Functional Divergence between Várzea and Igapó Forests: A Study of Functional Trait Diversity in the Colombian Orinoco Basin
Abstract
:1. Introduction
2. Materials and Methods
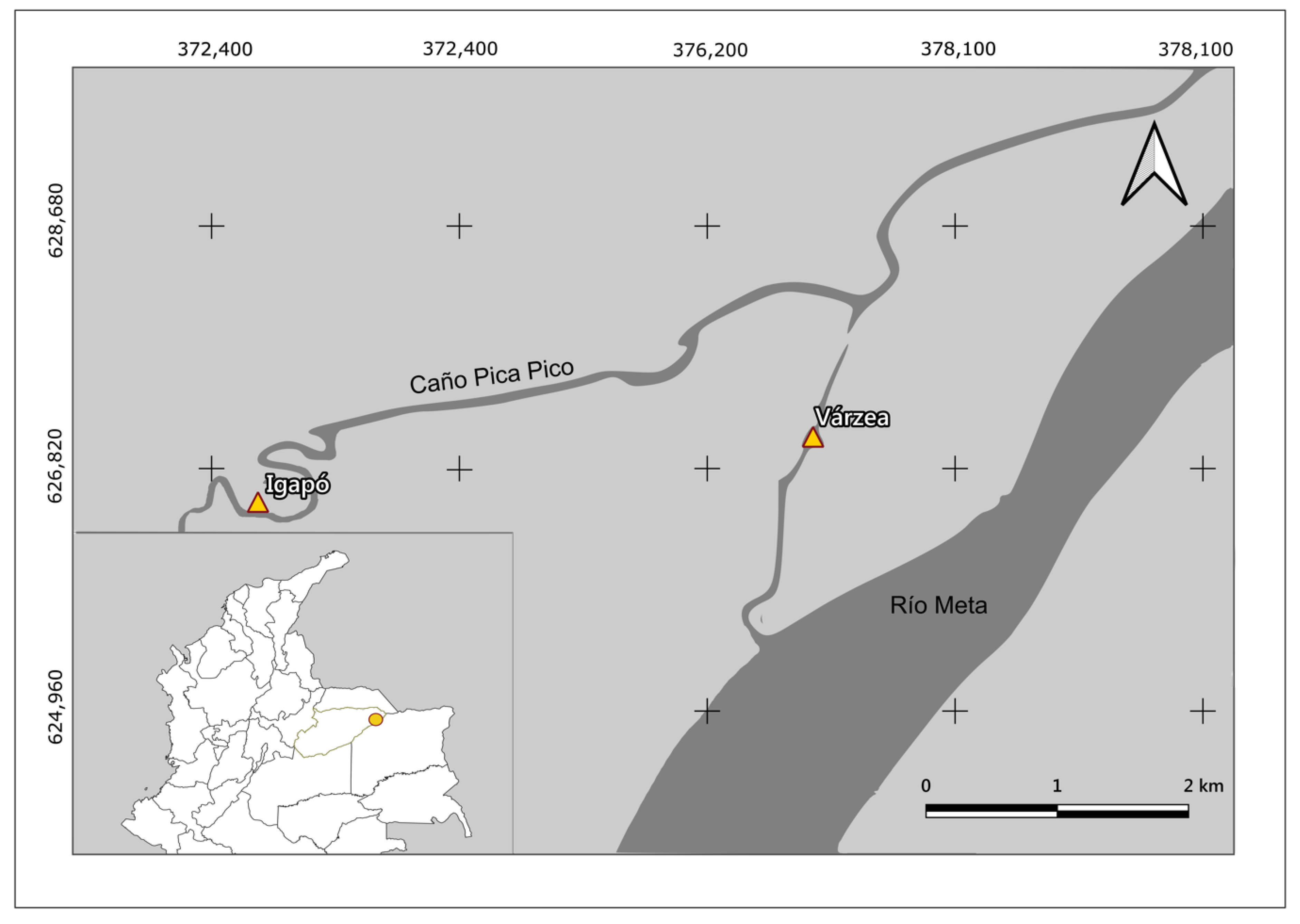
2.1. Study Area
2.2. Sampling and Measurement of Functional Traits
2.3. Statistical Analysis
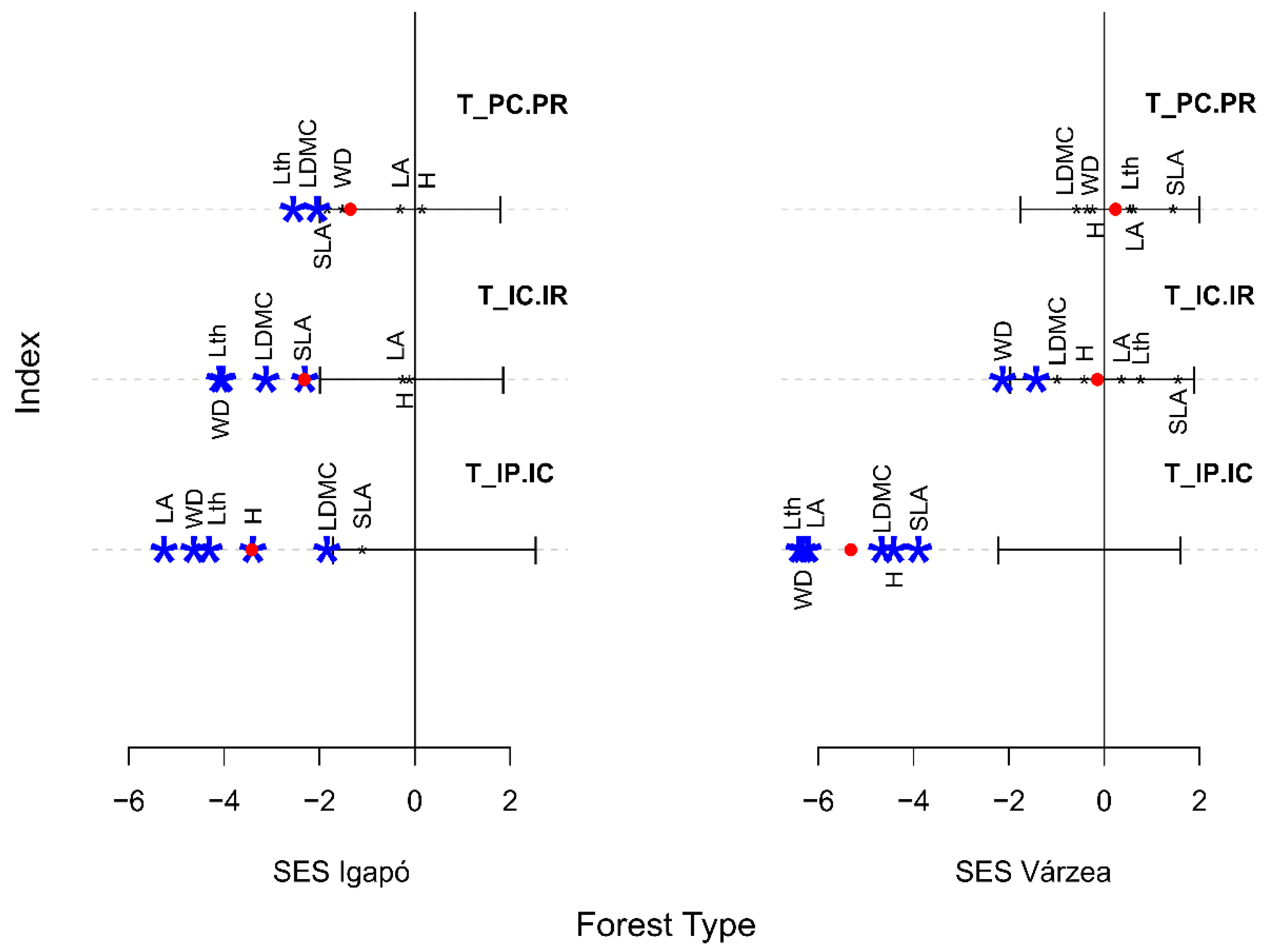
3. Results
3.1. Taxonomic Diversity of Várzea and Igapó Forests
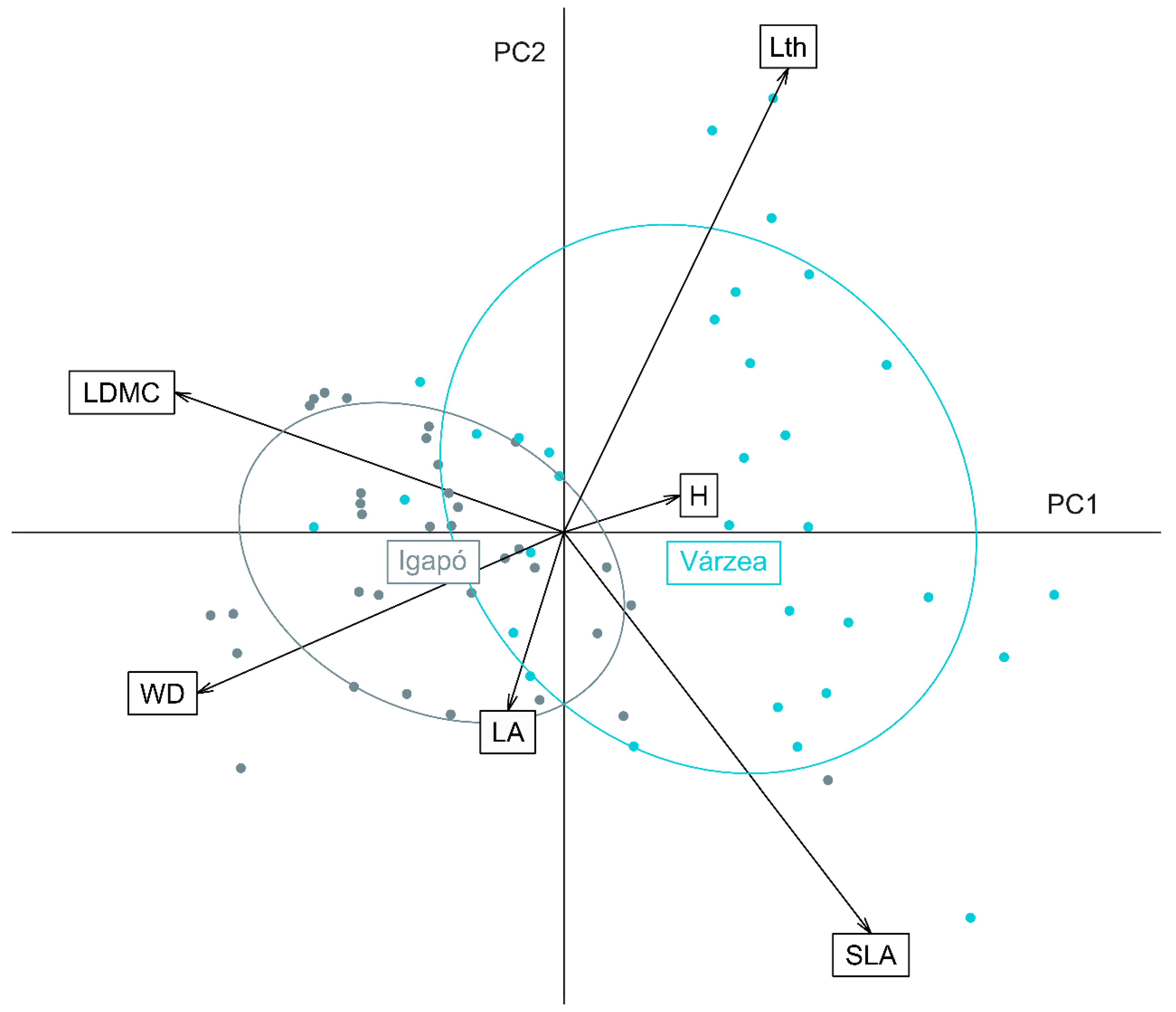
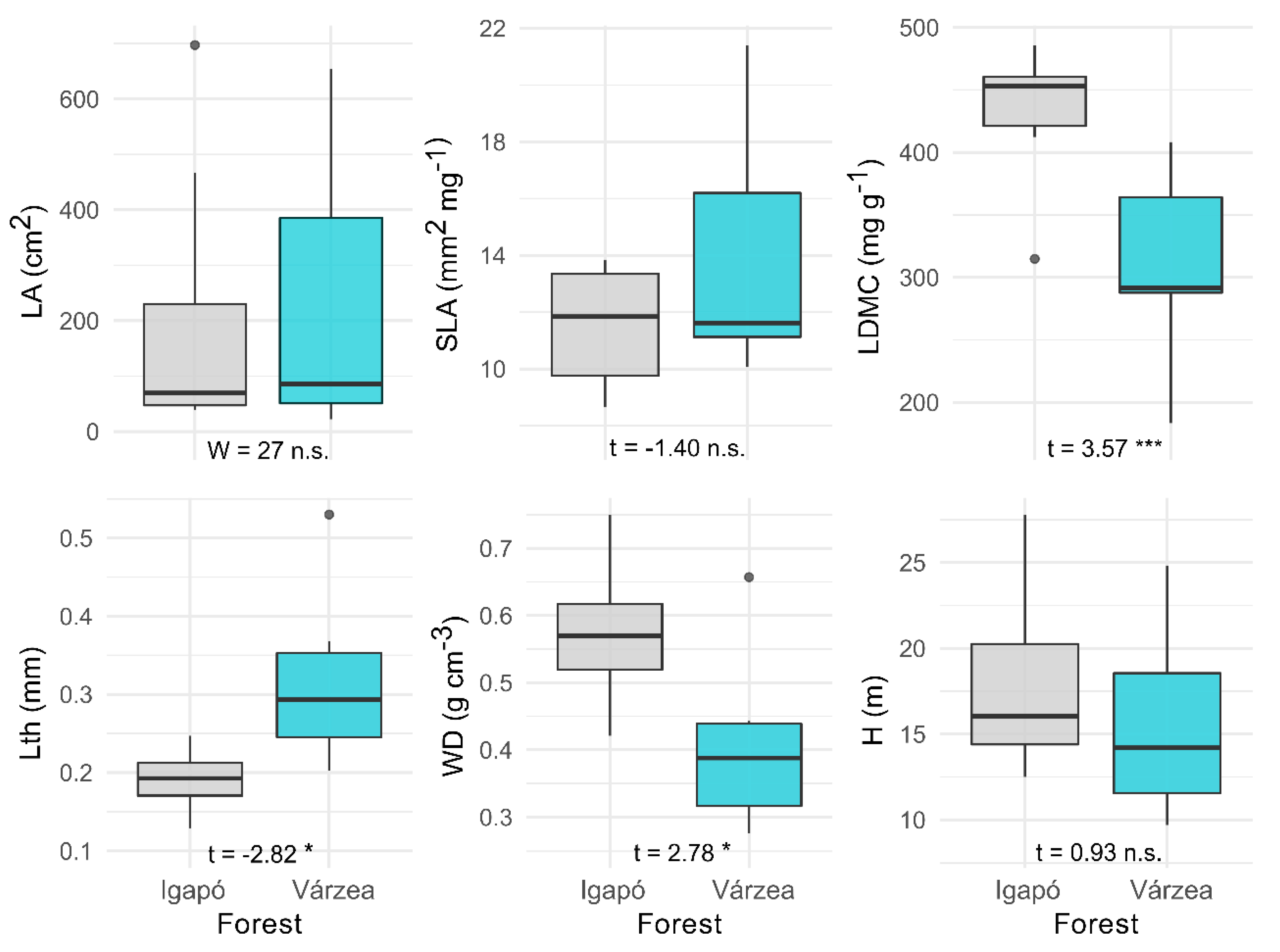
3.2. Functional Traits of Várzea and Igapó Forests
3.3. Components of the Functional Diversity of Várzea and Igapó Forests
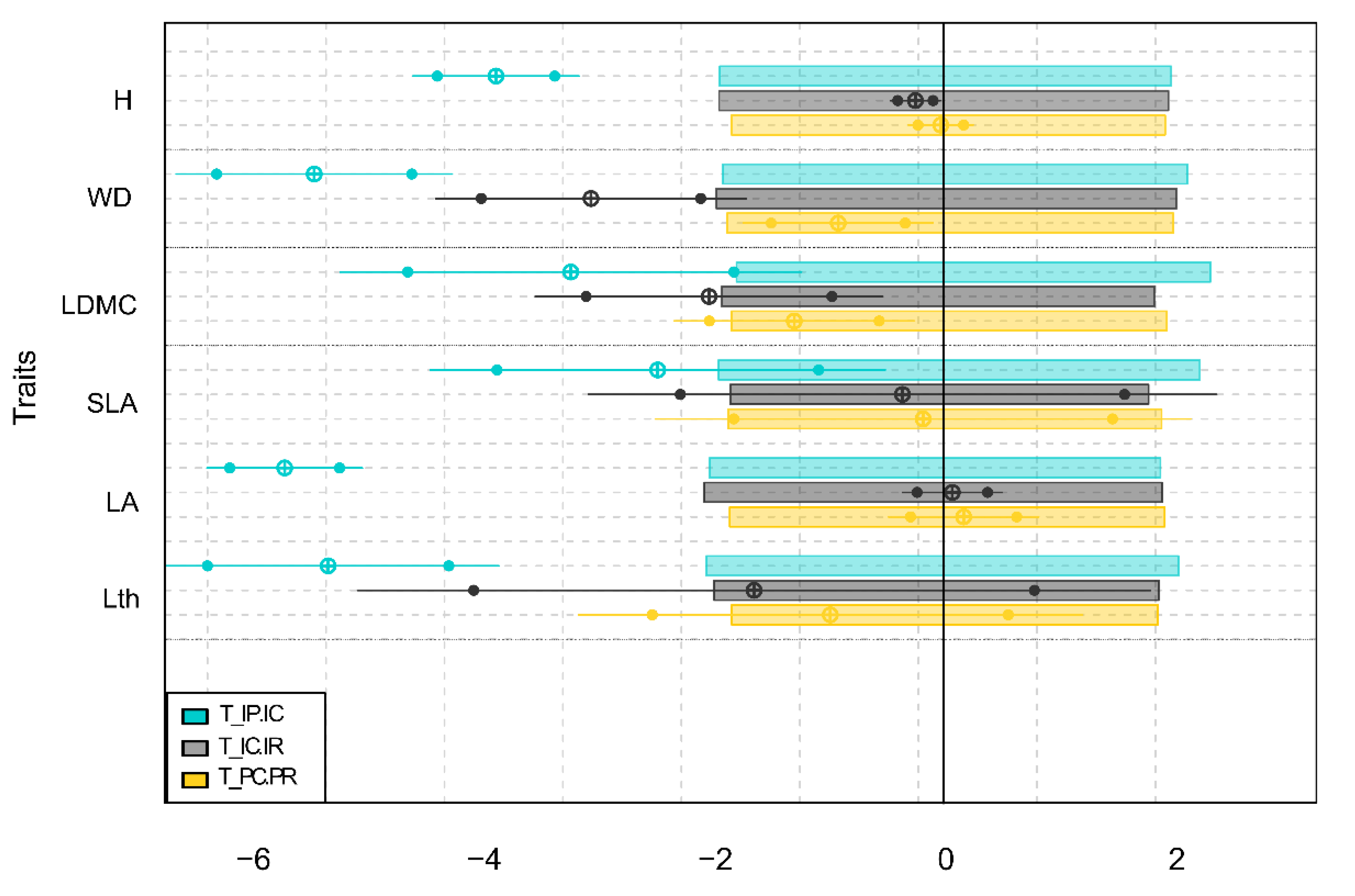
3.4. Internal and External Filters Influence on the Community Assembly of Flooded Forest
4. Discussion
4.1. Species Diversity in Várzea and Igapó Forests
4.2. Functional Divergence between Várzea and Igapó Forests
4.3. Effect of Internal and External Filters on Community Assembly and Functional Diversity of Flooded Forests
4.4. Orinoco and Amazon Basin Floodplain Forests
4.5. Orinoco Flooded Forests Compared to Other Ecosystems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haugaasen, T.; Peres, C.A. Floristic, edaphic and structural characteristics of flooded and unflooded forests in the lower Rio Purús region of central Amazonia, Brazil. Acta Amaz. 2006, 36, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, P.R.; Aldana, A.M.; Cárdenas, S.; Negret, P.J. Flooding and soil composition determine beta diversity of lowland forests in Northern South America. Biotropica 2018, 50, 568–577. [Google Scholar] [CrossRef]
