Soil Is a Net Source of Methane in Tropical African Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Study Site
2.2. Experimental Set up
2.3. Determination of Gas Exchange between Atmosphere and Soil or Water
2.4. Statistic Analysis
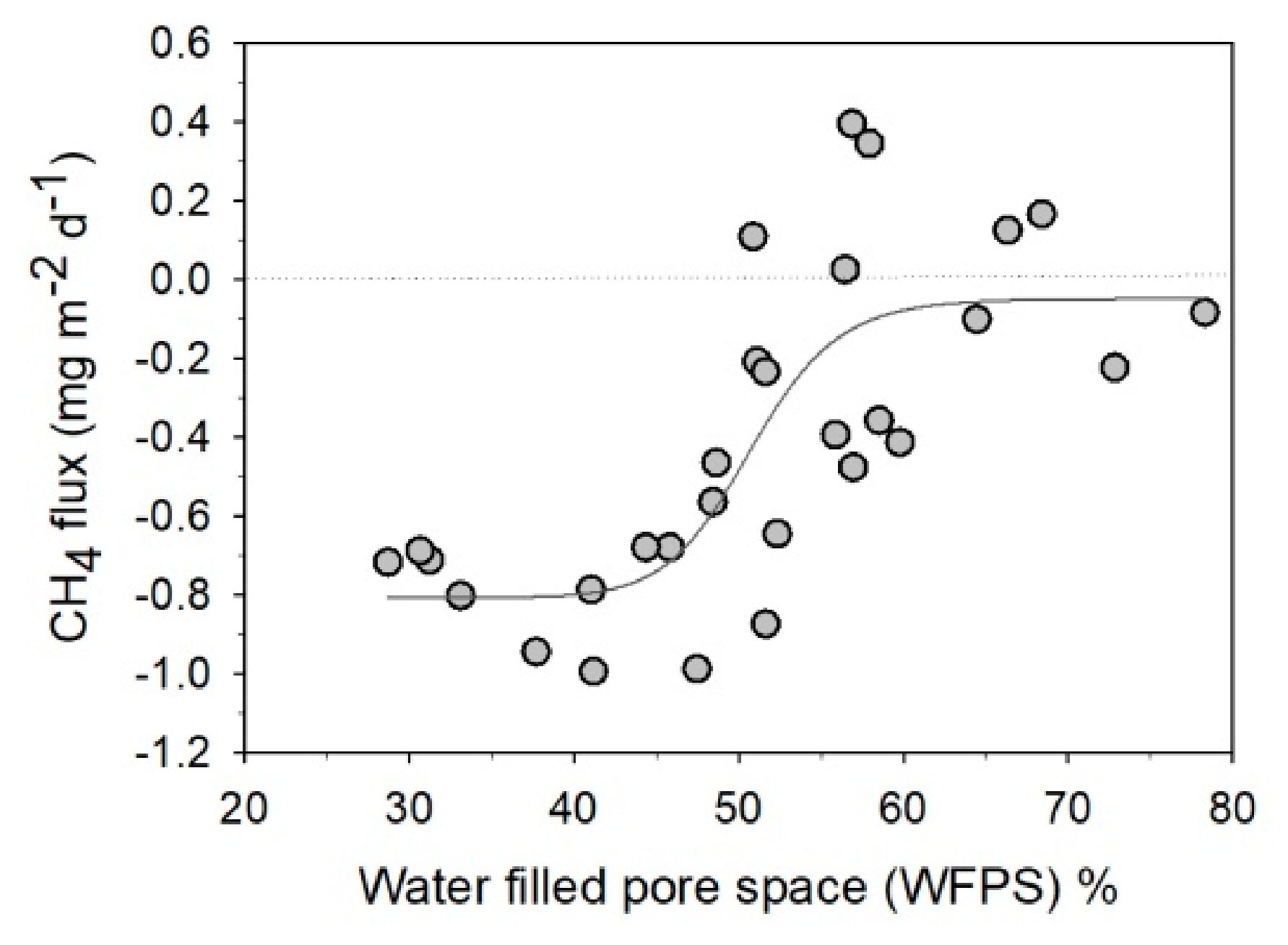
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Myhre, G.; Shindell, D.; Bréon, F.M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and Natural Radiative Forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 659–740. [Google Scholar] [CrossRef]
- Saunois, M.; Stavert, A.R.; Poulter, B.; Bousquet, P.; Canadell, J.G.; Jackson, R.B.; Raymond, P.A.; Dlugokencky, E.J.; Houweling, S.; Patra, P.K.; et al. The Global Methane Budget 2000–2017. Earth Syst. Sci. Data 2020, 12, 1561–1623. [Google Scholar] [CrossRef]
- Kirschke, S.; Bousquet, P.; Ciais, P.; Saunois, M.; Canadell, J.G.; Dlugokencky, E.; Bergamaschi, P.; Bergmann, D.; Blake, D.R.; Bruhwiler, L.M.P.; et al. Three decades of global methane sources and sinks. Nat. Geosci. 2013, 6, 813–823. [Google Scholar] [CrossRef]
- Saunois, M.; Bousquet, P.; Poulter, B.; Peregon, A.; Ciais, P.; Canadell, J.G.; Dlugokencky, E.; Etiope, G.; Bastviken, D.; Houweling, S.; et al. Variability and quasi-decadal changes in the methane budget over the period 2000–2012. Atmos. Chem. Phys. 2017, 17, 11135–11161. [Google Scholar] [CrossRef] [Green Version]
- Melton, J.R.; Wania, R.; Hodson, E.L.; Poulter, B.; Ringeval, B.; Spahni, R.; Bohn, T.; Avis, C.A.; Beerling, D.J.; Chen, G.; et al. Present state of global wetland extent and wetland methane modelling: Conclusions from a model inter-comparison project (WETCHIMP). Biogeosciences 2013, 10, 753–788. [Google Scholar] [CrossRef] [Green Version]
- Prigent, C.; Papa, F.; Aires, F.; Rossow, W.B.; Matthews, E. Global inundation dynamics inferred from multiple satellite observations, 1993–2000. J. Geophys. Res. Atmos. 2007, 112, 112. [Google Scholar] [CrossRef]
- Castaldi, S.; Ermice, A.; Strumia, S. Fluxes of N2O and CH4 from soils of savannas and seasonally-dry ecosystems. J. Biogeogr. 2006, 33, 401–415. [Google Scholar] [CrossRef]
- Castaldi, S.; De Grandcourt, A.; Rasile, A.; Skiba, U.; Valentini, R. CO2, CH4 and N2O fluxes from soil of a burned grassland in Central Africa. Biogeosciences 2010, 7, 3459–3471. [Google Scholar] [CrossRef] [Green Version]
- Keller, M.; Veldkamp, E.; Weitz, A.; Reiners, W.A. Effect of pasture age on soil trace-gas emissions from a deforested area of Costa Rica. Nature 1993, 365, 244–246. [Google Scholar] [CrossRef]
