Potential Pollution Sources from Agricultural Activities on Tropical Forested Floodplain Wetlands Revealed by Soil eDNA
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Soil Isotopes and Plant End-Members
2.3. eDNA Analyses
2.4. Data Analyses
3. Results
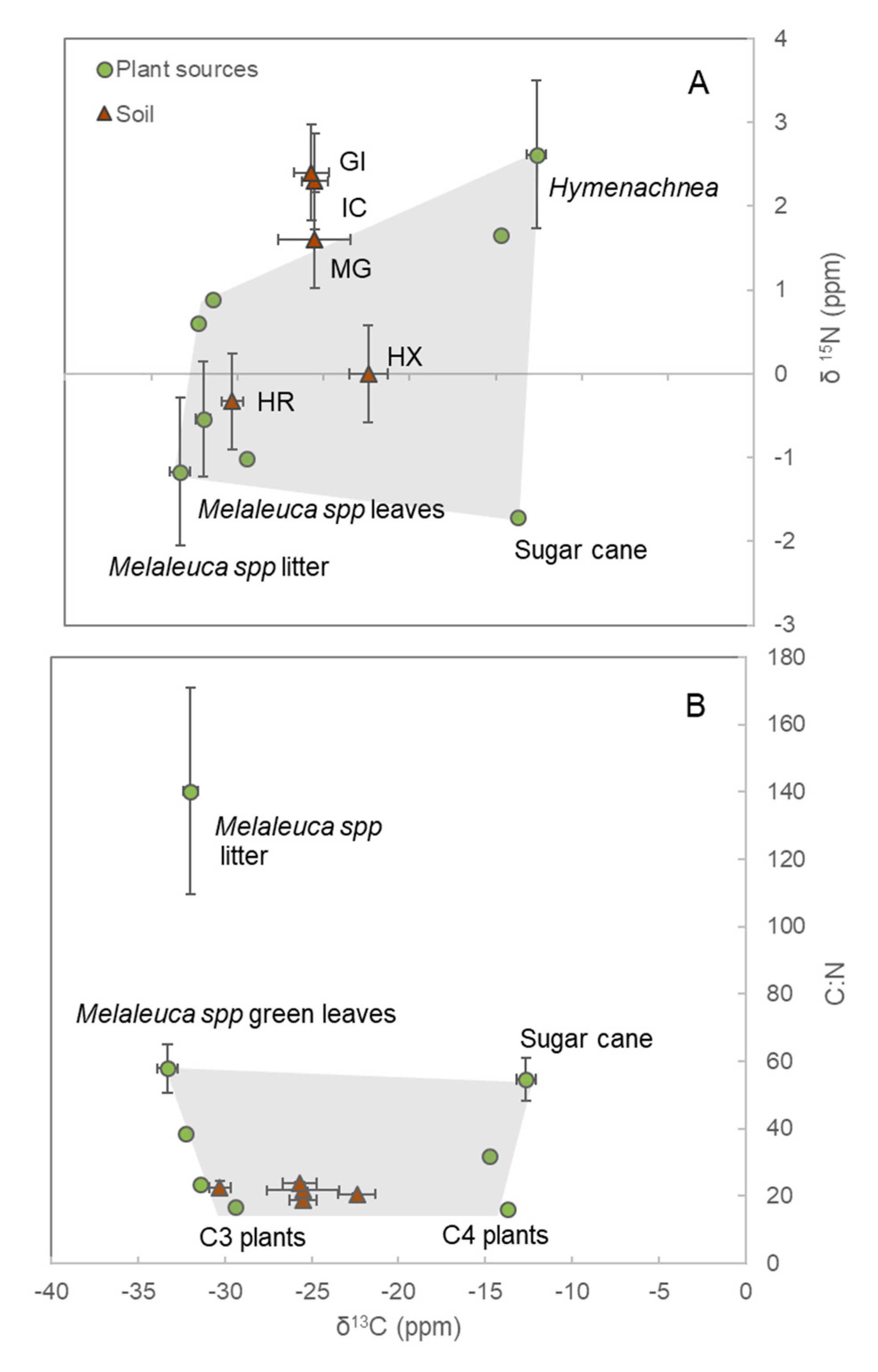
3.1. Soil Isotopes and End-Members
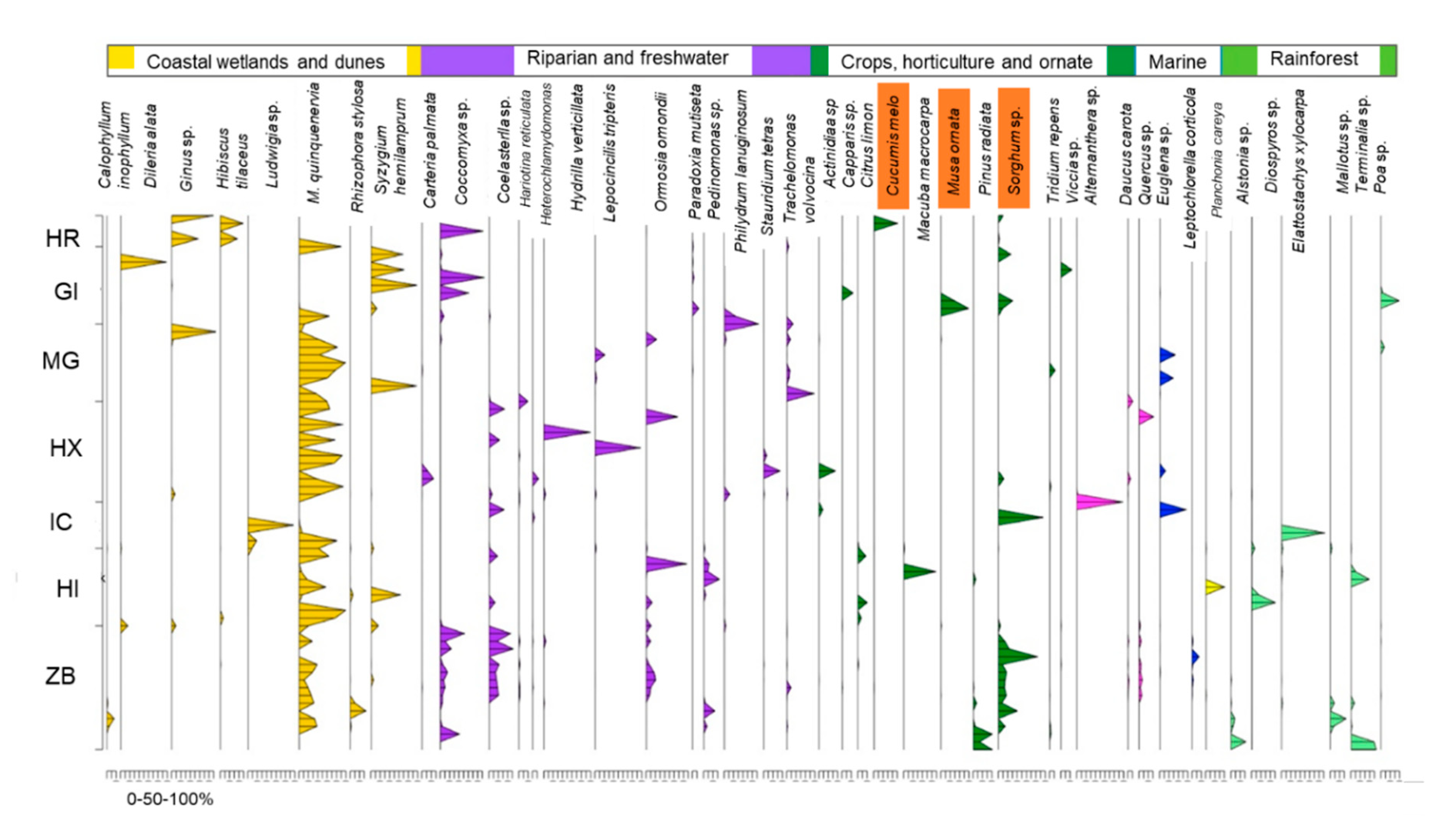
3.2. eDNA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Junk, W.F.; Bayley, P.B.; Sparks, R.E. The flood pulse concept in river-floodplain systems. Can. J. Fish. Aquat. Sci. 1989, 106, 110–127. [Google Scholar]
- Adame, M.; Pettit, N.; Valdez, D.; Ward, D.; Burford, M.; Bunn, S. The contribution of epiphyton to the primary production of tropical floodplain wetlands. Biotropica 2017, 49, 461–471. [Google Scholar] [CrossRef]
- Haugaasen, T.; Peres, C.A.; Journal, S.; Mar, N.; Haugaasen, T.; Peres, C.A. Mammal assemblage structure in Amazonian flooded and unflooded forests. J. Trop. Ecol. 2016, 21, 133–145. [Google Scholar] [CrossRef]
- Davis, A.; Pearson, R.; Brodie, J.; Butler, B. Review and conceptual models of agricultural impacts and water quality in waterways of the Great Barrier Reef catchment area. Mar. Freshw. Res. 2016, 68, 1–19. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Gosselink, J. Wetlands, 5th ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Adame, M.F.; Reef, R.; Wong, V.N.L.; Balcombe, S.R.; Turschwell, M.P.; Kavehei, E.; Rodríguez, D.C.; Kelleway, J.J.; Masque, P.; Ronan, M. Carbon and nitrogen sequestration of Melaleuca floodplain wetlands in tropical Australia. Ecosystems 2019, 23, 454–466. [Google Scholar] [CrossRef]
- Lougheed, V.L.; Mcintosh, M.D.; Parker, C.A.; Stevenson, R.J. Wetland degradation leads to homogenization of the biota at local and landscape scales. Freshw. Biol. 2008, 53, 2402–2413. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Gossilink, J.G. The value of wetlands: Importance of scale and landscape setting. Ecol. Econ. 2000, 35, 25–33. [Google Scholar] [CrossRef]
