Environmental Effects on Carbon Isotope Discrimination from Assimilation to Respiration in a Coniferous and Broad-Leaved Mixed Forest of Northeast China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Eddy Covariance and Meteorological Measurements
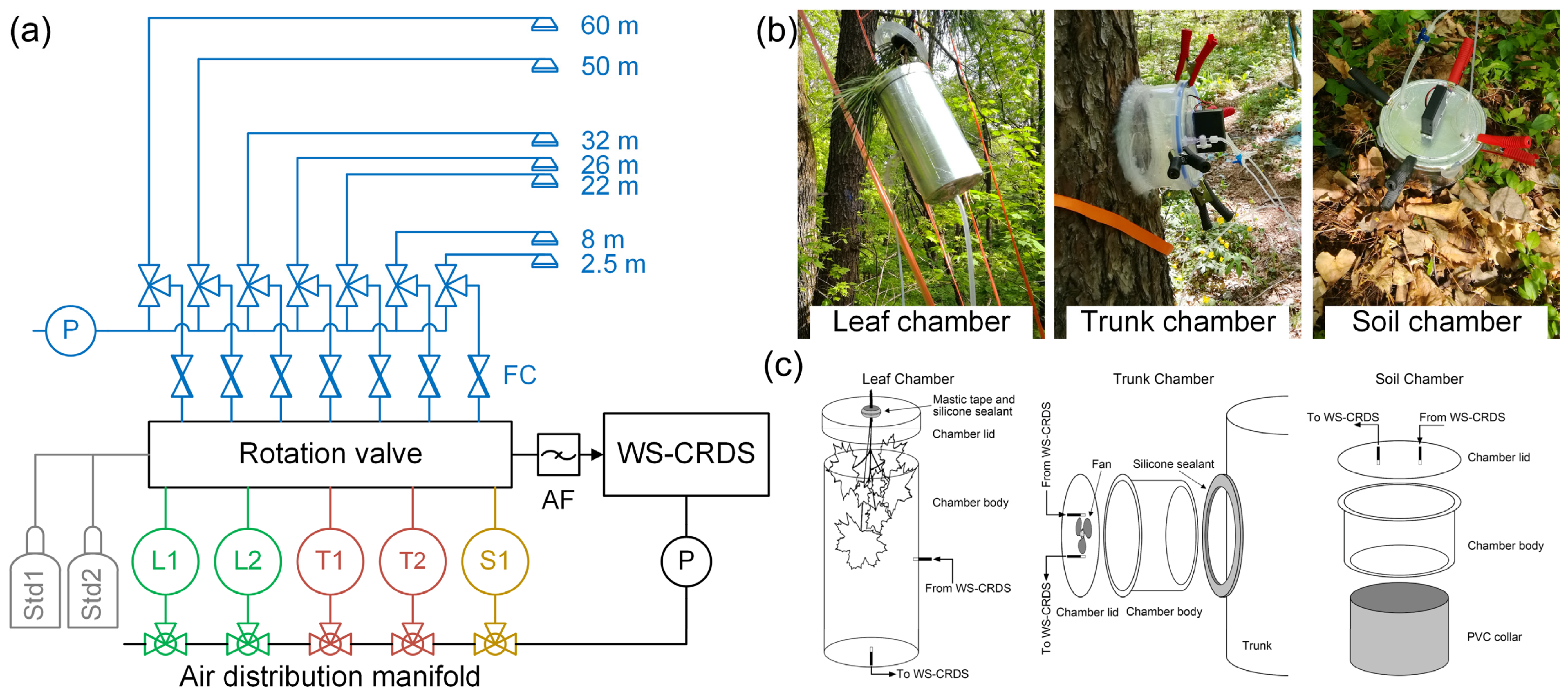
2.3. Canopy CO2 Concentration and Carbon Isotope Composition Profile
2.4. Carbon Isotope Composition of CO2 Respired from the Leaves, Trunks, Soil, and Ecosystem
2.5. Sampling and Carbon Isotope Analysis of the Ecosystem Compartments
2.6. Calculation of Photosynthetic Carbon Isotope Discrimination
2.7. Statistical Analyses
3. Results
3.1. Seasonal Variation of δ13Cair
3.2. Carbon Isotopic Compositions of Leaves and Photosynthetic Carbon Isotope Discrimination
3.3. Carbon Isotopic Compositions of Ecosystem Organic Pools and Respired CO2
3.4. Relationship between δ13CR and Environmental Factors
4. Discussion
4.1. Variations in Carbon Isotope Discrimination from Assimilation to Respiration
4.2. Seasonal Variations in the δ13CR and its Linkage to Environmental Factors
4.3. δ13Ceco Reveals Short-Term Links between Aboveground and Belowground Processes
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Farquhar, G.D.; O’Leary, M.H.; Berry, J.A. On the Relationship between Carbon Isotope Discrimination and the Intercellular Carbon Dioxide Concentration in Leaves. Funct. Plant Biol. 1982, 9, 121–137. [Google Scholar] [CrossRef]
- Cernusak, L.A.; Ubierna, N.; Winter, K.; Holtum, J.A.M.; Marshall, J.D.; Farquhar, G.D. Environmental and physiological determinants of carbon isotope discrimination in terrestrial plants. New Phytol. 2013, 200, 950–965. [Google Scholar] [CrossRef] [PubMed]
- Ehleringer, J.R.; Buchmann, N.; Flanagan, L.B. Carbon isotope ratios in belowground carbon cycle processes. Ecol. Appl. 2000, 10, 412–422. [Google Scholar] [CrossRef]
- Bowling, D.R.; Pataki, D.E.; Randerson, J.T. Carbon isotopes in terrestrial ecosystem pools and CO2 fluxes. New Phytol. 2008, 178, 24–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghashghaie, J.; Duranceau, M.; Badeck, F.-W.; Cornic, G.; Adeline, M.-T.; Deleens, E. δ13C of CO2 respired in the dark in relation to δ13C of leaf metabolites: Comparison between Nicotiana sylvestris and Helianthus annuus under drought. Plant Cell Environ. 2001, 24, 505–515. [Google Scholar] [CrossRef]
- Mortazavi, B.; Chanton, J.P.; Prater, J.L.; Oishi, A.C.; Oren, R.; Katul, G. Temporal variability in 13C of respired CO2 in a pine and a hardwood forest subject to similar climatic conditions. Oecologia 2005, 142, 57–69. [Google Scholar] [CrossRef]
- Prater, J.L.; Mortazavi, B.; Chanton, J.P. Diurnal variation of the δ13C of pine needle respired CO2 evolved in darkness. Plant Cell Environ. 2006, 29, 202–211. [Google Scholar] [CrossRef]
- Maunoury, F.; Berveiller, D.; Lelarge, C.; Pontailler, J.-Y.; Vanbostal, L.; Damesin, C. Seasonal, daily and diurnal variations in the stable carbon isotope composition of carbon dioxide respired by tree trunks in a deciduous oak forest. Oecologia 2007, 151, 268–279. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Gavrichkova, O. Time lag between photosynthesis and carbon dioxide efflux from soil: A review of mechanisms and controls. Glob. Chang. Biol. 2010, 16, 3386–3406. [Google Scholar] [CrossRef]