- Lasso, C.A.; Córdoba, D.; Morales-Betancourt, M.A. Áreas Clave para la Conservación de la Biodiversidad Dulceacuícola Amenazada en Colombia: Moluscos, Cangrejos, Peces, Tortugas, Crocodílidos, Aves y Mamiferos; Serie Editorial Recursos Hidrobiológicos y Pesqueros Continentales de Colombia; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2017; p. 353. [Google Scholar]
- Parolin, P.; Wittmann, F. Struggle in the flood: Tree responses to flooding stress in four tropical floodplain systems. AoB Plants 2010, 2010, plq003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petit, R.J.; Hampe, A. Some evolutionary consequences of being a tree. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 187–214. [Google Scholar]
- Parolin, P.D.; De Simone, O.; Haase, K.; Waldhoff, D.; Rottenberger, S.; Kuhn, U.; Junk, W.J. Central Amazonian floodplain forests: Tree adaptations in a pulsing system. Bot. Rev. 2004, 70, 357–380. [Google Scholar] [CrossRef]
- Junk, W.J.; Piedade, M.T.F. An Introduction to South American wetland forests: Distribution, definitions and general characterization. In Amazonian Floodplain Forests. Ecological Studies (Analysis and Synthesis); Junk, W., Piedade, M., Wittmann, F., Schöngart, J., Parolin, P., Eds.; Springer: Dordrecht, The Netherlands, 2010; Volume 210. [Google Scholar] [CrossRef]