- Vymazal, J.; Březinová, T. The use of constructed wetlands for removal of pesticides from agricultural runoff and drainage: A review. Environ. Int. 2015, 75, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Bruesewitz, D.A.; Hamilton, D.P.; Schipper, L.A. Denitrification potential in lake sediment increases across a gradient of catchment agriculture. Ecosystems 2011, 14, 341–352. [Google Scholar] [CrossRef]
- Houlahan, J.E.; Findlay, C.S. Estimating the “critical” distance at which adjacent land-use degrades wetland water and sediment quality. Landsc. Ecol. 2004, 19, 677–690. [Google Scholar] [CrossRef]
- Johnson, A.K.L.; Ebert, S.P. Quantifying inputs of pesticides to the Great Barrier Reef Marine Park—A case study in the Herbert River catchment of North-East Queensland. Mar. Pollut. Bull. 2000, 41, 302–309. [Google Scholar] [CrossRef]
- Bainbridge, Z.T.; Brodie, J.E.; Faithful, J.W.; Sydes, D.A.; Lewis, S.E. Identifying the land-based sources of suspended sediments, nutrients and pesticides discharged to the Great Barrier Reef from the Tully—Murray Basin, Queensland, Australia. Mar. Freshw. Res. 2009, 60, 1081–1090. [Google Scholar] [CrossRef]
- Kroon, F.J.; Hook, S.E.; Jones, D.; Metcalfe, S.; Henderson, B.; Smith, R.; Warne, M.S.J.; Turner, R.D.; McKeown, A.; Westcott, D.A. Altered transcription levels of endocrine associated genes in two fisheries species collected from the Great Barrier Reef catchment and lagoon. Mar. Environ. Res. 2015, 104, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Adame, M.F.; Arthington, A.H.; Waltham, N.; Hasan, S.; Selles, A.; Ronan, M. Managing threats and restoring wetlands within catchments of the Great Barrier Reef, Australia. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 829–839. [Google Scholar] [CrossRef]
