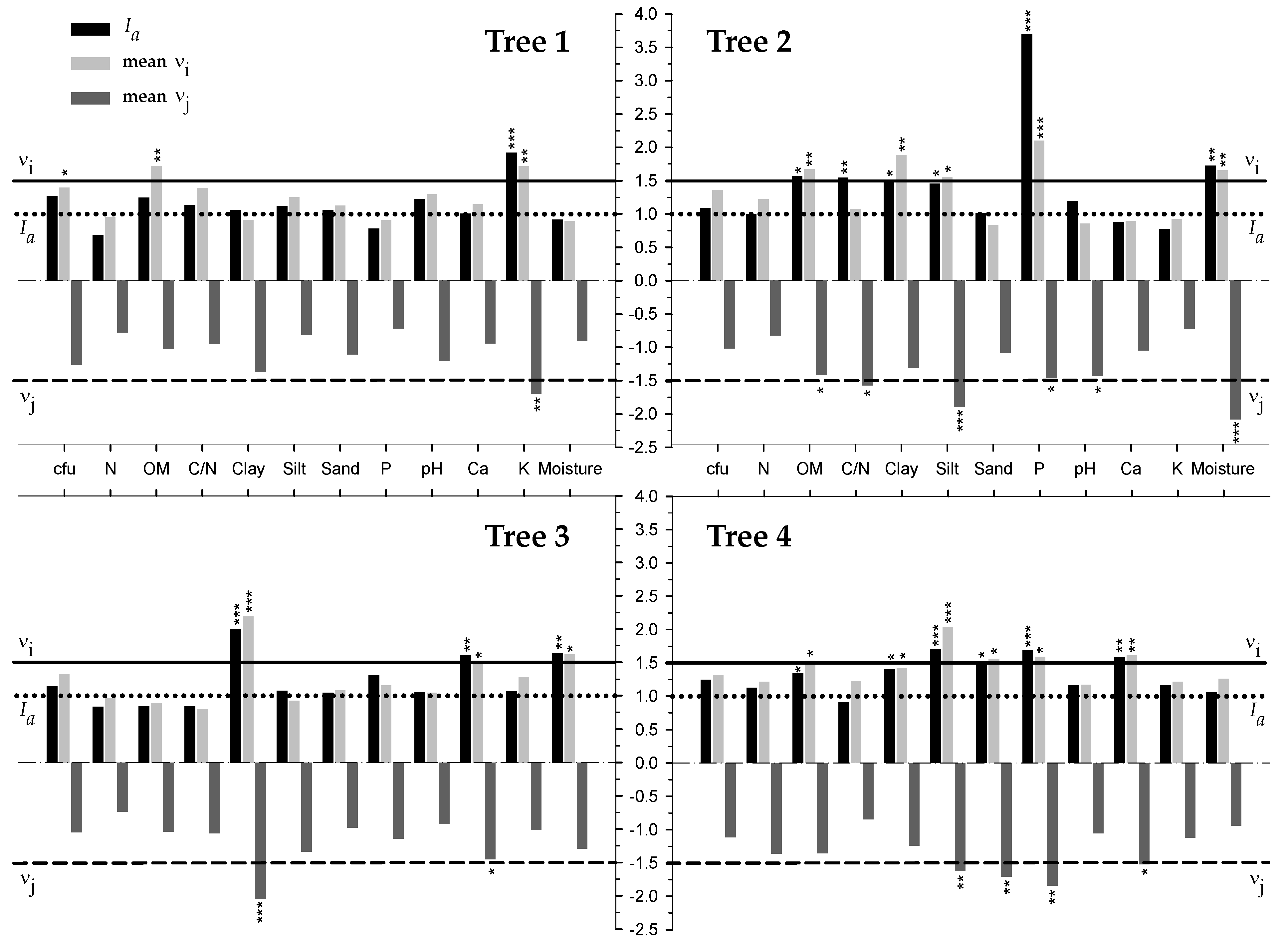
3.3. Spatial Analysis and Location of Edaphic Variables and Relationship with Cfu
3.4. Generalized Linear Mixed Model for Cfu
4. Discussion
4.1. The Abundance of Cfu and Soil Parameters are Influenced by the Canopy Cover
4.2. Concentrarion of Cfu was Influenced by the Spatial Distribution of Edaphic Variables
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiménez, J.J.; Serrano, M.S.; Vicente, M.; Fernández, P.; Trapero Casas, A.; Sánchez Hernández, E. Nuevas especies de Pythium que causan podredumbre radical de Quercus en España y Portugal. Bol. Sanid. Veg. Plagas 2008, 34, 549–562. [Google Scholar]
- Camilo-Alves, C.d.S.e.P.; Clara, M.I.E.d.; Ribeiro, N.M.C.d.A. Decline of Mediterranean oak trees and its association with Phytophthora cinnamomi: A review. Eur. J. For. Res. 2013, 132, 411–432. [Google Scholar] [CrossRef]
- Ruiz-Gómez, F.J.; Navarro Cerrillo, R.M.; Lara Gómez, M.A.; Sánchez-Cuesta, R. Aislamiento e identificación de oomicetos en focos de podredumbre radical de Andalucía y Extremadura. Cuad. Soc. Esp. Cienc. For. 2016, 43, 363–376. [Google Scholar] [CrossRef]
- González-Alonso, C. Analysis of the Oak Decline in Spain-La Seca. Bachelor’s Thesis, Forest Management, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2008. [Google Scholar]
- Vettraino, A.M.; Barzanti, G.P.; Bianco, M.C.; Ragazzi, A.; Capretti, P.; Paoletti, E.; Luisi, N.; Anselmi, N.; Vannini, A. Occurrence of Phytophthora species in oak stands in Italy and their association with declining oak trees. For. Pathol. 2002, 32, 19–28. [Google Scholar] [CrossRef]
- Pérez-Sierra, A.; López-García, C.; León, M.; García-Jiménez, J.; Abad-Campos, P.; Jung, T. Previously unrecorded low-temperature Phytophthora species associated with Quercus decline in a Mediterranean forest in eastern Spain. For. Pathol. 2013, 43, 331–339. [Google Scholar] [CrossRef]
- Navarro Cerrillo, R.M.; Fernández Rebollo, P.; Trapero, A.; Caetano, P.; Romero, M.A.; Sánchez, M.E.; Fernández Cancio, A.; Sánchez, I.; López Pantoja, G. Los Procesos de Decaimiento de Encinas y Alcornoques; Dirección General de Gestión del Medio Natural. Consejería de Medio Ambiente; Junta de Andalucía: Sevilla, Spain, 2004; ISBN 84-95785-89-7. [Google Scholar]
- Trapero, A.; Sánchez, M.E.; Pérez de Algaba, A.; Romero, M.A.; Navarro, N.; Varo, R.; Gutiérrez, J. Enfermedades de especies forestales en Andalucía. Agricultura 2000, 821, 822–824. [Google Scholar]