- Junk, W.J.; Piedade, M.T.F.; Parolin, P.; Wittmann, F.; Schöngart, J. Ecophysiology, biodiversity and sustainable management of central Amazonian floodplain forests: A synthesis. In Amazonian Floodplain Forests. Ecological Studies (Analysis and Synthesis); Junk, W., Piedade, M., Wittmann, F., Schöngart, J., Parolin, P., Eds.; Springer: Dordrecht, The Netherlands, 2010; Volume 210. [Google Scholar] [CrossRef]
- Ricaurte, L.F.; Patiño, J.E.; Zambrano, D.F.R.; Arias-G, J.C.; Acevedo, O.; Aponte, C.; Estupinan-Suarez, L.M. A classification system for colombian wetlands: An essential step forward in open environmental policy-making. Wetlands 2019, 39, 971–990. [Google Scholar] [CrossRef]
- Prance, G.T. Notes on the vegetation of Amazonia III. The terminology of Amazonian forest types subject to inundation. Brittonia 1979, 31, 26–38. [Google Scholar] [CrossRef]
- Melack, J.M.; Hess, L.L. Remote sensing of the distribution and extent of wetlands in the Amazon basin. In Amazonian Floodplain Forests. Ecological Studies (Analysis and Synthesis); Junk, W., Piedade, M., Wittmann, F., Schöngart, J., Parolin, P., Eds.; Springer: Dordrecht, The Netherlands, 2010; Volume 210, pp. 43–59. [Google Scholar] [CrossRef]
- Junk, W.J. Wetlands of tropical South America. In Wetlands of the World: Inventory, Ecology and Management Volume I; Handbook of Vegetation Science; Whigham, D.F., Dykyjová, D., Hejný, S., Eds.; Springer: Dordrecht, The Netherlands, 1993; Volume 15, pp. 679–739. [Google Scholar] [CrossRef]