- Vandergragt, M.; Warne, M.S.; Borschmann, G.; Johns, C.V. Pervasive pesticide contamination of wetlands in the Great Barrier Reef catchment area. J. Integr. Environ. Assess. Manag. 2020. [Google Scholar] [CrossRef]
- Brodie, J.E.; Mitchell, A.W. Nutrients in Australian tropical rivers: Changes with agricultural development and implications for receiving environments. Mar. Freshw. Res. 2005, 56, 279–302. [Google Scholar] [CrossRef]
- Costanzo, S.D.; O’Donohue, M.J.; Dennison, W.C.; Loneragan, N.R.; Thomas, M. A new approach for detecting and mapping sewage impacts. Mar. Pollut. Bull. 2001, 149–156. [Google Scholar] [CrossRef]
- Bahadori, M.; Chen, C.; Lewis, S.; Rezaei Rashti, M.; Cook, F.; Parnell, A.; Esfandbod, M.; Boyd, S. A novel approach of combining isotopic and geochemical signatures to differentiate the sources of sediments and particulate nutrients from different land uses. Sci. Total Environ. 2019, 655, 129–140. [Google Scholar] [CrossRef]
- Adame, M.F.; Wright, A.; Grinham, A.; Lobb, K.; Raymond, C.E.; Lovelock, C.E. Terrestrial-marine connectivity: Patterns of terrestrial soil carbon deposition in coastal sediments determined by analysis of glomalin related soil protein. Limnol. Oceanogr. 2012, 57, 1492–1502. [Google Scholar] [CrossRef]
- López-Merino, L.; Colás-Ruiz, N.R.; Adame, M.F.; Serrano, O.; Martínez Cortizas, A.; Mateo, M.A. A six thousand-year record of climate and land-use change from Mediterranean seagrass mats. J. Ecol. 2017, 105, 1267–1278. [Google Scholar] [CrossRef]
- Reef, R.; Atwood, T.B.; Samper-Villarreal, J.; Adame, M.F.; Sampayo, E.M.; Lovelock, C.E. Using eDNA to determine the source of organic carbon in seagrass meadows. Limnol. Oceanogr. 2017, 62, 1254–1265. [Google Scholar] [CrossRef]
- Australian Bureau of Meteorology. 2020. Available online: http://www.bom.gov.au/ (accessed on 10 May 2020).
- Queensland Governement. WetlandInfo. 2020. Available online: https://wetlandinfo.des.qld.gov.au/ (accessed on 10 May 2020).
