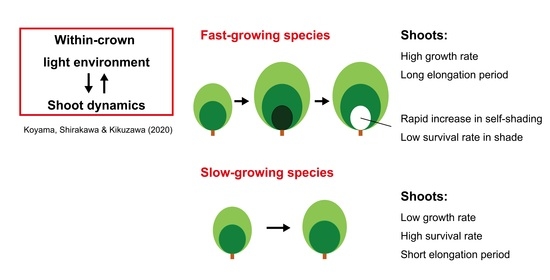
Redeployment of Shoots into Better-Lit Positions within the Crowns of Saplings of Five Species with Different Growth Patterns
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Study Sites and Sample Trees
2.3. Shoot Census
2.4. Leaf Census
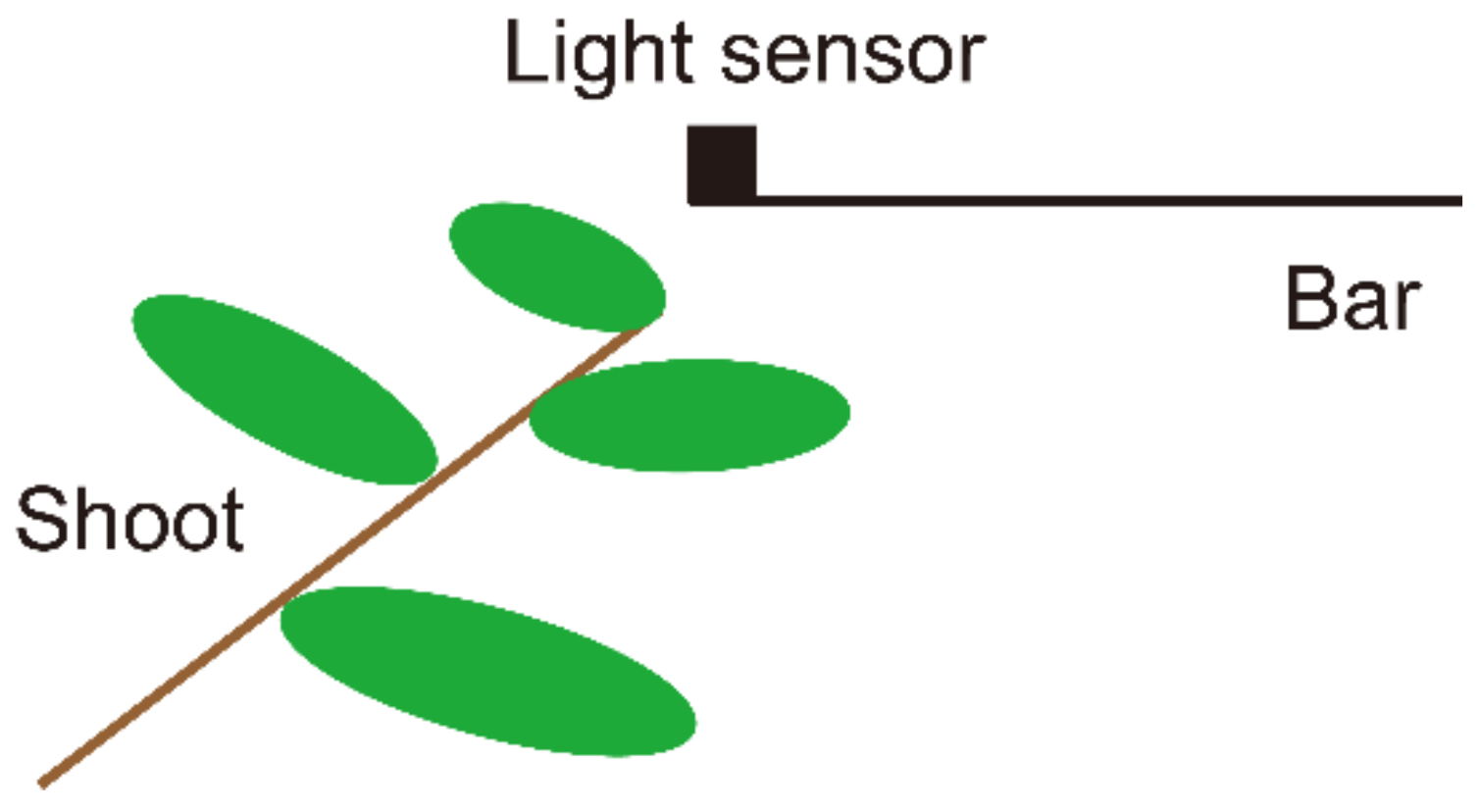
2.5. Measurement of Light Environments
3. Results
3.1. Growth Rate and Pattern
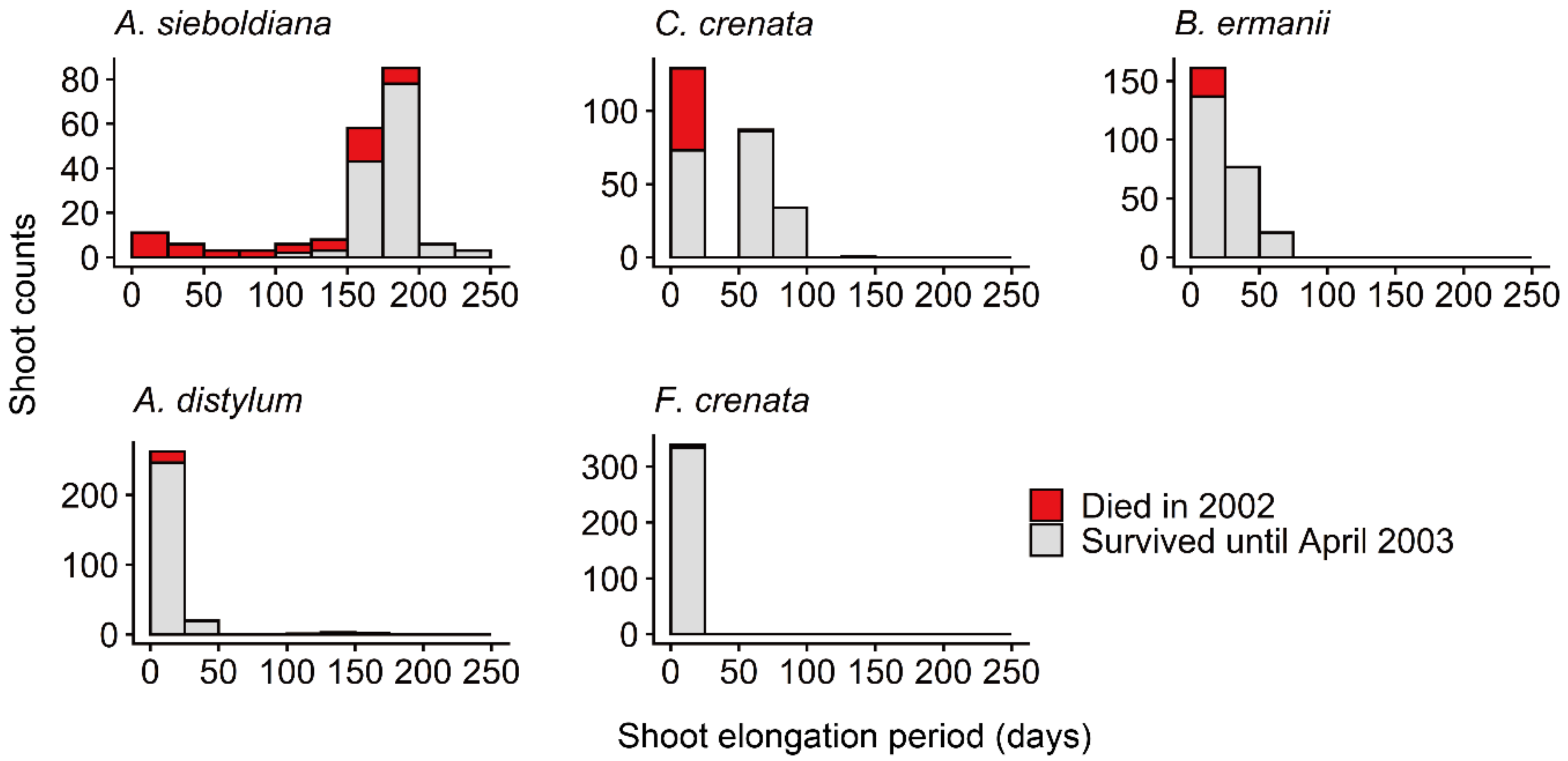
3.2. Shoot Survival
3.3. Light Environment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability Statement
References
- Sterck, F.J.; Schieving, F.; Lemmens, A.; Pons, T.L. Performance of trees in forest canopies: Explorations with a bottom-up functional-structural plant growth model. New Phytol. 2005, 166, 827–843. [Google Scholar] [CrossRef]
- Bazzaz, F.A.; Harper, J.L. Demographic analysis of the growth of Linum usitatissimum. New Phytol. 1977, 78, 193–208. [Google Scholar] [CrossRef]
- Niinemets, U.; Lukjanova, A. Total foliar area and average leaf age may be more strongly associated with branching frequency than with leaf longevity in temperate conifers. New Phytol. 2003, 158, 75–89. [Google Scholar] [CrossRef]
- Sterck, F.J.; Schieving, F. 3-D growth patterns of trees: Effects of carbon economy, meristem activity, and selection. Ecol. Monogr. 2007, 77, 405–420. [Google Scholar] [CrossRef]
- Beyer, R.; Letort, V.; Cournede, P.H. Modeling tree crown dynamics with 3D partial differential equations. Front. Plant Sci. 2014, 5, 329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laurans, M.; Vincent, G. Are inter- and intraspecific variations of sapling crown traits consistent with a strategy promoting light capture in tropical moist forest? Ann. Bot. 2016, 118, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Yamamoto, K.; Ushio, M. A lognormal distribution of the lengths of terminal twigs on self-similar branches of elm trees. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162395. [Google Scholar] [CrossRef]
- Rani, R.; Abramowicz, K.; Falster, D.S.; Sterck, F.; Brannstrom, A. Effects of bud-flushing strategies on tree growth. Tree Physiol. 2018, 38, 1384–1393. [Google Scholar] [CrossRef]
- Hikosaka, K. A model of dynamics of leaves and nitrogen in a plant canopy: An integration of canopy photosynthesis, leaf life span, and nitrogen use efficiency. Am. Nat. 2003, 162, 149–164. [Google Scholar] [CrossRef]
- Hikosaka, K. Leaf canopy as a dynamic system: Ecophysiology and optimality in leaf turnover. Ann. Bot. 2005, 95, 521–533. [Google Scholar] [CrossRef]
- Kaitaniemi, P.; Lintunen, A.; Sievänen, R. Power-law estimation of branch growth. Ecol. Model. 2020, 416, 108900. [Google Scholar] [CrossRef]
- Wang, F.; Kang, M.; Lu, Q.; Letort, V.; Han, H.; Guo, Y.; de Reffye, P.; Li, B. A stochastic model of tree architecture and biomass partitioning: Application to Mongolian Scots pines. Ann. Bot. 2011, 107, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, H.; Kikuzawa, K. Crown hollowing as a consequence of early shedding of leaves and shoots. Ecol. Res. 2009, 24, 839–845. [Google Scholar] [CrossRef]