- Ekblad, A.; Högberg, P. Natural abundance of 13C in CO2 respired from forest soils reveals speed of link between tree photosynthesis and root respiration. Oecologia 2001, 127, 305–308. [Google Scholar] [CrossRef]
- Damesin, C.; Lelarge, C. Carbon isotope composition of current-year shoots from Fagus sylvatica in relation to growth, respiration and use of reserves. Plant Cell Environ. 2003, 26, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Kuptz, D.; Matyssek, R.; Grams, T.E.E. Seasonal dynamics in the stable carbon isotope composition (δ13C) from non-leafy branch, trunk and coarse root CO2 efflux of adult deciduous (Fagus sylvatica) and evergreen (Picea abies) trees. Plant Cell Environ. 2011, 34, 363–373. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G.; Bowling, D.R.; Bond, B.J.; Irvine, J.; Law, B.E.; Anthoni, P.; Ehleringer, J.R. Response of the carbon isotopic content of ecosystem, leaf, and soil respiration to meteorological and physiological driving factors in a Pinus ponderosa ecosystem. Glob. Biogeochem. Cycles 2004, 18. [Google Scholar] [CrossRef]
- Werner, C.; Unger, S.; Pereira, J.S.; Maia, R.; David, T.S.; Kurz-Besson, C.; David, J.S.; Máguas, C. Importance of short-term dynamics in carbon isotope ratios of ecosystem respiration (δ13CR) in a Mediterranean oak woodland and linkage to environmental factors. New Phytol. 2006, 172, 330–346. [Google Scholar] [CrossRef]
- Barbour, M.M.; Hunt, J.E.; Kodama, N.; Laubach, J.; McSeveny, T.M.; Rogers, G.N.D.; Tcherkez, G.; Wingate, L. Rapid changes in δ13C of ecosystem-respired CO2 after sunset are consistent with transient 13C enrichment of leaf respired CO2. New Phytol. 2011, 190, 990–1002. [Google Scholar] [CrossRef] [PubMed]
- Bowling, D.R.; McDowell, N.G.; Bond, B.J.; Law, B.E.; Ehleringer, J.R. 13C content of ecosystem respiration is linked to precipitation and vapor pressure deficit. Oecologia 2002, 131, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Wingate, L.; Ogée, J.; Burlett, R.; Bosc, A.; Devaux, M.; Grace, J.; Loustau, D.; Gessler, A. Photosynthetic carbon isotope discrimination and its relationship to the carbon isotope signals of stem, soil and ecosystem respiration. New Phytol. 2010, 188, 576–589. [Google Scholar] [CrossRef]
- Zobitz, J.M.; Burns, S.P.; Reichstein, M.; Bowling, D.R. Partitioning net ecosystem carbon exchange and the carbon isotopic disequilibrium in a subalpine forest. Glob. Chang. Biol. 2008, 14, 1785–1800. [Google Scholar] [CrossRef]
- Chen, C.; Wei, J.; Wen, X.; Sun, X.; Guo, Q. Photosynthetic carbon isotope discrimination and effects on daytime NEE partitioning in a subtropical mixed conifer plantation. Agric. For. Meteorol. 2019, 272–273, 143–155. [Google Scholar] [CrossRef]
- Damesin, C.; Barbaroux, C.; Berveiller, D.; Lelarge, C.; Chaves, M.; Maguas, C.; Maia, R.; Pontailler, J.-Y. The carbon isotope composition of CO2 respired by trunks: Comparison of four sampling methods. Rapid Commun. Mass Spectrom. 2005, 19, 369–374. [Google Scholar] [CrossRef]
