Variability of Leaf Wetting and Water Storage Capacity of Branches of 12 Deciduous Tree Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Collection and Methodological Assumptions
2.2. Canopy Water Storage Capacity Measurement
2.3. Methods to Measure Inclination Angles and Determine Surface Free Energy
2.3.1. The Owens–Wendt Model
2.3.2. The Van Oss–Chaudhury–Good Model
2.3.3. The Zisman Method
2.4. Measurement of the Wax Content in the Leaves
2.5. Statistical Analyses
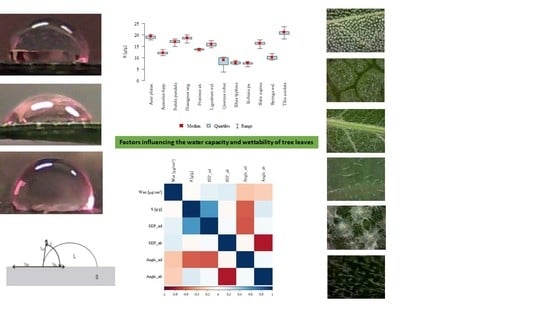
3. Results
3.1. The Influence of Factors: Species, Wax, SFP_ad, SFP_ab, Ang_ad, Ang_ab on Water Storage Capacity S
3.2. The Influence of Species on the Other Parameters
3.3. The Remaining Relationships
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A: Analysis of Leaf Surface Photographs
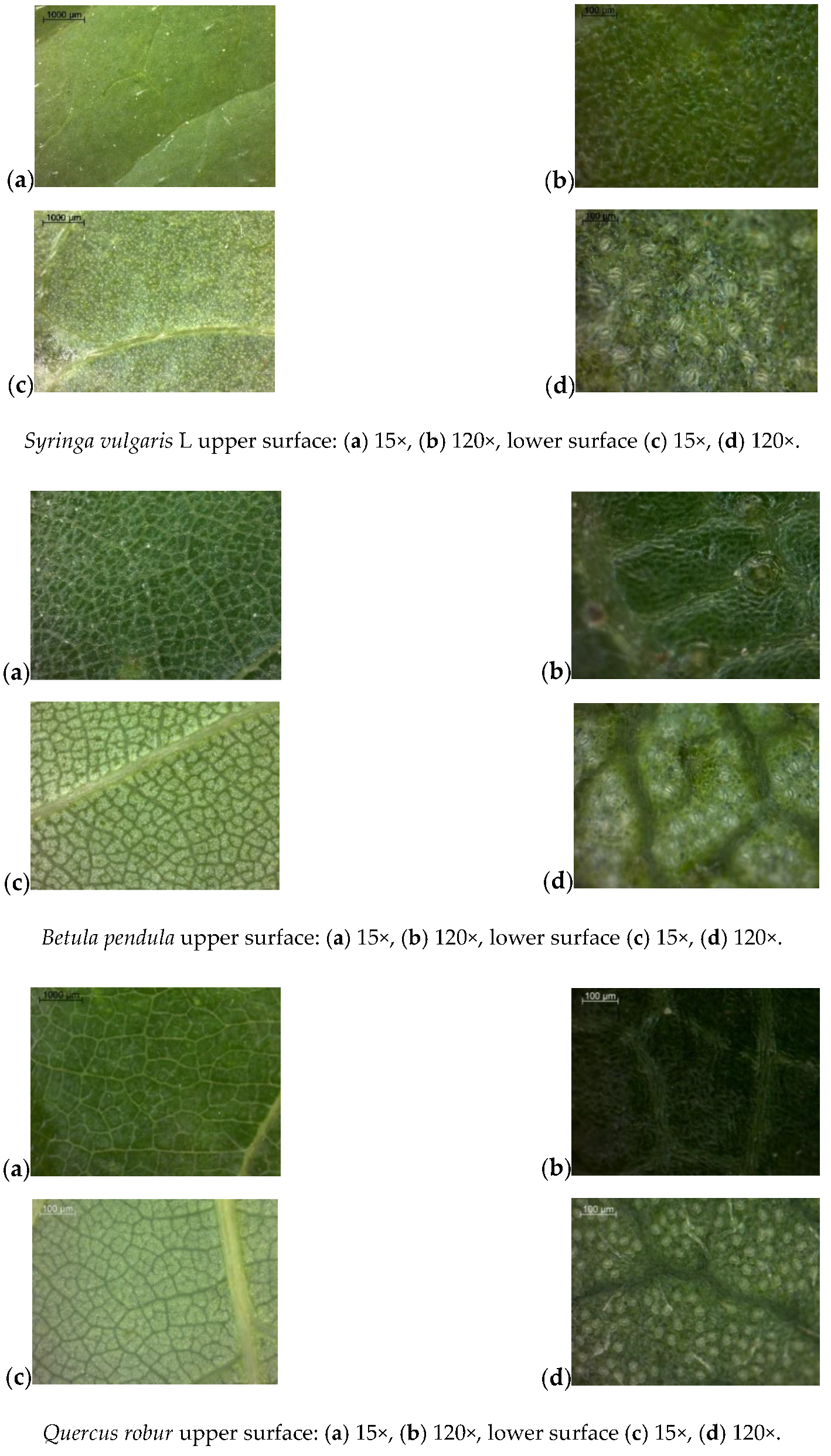

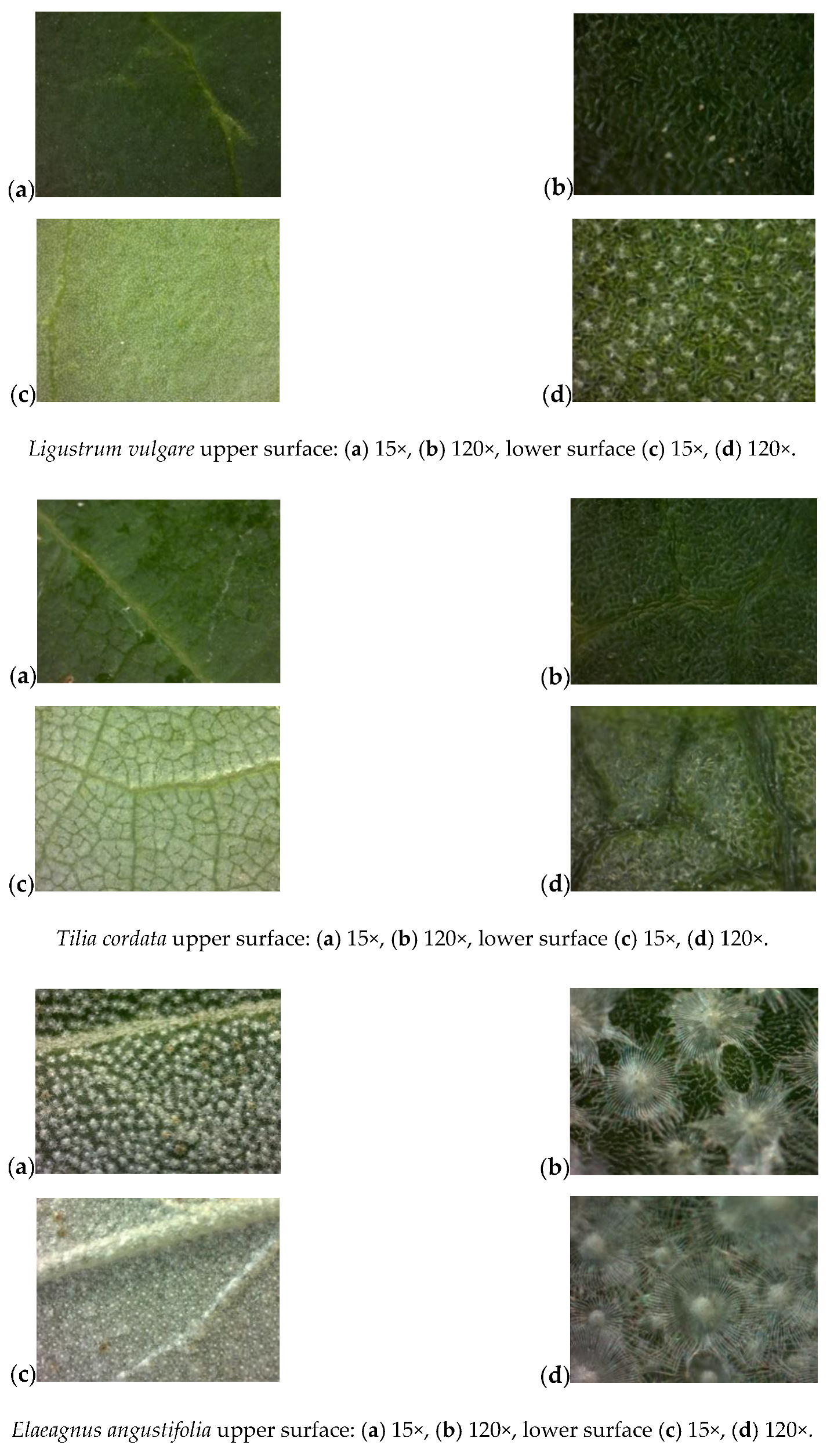
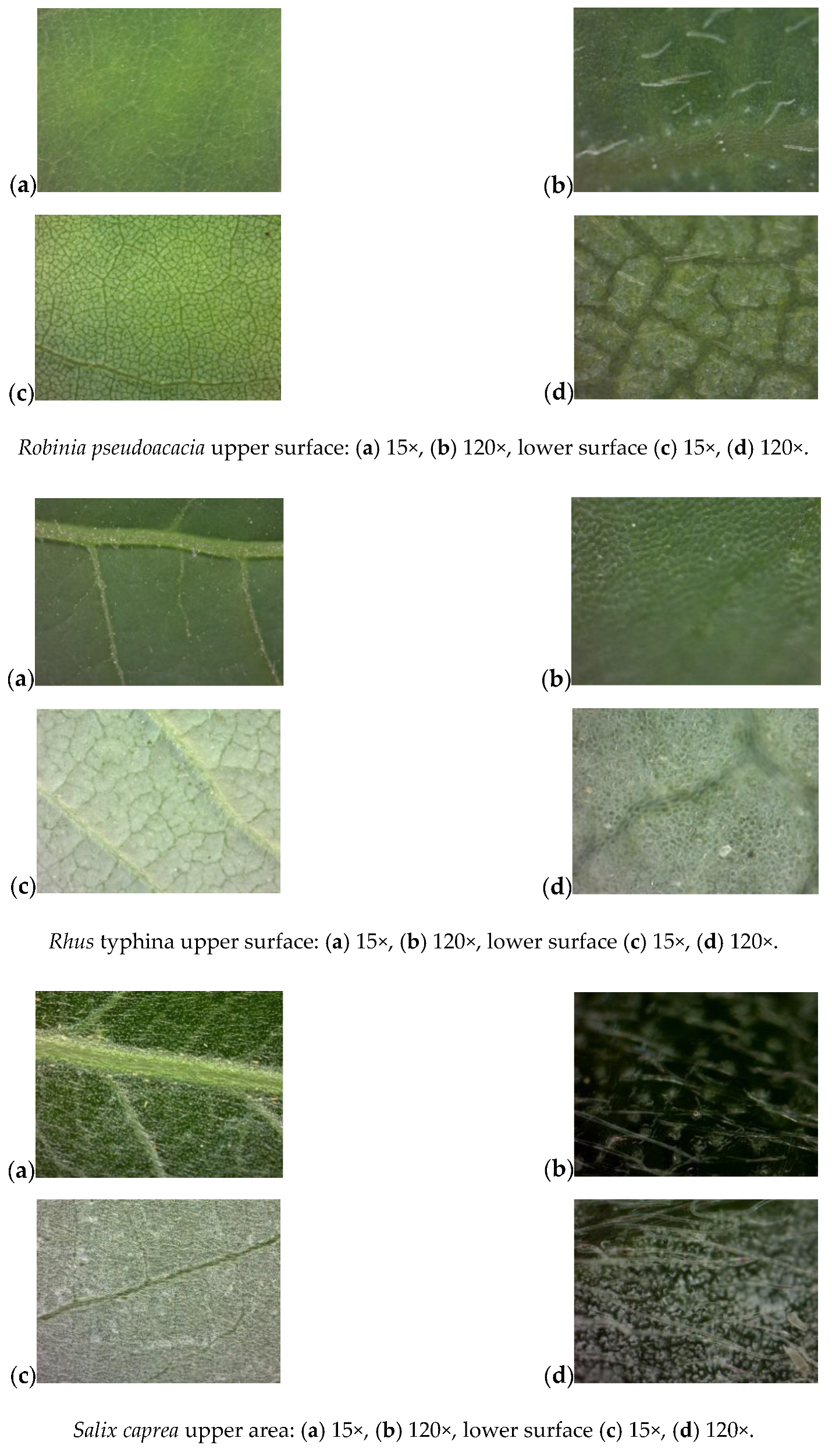
References
- Dunkerley, D.L. Evaporation of impact water droplets in interception processes: Historical precedence of the hypothesis and a brief literature overview. J. Hydrol. 2009, 376, 599–604. [Google Scholar] [CrossRef]
- Friesen, J.; Van Stan, J.T., II. Early European Observations of Precipitation Partitioning by Vegetation: A Synthesis and Evaluation of 19th Century Findings. Geosciences 2019, 9, 423. [Google Scholar] [CrossRef] [Green Version]
- Friesen, J.; Lundquist, J.; Van Stan, J.T. Evolution of forest precipitation water storage measurement methods. Hydrol. Process. 2014, 29, 2504–2520. [Google Scholar] [CrossRef]
- Papierowska, E.; Mazur, R.; Stańczyk, T.; Beczek, M.; Szewińska, J.; Sochan, A.; Ryżak, M.; Szatyłowicz, J.; Bieganowski, A. Influence of leaf surface wettability on the drop splash phenomenon. Biol. Agric. For. Meteorol. 2019, 279, 107762. [Google Scholar] [CrossRef]
- Holder, C.; Lauderbaugh, L.; Ginebra-Solanellas, R.; Webb, R. Changes in leaf inclination angle as an indicator of progression toward leaf surface storage during the rainfall interception process. J. Hydrol. 2020, 125070. [Google Scholar] [CrossRef]