- Koyama, K.; Kikuzawa, K. Is whole-plant photosynthetic rate proportional to leaf area? A test of scalings and a logistic equation by leaf demography census. Am. Nat. 2009, 173, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Hidaka, Y.; Ushio, M. Dynamic scaling in the growth of a non-branching plant, Cardiocrinum cordatum. PLoS ONE 2012, 7, e45317. [Google Scholar] [CrossRef]
- Ishii, H.; Asano, S. The role of crown architecture, leaf phenology and photosynthetic activity in promoting complementary use of light among coexisting species in temperate forests. Ecol. Res. 2010, 25, 715–722. [Google Scholar] [CrossRef]
- Bentley, L.P.; Stegen, J.C.; Savage, V.M.; Smith, D.D.; von Allmen, E.I.; Sperry, J.S.; Reich, P.B.; Enquist, B.J. An empirical assessment of tree branching networks and implications for plant allometric scaling models. Ecol. Lett. 2013, 16, 1069–1078. [Google Scholar] [CrossRef]
- Smith, D.D.; Sperry, J.S.; Enquist, B.J.; Savage, V.M.; McCulloh, K.A.; Bentley, L.P. Deviation from symmetrically self-similar branching in trees predicts altered hydraulics, mechanics, light interception and metabolic scaling. New Phytol. 2014, 201, 217–229. [Google Scholar] [CrossRef]
- Savage, V.M.; Bentley, L.P.; Enquist, B.J.; Sperry, J.S.; Smith, D.D.; Reich, P.B.; von Allmen, E.I. Hydraulic trade-offs and space filling enable better predictions of vascular structure and function in plants. Proc. Natl. Acad. Sci. USA 2010, 107, 22722–22727. [Google Scholar] [CrossRef]
- Enquist, B.J.; Bentley, L.P.; Shenkin, A.; Maitner, B.; Savage, V.; Michaletz, S.; Blonder, B.; Buzzard, V.; Espinoza, T.E.B.; Farfan-Rios, W.; et al. Assessing trait-based scaling theory in tropical forests spanning a broad temperature gradient. Glob. Ecol. Biogeogr. 2017, 26, 1357–1373. [Google Scholar] [CrossRef]
- Kurosawa, Y.; Mori, S.; Wang, M.; Ferrio, J.P.; Yamaji, K.; Koyama, K.; Haruma, T.; Doyama, K. Initial burst of root development with decreasing respiratory carbon cost in Fagus crenata Blume seedlings. Plant Spec. Biol. 2020. [Google Scholar] [CrossRef]
- Martin-Ducup, O.; Ploton, P.; Barbier, N.; Momo Takoudjou, S.; Mofack, G., II; Kamdem, N.G.; Fourcaud, T.; Sonké, B.; Couteron, P.; Pélissier, R. Terrestrial laser scanning reveals convergence of tree architecture with increasingly dominant crown canopy position. Funct. Ecol. 2020. [Google Scholar] [CrossRef]
- Lau, A.; Martius, C.; Bartholomeus, H.; Shenkin, A.; Jackson, T.; Malhi, Y.; Herold, M.; Bentley, L.P. Estimating architecture-based metabolic scaling exponents of tropical trees using terrestrial LiDAR and 3D modelling. For. Ecol. Manag. 2019, 439, 132–145. [Google Scholar] [CrossRef]
- Komiyama, A.; Nakagawa, M.; Kato, S. Common allometric relationships for estimating tree biomasses in cool temperate forests of Japan. J. Jpn. For. Soc. 2011, 93, 220–225. [Google Scholar] [CrossRef]
- Givnish, T.J. On the adaptive significance of leaf height in forest herbs. Am. Nat. 1982, 120, 353–381. [Google Scholar] [CrossRef]