- Oßwald, W.; Fleischmann, F.; Rigling, D.; Coelho, A.C.; Cravador, A.; Diez, J.; Dalio, R.J.; Horta Jung, M.; Pfanz, H.; Robin, C.; et al. Strategies of attack and defence in woody plant—Phytophthora interactions. For. Pathol. 2014, 44, 169–190. [Google Scholar] [CrossRef]
- Ruiz Gómez, F.; Pérez-de-Luque, A.; Sánchez-Cuesta, R.; Quero, J.; Navarro Cerrillo, R. Differences in the response to acute drought and Phytophthora cinnamomi Rands Infection in Quercus ilex L. seedlings. Forests 2018, 9, 634. [Google Scholar] [CrossRef]
- Jung, T.; Orlikowski, L.; Henricot, B.; Abad-Campos, P.; Aday, A.G.; Aguín Casal, O.; Bakonyi, J.; Cacciola, S.O.; Cech, T.; Chavarriaga, D.; et al. Widespread Phytophthora infestations in European nurseries put forest, semi-natural and horticultural ecosystems at high risk of Phytophthora diseases. For. Pathol. 2016, 46, 134–163. [Google Scholar] [CrossRef]
- Marais, L.J.; Menge, J.A.; Bender, G.S.; Faber, B. Phytophthora Root Rot; AvoResearch California Avocado Commission Publishing: Irvine, CA, USA, 2002; Volume 2, pp. 3–6. [Google Scholar]
- Zamora Rojas, E.; Andicoberry de los reyes, S.; Sánchez Clemente, M.E. Anexo A.1. IV. El decaimiento y la podredumbre radical en las dehesas andaluzas. In Ecosistemas de Dehesa: Desarrollo de Políticas y Herramientas Para la Gestión y Conservación de la Biodiversidad (Life Biodehesa Project); Consejería de Medio Ambiente y Ordenación del Territorio: Sevilla, Spain, 2014. [Google Scholar]
- Hüberli, D.; Tommerup, I.C.; Hardy, G.E.S.J. False-negative isolations or absence of lesions may cause mis-diagnosis of diseased plants infected with Phytophthora cinnamomi. Australas. Plant Pathol. 2000, 29, 164–169. [Google Scholar] [CrossRef]
- Ashton, M.S.; Larson, B.C. Germination and seedling growth of Quercus (section Erythrobalanus) across openings in a mixed-deciduous forest of southern New England, USA. For. Ecol. Manag. 1996, 80, 81–94. [Google Scholar] [CrossRef]
- Mauer, O.; Houškova, K.; Mikita, T. The root system of pedunculate oak (Quercus robur L.) at the margins of regenerated stands. J. For. Sci. 2017, 63, 22–33. [Google Scholar]
- Aponte, C.; Matías, L.; González-Rodríguez, V.; Castro, J.; García, L.V.; Villar, R.; Marañón, T. Soil nutrients and microbial biomass in three contrasting Mediterranean forests. Plant Soil 2014, 380, 57–72. [Google Scholar] [CrossRef]
- Carranca, C.; Castro, I.V.; Figueiredo, N.; Redondo, R.; Rodrigues, A.R.F.; Saraiva, I.; Maricato, R.; Madeira, M.A.V. Influence of tree canopy on N2 fixation by pasture legumes and soil rhizobial abundance in Mediterranean oak woodlands. Sci. Total Environ. 2015, 506–507, 86–94. [Google Scholar] [CrossRef]