- Karim, F.; Kinsey-Henderson, A.; Wallace, J.; Godfrey, P.; Arthington, A.H.; Pearson, R.G. Modelling hydrological connectivity of tropical floodplain wetlands via a combined natural and artificial stream network. Hydrol. Process. 2013, 28, 5696–5710. [Google Scholar] [CrossRef]
- Rasiah, V.; Armour, J.D.; Cogle, A.L.; Florentine, S.K. Nitrate import—Export dynamics in groundwater interacting with surface-water in a wet-tropical environment. Soil Res. 2010, 361–370. [Google Scholar] [CrossRef][Green Version]
- Queensland Government. QSpatial Catalogue. Australia. 2020. Available online: http://qldspatial.information.qld.gov.au/catalogue/custom/index.page (accessed on 20 July 2020).
- Finlayson, C.M. Plant ecology of Australia’s tropical floodplain wetlands: A review. Ann. Bot. 2005, 96, 541–555. [Google Scholar] [CrossRef]
- Turner, C.E.; Center, T.D.; Burrows, D.W.; Buckingham, G.R. Ecology and management of Melaleuca quinqenervia, an invader of wetlands in Florida, U.S.A. Wetl. Ecol. Manag. 1998, 5, 165–178. [Google Scholar] [CrossRef]
- Zhang, J.; Kobert, K.; Flouri, T. Stamatakis PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 1, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.; Haas, B.; Clemente, J.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 15, 2194–2200. [Google Scholar] [CrossRef]
- Caporaso, J.; Kuczynski, J.; Stomabaugh, J.; Bittinger, K.; Bushman, F.; Costello, E.; Fierer, N.; Peña, A.; Goodrich, J.; Gordon, J.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Fry, B. Alternative approaches for solving underdetermined isotope mixing problems. Mar. Ecol. Prog. Ser. 2013, 472, 1–13. [Google Scholar] [CrossRef]
- Adame, M.; Jardine, T.; Fry, B.; Valdez, D.; Linder, G.; Nadji, J.; Bunn, S. Estuarine crocodiles in a tropical coastal floodplain obtain nutrition from terrestrial prey. PLoS ONE 2018, e0200983. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.; Gregg, J. Uncertainty in source partitioning using stable isotopes. Oecologia 2011, 127, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Burrage, D.M.; Heron, M.L.; Hacker, J.M.; Stieglitz, T.C.; Steinberg, C.R.; Prytz, A. Evolution and dynamics of tropical river plumes in the Great Barrier Reef: An integrated remote sensing and in situ study. J. Geophys. Res. 2002, 107, 1–17. [Google Scholar] [CrossRef][Green Version]
- Lovelock, C.E.; Duarte, C.M. Dimensions of blue carbon and emerging perspectives. Biol. Lett. 2019, 15, 20180781. [Google Scholar] [CrossRef] [PubMed]
- Saintilan, N.; Rogers, K.; Mazumder, D.; Woodroffe, C. Allochthonous and autochthonous contributions to carbon accumulation and carbon store in southeastern Australian coast. Estuar. Coast. Shelf Sci. 2013, 128, 84–92. [Google Scholar] [CrossRef]
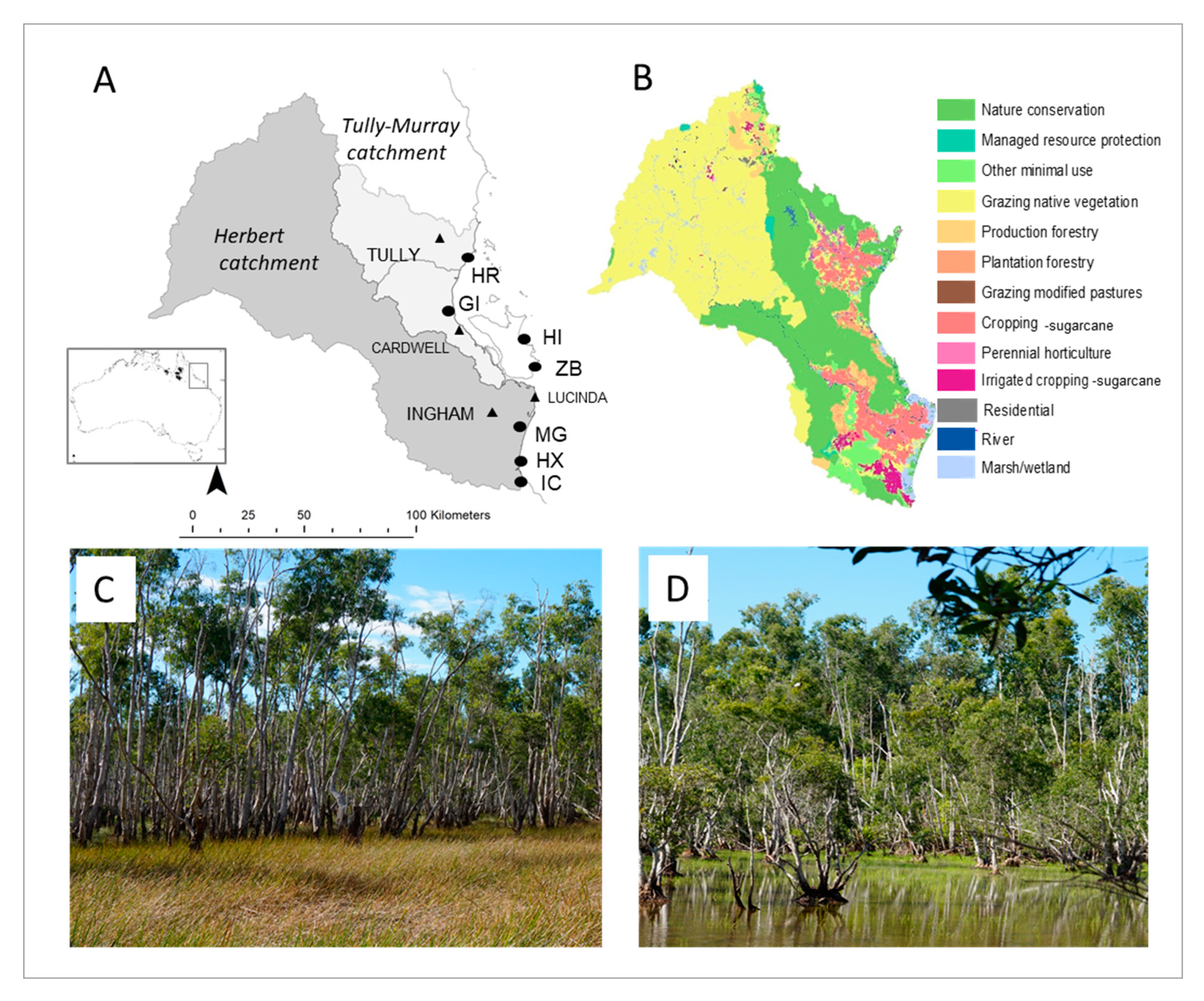
| Site | Catchment | Sub-Catchment | Nearby Land Use and Impacts | Vegetation | Hydrology |
|---|---|---|---|---|---|
| (HR) Hull River National Park | Tully-Murray River | Hull River | Grazing Sugarcane Damaged during cyclone in 2011 | M. viridiflora Rainforest | Permanently flooded |
| (GI) Girramay National Park | Whitfield-Dallachy Creek | Grazing Protected rainforest | M. quinquenerva Rainforest | Flooded by rain and overbank flows | |
| (MG) Mungalla | Herbert River | Palm Creek | Grazing Treatment plant discharge | M. quinquenerva | Flooded by nearby lagoon |
| (HX) Halifax Wetlands National Park | Trebonne Creek | Grazing Sugar cane Protected wetlands | M. quinquenerva Mangroves Marsh | Flooded by rain and tidal flows | |
| (IC) Insulator Creek | Insulator Creek | Sugar cane Protected rainforest | M. quinquenerva Mangroves Marsh | Flooded by rain and overbank flows | |
| (HI) East Hinchinbrook Island | Hinchinbrook Island | East Hinchinbrook | Protected rainforest and beach | M. quinquenerva Rainforest | Flooded by rain and groundwater |
| (ZB) Zoe Bay | Southeast Hinchinbrook | Protected wetlands and rainforests | M. quinquenerva Mangroves Marsh | Flooded by fringing tidal creek |