- Kammer, A.; Tuzson, B.; Emmenegger, L.; Knohl, A.; Mohn, J.; Hagedorn, F. Application of a quantum cascade laser-based spectrometer in a closed chamber system for real-time δ13C and δ18O measurements of soil-respired CO2. Agric. For. Meteorol. 2011, 151, 39–48. [Google Scholar] [CrossRef]
- Keeling, C.D. The concentration and isotopic abundances of atmospheric carbon dioxide in rural areas. Geochim. Cosmochim. Acta 1958, 13, 322–334. [Google Scholar] [CrossRef]
- Keeling, C.D. The concentration and isotopic abundances of carbon dioxide in rural and marine air. Geochim. Cosmochim. Acta 1961, 24, 277–298. [Google Scholar] [CrossRef]
- Zobitz, J.M.; Keener, J.P.; Schnyder, H.; Bowling, D.R. Sensitivity analysis and quantification of uncertainty for isotopic mixing relationships in carbon cycle research. Agric. For. Meteorol. 2006, 136, 56–75. [Google Scholar] [CrossRef]
- Chen, C.; Pang, J.; Wei, J.; Wen, X.; Sun, X. Inter-comparison of three models for δ13C of respiration with four regression approaches. Agric. For. Meteorol. 2017, 247, 229–239. [Google Scholar] [CrossRef]
- Guan, D.; Wu, J.; Zhao, X.; Han, S.; Yu, G.; Sun, X.; Jin, C. CO2 fluxes over an old, temperate mixed forest in northeastern China. Agric. For. Meteorol. 2006, 137, 138–149. [Google Scholar] [CrossRef]
- Kodama, N.; Barnard, R.L.; Salmon, Y.; Weston, C.; Ferrio, J.P.; Holst, J.; Werner, R.A.; Saurer, M.; Rennenberg, H.; Buchmann, N.; et al. Temporal dynamics of the carbon isotope composition in a Pinus sylvestris stand: From newly assimilated organic carbon to respired carbon dioxide. Oecologia 2008, 156, 737. [Google Scholar] [CrossRef]
- Brandes, E.; Kodama, N.; Whittaker, K.; Weston, C.; Rennenberg, H.; Keitel, C.; Adams, M.A.; Gessler, A. Short-term variation in the isotopic composition of organic matter allocated from the leaves to the stem of Pinus sylvestris: Effects of photosynthetic and postphotosynthetic carbon isotope fractionation. Glob. Chang. Biol. 2006, 12, 1922–1939. [Google Scholar] [CrossRef]
- Gessler, A.; Keitel, C.; Kodama, N.; Weston, C.; Winters, A.J.; Keith, H.; Grice, K.; Leuning, R.; Farquhar, G.D. δ13C of organic matter transported from the leaves to the roots in Eucalyptus delegatensis: Short-term variations and relation to respired CO2. Funct. Plant Biol. 2007, 34, 692–706. [Google Scholar] [CrossRef]
- Wen, X.F.; Meng, Y.; Zhang, X.Y.; Sun, X.M.; Lee, X. Evaluating calibration strategies for isotope ratio infrared spectroscopy for atmospheric 13CO2/12CO2 measurement. Atmos. Meas. Tech. 2013, 6, 1491–1501. [Google Scholar] [CrossRef]
- Pang, J.; Wen, X.; Sun, X.; Huang, K. Intercomparison of two cavity ring-down spectroscopy analyzers for atmospheric 13CO2/12CO2 measurement. Atmos. Meas. Tech. 2016, 9, 3879–3891. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Yang, Z.; Wu, G.; Yang, Y.; Lin, G. The relationship between soil CO2 efflux and its carbon isotopic composition under non-steady-state conditions. Agric. For. Meteorol. 2018, 256-257, 492–500. [Google Scholar] [CrossRef]
- Wehr, R.; Saleska, S.R. An improved isotopic method for partitioning net ecosystem–atmosphere CO2 exchange. Agric. For. Meteorol. 2015, 214–215, 515–531. [Google Scholar] [CrossRef] [Green Version]