- Allen, S.T.; Keim, R.F.; Barnard, H.R.; McDonnell, J.J.; Renée Brooks, J. The role of stable isotopes in understanding rainfall interception processes: A review. Wiley Interdisciplinary Reviews. Water 2017, 4. [Google Scholar] [CrossRef]
- Allen, S.T.; Aubrey, D.P.; Bader, M.Y.; Coenders-Gerrits, M.; Friesen, J.; Gutmann, E.D.; Guillemette, F.; Jiménez-Rodríguez, C.; Keim, R.F.; Klamerus-Iwan, A.; et al. Key questions on the evaporation and transport of intercepted precipitation. In Precipitation Partitioning by Vegetation. A Global Synthesis; Van Stan, J.T., II, Gutmann, E., Friesen, J., Eds.; Springer: New York, NY, USA, 2020; pp. 269–280. ISBN 978-3-030-29701-5. [Google Scholar]
- Rosado, B.H.P.; Holder, C.D. The significance of leaf water repellency in ecohydrological research: A review. Ecohydrology 2013, 6, 150–161. [Google Scholar] [CrossRef]
- Nanko, K.; Hotta, N.; Suzuki, M. Evaluating the influence of canopy species and meteorological factors on throughfall drop size distribution. J. Hydrol. 2006, 329, 422–431. [Google Scholar] [CrossRef]
- Holder, C.D. Effects of leaf hydrophobicity and water droplet retention on canopy storage capacity. Ecohydrology 2013, 6, 483–490. [Google Scholar] [CrossRef]
- Tomaszewski, D.; Zieliński, J. Epicuticular wax structures on stems and comparison between stems and leaves—A survey. Flora 2014, 209, 215–232. [Google Scholar] [CrossRef]
- Gülz, P.; Müller, E. Seasonal variation in the composition of epicuticular waxes of quevcus robur leaves. Z. Nat. C 1992, 47, 800–806. [Google Scholar] [CrossRef]
- Fernández, V.; Sancho-Knapik, D.; Guzmán, P.; Peguero-Pina, J.J.; Gil, L.; Karabourniotis, G.; Khayet, M.; Fasseas, C.; Heredia-Guerrero, J.A.; Heredia, A.; et al. Wettability, polarity and water absorption of Quercus ilex leaves: Effect of leaf side and age. Plant Physiol. 2014, 166, 168–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gart, S.; Mates, J.E.; Megaridis, C.M.; Jung, S. Droplet impacting a cantilever: A leaf-raindrop system. Phys. Rev. Appl. 2015, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sikorska, D.; Papierowska, E.; Szatyłowicz, J.; Sikorski, P.; Suprun, K.; Hopkins, R.J. Variation in leaf surface hydrophobicity of wetland plants: The role of plant traits in water retention. Wetlands 2017, 37, 997–1002. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Shi, H.; Li, Y.; Wang, Y. The effects of leaf roughness, surface free energy and work of adhesion on leafwater drop adhesion. PLoS ONE 2014, 9, e107062. [Google Scholar] [CrossRef]
- Weisensee, P.B.; Tian, J.; Miljkovic, N.; King, W.P. Water droplet impact on elastic superhydrophobic surfaces. Sci. Rep. 2016, 6, 30328. [Google Scholar] [CrossRef]
- Papierowska, E.; Szporak-Wasilewska, S.; Szewińska, J.; Szatyłowicz, J.; Debaene, G.; Utratna, M. Contact angle measurements and water drop behavior on leaf surface for several deciduous shrub and tree species from a temperate zone. Trees 2018, 32, 1253–1266. [Google Scholar] [CrossRef] [Green Version]
- Aryal, B.; Neuner, G. Leaf wettability decreases along an extreme altitudinal gradient. Oecologia 2010, 162, 1–9. [Google Scholar] [CrossRef]
- Klamerus-Iwan, A.; Błońska, E. Canopy storage capacity and wettability of leaves and needles: The effect of water temperature changes. J. Hydrol. 2018. [Google Scholar] [CrossRef]
- Popek, R.; Gawrońska, H.; Wrochna, M.; Gawroński, S.; Sæbø, A. Particulate matter on foliage of 13 woody species: Deposition on surfaces and phytosta-bilisation in waxes, a 3 year study. Int. J. Phytorem. 2012, 15, 245–256. [Google Scholar] [CrossRef]
- Pogorzelski, S.; Rochowski, P.; Szurkowski, J. Pinus sylvestris L. needle surface wettability parameters as indicators of atmospheric environment pollution impacts: Novel contact angle hysteresis methodology. Appl. Surf. Sci. 2014, 292, 857–866. [Google Scholar] [CrossRef]
- Stosch, A.K.; Solga, A.; Steiner, U.; Oerke, C.; Barthlott, W.; Cermann, Z. Efficiency of self-cleaning properties in wheat (TriticumaestivumL.). Appl. Bot. Food Qual. 2007, 81, 49–55. Available online: http://pub.jki.bund.de/index.php/JABFQ/article/view/2111 (accessed on 1 August 2020).