- Reiners, W.; Keller, M.; Gerow, K.G. Estimating Rainy Season Nitrous Oxide and Methane Fluxes Across Forest and Pasture Landscapes in Costa Rica. Water Air Soil Pollut. 1998, 105, 117–130. [Google Scholar] [CrossRef]
- Silver, W.L.; Lugo, A.; Keller, M. Soil oxygen availability and biogeochemistry along rainfall and topographic gradients in upland wet tropical forest soils. Biogeochemistry 1999, 44, 301–328. [Google Scholar] [CrossRef]
- Teh, Y.A.; Diem, T.; Jones, S.P.; Quispe, L.P.H.; Baggs, E.; Morley, N.; Richards, M.; Smith, P.; Meir, P. Methane and nitrous oxide fluxes across an elevation gradient in the tropical Peruvian Andes. Biogeosciences 2014, 11, 2325–2339. [Google Scholar] [CrossRef] [Green Version]
- Von Fischer, J.C.; Hedin, L.O. Separating methane production and consumption with a field-based isotope pool dilution technique. Glob. Biogeochem. Cycles 2002, 16, 8-1–8-13. [Google Scholar] [CrossRef]
- Von Fischer, J.C.; Hedin, L.O. Controls on soil methane fluxes: Tests of biophysical mechanisms using stable isotope tracers. Glob. Biogeochem. Cycles 2007, 21, 2007. [Google Scholar] [CrossRef] [Green Version]
- Verchot, L.V.; Davidson, E.A.; Cattânio, J.H.; Ackerman, I.L. Land-Use Change and Biogeochemical Controls of Methane Fluxes in Soils of Eastern Amazonia. Ecosystems 2000, 3, 41–56. [Google Scholar] [CrossRef]
- Teh, Y.A.; Silver, W.L.; Conrad, M.E. Oxygen effects on methane production and oxidation in humid tropical forest soils. Glob. Chang. Biol. 2005, 11, 1283–1297. [Google Scholar] [CrossRef]
- Simona, C.; Bertolini, T.; Valente, A.; Chiti, T.; Valentini, R. Nitrous oxide emissions from soil of an African rain forest in Ghana. Biogeosciences 2013, 10, 4179–4187. [Google Scholar] [CrossRef] [Green Version]
- Fattore, F.; Bertolini, T.; Materia, S.; Gualdi, S.; M’Bou, A.T.; Nicolini, G.; Valentini, R.; De Grandcourt, A.; Tedesco, D.; Castaldi, S. Seasonal trends of dry and bulk concentration of nitrogen compounds over a rain forest in Ghana. Biogeosciences 2014, 11, 3067–3081. [Google Scholar] [CrossRef]
- Ahn, P.M. Soils of the Lower Tano Basin, South-Western Ghana; Ministry of Food and Agriculture, Scientific Services Division, Soil and Land-use Survey Branch: Kumasi, Ghana, 1961. [Google Scholar]
- Chiti, T.; Certini, G.; Grieco, E.; Valentini, R. The role of soil in storing carbon in tropical rainforests: The case of Ankasa Park, Ghana. Plant Soil 2010, 331, 453–461. [Google Scholar] [CrossRef]
- Keller, M.; Weitz, A.M.; Bryan, B.; Rivera, M.M.; Silver, W.L. Soil-atmosphere nitrogen oxide fluxes: Effects of roots disturbance. J. Geophys. Res. 2000, 105, 17693–17698. [Google Scholar] [CrossRef]
