Evaluation of the Foliar Damage That Threatens a Millennial-Age Tree, Araucaria araucana (Molina) K. Koch, Using Leaf Waxes
Abstract
1. Introduction
2. Materials and Methods
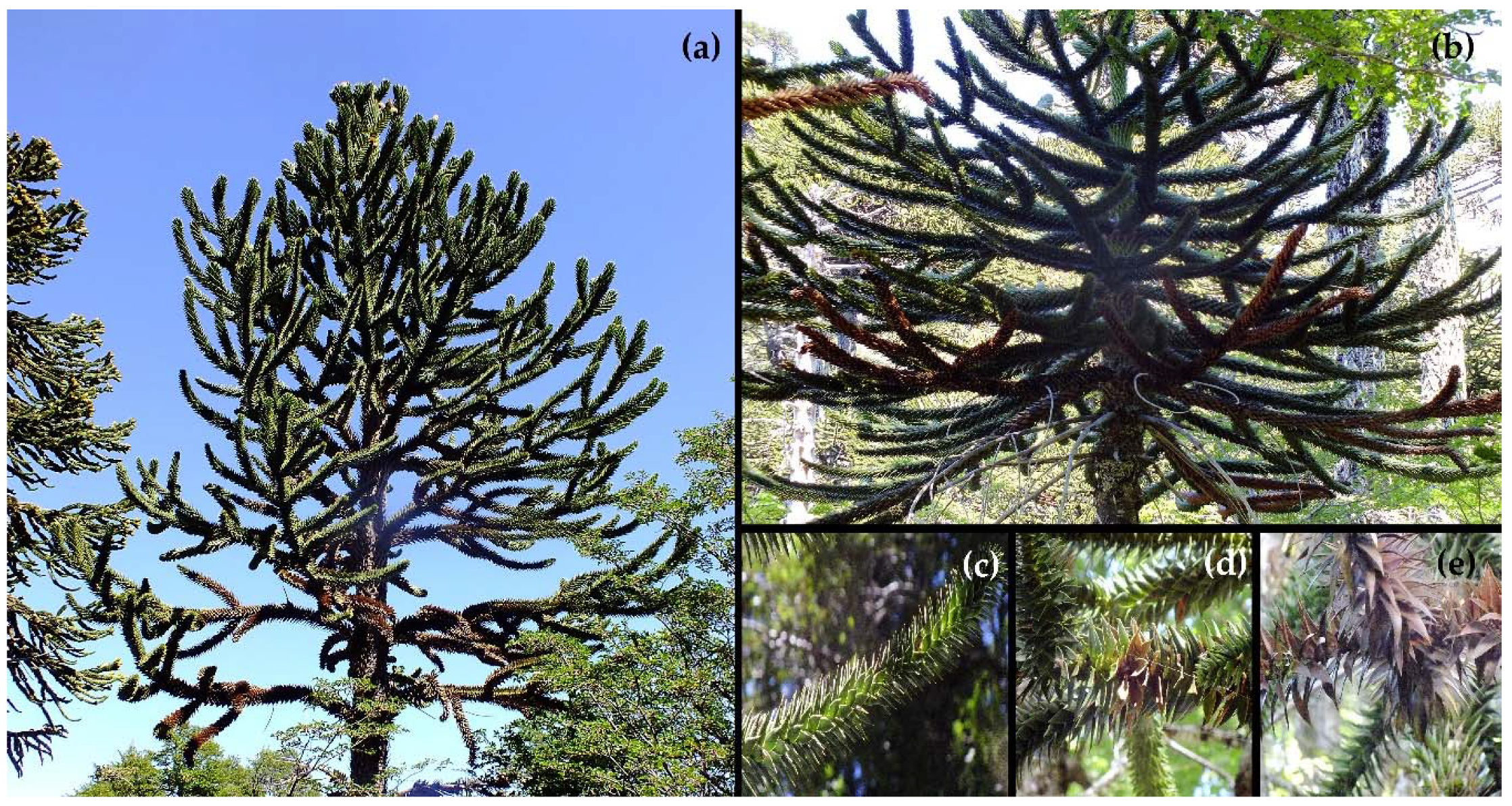
2.1. Study Site
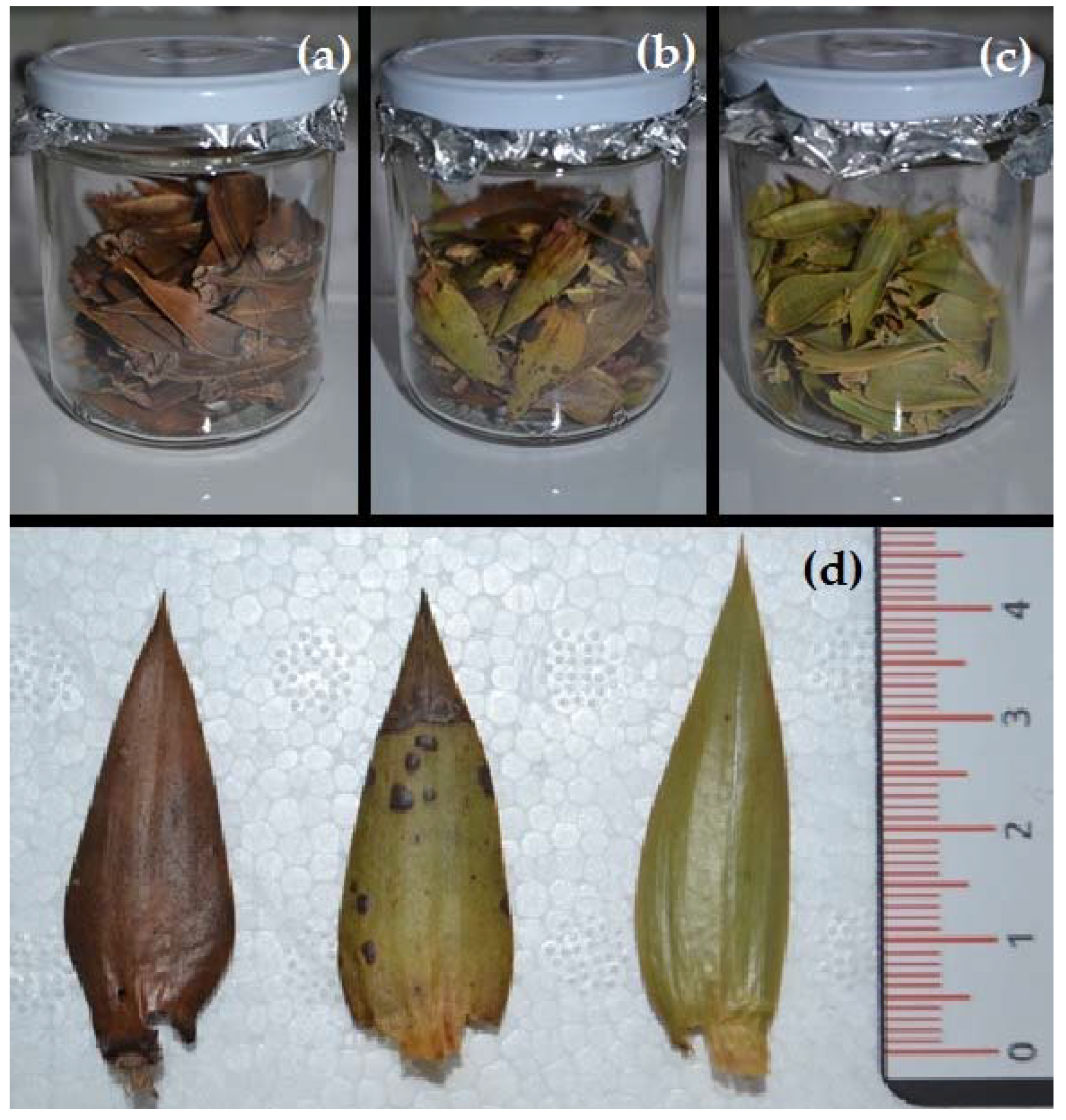
2.2. Sampling Protocol
2.3. Total Lipid Extraction
2.4. Total Lipid Separation
2.5. Derivatization
2.6. Leaf Wax Identification and Quantification
2.7. Statistical Analysis
3. Results
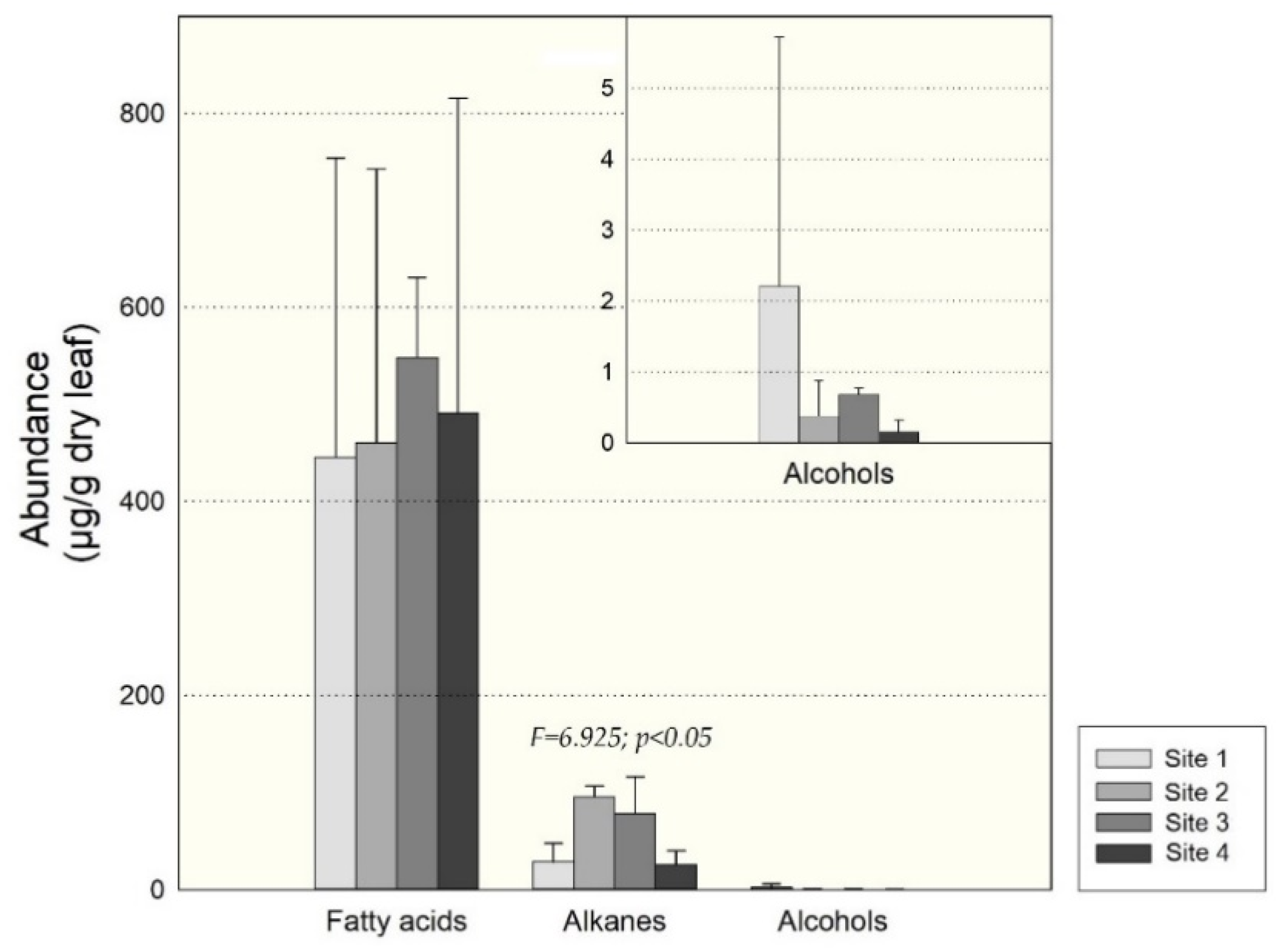
3.1. Leaf Waxes of Healthy A. araucana
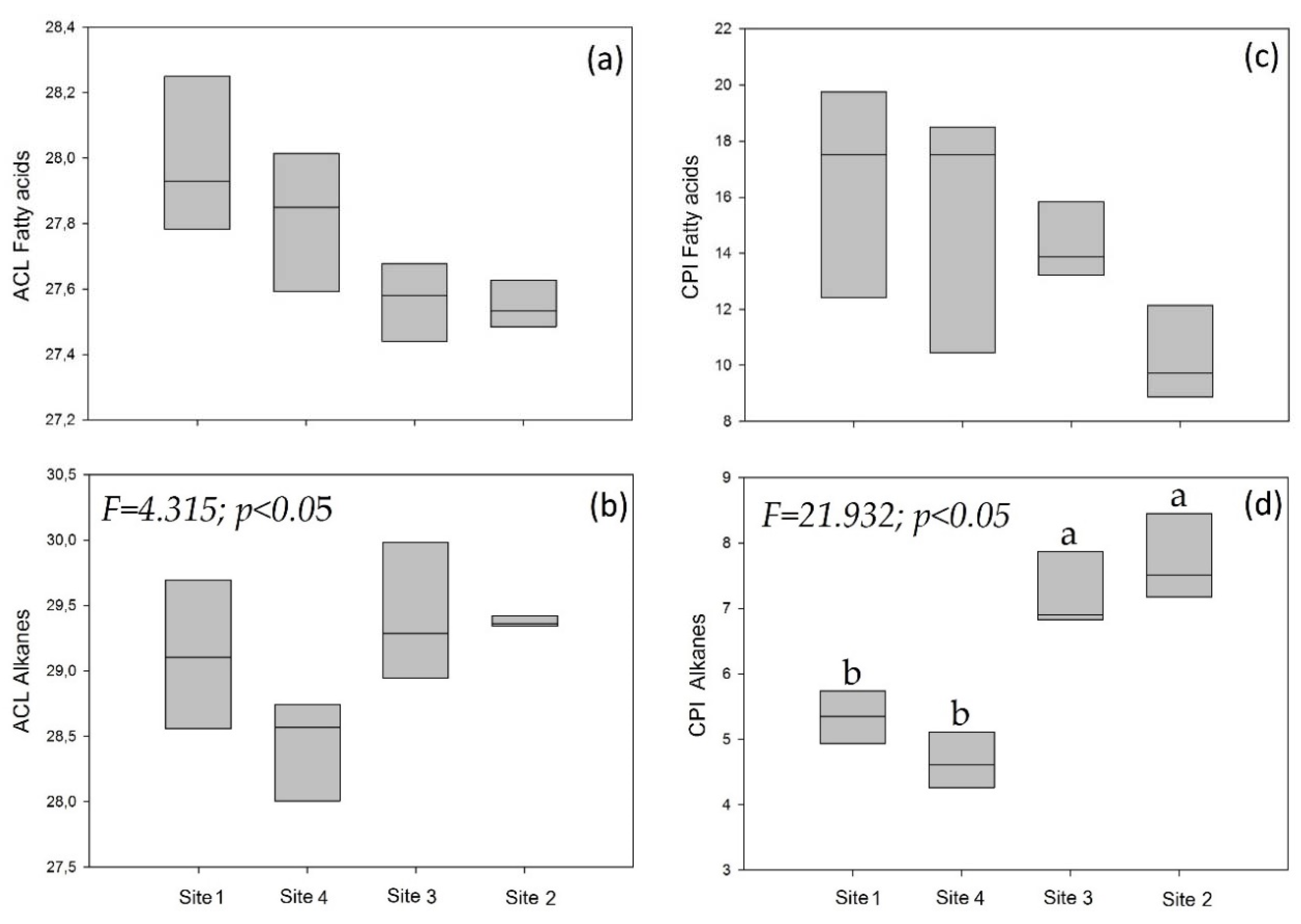
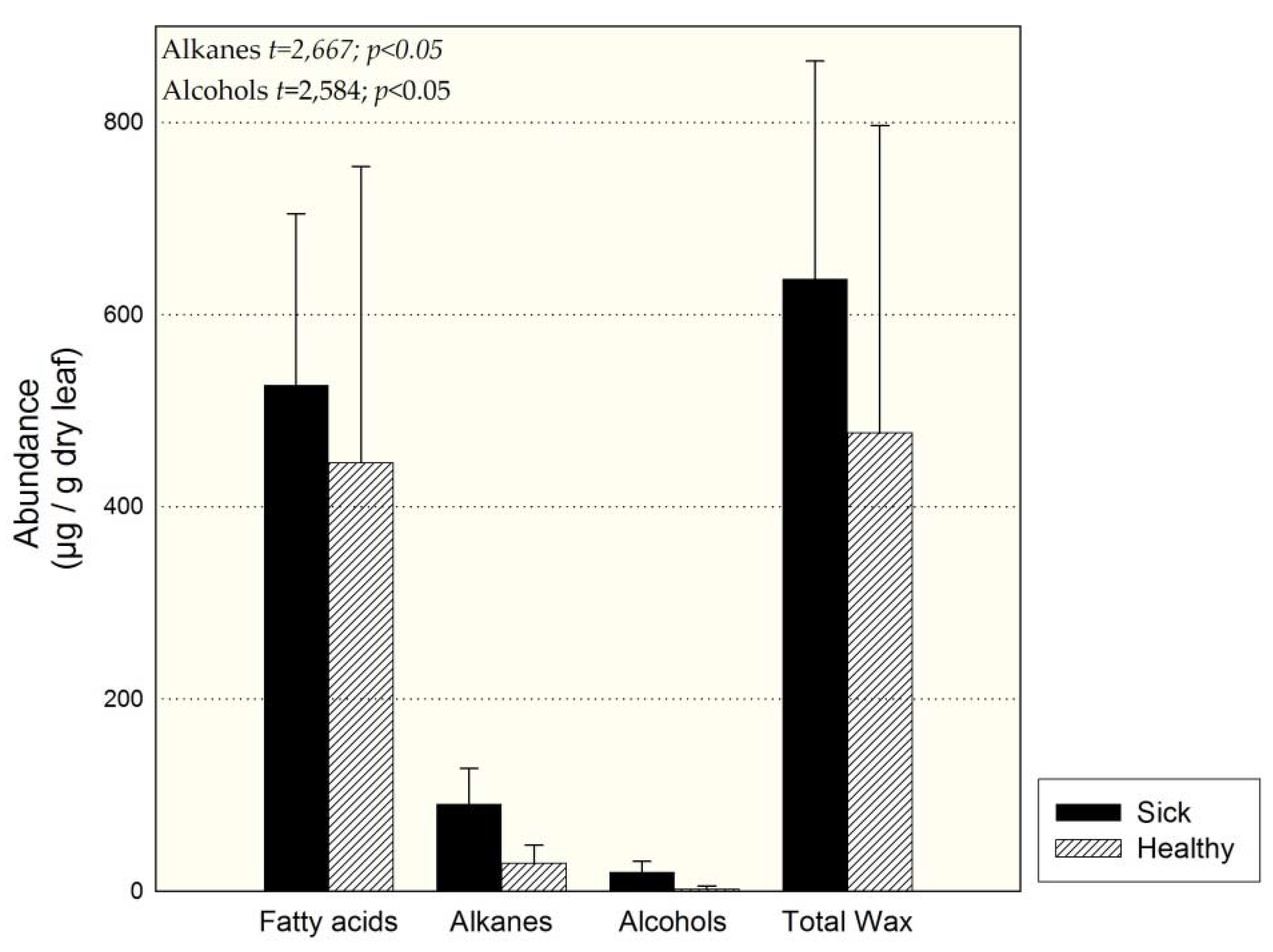
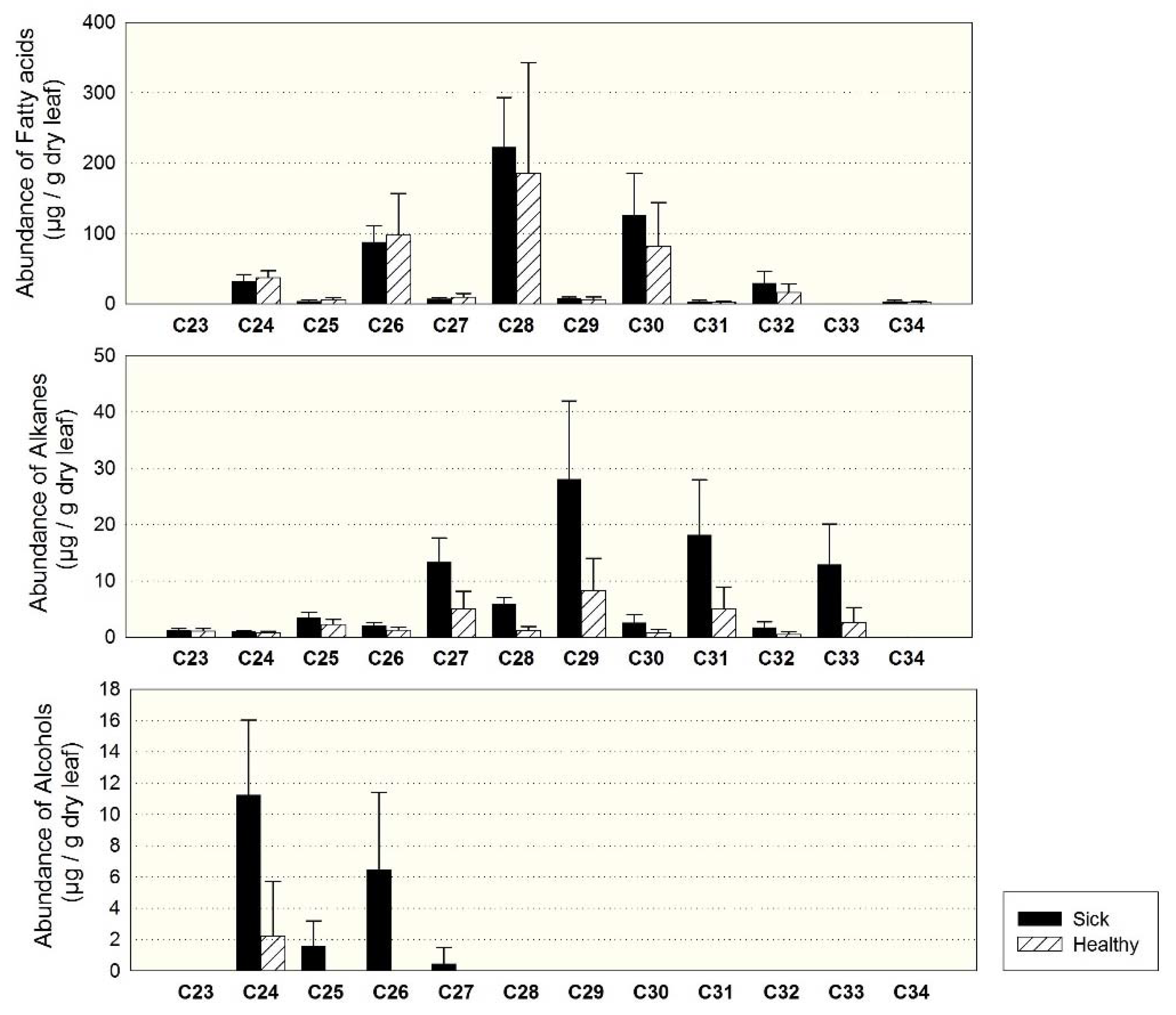
3.2. Individuals of Site 1 (Sick and Healthy)
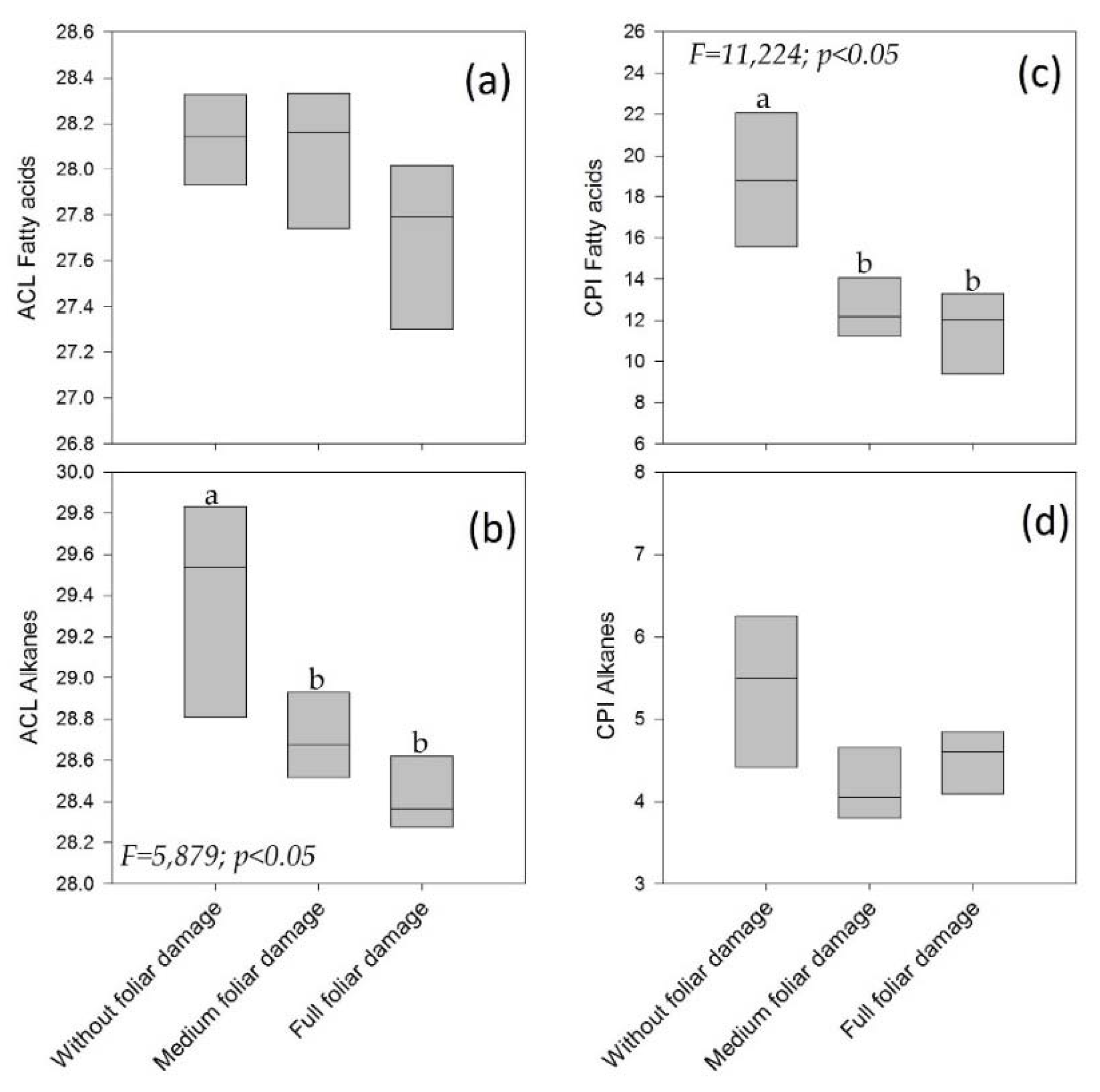
3.3. Sick Individuals At Site 1: Three Levels of Foliar Damage
4. Discussion
4.1. A. Araucana Leaf Wax Composition
4.2. Dominant Homologues of Leaf Waxes
4.3. Leaf Waxes of A. araucana from the Four Sites
4.4. Comparison of Sick and Healthy Individuals at Site 1
4.5. Potential Causes: Evidence of Leaf Wax Changes from Plant Diseases or Environmental Conditions
4.6. Sick Individuals: Three Levels of Foliar Damage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rodríguez, M.; Luebert, F.; Pliscoff, P. Sinopsis bioclimática y vegetacional de Chile. Rev. De Geogr. Norte Gd. 2008, 105–107. [Google Scholar]
- Veblen, T.T. Regeneration Patterns in Araucaria araucana Forests in Chile. J. Biogeogr. 1982, 9, 11–28. [Google Scholar] [CrossRef]
- Fuentes-Ramírez, A.; Arroyo-Vargas, P.; Fierro, A.D.; Pérez, F. Post-fire response of Araucaria araucana (Molina) K. Koch: Assessment of vegetative resprouting, seed production and germination. Gayana Botánica 2019, 76, 119–122. [Google Scholar] [CrossRef]
- González, M.E. Fire history data as reference information in ecological restoration. Dendrochronologia 2005, 22, 149–154. [Google Scholar] [CrossRef]
- Donoso, P.J.; Soto, D.P. Plantaciones con especies nativas en el centro-sur de Chile: Experiencias, desafíos y oportunidades. Rev. Bosque Nativ. 2010, 47, 10–17. [Google Scholar]
- Cóbar-Carranza, A.J.; García, R.A.; Pauchard, A.; Pena, E. Effect of Pinus contorta invasion on forest fuel properties and its potential implications on the fire regime of Araucaria araucana and Nothofagus antarctica forests. Biol. Invasion 2014, 16, 2273–2291. [Google Scholar] [CrossRef]
- Mundo, I.A.; Kitzberger, T.; Juñent, F.R.; Villalba, R.; Barrera, M.D. Fire history in the Araucaria araucana forests of Argentina: Human and climate influences. Int. J. Wildland Fire 2013, 22, 194–206. [Google Scholar] [CrossRef]
- Pena, E.; Hidalgo, M.; Langdon, B.; Pauchard, A. Management. Patterns of spread of Pinus contorta Dougl. ex Loud. invasion in a Natural Reserve in southern South America. For. Ecol. Manag. 2008, 256, 1049–1054. [Google Scholar] [CrossRef]