- Klamerus-Iwan, A.; Witek, W. Variability in the Wettability and Water Storage Capacity of Common Oak Leaves (Quercus robur L.). Water 2018, 10, 695. [Google Scholar] [CrossRef] [Green Version]
- Carlyle-Moses, D.E.; Gash, J.H. Rainfall interception loss by forest canopies. In Forest hydrology and biogeochemistry; Ecological Studies; Levia, D.F., Carlyle-Moses, D., Tanaka, T., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 216, pp. 407–423. [Google Scholar] [CrossRef]
- Porada, P.; Van Stan, J.T., II; Kleidon, A. Significant contribution of non-vascular vegetation to global rainfall interception. Nat. Geosci. 2018, 11, 563–567. [Google Scholar] [CrossRef]
- Doerr, G.J.; Kempthorne, D.M.; Mayo, L.C.; Forster, W.A.; Zabkiewicz, J.A.; McCue, S.W.; Belward, J.A.; Turner, I.W.; Hanan, J. Towards a model of spray-canopy interactions: Interception, shatter, bounce and retention of droplets on horizontal leaves. Ecol. Model. 2014, 290, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Nowak, D.J.; Coville, R.; Endreny, T.; Abdi, R.; Van Stan, J.T., II. Valuing urban tree impacts on precipitation partitioning. In Precipitation Partitioning by Vegetation: A Global Synthesis; Van Stan, J.T., Gutmann, E., Friesen, J., Eds.; Springer Nature: Berlin, Germany, 2020; pp. 253–268. [Google Scholar]
- Xiao, Q.; McPherson, E. Surface water storage capacity of twenty tree species in Davis, California. J. Environ. Qual. 2016, 45, 188–198. [Google Scholar] [CrossRef]
- Garcia-Estringana, P.; Alonso-Blázquez, N.; Alegre, J. Water storage capacity, stemflow and water funneling in Mediterranean shrubs. J. Hydrol. 2010, 389, 363–372. [Google Scholar] [CrossRef]
- Błońska, E.; Klamerus-Iwan, A.; Łagan, S.; Lasota, J. Changes to the water repellency and storage of different species of deadwood based on decomposition rate in a temperate climate. Ecohydrology 2018, 11, 1–8. [Google Scholar] [CrossRef]
- Good, R.J. Surface free energy of solids and liquids: Thermodynamics, molecular forces, and structure. J. Colloid Interface Sci. 1977, 59, 398–419. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Good, R.J.; Chaudhury, M.K. The role of van der Waals forces and hydrogen bonds in “hydrophobic interactions” between biopolymers and low energy surfaces. J. Colloid Interface Sci. 1986, 111, 378–390. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Ebercon, A.; Blum, A.; Jordan, W.R. A rapid colorimetric method for epicuticular wax content of sorghum leaves. Crop Sci. 1977, 17, 179–180. [Google Scholar] [CrossRef] [Green Version]
- Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Applied Statistics for the Behavioral Sciences, 5th ed.; Houghton Mifflin: Boston, MA, USA, 2003. [Google Scholar]