- Anten, N.P.R.; During, H.J. Is analysing the nitrogen use at the plant canopy level a matter of choosing the right optimization criterion? Oecologia 2011, 167, 293–303. [Google Scholar] [CrossRef]
- Boonman, A.; Anten, N.P.R.; Dueck, T.A.; Jordi, W.; van der Werf, A.; Voesenek, L.; Pons, T.L. Functional significance of shade-induced leaf senescence in dense canopies: An experimental test using transgenic tobacco. Am. Nat. 2006, 168, 597–607. [Google Scholar] [CrossRef]
- Field, C. Allocating leaf nitrogen for the maximization of carbon gain—Leaf age as a control on the allocation program. Oecologia 1983, 56, 341–347. [Google Scholar] [CrossRef]
- Oikawa, S.; Hikosaka, K.; Hirose, T. Leaf lifespan and lifetime carbon balance of individual leaves in a stand of an annual herb, Xanthium canadense. New Phytol. 2006, 172, 104–116. [Google Scholar] [CrossRef]
- Oikawa, S.; Hikosaka, K.; Hirose, T. Dynamics of leaf area and nitrogen in the canopy of an annual herb, Xanthium canadense. Oecologia 2005, 143, 517–526. [Google Scholar] [CrossRef]
- Kikuzawa, K. A cost-benefit analysis of leaf habit and leaf longevity of trees and their geographical pattern. Am. Nat. 1991, 138, 1250–1263. [Google Scholar] [CrossRef]
- Hikosaka, K.; Terashima, I.; Katoh, S. Effects of leaf age, nitrogen nutrition and photon flux-density on the distribution of nitrogen among leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading of leaves. Oecologia 1994, 97, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, K. Effects of leaf age, nitrogen nutrition and photon flux density on the organization of the photosynthetic apparatus in leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading of leaves. Planta 1996, 198, 144–150. [Google Scholar] [CrossRef]
- Franklin, O.; Ågren, G.I. Leaf senescence and resorption as mechanisms of maximizing photosynthetic production during canopy development at N limitation. Funct. Ecol. 2002, 16, 727–733. [Google Scholar] [CrossRef]
- Kikuzawa, K.; Lechowicz, M.J. Theories of Leaf Longevity. In Ecology of Leaf Longevity; Kikuzawa, K., Lechowicz, M.J., Eds.; Springer: Tokyo, Japan, 2011; pp. 41–56. [Google Scholar]
- Osada, N.; Takeda, H.; Kitajima, K.; Pearcy, R.W. Functional correlates of leaf demographic response to gap release in saplings of a shade-tolerant tree, Elateriospermum tapos. Oecologia 2003, 137, 181–187. [Google Scholar] [CrossRef]
- Escudero, A.; Mediavilla, S. Decline in photosynthetic nitrogen use efficiency with leaf age and nitrogen resorption as determinants of leaf life span. J. Ecol. 2003, 91, 880–889. [Google Scholar] [CrossRef]
- Tanaka, T.; Oikawa, S.; Kurokawa, C. Leaf shedding increases the photosynthetic rate of the canopy in N2-fixing and non-N2-fixing woody species. Tree Physiol. 2018, 38, 1903–1911. [Google Scholar] [CrossRef]
- Oikawa, S.; Hikosaka, K.; Hirose, T. Does leaf shedding increase the whole-plant carbon gain despite some nitrogen being lost with shedding? New Phytol. 2008, 178, 617–624. [Google Scholar] [CrossRef]
- Seiwa, K.; Kikuzawa, K.; Kadowaki, T.; Akasaka, S.; Ueno, N. Shoot life span in relation to successional status in deciduous broad-leaved tree species in a temperate forest. New Phytol. 2006, 169, 537–548. [Google Scholar] [CrossRef]