- Ruiz Gómez, F.J.R.; Navarro-Cerrillo, R.M.; Pérez-de-Luque, A.; Oβwald, W.; Vannini, A.; Morales-Rodríguez, C. Assessment of functional and structural changes of soil fungal and oomycete communities in holm oak declined dehesas through metabarcoding analysis. Sci. Rep. 2019, 9, 5315. [Google Scholar] [CrossRef]
- Gallardo, A. Effect of tree canopy on the spatial distribution of soil nutrients in a Mediterranean Dehesa. Pedobiologia 2003, 47, 117–125. [Google Scholar] [CrossRef]
- Cubera, E.; Moreno, G. Effect of land-use on soil water dynamic in dehesas of Central–Western Spain. Catena 2007, 71, 298–308. [Google Scholar] [CrossRef]
- Quero, J.L.; Maestre, F.T.; Ochoa, V.; García-Gómez, M.; Delgado-Baquerizo, M. On the importance of shrub encroachment by sprouters, climate, species richness and anthropic factors for ecosystem multifunctionality in semi-arid Mediterranean ecosystems. Ecosystems 2013, 16, 1248–1261. [Google Scholar] [CrossRef]
- Andivia, E.; Fernández, M.; Alejano, R.; Vázquez-Piqué, J. Tree patch distribution drives spatial heterogeneity of soil traits in cork oak woodlands. Ann. For. Sci. 2015, 72, 549–559. [Google Scholar] [CrossRef]
- Cappai, C.; Kemanian, A.R.; Lagomarsino, A.; Roggero, P.P.; Lai, R.; Agnelli, A.E.; Seddaiu, G. Small-scale spatial variation of soil organic matter pools generated by cork oak trees in Mediterranean agro-silvo-pastoral systems. Geoderma 2017, 304, 59–67. [Google Scholar] [CrossRef]
- Gómez-Aparicio, L.; Ibáñez, B.; Serrano, M.S.; De Vita, P.; Ávila, J.M.; Pérez-Ramos, I.M.; García, L.V.; Sánchez, M.E.; Marañón, T. Spatial patterns of soil pathogens in declining Mediterranean forests: Implications for tree species regeneration. New Phytol. 2012, 194, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, B.; Gómez-Aparicio, L.; Ávila, J.M.; Pérez-Ramos, I.M.; García, L.V.; Marañón, T. Impact of tree decline on spatial patterns of seedling-mycorrhiza interactions: Implications for regeneration dynamics in Mediterranean forests. For. Ecol. Manag. 2015, 353, 1–9. [Google Scholar] [CrossRef]
- Romero, M.A.; Sánchez, J.E.; Jiménez, J.J.; Belbahri, L.; Trapero, A.; Lefort, F.; Sánchez, M.E. New Pythium taxa causing root rot on Mediterranean Quercus species in South-West Spain and Portugal. J. Phytopathol. 2007, 155, 289–295. [Google Scholar] [CrossRef]
- Serrano, M.S.; De Vita, P.; Fernández-Rebollo, P.; Sánchez-Hernández, M.E. Calcium fertilizers induce soil suppressiveness to Phytophthora cinnamomi root rot of Quercus ilex. Eur. J. Plant Pathol. 2012, 132, 271–279. [Google Scholar] [CrossRef]