- R Core Team. R: A Language and Environment for Statisticalcomputing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 20 July 2020).
- Koch, K.; Barthlott, W. Superhydrophobic and superhydrophilic plant surfaces: An inspiration for biomimetic materials. Philos. Trans. R. Soc. A 2009, 367, 1487–1509. [Google Scholar] [CrossRef]
- Chang, M. Forest Hydrology: An Introduction to Water and Forests, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Holder, C.D.; Gibbes, C. Influence of leaf and canopy characteristics on rainfall interception and urban hydrology. HSJ 2016, 62, 182–190. [Google Scholar] [CrossRef]
- Li, J.; Cui, H.; Ma, Y.; Xun, L.; Li, Z.; Yang, Z.; Lu, H. Orchard spray study: A prediction model of droplet deposition states on leaf surfaces. Agronomy 2020, 10, 747. [Google Scholar] [CrossRef]
- Klamerus-Iwan, A.; Gloor, E.; Sadowska-Rociek, A.; Błońska, E.; Lasota, J.; Łagan, S. Linking the contents of hydrophobic PAHs with the canopy water storage capacity of coniferous trees. Environ. Pollut. 2018, 242, 1176–1184. [Google Scholar]
- Taylor, P. The wetting of leaf surfaces. Curr Opin Colloid. 2011, 16, 326–334. [Google Scholar] [CrossRef]
- Xue, D.; Zhang, X.; Lu, X. Molecular and evolutionary mechanisms of cuticular wax for plant drought tolerance. Front Plant Sci. 2017, 8, 621. [Google Scholar] [CrossRef]
- Sæbø, A.; Popek, R.; Nawrot, B.; Hanslin, H.M.; Gawronska, H.; Gawronski, S.W. Plant species differences in particulate matter accumulation on leaf surfaces. Sci. Total Environ. 2012, 427–428, 347–354. [Google Scholar] [CrossRef]
- Dzierżanowski, K.; Popek, R.; Gawrońska, H.; Sæbø, A.; Gawroński, S.W. Deposition of particulate matter of different size fractions on leaf surfaces and in waxes of urban forest species. Int. J. Phytoremediation 2011, 13, 1037–1046. [Google Scholar] [CrossRef]
- Dunkerley, D.L. A review of the effects of throughfall and stemflow on soil properties and soil erosion. In Precipitation Partitioning by Vegetation: A Global Synthesis; Van Stan, J.T., Gutmann, E., Friesen, J., Eds.; Springer Nature: Berlin, Germany, 2020; pp. 183–213. [Google Scholar]
- Klamerus-Iwan, A.; Błońska, E.; Lasota, J.; Waligórski, P.; Kalandyk, A. Seasonal variability of leaf water capacity and wettability under the influence of pollution in different city zones. Atmos. Pollut. Res. 2018. [Google Scholar] [CrossRef]
- Matuszko, D.; Węglarczyk, S. Effect of cloudiness on long-term variability in air temperature in Krakow. Int. J. Climatol. 2014, 34, 145–154. [Google Scholar] [CrossRef]