- Irion, G.; de Mello, J.A.; Morais, J.; Piedade, M.T.; Junk, W.J.; Garming, L. Development of the Amazon valley during the Middle to Late Quaternary: Sedimentological and climatological observations. In Amazonian Floodplain Forests. Ecological Studies (Analysis and Synthesis); Junk, W., Piedade, M., Wittmann, F., Schöngart, J., Parolin, P., Eds.; Springer: Dordrecht, The Netherlands, 2010; Volume 210, pp. 27–42. [Google Scholar] [CrossRef]
- Wittmann, F.; Schöngart, J.; Montero, J.C.; Motzer, T.; Junk, W.J.; Piedade, M.T.; Worbes, M. Tree species composition and diversity gradients in white-water forests across the Amazon Basin. J. Biogeogr. 2006, 33, 1334–1347. [Google Scholar] [CrossRef]
- Ferreira, L.V.; Prance, G.T. Structure and species richness of low-diversity floodplain forest on the Rio Tapajós, Eastern Amazonia, Brazil. Biodivers. Conserv. 1998, 7, 585–596. [Google Scholar] [CrossRef]
- Mori, G.B.; Schietti, J.; Poorter, L.; Piedade, M.T.F. Trait divergence and habitat specialization in tropical floodplain forests trees. PLoS ONE 2019, 14, e0212232. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Diaz, S.; Gamier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Urcelay, C. Corrigendum to: New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2016, 64, 715–716. [Google Scholar] [CrossRef] [Green Version]
- Cárdenas, S. Patrones Florísticos de los Planos de Inundación y Bosques de Tierra Firme: Efectos de Filtros Ambientales y Azar. Bachelor´s Thesis, Universidad de los Andes, Bogotá, Colombia, 2012. [Google Scholar]