- McAullife, C. Gas Chromatographic determination of solutes by multiple phase equilibration. Chem. Technol. 1971, 1, 46–51. [Google Scholar]
- Sanders, R. Compilation of Henry’s Law Constants for Inorganic and Organic Species of Potential Importance in Environmental Chemistry. 1999. Available online: https://www.ft.unicamp.br/~mariaacm/ST405/Lei%20de%20Henry.pdf (accessed on 10 October 2015).
- Vachon, D.; Prairie, Y.T. The ecosystem size and shape dependence of gas transfer velocity versus wind speed relationships in lakes. Can. J. Fish. Aquat. Sci. 2013, 70, 1757–1764. [Google Scholar] [CrossRef]
- Repo, M.E.; Huttunen, J.T.; Naumov, A.V.; Chichulin, A.V.; Lapshina, E.D.; Bleuten, W.; Martikainen, P.J. Release of CO2 and CH4 from small wetland lakes in western Siberia. Tellus B Chem. Phys. Meteorol. 2007, 59, 788–796. [Google Scholar] [CrossRef] [Green Version]
- Bergamaschi, P.; Frankenberg, C.; Meirink, J.F.; Krol, M.; Villani, M.G.; Houweling, S.; Dentener, F.; Dlugokencky, E.J.; Miller, J.B.; Gatti, L.V.; et al. Inverse modeling of global and regional CH4emissions using SCIAMACHY satellite retrievals. J. Geophys. Res. Space Phys. 2009, 114. [Google Scholar] [CrossRef] [Green Version]
- Megoniga, J.P.; Hines, M.E.; Visscher, P.T. Anaerobic Metabolism: Linkages to Trace Gases and Aerobic Processes; Schlesinger, W.H., Ed.; Elsevier-Pergamon: Oxford, UK, 2004; pp. 317–424. [Google Scholar]
- Bridgham, S.D.; Cadillo-Quiroz, H.; Keller, J.K.; Zhuang, Q. Methane emissions from wetlands: Biogeochemical, microbial, and modeling perspectives from local to global scales. Glob. Chang. Biol. 2013, 19, 1325–1346. [Google Scholar] [CrossRef]
- Deppe, M.; Knorr, K.-H.; McKnight, D.M.; Blodau, C. Effects of short-term drying and irrigation on CO2 and CH4 production and emission from mesocosms of a northern bog and an alpine fen. Biogeochemistry 2010, 100, 89–103. [Google Scholar] [CrossRef]
- Deppe, M.; McKnight, D.M.; Blodau, C. Effects of Short-Term Drying and Irrigation on Electron Flow in Mesocosms of a Northern Bog and an Alpine Fen. Environ. Sci. Technol. 2010, 44, 80–86. [Google Scholar] [CrossRef]
- Smith, K.A. Anaerobic Zones and Denitrification in Soil: Modelling and Measurement. In Denitrification in Soil and Sediment; Revsboech, N.P., Sørensen, J., Eds.; Plenum Press: New York, NY, USA, 1990; pp. 229–244. [Google Scholar]
- Malhi, Y.; Baldocchi, D.D.; Jarvis, P.G. The carbon balance of tropical, temperate and boreal forests. Plant Cell Environ. 1999, 22, 715–740. [Google Scholar] [CrossRef]