- Stoll, P.; Schmid, B. Plant foraging and dynamic competition between branches of Pinus sylvestris in contrasting light environments. J. Ecol. 1998, 86, 934–945. [Google Scholar] [CrossRef]
- Umeki, K.; Kikuzawa, K.; Sterck, F.J. Influence of foliar phenology and shoot inclination on annual photosynthetic gain in individual beech saplings: A functional–structural modeling approach. For. Ecol. Manag. 2010, 259, 2141–2150. [Google Scholar] [CrossRef]
- Umeki, K.; Seino, T.; Lim, E.-M.; Honjo, T. Patterns of shoot mortality in Betula platyphylla in northern Japan. Tree Physiol. 2006, 26, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, A. Shoot growth responses to light microenvironment and correlative inhibition in tree seedlings under a forest canopy. Tree Physiol. 2000, 20, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, D.; Tateno, M. Concentrative nitrogen allocation to sun-lit branches and the effects on whole-plant growth under heterogeneous light environments. Oecologia 2013, 172, 949–960. [Google Scholar] [CrossRef]
- Chen, L.; Sumida, A. Effects of light on branch growth and death vary at different organization levels of branching units in Sakhalin spruce. Trees 2018, 32, 1123–1134. [Google Scholar] [CrossRef]
- Sprugel, D.G. When branch autonomy fails: Milton’s Law of resource availability and allocation. Tree Physiol. 2002, 22, 1119–1124. [Google Scholar] [CrossRef]
- Yamanaka, T.; Okabe, H.; Kawai, S. Growth and nodulation in Alnus sieboldiana in response to Frankia inoculation and nitrogen treatments. Trees 2016, 30, 539–544. [Google Scholar] [CrossRef]
- Kamijo, T.; Kitayama, K.; Sugawara, A.; Urushimichi, S.; Sasai, K. Primary succession of the warm-temperate broad-leaved forest on a volcanic island, Miyake-jima, Japan. Folia Geobot. 2002, 37, 71–91. [Google Scholar] [CrossRef]
- Kikuzawa, K. Phenological and morphological adaptations to the light environment in two woody and two herbaceous plant species. Funct. Ecol. 2003, 17, 29–38. [Google Scholar] [CrossRef]
- Poorter, L.; Bongers, F. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 2006, 87, 1733–1743. [Google Scholar] [CrossRef]
- Gray, E.F.; Wright, I.J.; Falster, D.S.; Eller, A.S.D.; Lehmann, C.E.R.; Bradford, M.G.; Cernusak, L.A. Leaf:wood allometry and functional traits together explain substantial growth rate variation in rainforest trees. AoB Plants 2019, 11, plz024. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Cooke, J.; Cernusak, L.A.; Hutley, L.B.; Scalon, M.C.; Tozer, W.C.; Lehmann, C.E.R. Stem diameter growth rates in a fire-prone savanna correlate with photosynthetic rate and branch-scale biomass allocation, but not specific leaf area. Austral Ecol. 2019, 44, 339–350. [Google Scholar] [CrossRef]
- Seiwa, K.; Watanabe, A.; Irie, K.; Kanno, H.; Saitoh, T.; Akasaka, S. Impact of site-induced mouse caching and transport behaviour on regeneration in Castanea crenata. J. Veg. Sci. 2002, 13, 517–526. [Google Scholar] [CrossRef]