- Vadell, E.; de-Miguel, S.; Pemán, J. Large-scale reforestation and afforestation policy in Spain: A historical review of its underlying ecological, socioeconomic and political dynamics. Land Use Policy 2016, 55, 37–48. [Google Scholar] [CrossRef]
- Lehtijärvi, A.; Aday Kaya, A.G.; Woodward, S.; Jung, T.; Doğmuş Lehtijärvi, H.T. Oomycota species associated with deciduous and coniferous seedlings in forest tree nurseries of Western Turkey. For. Pathol. 2017, 47, e12363. [Google Scholar] [CrossRef]
- Burgess, T.I.; Scott, J.K.; Mcdougall, K.L.; Stukely, M.J.C.; Crane, C.; Dunstan, W.A.; Brigg, F.; Andjic, V.; White, D.; Rudman, T.; et al. Current and projected global distribution of Phytophthora cinnamomi, one of the world’s worst plant pathogens. Glob. Chang. Biol. 2017, 23, 1661–1674. [Google Scholar] [CrossRef]
- Eichhorn, J.; Roskams, P.; Potočić, N.; Timmermann, V.; Ferretti, M.; Mues, V.; Szepesi, A.; Durrant, D.; Seletković, I.; Schroeck, H.-W.; et al. Part IV: Visual assessment of crown condition and damaging agents. In UNECE ICP Forests Programme Co-ordinating Centre. Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2017; p. 54. ISBN 978-3-86576-162-0. [Google Scholar]
- Jeffers, S.N.; Martin, S.B. Comparison of two media selective for Phytophthora and Pythium species. Plant Dis. 1986, 70, 1038–1043. [Google Scholar] [CrossRef]
- Rohlf, F.J.; Sokal, R.R. Statistical Tables; W. H. Freeman: San Francisco, CA, USA, 1981. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Quero, J.L. La heterogeneidad en ecología: Herramientas de cuantificación y aplicaciones para la restauración. Acta Granatense 2006, 4, 107–114. [Google Scholar]
- Winder, L.; Alexander, C.; Griffiths, G.; Holland, J.; Woolley, C.; Perry, J. Twenty years and counting with SADIE: Spatial Analysis by Distance Indices software and review of its adoption and use. Rethink. Ecol. 2019, 4, 1–16. [Google Scholar] [CrossRef]
- Perry, J.N.; Dixon, P.M. A new method to measure spatial association for ecological count data. Écoscience 2002, 9, 133–141. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models using lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Schielzeth, H. Simple means to improve the interpretability of regression coefficients: Interpretation of regression coefficients. Methods Ecol. Evol. 2010, 1, 103–113. [Google Scholar] [CrossRef]
- Gea-Izquierdo, G.; Allen-Díaz, B.; San Miguel, A.; Canellas, I. How do trees affect spatio-temporal heterogeneity of nutrient cycling in Mediterranean annual grasslands? Ann. For. Sci. 2010, 67, 112. [Google Scholar] [CrossRef][Green Version]