- Yang, W.H.; McNicol, G.; Teh, Y.A.; Estera-Molina, K.; Wood, T.E.; Silver, W.L. Evaluating the Classical Versus an Emerging Conceptual Model of Peatland Methane Dynamics. Glob. Biogeochem. Cycles 2017, 31, 1435–1453. [Google Scholar] [CrossRef]
- Megonigal, J.P.; Whalen, S.C.; Tissue, D.T.; Bovard, B.D.; Allen, A.S.; Albert, D.B. A Plant-Soil-Atmosphere Microcosm for Tracing Radiocarbon from Photosynthesis through Methanogenesis. Soil Sci. Soc. Am. J. 1999, 63, 665–671. [Google Scholar] [CrossRef]
- King, J.Y.; Reeburgh, W.S.; Thieler, K.K.; Kling, G.W.; Loya, W.M.; Johnson, L.C.; Nadelhoffer, K.J. Pulse-labeling studies of carbon cycling in Arctic tundra ecosystems: The contribution of photosynthates to methane emission. Glob. Biogeochem. Cycles 2002, 16, 10–11. [Google Scholar] [CrossRef] [Green Version]
- Guenet, B.; Danger, M.; Abbadie, L.; Lacroix, G. Priming effect: Bridging the gap between terrestrial and aquatic ecology. Ecology 2010, 91, 2850–2861. [Google Scholar] [CrossRef] [PubMed]
- Hedin, L.O.; Von Fischer, J.C.; Ostrom, N.E.; Kennedy, B.P.; Brown, M.G.; Robertson, G.P. Thermodynamic Constraints on Nitrogen Transformations and Other Biogeochemical Processes at Soil-Stream Interfaces. Ecology 1998, 79, 684–703. [Google Scholar] [CrossRef] [Green Version]
- Belyea, L.R.; Baird, A.J. Beyond “The limits to peat bog growth”: Cross-scale feedback in peatland development. Ecol. Monogr. 2006, 76, 299–322. [Google Scholar] [CrossRef]
- Lohila, A.; Aalto, T.; Aurela, M.; Hatakka, J.; Tuovinen, J.-P.; Kilkki, J.; Penttilä, T.; Vuorenmaa, J.; Hänninen, P.; Sutinen, R.; et al. Large contribution of boreal upland forest soils to a catchment-scale CH 4 balance in a wet year. Geophys. Res. Lett. 2016, 43, 2946–2953. [Google Scholar] [CrossRef] [Green Version]
- Simona, C.; Ariangelo, D.P.R.; John, G.; Nina, N.; Ruben, M.; José, S.J. Nitrous oxide and methane fluxes from soils of the Orinoco savanna under different land uses. Glob. Chang. Biol. 2004, 10, 1947–1960. [Google Scholar] [CrossRef]
- Lindau, C.W.; Patrick, W.H., Jr.; DeLaune, R.D. Factors affecting methane production in flooded rice soils. In Agricultural Ecosystem Effects on Trace Gases and Global Climate Change; Karl, D.M., Ed.; American Society for Agronomy: Madison, WI, USA, 1993; pp. 157–165. [Google Scholar]
- Yagi, K.; Minami, K. Spatial and Temporal Variations of Methane Flux from a Rice Paddy Field; Oremland, R.S., Ed.; Biogeochemistry of Global Change; Chapman and Hall: New York, NY, USA, 1993; pp. 353–368. [Google Scholar]