- Llorens, P.; Gallart, F. A simplified method for forest water storage capacity measurement. J. Hydrol. 2000, 240, 131–144. [Google Scholar] [CrossRef]
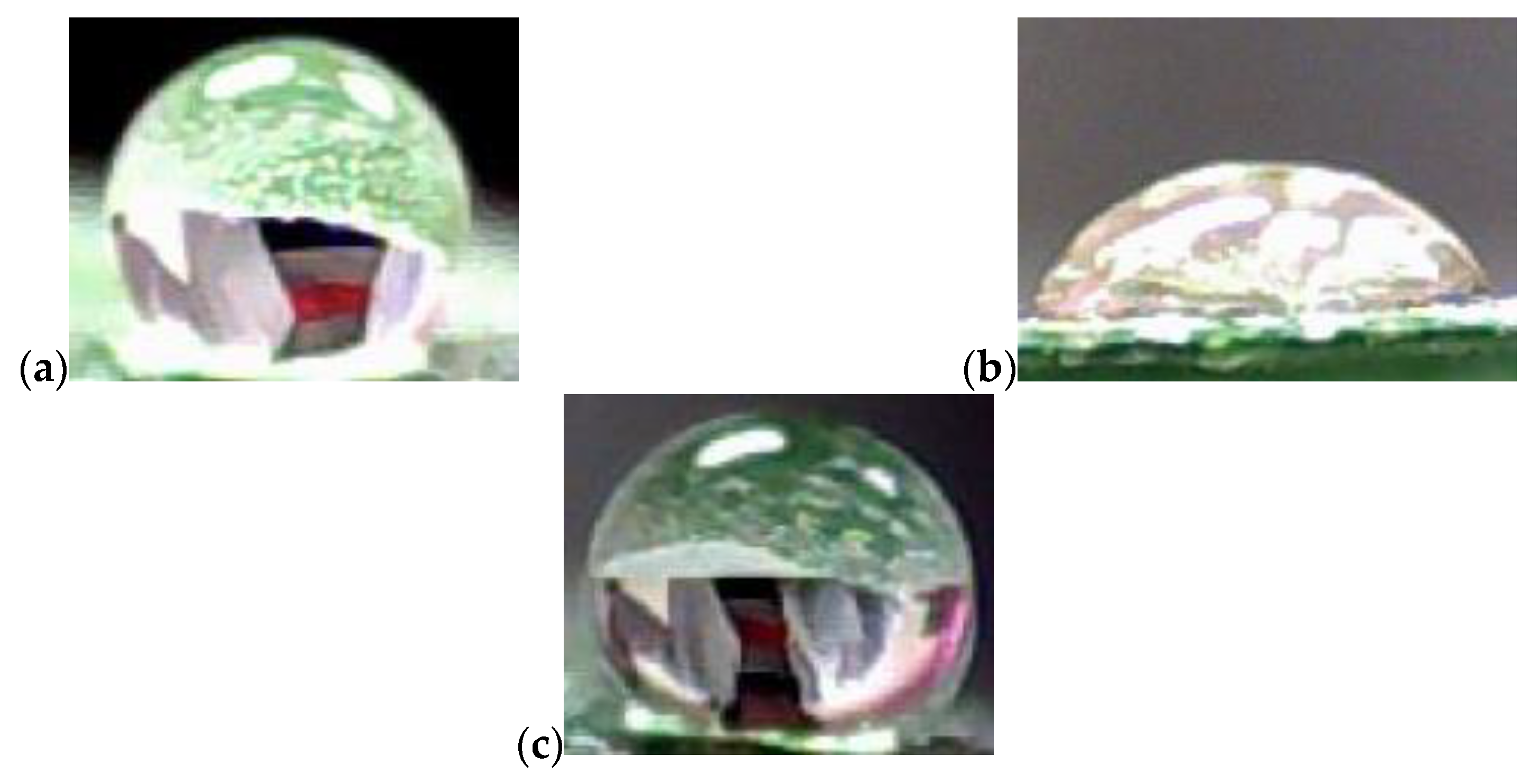


| Measuring Liquid | |||||
|---|---|---|---|---|---|
| [mJ/m2] | |||||
| Water | 72.8 | 21.8 | 51.0 | 25.5 | 25.5 |
| Glycerin | 64.0 | 34.0 | 30.0 | 3.9 | 57.4 |
| Diiodomethane | 50.8 | 50.8 | 0 | 0 | 0 |
| Variable | Standardized Parameter (Beta) | Regression Parameter (B) | 95% CI | p | ||
|---|---|---|---|---|---|---|
| (absolute term) | 9.304 | −0.494 | 19.101 | 0.062 | ||
| Wax [μg/cm2] | 0.236 | 0.067 | 0.02 | 0.114 | 0.006 | |
| SFP_ad | −0.131 | −0.074 | −0.172 | 0.025 | 0.141 | |
| SFP_ab | −0.175 | −0.101 | −0.23 | 0.028 | 0.125 | |
| Angle_ad | 0.001 | 0 | −0.042 | 0.042 | 0.987 | |
| Angle_ab | 0.046 | 0.019 | −0.03 | 0.068 | 0.45 | |
| species | Acer platan. | reference | ||||
| Aesculus hipp. | −0.266 | −4.391 | −7.34 | −1.442 | 0.004 | |
| Betula pendula | −0.297 | −4.905 | −8.188 | −1.622 | 0.004 | |
| Elaeagnus ang. | −0.017 | −0.287 | −1.564 | 0.99 | 0.656 | |
| Fraxinus ex. | −0.211 | −3.848 | −7.044 | −0.651 | 0.019 | |
| Ligustrum vul. | −0.13 | −2.246 | −5.306 | 0.814 | 0.148 | |
| Quercus robur | −0.608 | −11.104 | −12.866 | −9.342 | <0.001 | |
| Rhus typhina | −0.73 | −12.04 | −13.184 | −10.895 | <0.001 | |
| Robinia ps. | −0.845 | −13.941 | −16.656 | −11.227 | <0.001 | |
| Salix caprea | −0.168 | −2.769 | −5.698 | 0.16 | 0.064 | |
| Syringa vul. | −0.402 | −7.336 | −10.719 | −3.953 | <0.001 | |
| Tilia cordata | 0.131 | 2.164 | 0.803 | 3.525 | 0.002 | |
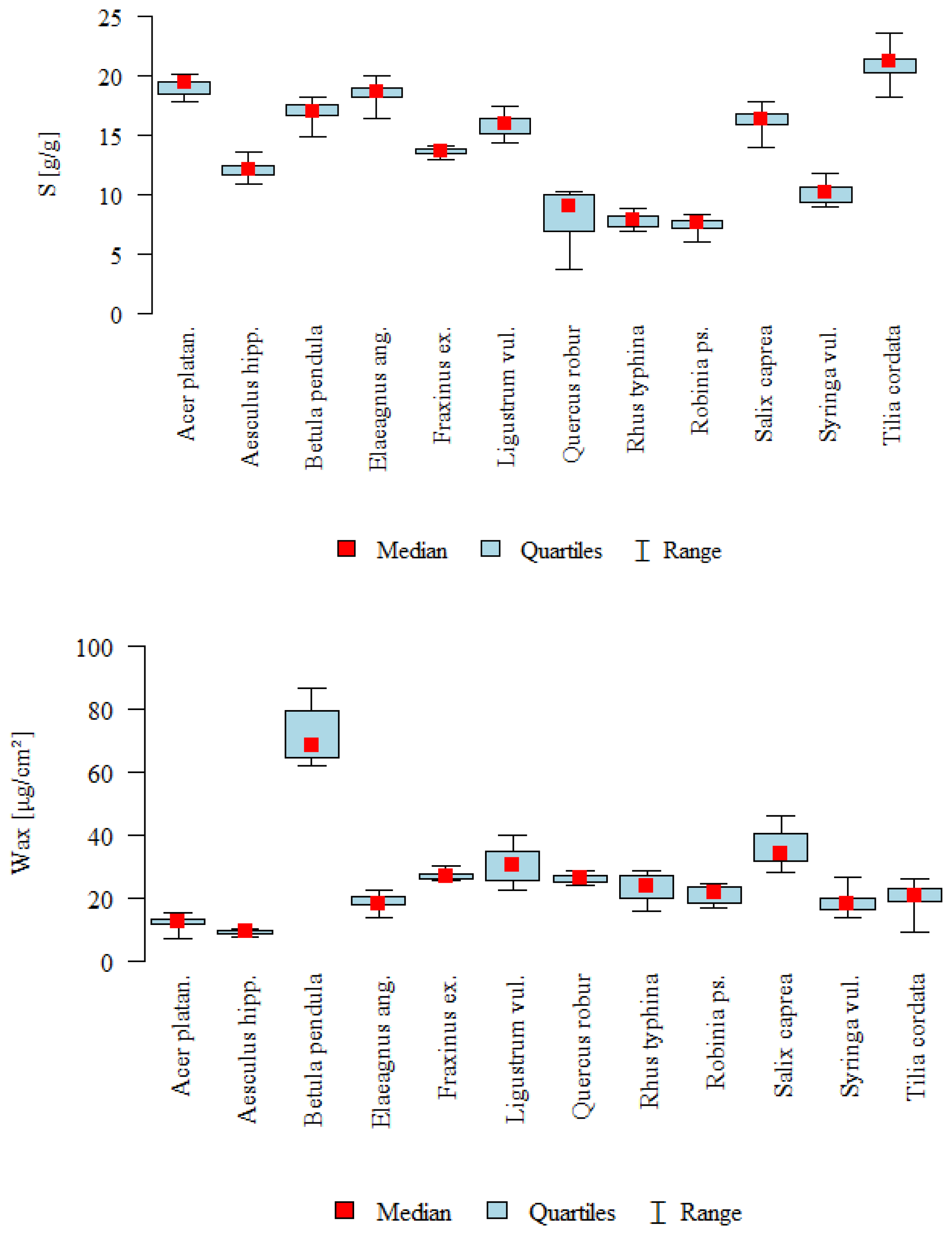
| Parameter | Type | N | Mean | SD | Median | min | max | Q1 | Q3 | p * |
|---|---|---|---|---|---|---|---|---|---|---|
| Wax [µg/cm2] | A | 10 | 12.33 | 2.23 | 12.37 | 7.09 | 15.39 | 11.75 | 13.46 | <0.001 |
| B | 10 | 9.14 | 0.73 | 9.43 | 7.87 | 10.14 | 8.8 | 9.53 | C > A, B, D, H, I, K, L | |
| C | 10 | 71.76 | 9.06 | 68.33 | 61.93 | 86.67 | 64.48 | 79.21 | J > A, B, D, I, K, L | |
| D | 10 | 18.6 | 2.66 | 18.43 | 13.81 | 22.58 | 17.7 | 20.38 | E, F, G > A, B | |
| E | 8 | 27.32 | 1.76 | 26.7 | 25.84 | 30.1 | 26.29 | 27.67 | H > B | |
| F | 9 | 30.46 | 5.62 | 30.38 | 22.38 | 39.68 | 25.84 | 34.98 | ||
| G | 8 | 26.25 | 1.39 | 26.35 | 24.31 | 28.54 | 25.16 | 27.02 | ||
| H | 10 | 23.48 | 4.36 | 23.85 | 16.03 | 28.8 | 20.16 | 27.3 | ||
| I | 10 | 21.13 | 2.88 | 21.92 | 16.9 | 24.73 | 18.5 | 23.39 | ||
| J | 10 | 35.81 | 5.81 | 34.12 | 27.98 | 45.85 | 31.64 | 40.65 | ||
| K | 8 | 18.86 | 3.97 | 17.94 | 13.88 | 26.69 | 16.54 | 20.2 | ||
| L | 10 | 19.47 | 5.76 | 20.68 | 9.01 | 26.1 | 18.77 | 23.11 | ||