- Tella, J.L.; Lambertucci, S.A.; Speziale, K.L.; Hiraldo, F.J. Conservation. Large-scale impacts of multiple co-occurring invaders on monkey puzzle forest regeneration, native seed predators and their ecological interactions. Glob. Ecol. Conserv. 2016, 6, 1–15. [Google Scholar] [CrossRef]
- CITES. Convention on International Trade in Endangered Species of Wild Fauna and Flora; Appendix I; CITES: Washington, DC, USA, 1973. [Google Scholar]
- N°43, D.S. Diario Oficial De La República De Chile, Santiago, Chile, 3 March 1990.
- N°29, D.S. Diario Oficial De La República De Chile, Santiago, Chile, 19 December 2018.
- Montaldo, P. Condiciones ecológicas y dasonómicas de la especie Araucaria araucana (Mol) Koch. Inst. For. Lat. Am. De Investig. Y Capacit. 1974, 46, 3–55. [Google Scholar]
- Aguilera-Betti, I.; Muñoz, A.A.; Stahle, D.; Figueroa, G.; Duarte, F.; González-Reyes, Á.; Christie, D.; Lara, A.; González, M.E.; Sheppard, P.R. The First Millennium-Age Araucaria Araucana in Patagonia. Tree-Ring Res. 2017, 73, 53–56. [Google Scholar] [CrossRef]
- Donoso, C. Bosques templados de Chile y Argentina. In Variación, Estructura Y Dinámica, 1st ed.; Editorial Universitaria: Santiago, Chile, 1994; pp. 372–379. [Google Scholar]
- Herrmann, T.M. Knowledge, values, uses and management of the Araucaria araucana forest by the indigenous Mapuche Pewenche people: A basis for collaborative natural resource management in southern Chile. Nat. Resour. Forum 2005, 29, 120–134. [Google Scholar] [CrossRef]
- Mellado-Mansilla, D.; Díaz, I.A.; Godoy-Güinao, J.; Ortega-Solís, G.; Moreno-Gonzalez, R. Bosque Pehuén Park's Flora: A Contribution to the Knowledge of the Andean Montane Forests in the Araucanía Region, Chile. Nat. Areas J. 2018, 38, 298–311. [Google Scholar] [CrossRef]
- Saavedra, A.; Willhite, E. Observaciones Y Recomendaciones Relacionadas Con La Muerte de Ramas Y Follaje (Daño Foliar De La Araucaria) En Araucaria Araucana En Los Parques Nacionales Del Sur—Centro De Chile; Technical report; US Forest Service: Washington, DC, USA, 2017. [CrossRef]
- CONAF. Available online: http://www.conaf.cl/plan-nacional-de-conservacion-para-frenar-aumento-de-araucarias-contagiadas/ (accessed on 8 September 2019).
- CONAF. Available online: http://www.conaf.cl/consejo-de-la-sociedad-civil-conaf-se-interioriza-de-dano-que-afecta-a-araucarias/ (accessed on 8 September 2019).
- Jetter, R.; Kunst, L.; Samuels, A.L. Composition of plant cuticular waxes. Biol. Plant Cuticle 2008, 23, 145–181. [Google Scholar]
- Bargel, H.; Koch, K.; Cerman, Z.; Neinhuis, C. Structure—Function relationships of the plant cuticle and cuticular waxes—A smart material? Funct. Plant Biol. 2006, 33, 893–910. [Google Scholar] [CrossRef]
- Jeffree, C. The fine structure of the plant cuticle. Biol. Plant Cuticle 2006, 23, 11–125. [Google Scholar]