- Simón, N.; Montes, F.; Díaz-Pinés, E.; Benavides, R.; Roig, S.; Rubio, A. Spatial distribution of the soil organic carbon pool in a Holm oak dehesa in Spain. Plant Soil 2013, 366, 537–549. [Google Scholar] [CrossRef]
- Sánchez, M.; Caetano, P.; Ferraz, J.; Trapero, A. Phytophthora disease of Quercus ilex in south-western Spain. For. Pathol. 2002, 32, 5–18. [Google Scholar] [CrossRef]
- González, M.; Romero, M.; Ramo, C.; Serrano, M.S.; Sánchez, M.E. Control of Phytophthora Root Rot on Mediteranean Quercus spp. Using Fosetyl-Al Trunk Injections. M; Firenze University Press: Firenze, Italy, 2017. [Google Scholar]
- Navarro-Cerrillo, R.M.; Varo-Martínez, M.Á.; Acosta, C.; Palacios Rodriguez, G.; Sánchez-Cuesta, R.; Ruiz Gómez, F.J. Integration of WorldView-2 and airborne laser scanning data to classify defoliation levels in Quercus ilex L. dehesas affected by root rot mortality: Management implications. For. Ecol. Manag. 2019, 451, 117564. [Google Scholar] [CrossRef]
- Maestre, F.T.; Quero, J.L. Análisis espacial mediante índices de distancia (SADIE). In Introducción al Análisis Espacial de Datos en Ecología y Ciencias Ambientales: Métodos y Aplicaciones; Universidad Rey Juan Carlos: Madrid, Spain, 2008; pp. 637–648. ISBN 978-84-9849-308-5. [Google Scholar]
- Sánchez, M.E.; Caetano, P.; Ferranz, J.; Trapero Casas, A. El decaimiento y muerte de encinas en tres dehesas de la provincia de Huelva. Bol. Sanid. Veg. Plagas 2000, 26, 447–464. [Google Scholar]
- Rodríguez, M.; Sánchez, M.E.; Trapero, A. Desarrollo de un método eficaz para la cuantificación de Phytophthora cinnamomi en muestras de suelo. In Proceedings of the XII Congreso de la Sociedad Española de Fitopatología, Girona, Spain, 26 September–1 October 2004. [Google Scholar]
- Serrano, M.S.; Leal, R.; Vita, P.D.; Fernández-Rebollo, P.; Sánchez, M.E. Control of Phytophthora cinnamomi by soil application of calcium fertilizers in a Dehesa ecosystem in Spain. Integr. Prot. Oak For. IOBC-WPRS Bull. 2014, 101, 139–143. [Google Scholar]
- Canadell, J.; Rodà, F. Root biomass of Quercus ilex in a montane Mediterranean forest. Can. J. For. Res. 1991, 21, 1771–1778. [Google Scholar] [CrossRef][Green Version]
- Silva, J.S.; Rego, F.C. Root to shoot relationships in Mediterranean woody plants from Central Portugal. Biologia (Bratislava) 2004, 59, 109–115. [Google Scholar]