| S [g/g] | A | 10 | 19 | 0.71 | 19.34 | 17.84 | 20.02 | 18.47 | 19.4 | <0.001 |
| B | 10 | 12.14 | 0.81 | 12.07 | 10.89 | 13.62 | 11.6 | 12.39 | L > B, E, G, H, I, K | |
| C | 10 | 16.92 | 0.95 | 16.94 | 14.8 | 18.12 | 16.68 | 17.57 | A, D > B, G, H, I, K | |
| D | 10 | 18.48 | 1.01 | 18.62 | 16.34 | 19.92 | 18.11 | 18.91 | C > G, H, I | |
| E | 8 | 13.59 | 0.39 | 13.66 | 12.9 | 14.03 | 13.47 | 13.88 | F, J > H, I | |
| F | 9 | 15.79 | 0.92 | 15.87 | 14.38 | 17.34 | 15.09 | 16.32 | ||
| G | 8 | 8.07 | 2.51 | 9.07 | 3.67 | 10.29 | 6.92 | 9.94 | ||
| H | 10 | 7.81 | 0.62 | 7.93 | 6.97 | 8.85 | 7.27 | 8.24 | ||
| I | 10 | 7.43 | 0.68 | 7.58 | 6.08 | 8.34 | 7.21 | 7.87 | ||
| J | 10 | 16.1 | 1.18 | 16.37 | 13.89 | 17.77 | 15.92 | 16.78 | ||
| K | 8 | 10.1 | 0.94 | 10.23 | 8.97 | 11.78 | 9.3 | 10.58 | ||
| L | 10 | 21.02 | 1.47 | 21.19 | 18.23 | 23.48 | 20.19 | 21.38 | ||
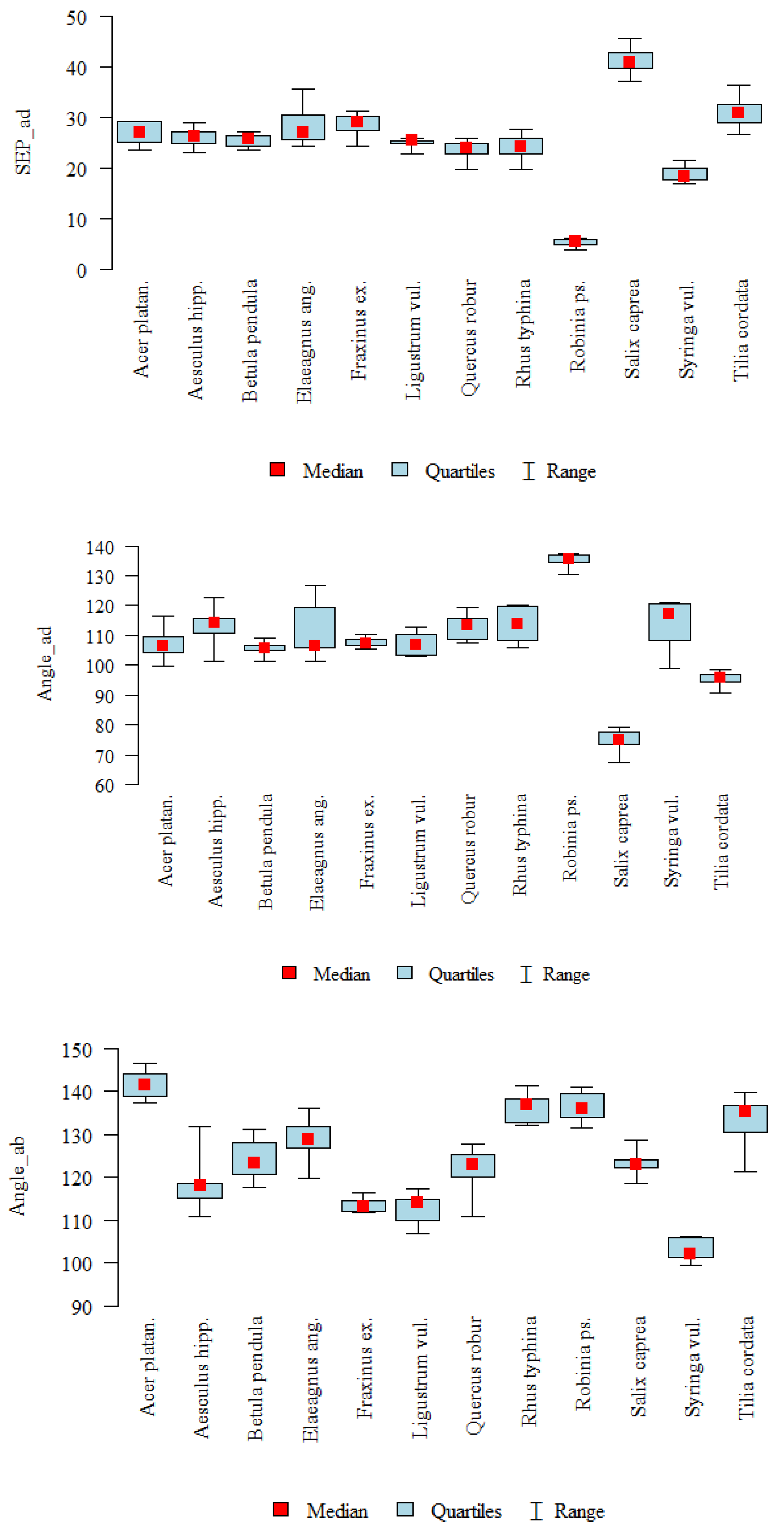
| SEP_ad | A | 10 | 26.95 | 2.28 | 27.04 | 23.62 | 29.27 | 25.13 | 29.23 | <0.001 |
| B | 10 | 26.05 | 1.88 | 26.22 | 23.12 | 28.81 | 24.77 | 27 | J > B, C, F, G, H, I, K | |
| C | 10 | 25.37 | 1.23 | 25.68 | 23.43 | 27.03 | 24.38 | 26.32 | L > G, H, I, K | |
| D | 10 | 28.1 | 3.66 | 26.91 | 24.24 | 35.56 | 25.5 | 30.33 | A, C, D > I, K | |
| E | 8 | 28.56 | 2.21 | 28.98 | 24.28 | 31.23 | 27.41 | 30.1 | B > I | |
| F | 9 | 25.03 | 0.87 | 25.35 | 22.88 | 25.86 | 24.92 | 25.44 | ||
| G | 8 | 23.61 | 1.94 | 23.83 | 19.74 | 25.83 | 22.75 | 24.95 | ||
| H | 10 | 24.09 | 2.47 | 24.13 | 19.73 | 27.56 | 22.82 | 25.84 | ||
| I | 10 | 5.31 | 0.73 | 5.39 | 3.9 | 6.25 | 4.85 | 5.81 | ||
| J | 10 | 41.08 | 2.58 | 40.81 | 36.99 | 45.59 | 39.71 | 42.78 | ||
| K | 8 | 18.79 | 1.55 | 18.2 | 16.83 | 21.37 | 17.78 | 19.97 | ||
| L | 10 | 30.95 | 2.76 | 30.84 | 26.67 | 36.26 | 28.89 | 32.54 | ||
| SEP_ab | A | 10 | 7.4 | 1.42 | 7.64 | 5.5 | 9.49 | 6.21 | 8.43 | <0.001 |
| B | 10 | 26.14 | 2.68 | 26.01 | 22.45 | 29.87 | 23.9 | 28.29 | B, E, K > A, H, I, J, L | |
| C | 10 | 16.84 | 1.46 | 17.3 | 14.27 | 18.74 | 15.71 | 17.76 | F > A, H, I, L | |
| D | 10 | 10.74 | 1.14 | 10.96 | 9.09 | 12.73 | 9.76 | 11.18 | C > A, H, I | |
| E | 8 | 26.46 | 1.54 | 26.21 | 24.22 | 28.73 | 25.74 | 27.02 | G > I | |
| F | 9 | 25.08 | 1.94 | 25.07 | 22.57 | 27.87 | 24.11 | 26.17 | ||
| G | 8 | 13.63 | 2.61 | 12.62 | 10.8 | 17.45 | 11.68 | 16.19 | ||
| H | 10 | 7.53 | 1.66 | 7.26 | 5.38 | 10.63 | 6.54 | 8.53 | ||
| I | 10 | 4.72 | 1.06 | 4.62 | 3.45 | 7.1 | 3.98 | 4.92 | ||
| J | 10 | 10.45 | 0.64 | 10.43 | 9.11 | 11.49 | 10.24 | 10.68 | ||
| K | 8 | 26.06 | 0.75 | 26.05 | 25.1 | 27.48 | 25.74 | 26.33 | ||
| L | 10 | 8.93 | 1.72 | 8.59 | 7.02 | 12.78 | 7.89 | 9.12 | ||