- Jetter, R.; Riederer, M. Localization of the transpiration barrier in the epi-and intracuticular waxes of eight plant species: Water transport resistances are associated with fatty acyl rather than alicyclic components. Plant Physiol. 2016, 170, 921–934. [Google Scholar] [CrossRef]
- Samuels, L.; Kunst, L.; Jetter, R. Sealing plant surfaces: Cuticular wax formation by epidermal cells. Annu. Rev. Plant Biol. 2008, 59, 683–707. [Google Scholar] [CrossRef]
- Ebada, S.S.; Talaat, A.N.; Labib, R.M.; Mándi, A.; Kurtán, T.; Müller, W.E.; Singab, A.; Proksch, P. Cytotoxic labdane diterpenes and bisflavonoid atropisomers from leaves of Araucaria bidwillii. Tetrahedron 2017, 73, 3048–3055. [Google Scholar] [CrossRef]
- Leide, J.; Hildebrandt, U.; Vogg, G.; Riederer, M. The positional sterile (ps) mutation affects cuticular transpiration and wax biosynthesis of tomato fruits. J. Plant Physiol. 2011, 168, 871–877. [Google Scholar] [CrossRef]
- Riederer, M. Thermodynamics of the water permeability of plant cuticles: Characterization of the polar pathway. J. Exp. Bot. 2006, 57, 2937–2942. [Google Scholar] [CrossRef] [PubMed]
- Stark, R.E.; Tian, S. The cutin biopolymer matrix. In Biology of the Plant Cuticle; Riederer, M., Müller, C., Eds.; Blackwell Publishing: Oxford, UK, 2006; Volume 23, pp. 126–144. [Google Scholar]
- Dragota, S.; Riederer, M. Comparative study on epicuticular leaf waxes of Araucaria araucana, Agathis robusta and Wollemia nobilis (Araucariaceae). Aust. J. Bot. 2009, 56, 644–650. [Google Scholar] [CrossRef]
- Sharma, P.; Kothari, S.L.; Rathore, M.; Gour, V. Properties, variations, roles, and potential applications of epicuticular wax: A review. Turk. J. Bot. 2018, 42, 135–149. [Google Scholar] [CrossRef]
- Tafolla-Arellano, J.C.; González-León, A.; Tiznado-Hernández, M.E.; Zacarías García, L.; Báez-Sañudo, R. Composición, fisiología y biosíntesis de la cutícula en plantas. Rev. Fitotec. Mex. 2013, 36, 3–12. [Google Scholar]
- Kerstiens, G.; Roberts, K. Cuticle. In Handbook of Plant Science; Roberts, K., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2007; pp. 151–153. [Google Scholar]
- Meyers, P.A.; Ishiwatari, R. Lacustrine organic geochemistry—An overview of indicators of organic matter sources and diagenesis in lake sediments. Org. Geochem. 1993, 20, 867–900. [Google Scholar] [CrossRef]
- Sachse, D.; Radke, J.; Gleixner, G. δD values of individual n-alkanes from terrestrial plants along a climatic gradient–Implications for the sedimentary biomarker record. Org. Geochem. 2006, 37, 469–483. [Google Scholar] [CrossRef]
- Bondada, B.R.; Oosterhuis, D.M.; Murphy, J.B.; Kim, K.S. Effect of water stress on the epicuticular wax composition and ultrastructure of cotton (Gossypium hirsutum L.) leaf, bract, and boll. Environ. Exp. Bot. 1996, 36, 61–69. [Google Scholar] [CrossRef]
- Kim, K.S.; Park, S.H.; Jenks, M.A. Changes in leaf cuticular waxes of sesame (Sesamum indicum L.) plants exposed to water deficit. J. Plant Physiol. 2007, 164, 1134–1143. [Google Scholar] [CrossRef]