- Lopez, B.; Sabate, S.; Gracia, C. Fine roots dynamics in a Mediterranean forest: Effects of drought and stem density. Tree Physiol. 1998, 18, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, D.J.; Bowker, M.A.; Maestre, F.T.; Roger, E.; Reynolds, J.F.; Whitford, W.G. Impacts of shrub encroachment on ecosystem structure and functioning: Towards a global synthesis. Ecol. Lett. 2011, 14, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Corcobado, T. Influencia de Phytophthora cinnamomi Rands en el Decaimiento de Quercus ilex L. y su Relación con las Propiedades del Suelo y las Ectomicorrizas. Ph.D. Thesis, Universidad de Extremadura, Plasencia, Spain, 2013. [Google Scholar]
- García Moreno, A.M.; Fernández Rebollo, P.; Ortiz Berrocal, F.; Carbonero Muñoz, M.D. Podredumbre Radical, Descripción y Control Aplicado a Los Ecosistemas de Dehesa; Instituto de Investigación y Formación Agraria y Pesquera, Consejería de Agricultura, Pesca y Desarrollo Rural, Junta de Andalucía: Córdoba, Spain, 2016. [Google Scholar]
- Moreira, A.C.; Martins, J.M.S. Influence of site factors on the impact of Phytophthora cinnamomi in cork oak stands in Portugal. For. Pathol. 2005, 35, 145–162. [Google Scholar] [CrossRef]
- Satyaprakash, M.; Nikitha, T.; Reddi, E.U.B.; Sadhana, B.; Vani, S.S. Phosphorous and phosphate solubilising bacteria and their role in plant nutrition. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2133–2144. [Google Scholar]
- Rodà, F.; Retana, J.; Gracia, C.A.; Bellot, J. Ecology of Mediterranean Evergreen Oak Forests; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1999; Volume 137, ISBN 3-540-65019-9. [Google Scholar]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Plant-soil interactions in Mediterranean forest and shrublands: Impacts of climatic change. Plant Soil 2013, 365, 1–33. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Gallardo, A.; Bowker, M.A.; Wallenstein, M.D.; Quero, J.L.; Ochoa, V.; Gozalo, B.; García-Gómez, M.; Soliveres, S.; et al. Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature 2013, 502, 672–676. [Google Scholar] [CrossRef]
- Aber, J.A.; Melillo, J.M. Terrestrial Ecosystems, 2nd ed.; Brooks/Cole Publishing: Pacific Grove, CA, USA, 2001; ISBN 978-0-12-041755-1. [Google Scholar]
- Brown, R.B. Soil Texture; Soil and Water Science Department, Florida Cooperative Extension Service, Institute of Food and Agriculture Sciences, University of Florida: Gainesville, FL, USA, 2003. [Google Scholar]
- Serrano, M.S.; Fernández-Rebollo, P.; De Vita, P.; Sánchez, M.E. Calcium mineral nutrition increases the tolerance of Quercus ilex to Phytophthora root disease affecting oak rangeland ecosystems in Spain. Agrofor. Syst. 2013, 87, 173–179. [Google Scholar] [CrossRef]