- Brugnoli, E.; Farquhar, G.D. Photosynthetic fractionation of carbon isotopes. In Photosynthesis: Physiology and Metabolism; Leegood, R.C., Sharkey, T.D., von Caemmerer, S., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 399–434. [Google Scholar] [CrossRef]
- Brooks, A.; Farquhar, G.D. Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Planta 1985, 165, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Tcherkez, G.; Schäufele, R.; Nogués, S.; Piel, C.; Boom, A.; Lanigan, G.; Barbaroux, C.; Mata, C.; Elhani, S.; Hemming, D.; et al. On the 13C/12C isotopic signal of day and night respiration at the mesocosm level. Plant Cell Environ. 2010, 33, 900–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowling, D.R.; Tans, P.P.; Monson, R.K. Partitioning net ecosystem carbon exchange with isotopic fluxes of CO2. Glob. Chang. Biol. 2001, 7, 127–145. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Field, C.B.; Lin, Z.-f.; Kuo, C.-y. Leaf carbon isotope and mineral composition in subtropical plants along an irradiance cline. Oecologia 1986, 70, 520–526. [Google Scholar] [CrossRef]
- Brooks, J.R.; Flanagan, L.B.; Varney, G.T.; Ehleringer, J.R. Vertical gradients in photosynthetic gas exchange characteristics and refixation of respired CO2 within boreal forest canopies. Tree Physiol. 1997, 17, 1–12. [Google Scholar] [CrossRef]
- Tan, W.; Wang, G.; Han, J.; Liu, M.; Zhou, L.; Luo, T.; Cao, Z.; Cheng, S. δ13C and water-use efficiency indicated by δ13C of different plant functional groups on Changbai Mountains, Northeast China. Chin. Sci. Bull. 2009, 54, 1759–1764. [Google Scholar] [CrossRef] [Green Version]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Scartazza, A.; Moscatello, S.; Matteucci, G.; Battistelli, A.; Brugnoli, E. Seasonal and inter-annual dynamics of growth, non-structural carbohydrates and C stable isotopes in a Mediterranean beech forest. Tree Physiol. 2013, 33, 730–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinne, K.T.; Saurer, M.; Kirdyanov, A.V.; Bryukhanova, M.V.; Prokushkin, A.S.; Churakova, O.V.; Siegwolf, R.T.W. Examining the response of needle carbohydrates from Siberian larch trees to climate using compound-specific δ13C and concentration analyses. Plant Cell Environ. 2015, 38, 2340–2352. [Google Scholar] [CrossRef]
- Jäggi, M.; Saurer, M.; Fuhrer, J.; Siegwolf, R. The relationship between the stable carbon isotope composition of needle bulk material, starch, and tree rings in Picea abies. Oecologia 2002, 131, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Cernusak, L.A.; Marshall, J.D.; Comstock, J.P.; Balster, N.J. Carbon isotope discrimination in photosynthetic bark. Oecologia 2001, 128, 24–35. [Google Scholar] [CrossRef]
- Badeck, F.-W.; Tcherkez, G.; Nogués, S.; Piel, C.; Ghashghaie, J. Post-photosynthetic fractionation of stable carbon isotopes between plant organs—A widespread phenomenon. Rapid Commun. Mass Spectrom. 2005, 19, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Gessler, A. Diel variations in the carbon isotope composition of respired CO2 and associated carbon sources: A review of dynamics and mechanisms. Biogeosciences 2011, 8, 2437–2459. [Google Scholar] [CrossRef] [Green Version]