| Angle_ad | A | 10 | 107.21 | 4.7 | 106.55 | 99.52 | 116.4 | 104.41 | 109.63 | <0.001 |
| B | 10 | 113.11 | 5.96 | 114.22 | 101.16 | 122.8 | 110.9 | 115.67 | I > A, C, D, E, F, J, L | |
| C | 10 | 105.56 | 2.26 | 105.66 | 101.35 | 109.16 | 105.01 | 106.73 | B, G, H, K > J, L | |
| D | 10 | 111.7 | 9.45 | 106.65 | 101.16 | 126.66 | 105.8 | 119.31 | D > J | |
| E | 8 | 107.62 | 1.62 | 107.38 | 105.61 | 110.2 | 106.51 | 108.88 | ||
| F | 9 | 107.03 | 3.72 | 106.79 | 103.15 | 112.72 | 103.4 | 110.31 | ||
| G | 8 | 112.92 | 4.41 | 113.47 | 107.51 | 119.48 | 108.67 | 115.65 | ||
| H | 10 | 113.65 | 6.15 | 113.87 | 105.9 | 120.03 | 108.21 | 119.57 | ||
| I | 10 | 135.14 | 2.25 | 135.5 | 130.28 | 137.44 | 134.62 | 136.88 | ||
| J | 10 | 74.47 | 3.95 | 74.86 | 67.44 | 79.18 | 73.36 | 77.66 | ||
| K | 8 | 114.05 | 8.05 | 117.09 | 98.82 | 121 | 108.41 | 120.71 | ||
| L | 10 | 95.22 | 2.42 | 95.76 | 90.59 | 98.46 | 94.32 | 96.9 | ||
| Angle_ab | A | 10 | 141.53 | 3.25 | 141.5 | 137.14 | 146.57 | 138.71 | 143.9 | <0.001 |
| B | 10 | 118.35 | 6.01 | 118.04 | 110.89 | 131.86 | 115.32 | 118.53 | A > B, C, E, F, G, J, K | |
| C | 10 | 124.07 | 4.72 | 123.25 | 117.73 | 131.01 | 120.64 | 127.96 | H, I > B, E, F, K | |
| D | 10 | 129.06 | 4.81 | 128.73 | 119.85 | 135.94 | 126.69 | 131.63 | L > E, F, K | |
| E | 8 | 113.61 | 1.67 | 113.26 | 111.88 | 116.53 | 112.2 | 114.71 | D > F, K | |
| F | 9 | 112.62 | 3.51 | 114.04 | 106.94 | 117.38 | 110.12 | 114.76 | ||
| G | 8 | 121.42 | 6.22 | 122.89 | 110.8 | 127.66 | 120 | 125.42 | ||
| H | 10 | 136.22 | 3.51 | 136.76 | 132.1 | 141.29 | 132.65 | 138.27 | ||
| I | 10 | 136.37 | 3.8 | 135.97 | 131.57 | 141.07 | 133.81 | 139.55 | ||
| J | 10 | 123.05 | 2.86 | 123.05 | 118.61 | 128.62 | 122.28 | 124.18 | ||
| K | 8 | 103.1 | 2.61 | 102.22 | 99.59 | 106.23 | 101.48 | 105.99 | ||
| L | 10 | 132.56 | 6.33 | 135.19 | 121.25 | 139.76 | 130.37 | 136.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anna, K.-I.; Sylwia, Ł.; Marcin, Z.; Ewa, S.-O.; Wojtan, B. Variability of Leaf Wetting and Water Storage Capacity of Branches of 12 Deciduous Tree Species. Forests 2020, 11, 1158. https://doi.org/10.3390/f11111158
Anna K-I, Sylwia Ł, Marcin Z, Ewa S-O, Wojtan B. Variability of Leaf Wetting and Water Storage Capacity of Branches of 12 Deciduous Tree Species. Forests. 2020; 11(11):1158. https://doi.org/10.3390/f11111158
Chicago/Turabian StyleAnna, Klamerus-Iwan, Łagan Sylwia, Zarek Marcin, Słowik-Opoka Ewa, and Bartłomiej Wojtan. 2020. "Variability of Leaf Wetting and Water Storage Capacity of Branches of 12 Deciduous Tree Species" Forests 11, no. 11: 1158. https://doi.org/10.3390/f11111158