- Gómez, Y.A. Influencia de los Nutrientes del Suelo y Otros Factores Abióticos en la Distribución de Especies en Bosques de Igapó y Várzea, Casanare. Bachelor’s Thesis, Universidad de los Andes, Bogotá, Colombia, 2017. [Google Scholar]
- Garnier, E.; Navas, M.L.; Grigulis, K. Plant Functional Diversity: Organism Traits, Community Structure, and Ecosystem Properties; Oxford University Press: Oxford, UK, 2016. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Costa, F.R.; van Baalen, E.; de Jonge, A.; Bittencourt, P.R.; Almanza, Y.; Guimaraes, Z.T. Embolism resistance drives the distribution of Amazonian rainforest tree species along hydro-topographic gradients. New Phytol. 2019, 221, 1457–1465. [Google Scholar] [CrossRef] [Green Version]
- Reich, P.B. The world-wide ‘fast–slow’plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Lortie, C.J.; Brooker, R.W.; Choler, P.; Kikvidze, Z.; Michalet, R.; Pugnaire, F.I.; Callaway, R.M. Rethinking plant community theory. Oikos 2004, 107, 433–438. [Google Scholar] [CrossRef]
- Violle, C.; Enquist, B.J.; McGill, B.J.; Jiang, L.; Albert, C.H.; Hulshof, C.; Messier, J. The return of the variance: Intraspecific variability in community ecology. Trends Ecol. Evol. 2012, 27, 244–252. [Google Scholar] [CrossRef]
- Godoy, J.R.; Petts, G.; Salo, J. Riparian flooded forests of the Orinoco and Amazon basins: A comparative review. Biodivers. Conserv. 1999, 8, 551–586. [Google Scholar] [CrossRef]