- Koike, T. Leaf structure and photosynthetic performance as related to the forest succession of deciduous broad-leaved trees. Plant Spec. Biol. 1988, 3, 77–87. [Google Scholar] [CrossRef]
- Cao, K.F.; Ohkubo, T. Allometry, root/shoot ratio and root architecture in understory saplings of deciduous dicotyledonous trees in central Japan. Ecol. Res. 1998, 13, 217–227. [Google Scholar] [CrossRef]
- Ohkubo, T.; Kaji, M.; Hamaya, T. Structure of primary Japanese beech (Fagus japonica maxim.) forests in the Chichibu Mountains, central Japan, with special reference to regeneration processes. Ecol. Res. 1988, 3, 101–116. [Google Scholar] [CrossRef]
- Sakai, S. Patterns of branching and extension growth of vigorous saplings of Japanese Acer species in relation to their regeneration strategies. Can. J. Bot. 1987, 65, 1578–1585. [Google Scholar] [CrossRef]
- Miyashita, A.; Tateno, M. A novel index of leaf RGR predicts tree shade tolerance. Funct. Ecol. 2014, 28, 1321–1329. [Google Scholar] [CrossRef]
- Okaura, T.; Harada, K. Phylogeographical structure revealed by chloroplast DNA variation in Japanese beech (Fagus crenata Blume). Heredity 2002, 88, 322. [Google Scholar] [CrossRef]
- Koyama, K.; Kikuzawa, K. Reduction of photosynthesis before midday depression occurred: Leaf photosynthesis of Fagus crenata in a temperate forest in relation to canopy position and a number of days after rainfall. Ecol. Res. 2011, 26, 999–1006. [Google Scholar] [CrossRef]
- Koyama, K.; Kikuzawa, K. Can we estimate forest gross primary production from leaf lifespan? A test in a young Fagus crenata forest. J. Ecol. Field Biol. 2010, 33, 253–260. [Google Scholar] [CrossRef]
- Koyama, K.; Kikuzawa, K. Geometrical similarity analysis of photosynthetic light response curves, light saturation and light use efficiency. Oecologia 2010, 164, 53–63. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Watanabe, M.; Hiroshima, H.; Kinose, Y.; Okabe, S.; Izuta, T. Nitrogen use efficiency for growth of Fagus crenata seedlings under elevated ozone and different soil nutrient conditions. Forests 2020, 11, 371. [Google Scholar] [CrossRef]
- Koyama, K.; Kikuzawa, K. Intraspecific variation in leaf life span for the semi-evergreen liana Akebia trifoliata is caused by both seasonal and aseasonal factors in a temperate forest. J. Ecol. Field Biol. 2008, 31, 207–211. [Google Scholar] [CrossRef][Green Version]
- Hollinger, D.Y. Optimality and nitrogen allocation in a tree canopy. Tree Physiol. 1996, 16, 627–634. [Google Scholar] [CrossRef]
- Muraoka, H.; Hirota, H.; Matsumoto, J.; Nishimura, S.; Tang, Y.; Koizumi, H.; Washitani, I. On the convertibility of different microsite light availability indices, relative illuminance and relative photon flux density. Funct. Ecol. 2001, 15, 798–803. [Google Scholar] [CrossRef]