- Ghashghaie, J.; Badeck, F.W. Opposite carbon isotope discrimination during dark respiration in leaves versus roots—A review. New Phytol. 2014, 201, 751–769. [Google Scholar] [CrossRef]
- Millard, P.; Midwood, A.J.; Hunt, J.E.; Barbour, M.M.; Whitehead, D. Quantifying the contribution of soil organic matter turnover to forest soil respiration, using natural abundance δ13C. Soil Biol. Biochem. 2010, 42, 935–943. [Google Scholar] [CrossRef]
- Ngao, J.; Cotrufo, M.F. Carbon isotope discrimination during litter decomposition can be explained by selective use of substrate with differing δ13C. Biogeosci. Discuss. 2011, 8, 51–82. [Google Scholar] [CrossRef]
- ŠantRůČková, H.; Bird, M.I.; Lloyd, J. Microbial processes and carbon-isotope fractionation in tropical and temperate grassland soils. Funct. Ecol. 2000, 14, 108–114. [Google Scholar] [CrossRef]
- Dijkstra, P.; Ishizu, A.; Doucett, R.; Hart, S.C.; Schwartz, E.; Menyailo, O.V.; Hungate, B.A. 13C and 15N natural abundance of the soil microbial biomass. Soil Biol. Biochem. 2006, 38, 3257–3266. [Google Scholar] [CrossRef]
- Werth, M.; Kuzyakov, Y. 13C fractionation at the root–microorganisms–soil interface: A review and outlook for partitioning studies. Soil Biol. Biochem. 2010, 42, 1372–1384. [Google Scholar] [CrossRef]
- Goffin, S.; Aubinet, M.; Maier, M.; Plain, C.; Schack-Kirchner, H.; Longdoz, B. Characterization of the soil CO2 production and its carbon isotope composition in forest soil layers using the flux-gradient approach. Agric. For. Meteorol. 2014, 188, 45–57. [Google Scholar] [CrossRef]
- Formánek, P.; Ambus, P. Assessing the use of δ13C natural abundance in separation of root and microbial respiration in a Danish beech (Fagus sylvatica L.) forest. Rapid Commun. Mass Spectrom. 2004, 18, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Bowling, D.R.; Egan, J.E.; Hall, S.J.; Risk, D.A. Environmental forcing does not induce diel or synoptic variation in the carbon isotope content of forest soil respiration. Biogeosciences 2015, 12, 5143–5160. [Google Scholar] [CrossRef] [Green Version]
- Duranceau, M.; Ghashghaie, J.; Badeck, F.; Deleens, E.; Cornic, G. δ13C of CO2 respired in the dark in relation to δ13C of leaf carbohydrates in Phaseolus vulgaris L. under progressive drought. Plant Cell Environ. 1999, 22, 515–523. [Google Scholar] [CrossRef]
- Loader, N.J.; McCarroll, D.; Gagen, M.; Robertson, I.; Jalkanen, R. Extracting climatic information from stable isotopes in tree rings. In Terrestrial Ecology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 1, pp. 25–48. [Google Scholar]
- Fessenden, J.E.; Ehleringer, J.R. Temporal variation in δ13C of ecosystem respiration in the Pacific Northwest: Links to moisture stress. Oecologia 2003, 136, 129–136. [Google Scholar] [CrossRef]
- Ekblad, A.; Boström, B.; Holm, A.; Comstedt, D. Forest soil respiration rate and δ13C is regulated by recent above ground weather conditions. Oecologia 2005, 143, 136–142. [Google Scholar] [CrossRef]