- Sampangi-Ramaiah, M.H.; Ravishankar, K.V.; Seetharamaiah, S.K.; Roy, T.K.; Hunashikatti, L.R.; Rekha, A.; Shilpa, P. Barrier against water loss: Relationship between epicuticular wax composition, gene expression and leaf water retention capacity in banana. Funct. Plant Biol. 2016, 43, 492–501. [Google Scholar] [CrossRef]
- Huggins, T.; Mohammed, S.; Sengodon, P.; Ibrahim, A.; Tilley, M.; Hays, D. Changes in leaf epicuticular wax load and its effect on leaf temperature and physiological traits in wheat cultivars (Triticum aestivum L.) exposed to high temperatures during anthesis. J. Agron. Crop. Sci. 2018, 204, 49–61. [Google Scholar] [CrossRef]
- Grill, D.; Pfeifhofer, H.; Halbwachs, G.; Waltinger, H. Investigations on epicuticular waxes of differently damaged spruce needles. Eur. J. For. Pathol. 1987, 17, 246–255. [Google Scholar] [CrossRef]
- Rai, A.; Kulshreshtha, K.; Srivastava, P.; Mohanty, C. Leaf surface structure alterations due to particulate pollution in some common plants. Environmentalist 2010, 30, 18–23. [Google Scholar] [CrossRef]
- Bernard, A.; Joubès, J. Arabidopsis cuticular waxes: Advances in synthesis, export and regulation. Prog. Lipid Res. 2013, 52, 110–129. [Google Scholar] [CrossRef] [PubMed]
- Jetter, R.; Kunst, L. Plant surface lipid biosynthetic pathways and their utility for metabolic engineering of waxes and hydrocarbon biofuels. Plant J. 2008, 54, 670–683. [Google Scholar] [CrossRef]
- Joubès, J.; Domergue, F. Biosynthesis of the plant cuticle. In Hydrocarbons, Oils and Lipids: Diversity, Origin, Chemistry and Fat; Wilke, H., Ed.; Springer International Publishing: New York, NY, USA, 2018; pp. 1–19. [Google Scholar]
- Kunst, L.; Samuels, A. Biosynthesis and secretion of plant cuticular wax. Prog. Lipid Res. 2003, 42, 51–80. [Google Scholar] [CrossRef]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281. [Google Scholar] [CrossRef]
- Rafii, Z.A.; Dodd, R. Ecology. Genetic diversity among coastal and Andean natural populations of Araucaria araucana (Molina) K. Koch. Biochem. Syst. Ecol. 1998, 26, 441–451. [Google Scholar] [CrossRef]
- CONAF. Plan De Manejo Parque Nacional Tolhuaca, Santiago: Departamento De Planificacion Y Desarrollo; CONAF: Santiago, Chile, 2014. [Google Scholar]
- Pu, Z.; Weiguo, L. Effect of plant life form on relationship between δD values of leaf wax n-alkanes and altitude along Mount Taibai, China. Org. Geochem. 2011, 42, 100–107. [Google Scholar] [CrossRef]
- Feakins, S.J.; Peters, T.; Wu, M.S.; Shenkin, A.; Salinas, N.; Girardin, C.A.; Bentley, L.P.; Blonder, B.; Enquist, B.J.; Martin, R. Production of leaf wax n-alkanes across a tropical forest elevation transect. Org. Geochem. 2016, 100, 89–100. [Google Scholar] [CrossRef]
- Dodd, R.S.; Afzal-Rafii, Z. Habitat-related adaptive properties of plant cuticular lipids. Evolution 2000, 54, 1438–1445. [Google Scholar] [CrossRef]