- Muraoka, H.; Tang, Y.; Koizumi, H.; Washitani, I. Combined effects of light and water availability on photosynthesis and growth of Arisaema heterophyllum in the forest understory and an open site. Oecologia 1997, 112, 26–34. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Reich, P.B. The world-wide ‘fast–slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Roumet, C.; Birouste, M.; Picon-Cochard, C.; Ghestem, M.; Osman, N.; Vrignon-Brenas, S.; Cao, K.-f.; Stokes, A. Root structure–function relationships in 74 species: Evidence of a root economics spectrum related to carbon economy. New Phytol. 2016, 210, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Coley, P.D.; Bryant, J.P.; Chapin, F.S. Resource availability and plant antiherbivore defense. Science 1985, 230, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Poorter, H. Inherent variation in growth rate between higher plants: A search for physiological causes and ecological consequences. Adv. Ecol. Res. 1992, 23, 187–261. [Google Scholar]
- Freschet, G.T.; Cornelissen, J.H.C.; van Logtestijn, R.S.P.; Aerts, R. Evidence of the ‘plant economics spectrum’ in a subarctic flora. J. Ecol. 2010, 98, 362–373. [Google Scholar] [CrossRef]
- Iida, Y.; Sun, I.F.; Price, C.A.; Chen, C.T.; Chen, Z.S.; Chiang, J.M.; Huang, C.L.; Swenson, N.G. Linking leaf veins to growth and mortality rates: An example from a subtropical tree community. Ecol. Evol. 2016, 6, 6085–6096. [Google Scholar] [CrossRef]
- Wright, S.J.; Kitajima, K.; Kraft, N.J.B.; Reich, P.B.; Wright, I.J.; Bunker, D.E.; Condit, R.; Dalling, J.W.; Davies, S.J.; Díaz, S.; et al. Functional traits and the growth-mortality trade-off in tropical trees. Ecology 2010, 91, 3664–3674. [Google Scholar] [CrossRef]
- Karst, A.L.; Lechowicz, M.J. Are correlations among foliar traits in ferns consistent with those in the seed plants? New Phytol. 2007, 173, 306–312. [Google Scholar] [CrossRef]
- Waite, M.; Sack, L. How does moss photosynthesis relate to leaf and canopy structure? Trait relationships for 10 Hawaiian species of contrasting light habitats. New Phytol. 2010, 185, 156–172. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From tropics to tundra: Global convergence in plant functioning. Proc. Natl. Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. Leaf age and season influence the relationships between leaf nitrogen, leaf mass per area and photosynthesis in maple and oak trees. Plant Cell Environ. 1991, 14, 251–259. [Google Scholar] [CrossRef]
- Shiodera, S.; Rahajoe, J.S.; Kohyama, T. Variation in longevity and traits of leaves among co-occurring understorey plants in a tropical montane forest. J. Trop. Ecol. 2008, 24, 121–133. [Google Scholar] [CrossRef]
- Saura-Mas, S.; Shipley, B.; Lloret, F. Relationship between post-fire regeneration and leaf economics spectrum in Mediterranean woody species. Funct. Ecol. 2009, 23, 103–110. [Google Scholar] [CrossRef]
- Vincent, G. Leaf life span plasticity in tropical seedlings grown under contrasting light regimes. Ann. Bot. 2006, 97, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.; Walters, M.; Ellsworth, D. Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol. Monogr. 1992, 62, 365–392. [Google Scholar] [CrossRef]
- Baas, P.; Ewers, F.W.; Davis, S.D.; Wheeler, E.A. Evolution of xylem physiology. In The Evolution of Plant Physiology; Hemsley, A.R., Poole, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 273–295. [Google Scholar]