- SIAC Sistema de Información Ambiental de Colombia. Available online: http://www.siac.gov.co/catalogo-de-mapas (accessed on 20 September 2020).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.B.; Wiemann, M.C. Measuring wood specific gravity… correctly. Am. J. Bot. 2010, 97, 519–524. [Google Scholar] [CrossRef] [Green Version]
- Schleuter, D.; Daufresne, M.; Massol, F.; Argillier, C. A user’s guide to functional diversity indices. Ecol. Monogr. 2010, 80, 469–484. [Google Scholar] [CrossRef] [Green Version]
- Cornwell, W.K.; Schwilk, D.W.; Ackerly, D.D. A trait-based test for habitat filtering: Convex hull volume. Ecology 2006, 87, 1465–1471. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laliberté, E.; Legendre, P. A distance based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Taudière, A.; Violle, C. cati: A R package using functional traits to detect and quantify multi-level community assembly processes. Ecography 2016, 39, 699–708. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Solymos, P. Vegan: Community Ecology Package. R Package Version 2.5–6. Available online: https://cran.r-project.org/web/packages/vegan/ (accessed on 15 April 2020).
- Dray, S.; Dufour, A.B. The ade4 Package: Implementing the Duality Diagram for Ecologists. R Package Version 1.7–15. Available online: https://cran.r-project.org/web/packages/ade4/ (accessed on 15 April 2020).