- Bush, R.T.; McInerney, F. Influence of temperature and C4 abundance on n-alkane chain length distributions across the central USA. Org. Geochem. 2015, 79, 65–73. [Google Scholar] [CrossRef]
- Diefendorf, A.F.; Leslie, A.B.; Wing, S. Leaf wax composition and carbon isotopes vary among major conifer groups. Geochim. Et Cosmochim. Acta 2015, 170, 145–156. [Google Scholar] [CrossRef]
- Russell, J.M.; Werne, J.P. The use of solid phase extraction columns in fatty acid purification. Org. Geochem. 2007, 38, 48–51. [Google Scholar] [CrossRef]
- Lee, D.; Lee, W.; Jordan, G.; Barreda, V. The Cenozoic history of New Zealand temperate rainforests: Comparisons with southern Australia and South America. N. Z. J. Bot. 2016, 54, 100–127. [Google Scholar] [CrossRef]
- Panti, C.; Pujana, R.R.; Zamaloa, M.C.; Romero, E.J. Araucariaceae macrofossil record from South America and Antarctica. Alcheringa Australas. J. Palaeontol. 2012, 36, 1–22. [Google Scholar] [CrossRef]
- Herrmann, T.M.; Torri, M. Changing forest conservation and management paradigms: Traditional ecological knowledge systems and sustainable forestry: Perspectives from Chile and India. Int. J. Sustain. Dev. World Ecol. 2009, 16, 392–403. [Google Scholar] [CrossRef]
- Müller, C.; Riederer, M. Plant surface properties in chemical ecology. J. Chem. Ecol. 2005, 31, 2621–2651. [Google Scholar] [CrossRef]
- Marco, J.A. Química De Los Productos Naturales: Aspectos Fundamentales Del Metabolismo Secundario, 1st ed.; Editorial Síntesis: Madrid, Spain, 2006; pp. 40–60. [Google Scholar]
- Diefendorf, A.F.; Freeman, K.H.; Wing, S.L.; Graham, H. Production of n-alkyl lipids in living plants and implications for the geologic past. Geochim. Et Cosmochim. Acta 2011, 75, 7472–7485. [Google Scholar] [CrossRef]
- Freimuth, E.J.; Diefendorf, A.F.; Lowell, T. Hydrogen isotopes of n-alkanes and n-alkanoic acids as tracers of precipitation in a temperate forest and implications for paleorecords. Geochim. Et Cosmochim. Acta 2017, 206, 166–183. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Lei, X.; Tian, W.; Cao, J.; Jiang, W.; Wang, M.J. Effects of wax coating on the moisture loss of cucumbers at different storage temperatures. J. Food Qual. 2018, 2018, 9351821. [Google Scholar] [CrossRef]
- Jetter, R.; Schäffer, S.J. Chemical composition of the Prunus laurocerasus leaf surface. Dynamic changes of the epicuticular wax film during leaf development. Plant Physiol. 2001, 126, 1725–1737. [Google Scholar] [CrossRef] [PubMed]
- Buschhaus, C.; Herz, H.; Jetter, R. Chemical composition of the epicuticular and intracuticular wax layers on adaxial sides of Rosa canina leaves. Ann. Bot. 2007, 100, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Dodd, R.S.; Rafii, Z.A.; Power, A. Ecotypic adaptation in Austrocedrus chilensis in cuticular hydrocarbon composition. N. Phytol. 1998, 138, 699–708. [Google Scholar] [CrossRef]