- Rosas, T.; Mencuccini, M.; Barba, J.; Cochard, H.; Saura-Mas, S.; Martínez-Vilalta, J. Adjustments and coordination of hydraulic, leaf and stem traits along a water availability gradient. New Phytol. 2019, 223, 632–646. [Google Scholar] [CrossRef]
- Mooney, H.A.; Gulmon, S.L. Environmental and evolutionary constraints on the photosynthetic characteristics of higher plants. In Topics in Plant Population Biology; Solbrig, O.T., Jain, S., Johnson, G.B., Raven, P.H., Eds.; Macmillan Education: London, UK, 1979; pp. 316–337. [Google Scholar]
- Onoda, Y.; Westoby, M.; Adler, P.B.; Choong, A.M.; Clissold, F.J.; Cornelissen, J.H.; Diaz, S.; Dominy, N.J.; Elgart, A.; Enrico, L.; et al. Global patterns of leaf mechanical properties. Ecol. Lett. 2011, 14, 301–312. [Google Scholar] [CrossRef]
- Shipley, B.; Lechowicz, M.J.; Wright, I.; Reich, P.B. Fundamental trade-offs generating the worldwide leaf economics spectrum. Ecology 2006, 87, 535–541. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Díaz-Espejo, A.; Flexas, J.; Galmés, J.; Warren, C.R. Role of mesophyll diffusion conductance in constraining potential photosynthetic productivity in the field. J. Exp. Bot. 2009, 60, 2249–2270. [Google Scholar] [CrossRef]
- Chabot, B.F.; Hicks, D.J. The ecology of leaf life spans. Ann. Rev. Ecol. Syst. 1982, 13, 229–259. [Google Scholar] [CrossRef]
- Osada, N.; Oikawa, S.; Kitajima, K. Implications of life span variation within a leaf cohort for evaluation of the optimal timing of leaf shedding. Funct. Ecol. 2015, 29, 308–314. [Google Scholar] [CrossRef]
- Oikawa, S.; Suno, K.; Osada, N. Inconsistent intraspecific pattern in leaf life span along nitrogen-supply gradient. Am. J. Bot. 2017, 104, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Osada, N.; Takeda, H.; Furukawa, A.; Awang, M. Leaf dynamics and maintenance of tree crowns in a Malaysian rain forest stand. J. Ecol. 2001, 89, 774–782. [Google Scholar] [CrossRef]
- Reich, P.B.; Wright, I.J.; Cavender-Bares, J.; Craine, J.M.; Oleksyn, J.; Westoby, M.; Walters, M.B. The evolution of plant functional variation: Traits, spectra, and strategies. Int. J. Plant Sci. 2003, 164, S143–S164. [Google Scholar] [CrossRef]
- Kikuzawa, K.; Ackerly, D. Significance of leaf longevity in plants. Plant Spec. Biol. 1999, 14, 39–45. [Google Scholar] [CrossRef]
- Eissenstat, D.; Wells, C.; Yanai, R.; Whitbeck, J. Building roots in a changing environment: Implications for root longevity. New Phytol. 2000, 147, 33–42. [Google Scholar] [CrossRef]
- Fujiki, D.; Kikuzawa, K. Stem turnover strategy of multiple-stemmed woody plants. Ecol. Res. 2006, 21, 380–386. [Google Scholar] [CrossRef]
- Verbeeck, H.; Bauters, M.; Jackson, T.; Shenkin, A.; Disney, M.; Calders, K. Time for a plant structural economics spectrum. Front. For. Glob. Chang. 2019, 2, 43. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Akiyama, T.; Hashimoto, Y.; Inatomi, M.; Sakai, T.; Jia, S.; Mo, W.; Tsuda, S.; Koizumi, H. Biometric based estimates of net primary production (NPP) in a cool-temperate deciduous forest stand beneath a flux tower. Agric. For. Meteorol. 2005, 134, 27–38. [Google Scholar] [CrossRef]