- Laliberté, A.E.; Legendre, P.; Shipley, B.; Laliberté, M.E. FD: Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology. R Package: 0–12. Available online: https://cran.r-project.org/web/packages/FD/ (accessed on 25 April 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; Version 3.6.3; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://cran.r-project.org/bin/windows/base/old/3.6.3/ (accessed on 30 March 2020).
- Parolin, P.; Ferreira, L.V. Are there differences in specific wood gravities between trees in várzea and igapó (Central Amazonia). Ecotropica 1998, 4, 25–32. [Google Scholar]
- González-Abella, J.S. Dinámica, Estructura y Diversidad de Bosques de Galería de la Reserva de Tomo Grande, Vichada. Bachelor’s Thesis, Universidad de los Andes, Bogotá, Colombia, 2015. [Google Scholar]
- Lopez, O.R.; Kursar, T.A. Flood tolerance of four tropical tree species. Tree Physiol. 1999, 19, 925–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junk, W.J. Flood tolerance and tree distribution in central Amazonian floodplain. In Tropical Forests; Botanical Dynamics, Speciation and Diversity; Holm-Nielsen, L.B., Nielsen, I.C., Baslev, H., Eds.; Academic Press: San Diego, CA, USA, 1989; p. 645. [Google Scholar] [CrossRef]
- Umaña, M.N.; Norden, N.; Cano, Á.; Stevenson, P.R. Determinants of plant community assembly in a mosaic of landscape units in Central Amazonia: Ecological and phylogenetic perspectives. PLoS ONE 2012, 7, e45199. [Google Scholar] [CrossRef]
- Correa, H.D.; Ruíz, S.L.; Arevalo, L.M. Plan de Acción en Biodiversidad de la Cuenca del Orinoco 2005–2015: Propuesta Técnica; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2006. [Google Scholar]
- Cano, A.; Stevenson, P.R. Diversidad y composición florística de tres tipos de bosque en la Estación Biológica Caparú, Vaupés. Colomb. For. 2009, 12, 63–80. [Google Scholar] [CrossRef]
- Kubitzki, K. The ecogeographical differentiation of Amazonian inundation forests. Plant Syst. Evol. 1989, 162, 285–304. [Google Scholar] [CrossRef]
- Worbes, M. The forest ecosystem of the floodplains. In The Central Amazon Floodplain. Ecological Studies (Analysis and Synthesis); Junk, W.J., Ed.; Springer: Berlin/Heidelberg, Germany, 1997; Volume 126, pp. 223–265. [Google Scholar] [CrossRef]
- Schmidt, G.W. Primary production of phytoplankton in the three types of Amazonian waters. IV. On the primary productivity of phytoplankton in a bay of the lower Rio Negro (Amazonas, Brazil). Amaz. Limnol. Oecol. Reg. Syst. Flum. Amaz. 1976, 5, 517–528. [Google Scholar]
- Parolin, P.D.; Worbes, M. Wood density of trees in black water floodplains of Rio Jaú National Park, Amazonia, Brazil. Acta Amaz. 2000, 30, 441–448. [Google Scholar] [CrossRef] [Green Version]
- De Bello, F.; LepŠ, J.A.N.; Sebastià, M.T. Predictive value of plant traits to grazing along a climatic gradient in the Mediterranean. J. Appl. Ecol. 2005, 42, 824–833. [Google Scholar] [CrossRef]
- Pinay, G.; Fabre, A.; Vervier, P.; Gazelle, F. Control of C, N, P distribution in soils of riparian forests. Landsc. Ecol. 1992, 6, 121–132. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and soil acidity. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 475–490. [Google Scholar] [CrossRef] [Green Version]
- Mason, N.W.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Tilman, D. Biodiversity: Population versus ecosystem stability. Ecology 1996, 77, 350–363. [Google Scholar] [CrossRef]
- Maracahipes, L.; Carlucci, M.B.; Lenza, E.; Marimon, B.S.; Marimon, B.H., Jr.; Guimarães, F.A.; Cianciaruso, M.V. How to live in contrasting habitats? Acquisitive and conservative strategies emerge at inter-and intraspecific levels in savanna and forest woody plants. Perspect. Plant Ecol. Evol. Syst. 2018, 34, 17–25. [Google Scholar] [CrossRef]
- Poorter, L.; Rozendaal, D.M.; Bongers, F.; de Almeida-Cortez, J.S.; Zambrano, A.M.A.; Álvarez, F.S.; Bentos, T.V. Wet and dry tropical forests show opposite successional pathways in wood density but converge over time. Nat. Ecol. Evol. 2019, 3, 928–934. [Google Scholar] [CrossRef]
- Thomas, E.; Alcazar, C.; Moscoso-Higuita, L.G.; Osorio, L.F.; Salgado-Negret, B.; Gonzalez, M.; Ramirez, W. The Importance of Species Selection and Seed Sourcing in Forest Restoration for Enhancing Adaptive Potential to Climate Change: Colombian Tropical Dry Forest as a Model; Secretariat of the Convention on Biological Diversity: Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Kattge, J.; Diaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Bönisch, G.; Cornelissen, J.H.C. TRY—A Global Database of Plant Traits. Available online: https://www.try-db.org/TryWeb/Home.php (accessed on 10 May 2020).