- Guo, N.; Gao, J.; He, Y.; Zhang, Z.; Guo, Y. Variations in leaf epicuticular n-alkanes in some Broussonetia, Ficus and Humulus species. Biochem. Syst. Ecol. 2014, 54, 150–156. [Google Scholar] [CrossRef]
- Hoffmann, B.; Kahmen, A.; Cernusak, L.A.; Arndt, S.K.; Sachse, D. Abundance and distribution of leaf wax n-alkanes in leaves of Acacia and Eucalyptus trees along a strong humidity gradient in northern Australia. Org. Geochem. 2013, 62, 62–67. [Google Scholar] [CrossRef]
- Tassone, E.E.; Lipka, A.E.; Tomasi, P.; Lohrey, G.T.; Qian, W.; Dyer, J.M.; Gore, M.A.; Jenks, M. Products. Chemical variation for leaf cuticular waxes and their levels revealed in a diverse panel of Brassica napus L. Industrial Crop. Prod. 2016, 79, 77–83. [Google Scholar] [CrossRef]
- Shepherd, T.; Wynne-Griffiths, D. The effects of stress on plant cuticular waxes. N. Phytol. 2006, 171, 469–499. [Google Scholar] [CrossRef]
- Bianchi, T.S.; Canuel, E.A. Oceanography. In Chemical Biomarkers in Aquatic Ecosystems; Parrish, C.C., Ed.; Princeton University Press: Princeton, NJ, USA, 2011; Volume 25, pp. 304–305. [Google Scholar]
- Le Provost, G.; Domergue, F.; Lalanne, C.; Campos, P.R.; Grosbois, A.; Bert, D.; Meredieu, C.; Danjon, F.; Plomion, C.; Gion, J. Soil water stress affects both cuticular wax content and cuticle-related gene expression in young saplings of maritime pine (Pinus pinaster Ait). BMC Plant Biol. 2013, 13, 95. [Google Scholar] [CrossRef]
- Costaglioli, P.; Joubès, J.; Garcia, C.; Stef, M.; Arveiler, B.; Lessire, R.; Garbay, B. Profiling candidate genes involved in wax biosynthesis in Arabidopsis thaliana by microarray analysis. Biochim. Et Biophys. Acta Mol. Cell Biol. Lipids. 2005, 1734, 247–258. [Google Scholar] [CrossRef]
- Guo, J.; Xu, W.; Yu, X.; Shen, H.; Li, H.; Cheng, D.; Liu, A.; Liu, J.; Liu, C.; Zhao, S. Cuticular wax accumulation is associated with drought tolerance in wheat near-isogenic lines. Front. Plant Sci. 2016, 7, 1809. [Google Scholar] [CrossRef]
- Liu, X.; Feakins, S.J.; Dong, X.; Xue, Q.; Marek, T.; Leskovar, D.I.; Neely, C.B.; Ibrahim, A. Experimental study of leaf wax n-alkane response in winter wheat cultivars to drought conditions. Org. Geochem. 2017, 113, 210–223. [Google Scholar] [CrossRef]
- Belding, R.D.; Sutton, T.B.; Blankenship, S.M.; Young, E. Relationship between apple fruit epicuticular wax and growth of Peltaster fructicola and Leptodontidium elatius, two fungi that cause sooty blotch disease. Plant Dis. 2000, 84, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Blakeman, J. The chemical environment of leaf surfaces with special reference to spore germination of pathogenic fungi. Pestic. Sci. 1973, 4, 575–588. [Google Scholar] [CrossRef]
- Žnidarčič, D.; Valič, N.; Trdan, S. Epicuticular wax content in the leaves of cabbage (Brassica oleracea L. var. capitata) as a mechanical barrier against three insect pests. Acta Agric. Slov. 2008, 91, 361–370. [Google Scholar]