- Zentmyer, G.A. Phytophthora Cinnamomi and the Diseases It Causes; Monograph, American Phytopathological Society: Riverside, CA, USA, 1980; ISBN 0-89054-030-6. [Google Scholar]
- Gallardo, A.; Rodríguez-Saucedo, J.J.; Covelo, F.; Fernández-Alés, R. Soil nitrogen heterogeneity in a Dehesa ecosystem. Plant Soil 2000, 222, 71–82. [Google Scholar] [CrossRef]
- Hardham, A.R. Phytophthora cinnamomi. Mol. Plant Pathol. 2005, 6, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Cerri, M.; Sapkota, R.; Coppi, A.; Ferri, V.; Foggi, B.; Gigante, D.; Lastrucci, L.; Selvaggi, R.; Venanzoni, R.; Nicolaisen, M.; et al. Oomycete communities associated with reed die-back syndrome. Front. Plant Sci. 2017, 8, 1550. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, W.A.; Rudman, T.; Shearer, B.L.; Moore, N.A.; Paap, T.; Calver, M.C.; Dell, B.; Hardy, G.E.S.J. Containment and spot eradication of a highly destructive, invasive plant pathogen (Phytophthora cinnamomi) in natural ecosystems. Biol. Invasions 2010, 12, 913–925. [Google Scholar] [CrossRef]
- Garrido Valero, M.S. Interpretación de Análisis de Suelos; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 1994; ISBN 84-341-0810-0. [Google Scholar]
- Tecon, R.; Or, D. Biophysical processes supporting the diversity of microbial life in soil. FEMS Microbiol. Rev. 2017, 41, 599–623. [Google Scholar] [CrossRef]
- Jeon, S.; Krasnow, C.S.; Kirby, C.K.; Granke, L.L.; Hausbeck, M.K.; Zhang, W. Transport and retention of Phytophthora capsici zoospores in saturated porous media. Environ. Sci. Technol. 2016, 50, 9270–9278. [Google Scholar] [CrossRef]

| Position Respect to Crown Cover | ||||||
|---|---|---|---|---|---|---|
| Tree | Mean cfu n = 33 | I n = 8 | T n = 8 | O n = 8 | IC n = 11 | OC n = 22 |
| 1 | 12 ± 3 | 22 ± 5 a | 6 ± 4 b | 5 ± 2 b | 23 ± 7 a | 6 ± 2 b |
| 2 | 13 ± 5 | 34 ± 13 a | 4 ± 3 b | 3 ± 2 b | 26 ± 10 a | 7 ± 6 b |
| 3 | 19 ± 8 | 38 ± 20 a | 39 ± 26 a | 0 ± 0 b | 44 ± 21 a | 5 ± 4 b |
| 4 | 44 ± 10 | 119 ± 26 a | 49 ± 11 b | 16 ± 6 c | 100 ± 22 a | 16 ± 4 b |
| Mean | 22 ± 4 | 53 ± 11 a | 24 ± 8 b | 6 ± 2 b | 48 ± 9 a | 9 ± 2 b |
| Position with Respect to Crown Cover | ||||||
|---|---|---|---|---|---|---|
| Variable | I n = 32 | T n = 32 | O n = 32 | IC n = 44 | OC n = 88 | Corr. coef. (ρ) |
| N (%) | 0.19 ± 0.01 a | 0.18 ± 0.01 a | 0.18 ± 0.01 a | 0.19 ± 0.01 a | 0.17 ± 0.01 a | −0.045 |
| OM (%) | 1.91 ± 0.11 b | 1.47 ± 0.12 b | 1.77 ± 0.11 a | 1.78 ± 0.08 a | 1.61 ± 0.07 a | −0.008 |
| C/N | 6.67 ± 0.52 a | 5.13 ± 0.49 a | 6.25 ± 0.54 a | 6.09 ± 0.39 a | 5.98 ± 0.36 a | 0.020 |