- Lebrija-Trejos, E.; Pérez-García, E.A.; Meave, J.A.; Bongers, F.; Poorter, L. Functional traits and environmental filtering drive community assembly in a species-rich tropical system. Ecology 2010, 91, 386–398. [Google Scholar] [CrossRef] [Green Version]
- Paine, C.T.; Baraloto, C.; Chave, J.; Hérault, B. Functional traits of individual trees reveal ecological constraints on community assembly in tropical rain forests. Oikos 2011, 120, 720–727. [Google Scholar] [CrossRef]
- Wright, S.J.; Kitajima, K.; Kraft, N.J.; Reich, P.B.; Wright, I.J.; Bunker, D.E.; Engelbrecht, B.M. Functional traits and the growth–mortality trade-off in tropical trees. Ecology 2010, 91, 3664–3674. [Google Scholar] [CrossRef]
- Prado-Junior, J.A.; Schiavini, I.; Vale, V.S.; Arantes, C.S.; van der Sande, M.T.; Lohbeck, M.; Poorter, L. Conservative species drive biomass productivity in tropical dry forests. J. Ecol. 2016, 104, 817–827. [Google Scholar] [CrossRef] [Green Version]
- Karrenberg, S.; Edwards, P.J.; Kollmann, J. The life history of Salicaceae living in the active zone of floodplains. Freshw. Biol. 2002, 47, 733–748. [Google Scholar] [CrossRef]
- Junk, W.J. Structure and Function of the large central Amazonian river floodplains: Synthesis and discussion. In The Central Amazon Floodplain. Ecological Studies (Analysis and Synthesis); Junk, W.J., Ed.; Springer: Berlin/Heidelberg, Germany, 1997; Volume 126. [Google Scholar] [CrossRef]
- Goulding, M. The Fishes and the Forest, Explorations in Amazonian Natural History; University of California Press: Berkeley, CA, USA, 1980. [Google Scholar]
| Index | Description | Formula | |
|---|---|---|---|
| Functional richness [30,31] | FRic | Convex volume | Quickhull Algorithm |
| Functional evenness [31] | FEve | Sum of the length of the MST branches weighted by the relative abundance of the species. | |
| Functional divergence [31] | FDiv | Weighted mean distance of traits of individual species to the weighted centroid of all species. | |
| Functional dispersion [32] | FDis | Deviation of the average distance from the center of gravity weighted by relative abundance. | |
| Community-weighted mean | CWM | Average expected value of a trait in a community. |
| # Individuals | Richness | Fisher’s α | Shannon | Simpson | |
|---|---|---|---|---|---|
| Várzea | 552 | 24 | 6.32 | 1.63 | 0.66 |
| Igapó | 491 | 29 | 8.82 | 1.85 | 0.66 |
| LA (cm2) | SLA (mm2 mg−1) | LDMC (mg g−1) | Lth (mm) | WD (g cm−3) | H (m) | |
|---|---|---|---|---|---|---|
| Várzea | 97.44 (275.01) | 17.55 (4.13) | 251.16 (74.58) | 0.33 (0.11) | 0.42 (0.13) | 13.68 (5.38) |
| Igapó | 484.40 (247.73) | 10.98 (2.03) | 465.30 (53.24) | 0.19 (0.04) | 0.68 (0.10) | 15.80 (5.91) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonilla, D.; Aldana, A.M.; Cárdenas, S.; Sanchez, A. Functional Divergence between Várzea and Igapó Forests: A Study of Functional Trait Diversity in the Colombian Orinoco Basin. Forests 2020, 11, 1172. https://doi.org/10.3390/f11111172
Bonilla D, Aldana AM, Cárdenas S, Sanchez A. Functional Divergence between Várzea and Igapó Forests: A Study of Functional Trait Diversity in the Colombian Orinoco Basin. Forests. 2020; 11(11):1172. https://doi.org/10.3390/f11111172
Chicago/Turabian StyleBonilla, Diana, Ana M. Aldana, Sasha Cárdenas, and Adriana Sanchez. 2020. "Functional Divergence between Várzea and Igapó Forests: A Study of Functional Trait Diversity in the Colombian Orinoco Basin" Forests 11, no. 11: 1172. https://doi.org/10.3390/f11111172
APA StyleBonilla, D., Aldana, A. M., Cárdenas, S., & Sanchez, A. (2020). Functional Divergence between Várzea and Igapó Forests: A Study of Functional Trait Diversity in the Colombian Orinoco Basin. Forests, 11(11), 1172. https://doi.org/10.3390/f11111172