- Marcell, L.M.; Beattie, G.A. Effect of leaf surface waxes on leaf colonization by Pantoea agglomerans and Clavibacter michiganensis. Mol. Plant Microbe Interact. 2002, 15, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Schwab, M.; Noga, G.; Barthlott, W. The significance of epicuticular waxes for defence of pathogens as shown for Botrytis cinerea infections of kohlrabi and pea plants. Gartenbauwissenschaft 1995, 60, 102–109. [Google Scholar]
- Baldotto, L.E.; Olivares, F.L. Phylloepiphytic interaction between bacteria and different plant species in a tropical agricultural system. Can. J. Microbiol. 2008, 54, 918–931. [Google Scholar] [CrossRef]
- Gentry, G.L.; Barbosa, P.J. Effects of leaf epicuticular wax on the movement, foraging behavior, and attack efficacy of Diaeretiella rapae. Entomol. Exp. Et Appl. 2006, 121, 115–122. [Google Scholar] [CrossRef]
- Kosma, D.K.; Parsons, E.P.; Isaacson, T.; Lü, S.; Rose, J.K.; Jenks, M. Fruit cuticle lipid composition during development in tomato ripening mutants. Physiol. Plant. 2010, 139, 107–117. [Google Scholar] [CrossRef]
- Seo, P.J.; Park, C. Cuticular wax biosynthesis as a way of inducing drought resistance. Plant Signal. Behav. 2011, 6, 1043–1045. [Google Scholar] [CrossRef]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morphological traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef] [PubMed]
- Bliedtner, M.; Schäfer, I.K.; Zech, R.; von Suchodoletz, H. Leaf wax n-alkanes in modern plants and topsoils from eastern Georgia (Caucasus)—Implications for reconstructing regional paleovegetation. Biogeosciences 2018, 15, 3927–3936. [Google Scholar] [CrossRef]
- Tu, T.T.; Egasse, C.; Anquetil, C.; Zanetti, F.; Zeller, B.; Huon, S.; Derenne, S. Leaf lipid degradation in soils and surface sediments: A litterbag experiment. Org. Geochem. 2017, 104, 35–41. [Google Scholar]

| Fatty Acids | Site | C23 | C24 | C25 | C26 | C27 | C28 | C29 | C30 | C31 | C32 | C33 | C34 | ACL | CPI | Total |
| Healthy | 1 | 3.8 | 37.5 | 6.1 | 97.6 | 9.5 | 185.7 | 6.1 | 82.3 | 2.3 | 16.5 | 0.4 | 2.2 | 27.5 | 14.1 | 450.0 ± 308.6 |
| Healthy | 2 | 6.5 | 45.5 | 10.8 | 89.0 | 13.1 | 208.5 | 9.3 | 62.9 | 2.3 | 10.2 | 0.2 | 2.7 | 27.5 | 10.2 | 461.0 ± 281.4 |
| Healthy | 3 | 5.3 | 52.4 | 9.1 | 119.4 | 10.5 | 241.7 | 8.0 | 84.8 | 2.5 | 12.7 | 0.7 | 1.4 | 27.6 | 14.3 | 548.4 ±82.6 |
| Healthy | 4 | 4.0 | 32.9 | 5.9 | 95.1 | 9.2 | 219.5 | 7.1 | 94.0 | 2.8 | 17.9 | 0.4 | 2.5 | 27.8 | 15.5 | 491.2 ± 324.4 |
| Sick: without foliar damage | 1 | 2.8 | 32.6 | 4.4 | 88.1 | 7.8 | 223.0 | 8.0 | 125.8 | 3.5 | 30.2 | 0.0 | 3.4 | 28.1 | 19.8 | 529.6 ± 178.2 |
| Sick: medium foliar damage | 1 | 7.0 | 65.6 | 11.8 | 109.1 | 12.2 | 208.0 | 11.8 | 171.2 | 5.5 | 40.5 | 0.0 | 3.6 | 28.1 | 13.5 | 646.3 ± 44.1 |
| Sick: full foliar damage | 1 | 8.3 | 65.6 | 10.3 | 89.9 | 8.6 | 147.6 | 7.4 | 110.5 | 3.4 | 23.4 | 0.0 | 1.8 | 27.7 | 13.0 | 476.7 ± 128.6 |