| Sites | BD (g cm−3) | N (%) | C (%) | C:N | δ13C (‰) | δ 15N (‰) |
|---|---|---|---|---|---|---|
| HR | 0.34 ± 0.05 | 0.78 ± 0.04 | 14.3 ± 1.4 | 22.4 ± 2.2 | −30.3 ± 0.6 | −0.3 ± 0.6 |
| GI | 0.59 ± 0.02 | 0.34 ± 0.02 | 4.1 ± 0.4 | 23.8 ± 0.4 | −25.7 ± 1.0 | 2.4 ± 0.4 |
| MG | 0.61 ± 0.07 | 0.44 ± 0.15 | 7.6 ± 2.8 | 21.7 ± 0.7 | −25.5 ± 2.1 | 1.6 ± 0.9 |
| HX | 0.84 ± 0.12 | 0.06 ± 0.01 | 1.0 ± 0.2 | 20.6 ± 0.3 | −22.4 ± 1.1 | n.d. |
| IC | 0.65 ± 0.10 | 0.54 ± 0.14 | 7.8 ± 2.2 | 18.7 ± 1.2 | −25.5 ± 0.8 | 2.3 ± 0.4 |
| Site | Native Trees | Grass/Shrubs | Macrophyte | Microalgae | Non-Native | Cultivated |
|---|---|---|---|---|---|---|
| HR | 21.5 | 0.03 | 0.01 | 0.04 | 0.01 | 16.0 |
| GI | 50.7 | 4.9 | 0.1 | 20.0 | 0.1 | 20.7 |
| MG | 57.8 | 0.8 | 17.2 | 18.9 | 0.9 | 1.4 |
| HX | 57.1 | 0.2 | 9.6 | 22.1 | n.d. | 6.8 |
| IC | 30.6 | 35.7 | 0.02 | 15.1 | 0.01 | 17.4 |
| HI | 69.6 | 1.9 | 0.03 | 4.0 | 0.3 | 7.5 |
| ZB | 35.8 | 0.1 | 0.6 | 16.7 | 0.5 | 24.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adame, M.F.; Reef, R. Potential Pollution Sources from Agricultural Activities on Tropical Forested Floodplain Wetlands Revealed by Soil eDNA. Forests 2020, 11, 892. https://doi.org/10.3390/f11080892
Adame MF, Reef R. Potential Pollution Sources from Agricultural Activities on Tropical Forested Floodplain Wetlands Revealed by Soil eDNA. Forests. 2020; 11(8):892. https://doi.org/10.3390/f11080892
Chicago/Turabian StyleAdame, Maria Fernanda, and Ruth Reef. 2020. "Potential Pollution Sources from Agricultural Activities on Tropical Forested Floodplain Wetlands Revealed by Soil eDNA" Forests 11, no. 8: 892. https://doi.org/10.3390/f11080892
APA StyleAdame, M. F., & Reef, R. (2020). Potential Pollution Sources from Agricultural Activities on Tropical Forested Floodplain Wetlands Revealed by Soil eDNA. Forests, 11(8), 892. https://doi.org/10.3390/f11080892