| Clay † (%) | 27.17 ± 1.01 b | 28.21 ± 0.90 a,b | 26.85 ± 1.33 a | 25.43 ± 0.84 a,* | 28.66 ± 0.67 b,* | −0.398 *** |
| Silt (%) | 37.28 ± 1.36 a | 38.86 ± 1.16 a | 37.68 ± 1.28 a | 38.49 ± 1.23 a | 37.25 ± 0.69 a | −0.026 |
| Sand (%) | 35.54 ± 2.03 a | 32.93 ± 1.34 a | 35.48 ± 2.17 a | 36.08 ± 1.72 a | 34.09 ± 1.07 a | 0.242 ** |
| P † (mg/kg) | 32.45 ± 9.05 b | 7.41 ± 3.38 b | 19.58 ± 6.94 a | 27.07 ± 6.55 a,* | 12.19 ± 3.31 b,* | 0.109 |
| pH | 5.13 ± 0.06 a | 5.17 ± 0.04 a | 5.20 ± 0.05 a | 5.13 ± 0.04 a | 5.19 ± 0.03 a | 0.319 *** |
| Ca (meq/100 g) | 0.70 ± 0.04 a | 0.59 ± 0.03 a | 0.73 ± 0.05 a | 0.64 ± 0.03 a | 0.67 ± 0.03 a | 0.374 *** |
| K † (meq/100 g) | 0.50 ± 0.13 a | 0.20 ± 0.02 a | 0.22 ± 0.05 a | 0.41 ± 0.09 a,* | 0.19 ± 0.02 b,* | 0.352 *** |
| Moisture † (%) | 15.04 ± 1.24 a | 15.75 ± 0.65 a | 15.24 ± 0.77 a | 15.25 ± 0.84 a | 15.30 ± 0.46 a | 0.066 |
| Single Effects Influence | Df | AIC | P | |
|---|---|---|---|---|
| None | 2703.8 | |||
| Random effects | Tree | 6 | 4876.2 | <0.001 |
| Position | 6 | 4103.0 | <0.001 | |
| Fixed effects | Silt | 1 | 2704.8 | 0.082 |
| K | 1 | 2754.4 | <0.001 | |
| P | 1 | 2933.8 | <0.001 | |
| Moisture | 1 | 3864.5 | <0.001 | |
| Estimate | Std. Error | z Value | P | |
|---|---|---|---|---|
| (Intercept) | 2.501 | 0.957 | 2.612 | <0.01 |
| Silt | 0.044 | 0.026 | 1.737 | <0.05 |
| P | 0.803 | 0.051 | 15.877 | <0.001 |
| K | −0.188 | 0.027 | −6.983 | <0.001 |
| Moisture | 1.081 | 0.032 | 33.881 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Cuesta, R.; Navarro-Cerrillo, R.M.; Quero, J.L.; Ruiz-Gómez, F.J. Small-Scale Abiotic Factors Influencing the Spatial Distribution of Phytophthora cinnamomi under Declining Quercus ilex Trees. Forests 2020, 11, 375. https://doi.org/10.3390/f11040375
Sánchez-Cuesta R, Navarro-Cerrillo RM, Quero JL, Ruiz-Gómez FJ. Small-Scale Abiotic Factors Influencing the Spatial Distribution of Phytophthora cinnamomi under Declining Quercus ilex Trees. Forests. 2020; 11(4):375. https://doi.org/10.3390/f11040375
Chicago/Turabian StyleSánchez-Cuesta, Rafael, Rafael M. Navarro-Cerrillo, José L. Quero, and Francisco J. Ruiz-Gómez. 2020. "Small-Scale Abiotic Factors Influencing the Spatial Distribution of Phytophthora cinnamomi under Declining Quercus ilex Trees" Forests 11, no. 4: 375. https://doi.org/10.3390/f11040375
APA StyleSánchez-Cuesta, R., Navarro-Cerrillo, R. M., Quero, J. L., & Ruiz-Gómez, F. J. (2020). Small-Scale Abiotic Factors Influencing the Spatial Distribution of Phytophthora cinnamomi under Declining Quercus ilex Trees. Forests, 11(4), 375. https://doi.org/10.3390/f11040375