| Alkanes | Site | C23 | C24 | C25 | C26 | C27 | C28 | C29 | C30 | C31 | C32 | C33 | C34 | ACL | CPI | Total |
| Healthy | 1 | 1.1 | 0.8 | 2.3 | 1.2 | 5.1 | 1.2 | 8.3 | 0.8 | 5.0 | 0.5 | 2.7 | 0.0 | 28.6 | 5.3 | 28.9 ± 18.8 |
| Healthy | 2 | 0.9 | 0.6 | 3.1 | 1.6 | 16.9 | 3.7 | 33.8 | 2.9 | 18.6 | 1.8 | 11.7 | 0.4 | 29.4 | 7.7 | 96.1 ± 10.7 |
| Healthy | 3 | 1.0 | 0.7 | 3.4 | 1.5 | 13.2 | 3.1 | 26.4 | 2.4 | 15.6 | 1.6 | 9.5 | 0.1 | 29.4 | 7.2 | 78.4 ± 38.2 |
| Healthy | 4 | 1.2 | 1.0 | 2.8 | 1.4 | 5.2 | 1.1 | 6.1 | 0.7 | 3.7 | 0.4 | 2.6 | 0.1 | 28.4 | 4.7 | 26.2 ± 14.0 |
| Sick: without foliar damage | 1 | 1.|3 | 1.0 | 3.6 | 2.1 | 13.4 | 6.0 | 28.2 | 2.6 | 18.2 | 1.8 | 13.0 | 0.4 | 29.4 | 5.6 | 91.5 ± 36.9 |
| Sick: medium foliar damage | 1 | 2.4 | 1.7 | 5.6 | 2.6 | 12.7 | 6.9 | 21.0 | 1.8 | 11.3 | 0.8 | 5.6 | 0.2 | 28.7 | 4.3 | 72.4 ± 19.0 |
| Sick: full foliar damage | 1 | 2.6 | 1.8 | 7.6 | 2.8 | 12.1 | 5.3 | 19.1 | 1.7 | 9.9 | 0.7 | 4.5 | 0.2 | 28.4 | 4.5 | 68.1 ± 13.5 |
| Alcohols | Site | C23 | C24 | C25 | C26 | C27 | C28 | C29 | C30 | C31 | C32 | C33 | C34 | Total | ||
| Healthy | 1 | 0.0 | 2.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | - | - | 2.2 ± 3.5 |
| Healthy | 2 | 0.0 | 0.3 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | - | - | 0.4 ± 0.5 |
| Healthy | 3 | 0.0 | 0.4 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | - | - | 0.7 ± 0.1 |
| Healthy | 4 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | - | - | 0.2 ± 0.2 |
| Sick: without foliar damage | 1 | 0.0 | 11.3 | 1.6 | 6.5 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | - | - | 19.8 ± 11.1 |
| Sick: medium foliar damage | 1 | 0.0 | 14.6 | 1.9 | 5.4 | 0.5 | 1.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | - | - | 23.6 ± 13.2 |
| Sick: full foliar damage | 1 | 0.0 | 12.8 | 1.1 | 9.1 | 0.5 | 2.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | - | - | 25.8 ± 21.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cifuentes, G.; Contreras, S.; Cerda-Peña, C. Evaluation of the Foliar Damage That Threatens a Millennial-Age Tree, Araucaria araucana (Molina) K. Koch, Using Leaf Waxes. Forests 2020, 11, 59. https://doi.org/10.3390/f11010059
Cifuentes G, Contreras S, Cerda-Peña C. Evaluation of the Foliar Damage That Threatens a Millennial-Age Tree, Araucaria araucana (Molina) K. Koch, Using Leaf Waxes. Forests. 2020; 11(1):59. https://doi.org/10.3390/f11010059
Chicago/Turabian StyleCifuentes, Gerald, Sergio Contreras, and Carol Cerda-Peña. 2020. "Evaluation of the Foliar Damage That Threatens a Millennial-Age Tree, Araucaria araucana (Molina) K. Koch, Using Leaf Waxes" Forests 11, no. 1: 59. https://doi.org/10.3390/f11010059
APA StyleCifuentes, G., Contreras, S., & Cerda-Peña, C. (2020). Evaluation of the Foliar Damage That Threatens a Millennial-Age Tree, Araucaria araucana (Molina) K. Koch, Using Leaf Waxes. Forests, 11(1), 59. https://doi.org/10.3390/f11010059