- Knohl, A.; Werner, R.A.; Brand, W.A.; Buchmann, N. Short-term variations in δ13C of ecosystem respiration reveals link between assimilation and respiration in a deciduous forest. Oecologia 2005, 142, 70–82. [Google Scholar] [CrossRef]
- Scartazza, A.; Mata, C.; Matteucci, G.; Yakir, D.; Moscatello, S.; Brugnoli, E. Comparisons of δ13C of photosynthetic products and ecosystem respiratory CO2 and their responses to seasonal climate variability. Oecologia 2004, 140, 340–351. [Google Scholar] [CrossRef]
- McDowell, N.G.; Bowling, D.R.; Schauer, A.; Irvine, J.; Bond, B.J.; Law, B.E.; Ehleringer, J.R. Associations between carbon isotope ratios of ecosystem respiration, water availability and canopy conductance. Glob. Chang. Biol. 2004, 10, 1767–1784. [Google Scholar] [CrossRef]
- Bowling, D.R.; Burns, S.P.; Conway, T.J.; Monson, R.K.; White, J.W.C. Extensive observations of CO2 carbon isotope content in and above a high-elevation subalpine forest. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Schaeffer, S.M.; Anderson, D.E.; Burns, S.P.; Monson, R.K.; Sun, J.; Bowling, D.R. Canopy structure and atmospheric flows in relation to the δ13C of respired CO2 in a subalpine coniferous forest. Agric. For. Meteorol. 2008, 148, 592–605. [Google Scholar] [CrossRef]





| Pearson’s Correlation Coefficient (r) | δ13CR (‰) | ||||
|---|---|---|---|---|---|
| Pk Leaf | Am Leaf | Pk Trunk | Fm Trunk | Soil | |
| Air temperature (°C) | −0.035 | 0.009 | −0.363 ** | −0.443 ** | 0.234 |
| VPD (kPa) | 0.110 | 0.086 | −0.146 | −0.120 | 0.101 |
| Global radiation (W m−2) | 0.166 | 0.242 | 0.244 | 0.156 | 0.312 * |
| Soil temperature (°C) | −0.199 | −0.101 | −0.462 ** | −0.513 ** | 0.092 |
| Soil moisture (m3 m−3) | −0.000 | −0.056 | −0.484 ** | −0.469 ** | 0.153 |
| Lag | r | |
|---|---|---|
| Air temperature (°C) | 0 | −0.256 * |
| VPD (kPa) | 10 | −0.280 * |
| Global radiation (W m−2) | 0 | −0.217 |
| Soil temperature (°C) | 10 | 0.085 |
| Soil moisture (m3 m−3) | 10 | 0.295 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diao, H.; Wang, A.; Yuan, F.; Guan, D.; Dai, G.; Wu, J. Environmental Effects on Carbon Isotope Discrimination from Assimilation to Respiration in a Coniferous and Broad-Leaved Mixed Forest of Northeast China. Forests 2020, 11, 1156. https://doi.org/10.3390/f11111156
Diao H, Wang A, Yuan F, Guan D, Dai G, Wu J. Environmental Effects on Carbon Isotope Discrimination from Assimilation to Respiration in a Coniferous and Broad-Leaved Mixed Forest of Northeast China. Forests. 2020; 11(11):1156. https://doi.org/10.3390/f11111156
Chicago/Turabian StyleDiao, Haoyu, Anzhi Wang, Fenghui Yuan, Dexin Guan, Guanhua Dai, and Jiabing Wu. 2020. "Environmental Effects on Carbon Isotope Discrimination from Assimilation to Respiration in a Coniferous and Broad-Leaved Mixed Forest of Northeast China" Forests 11, no. 11: 1156. https://doi.org/10.3390/f11111156
APA StyleDiao, H., Wang, A., Yuan, F., Guan, D., Dai, G., & Wu, J. (2020). Environmental Effects on Carbon Isotope Discrimination from Assimilation to Respiration in a Coniferous and Broad-Leaved Mixed Forest of Northeast China. Forests, 11(11), 1156. https://doi.org/10.3390/f11111156