- Pangala, S.R.; Enrich-Prast, A.; Basso, L.S.; Peixoto, R.B.; Bastviken, D.; Hornibrook, E.R.C.; Gatti, L.V.; Marotta, H.; Calazans, L.S.B.; Sakuragui, C.M.; et al. Large emissions from floodplain trees close the Amazon methane budget. Nature 2017, 552, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Carmo, J.B.D.; Keller, M.; Dias, J.D.; De Camargo, P.B.; Crill, P. A source of methane from upland forests in the Brazilian Amazon. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.B.; Gatti, L.V.; D’Amelio, M.T.S.; Crotwell, A.M.; Dlugokencky, E.J.; Bakwin, P.; Artaxo, P.; Tans, P.P. Airborne measurements indicate large methane emissions from the eastern Amazon basin. Geophys. Res. Lett. 2007, 34, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Querino, C.A.S.; Smeets, C.J.P.P.; Vigano, I.; Holzinger, R.; Moura, V.; Gatti, L.; Martinewski, A.; Manzi, A.O.; De Araújo, A.C.; Röckmann, T. Methane flux, vertical gradient and mixing ratio measurements in a tropical forest. Atmos. Chem. Phys. 2011, 11, 7943–7953. [Google Scholar] [CrossRef] [Green Version]






| Flux Class mg CH4 m−2 d−1 | Counts | Frequency % |
|---|---|---|
| <0 | 162 | 53.3 |
| 0 | 17 | 5.6 |
| 0–1 | 40 | 13.2 |
| 1–10 | 44 | 14.5 |
| 10–50 | 19 | 6.2 |
| 50–100 | 9 | 3.0 |
| 100–1000 | 13 | 3.6 |
| >1000 | 2 | 0.7 |
| Day 1 | Day 2 | Day 3 | |
|---|---|---|---|
| Water height (cm) above soil surface | 10 | 8 | 6 |
| CH4 concentration in water (M) | 0.06 ± 0.03 | 0.51 ± 0.13 | 0.43 ± 0.09 |
| % CH4 saturation ± 1SD (10) | (6.39 ± 3.16) × 104 | (4.65 ± 1.24) × 105 | (4. 57 ± 0.01) × 105 |
| Diffusive CH4 fluxes (mg CH4 m−2 d−1 ± 1 SD) | |||
| Model 1 [24] | 5.34 ± 2.65 | 42.41 ± 11.32 | 36.02 ± 7.30 |
| Model 2 [25] | 7.77 ± 3.85 | 61.72 ± 13.87 | 52.43 ± 10.63 |
| Model 3 (Fick’s model) | 16.08 ± 9.97 | 127.26 ± 33.96 | 108.39 ± 22.00 |
| Mean of CH4 flux model ensemble mg CH4 m−2 d−1 ± 1 SD | 9.73 ± 5.63 | 77.13 ± 44.47 | 65.61 ± 37.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castaldi, S.; Bertolini, T.; Nicolini, G.; Valentini, R. Soil Is a Net Source of Methane in Tropical African Forests. Forests 2020, 11, 1157. https://doi.org/10.3390/f11111157
Castaldi S, Bertolini T, Nicolini G, Valentini R. Soil Is a Net Source of Methane in Tropical African Forests. Forests. 2020; 11(11):1157. https://doi.org/10.3390/f11111157
Chicago/Turabian StyleCastaldi, Simona, Teresa Bertolini, Giacomo Nicolini, and Riccardo Valentini. 2020. "Soil Is a Net Source of Methane in Tropical African Forests" Forests 11, no. 11: 1157. https://doi.org/10.3390/f11111157
APA StyleCastaldi, S., Bertolini, T., Nicolini, G., & Valentini, R. (2020). Soil Is a Net Source of Methane in Tropical African Forests. Forests, 11(11), 1157. https://doi.org/10.3390/f11111157