- Ameztegui, A.; Paquette, A.; Shipley, B.; Heym, M.; Messier, C.; Gravel, D. Shade tolerance and the functional trait: Demography relationship in temperate and boreal forests. Funct. Ecol. 2017, 31, 821–830. [Google Scholar] [CrossRef]
- Ishii, H.; Ford, E.D. The role of epicormic shoot production in maintaining foliage in old Pseudotsuga menziesii (Douglas-fir) trees. Can. J. Bot. 2001, 79, 251–264. [Google Scholar]
- Sumida, A.; Terazawa, I.; Togashi, A.; Komiyama, A. Spatial arrangement of branches in relation to slope and neighbourhood competition. Ann. Bot. 2002, 89, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Guillemot, J.; Kunz, M.; Schnabel, F.; Fichtner, A.; Madsen, C.P.; Gebauer, T.; Härdtle, W.; von Oheimb, G.; Potvin, C. Neighbourhood-mediated shifts in tree biomass allocation drive overyielding in tropical species mixtures. New Phytol. 2020, 228, 1256–1268. [Google Scholar] [CrossRef] [PubMed]

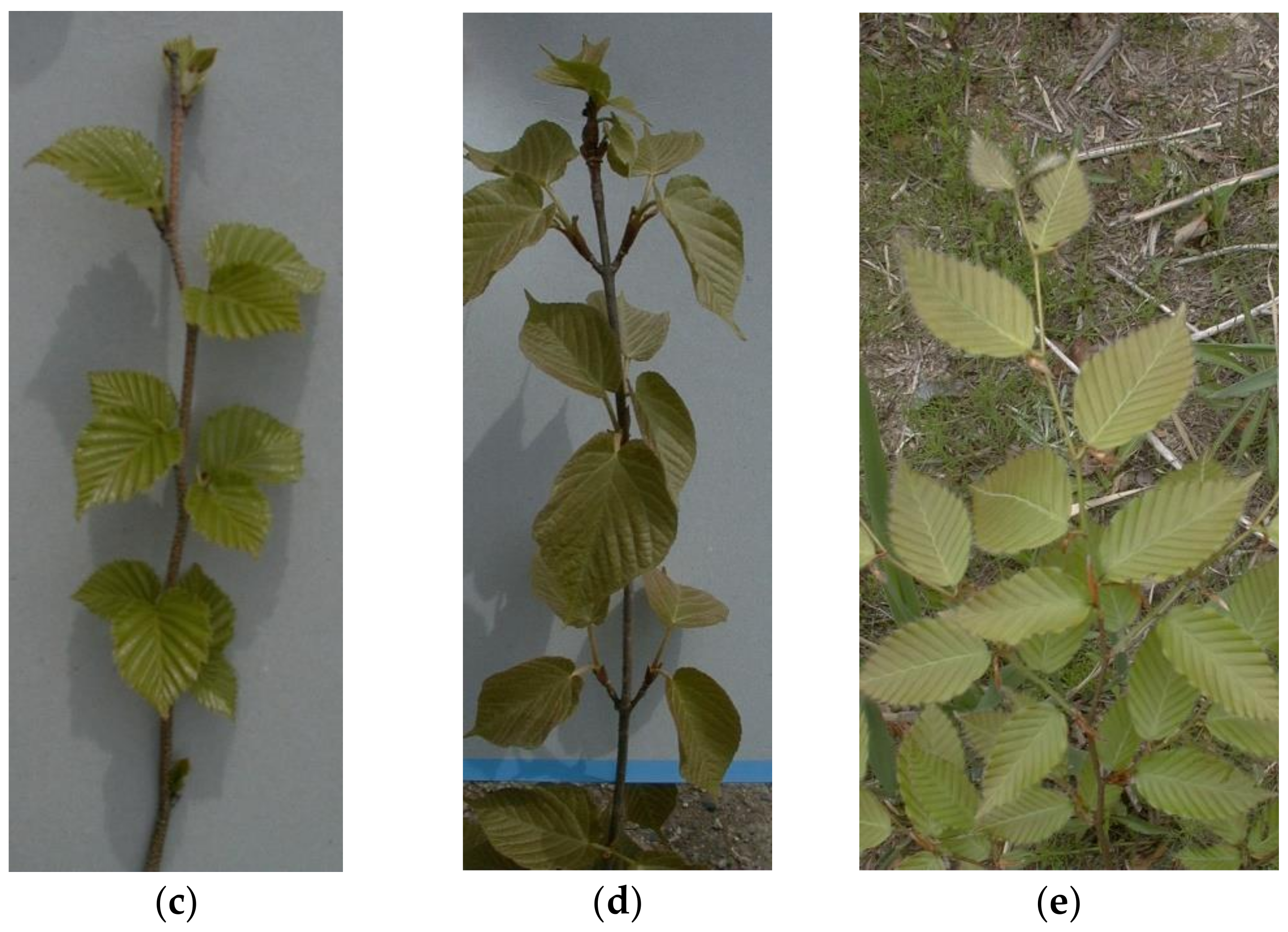
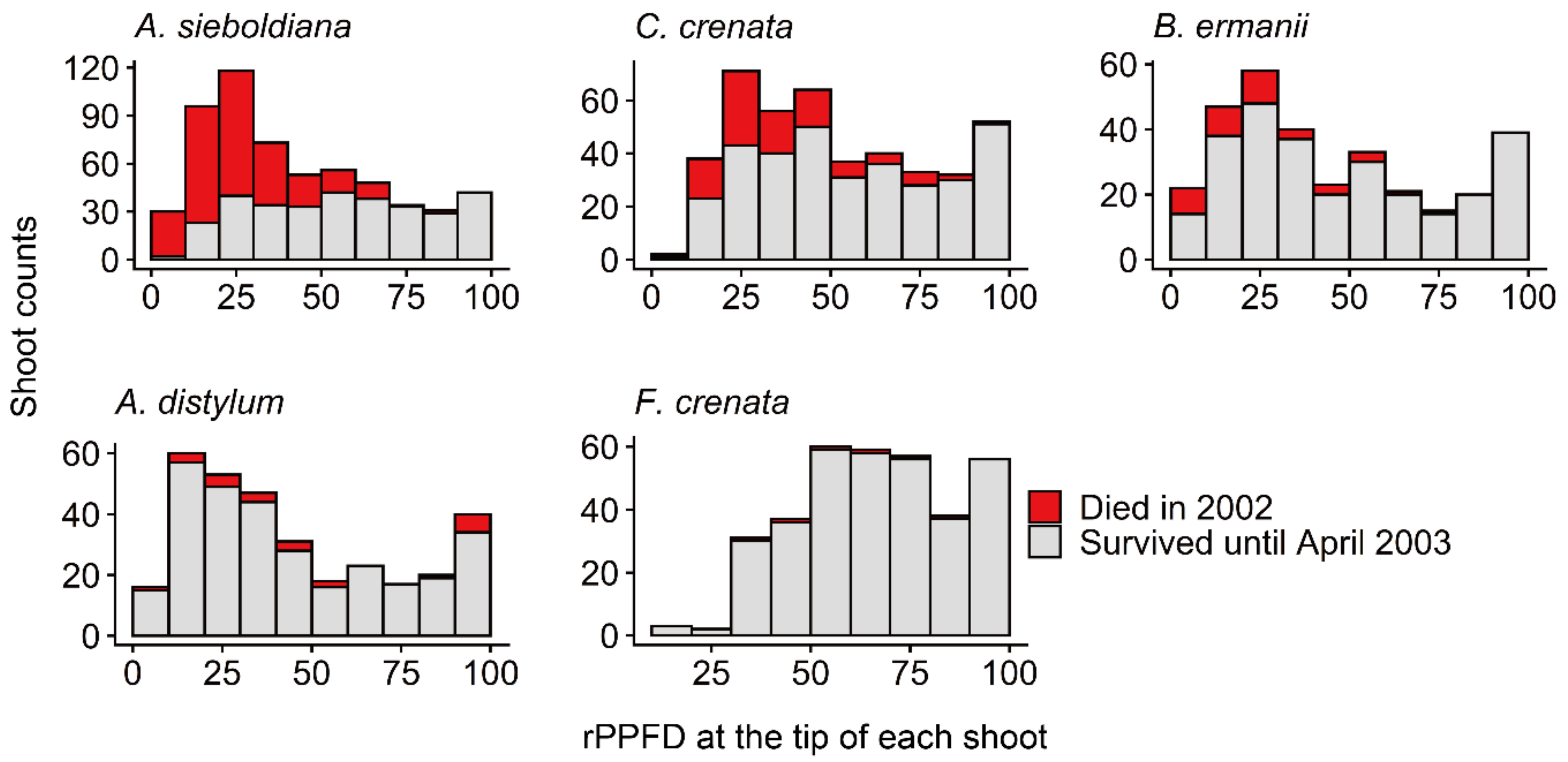
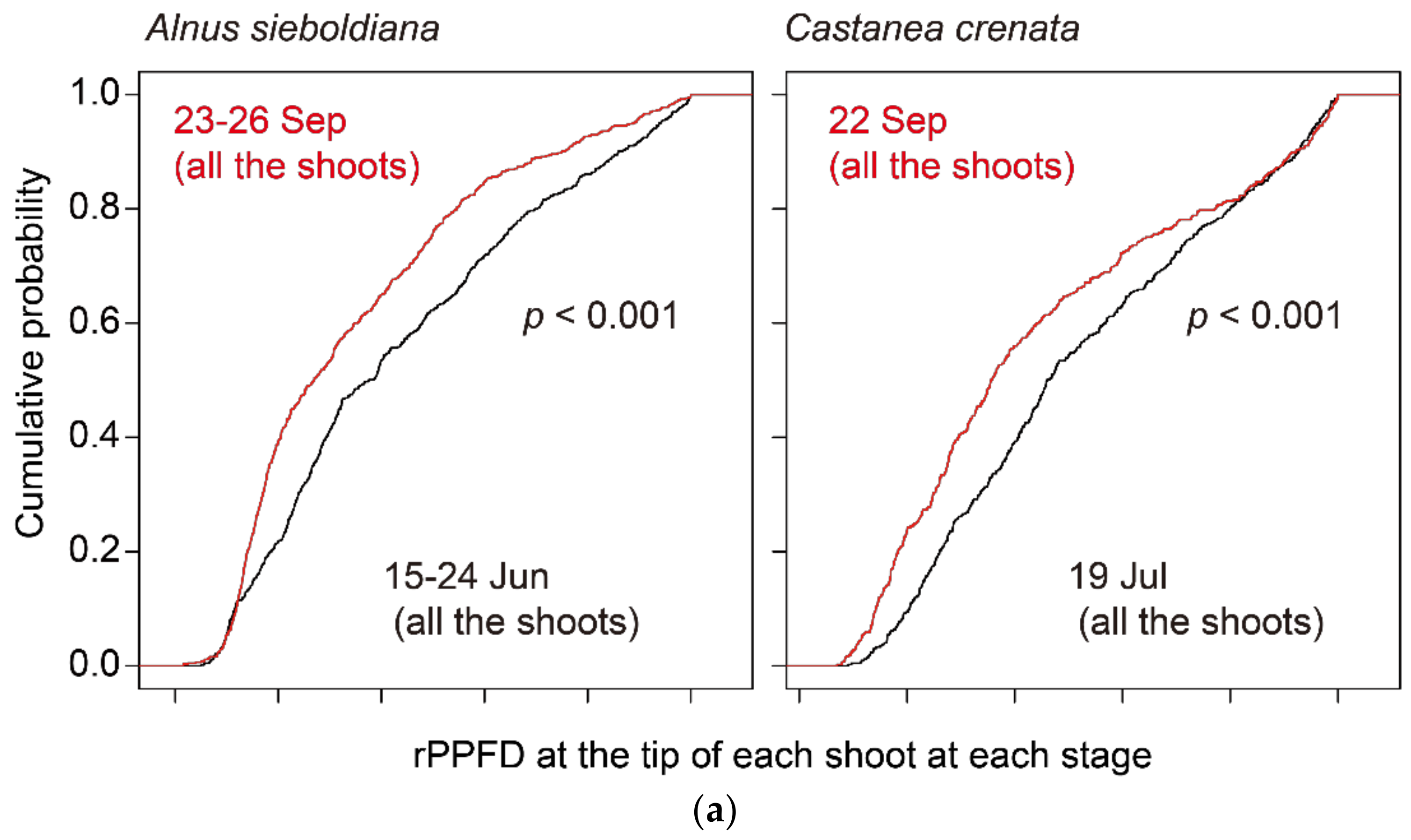
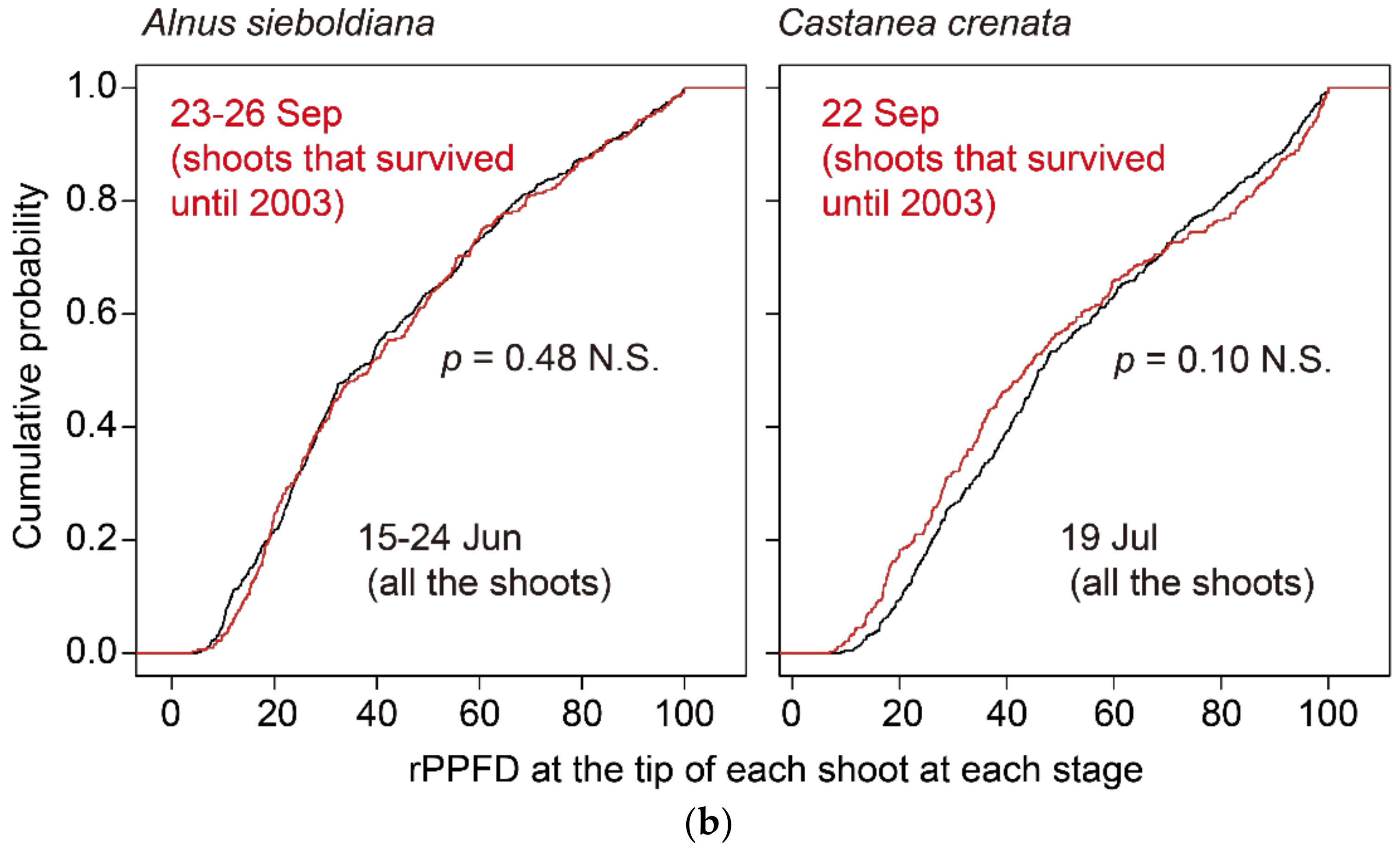
| Alnus sieboldiana | Castanea crenata | Betula ermanii | Acer distylum | Fagus crenata | |
|---|---|---|---|---|---|
| No. of saplings | 3 | 3 | 3 | 6 | 4 |
| Tree height (m) | 0.81–1.03 | 0.95–1.13 | 0.61–0.81 | 0.86–1.72 | 0.81–1.02 |
| No. of shoots for the shoot census 1 | 581 | 425 | 318 | 325 | 343 |
| No. of shoots for the leaf census 1 | 189 | 251 | 259 | 288 | 339 |
| A. sieboldiana | C. crenata | B. ermanii | A. distylum | F. crenata | |
|---|---|---|---|---|---|
| Species category | Fast | Fast | Medium | Slow | Slow |
| Annual shoot elongation 1,2 (cm year−1) | 26.9 | 8.0 | 4.4 | 1.5 | 2.2 |
| Shoot elongation period (days) 1 | 156 | 33 | 18 | 8 | 3.5 |
| Number of daughter buds per shoot 1,2,3 | 5.1 | 2.7 | 1.9 | 1.1 | 1.1 |
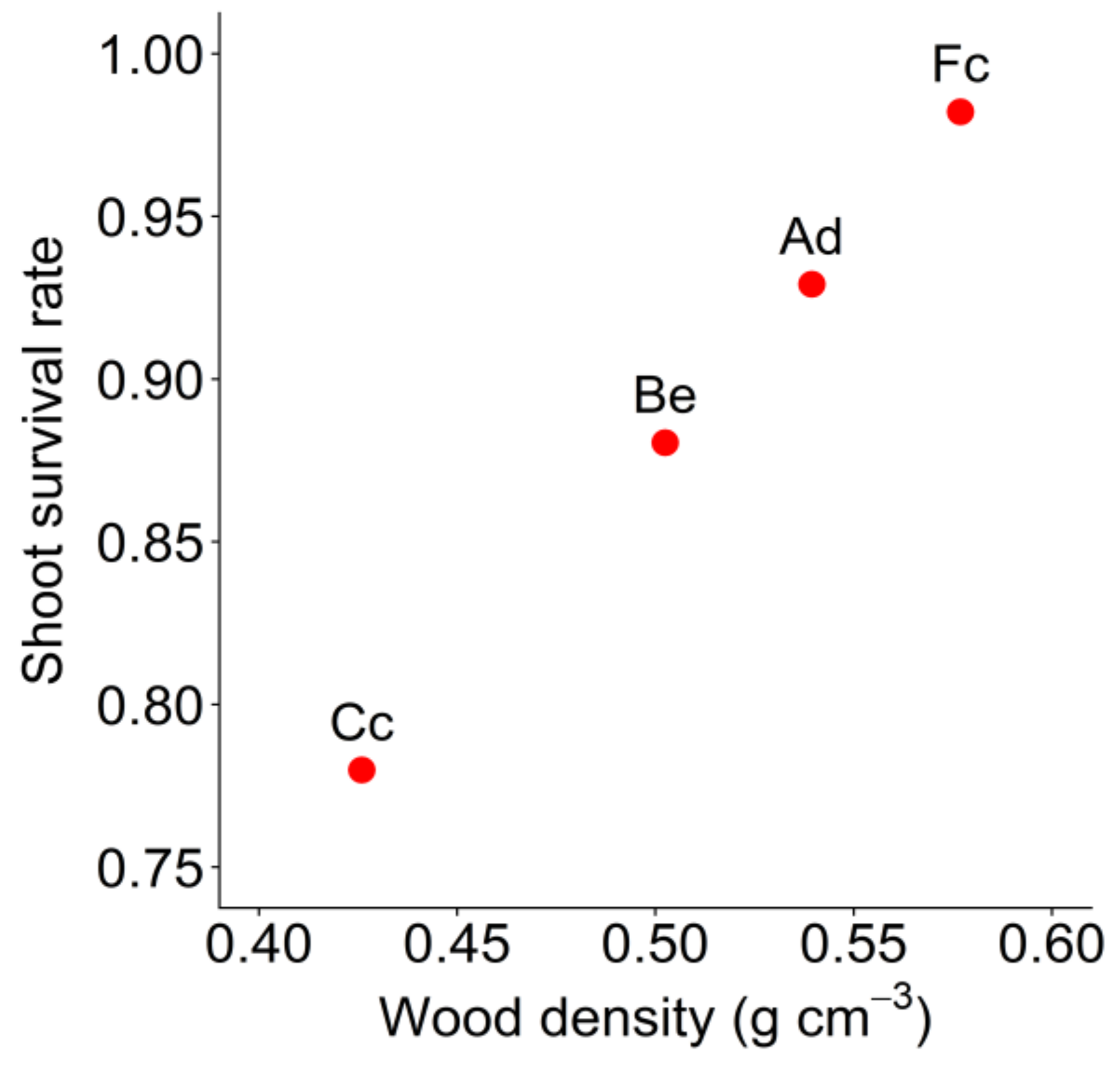
| Shoot survival rate | 0.54 | 0.78 | 0.88 | 0.93 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koyama, K.; Shirakawa, H.; Kikuzawa, K. Redeployment of Shoots into Better-Lit Positions within the Crowns of Saplings of Five Species with Different Growth Patterns. Forests 2020, 11, 1301. https://doi.org/10.3390/f11121301
Koyama K, Shirakawa H, Kikuzawa K. Redeployment of Shoots into Better-Lit Positions within the Crowns of Saplings of Five Species with Different Growth Patterns. Forests. 2020; 11(12):1301. https://doi.org/10.3390/f11121301
Chicago/Turabian StyleKoyama, Kohei, Hiroyuki Shirakawa, and Kihachiro Kikuzawa. 2020. "Redeployment of Shoots into Better-Lit Positions within the Crowns of Saplings of Five Species with Different Growth Patterns" Forests 11, no. 12: 1301. https://doi.org/10.3390/f11121301
APA StyleKoyama, K., Shirakawa, H., & Kikuzawa, K. (2020). Redeployment of Shoots into Better-Lit Positions within the Crowns of Saplings of Five Species with Different Growth Patterns. Forests, 11(12), 1301. https://doi.org/10.3390/f11121301