Climatic Correlates of White Pine Blister Rust Infection in Whitebark Pine in the Greater Yellowstone Ecosystem
Abstract
:1. Introduction
2. Materials and Methods
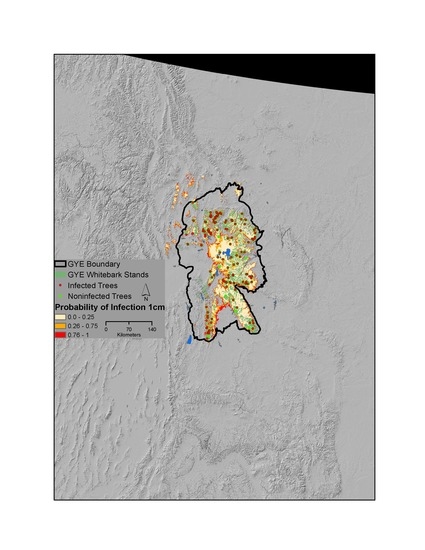
2.1. Study Area
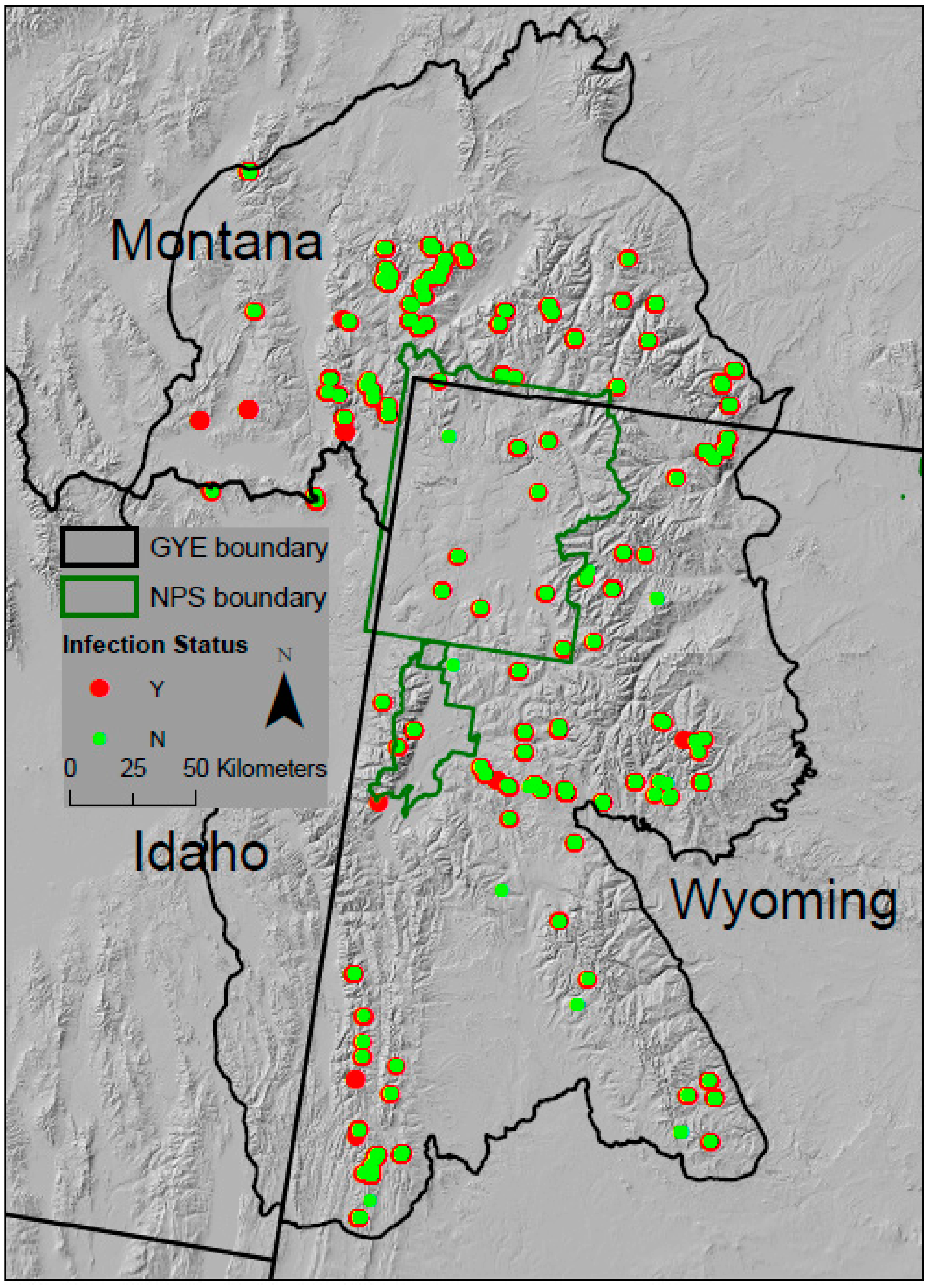
2.2. Field Sampling for Infection Status
2.3. Climate and Location Variables
2.4. Data Analysis
3. Results
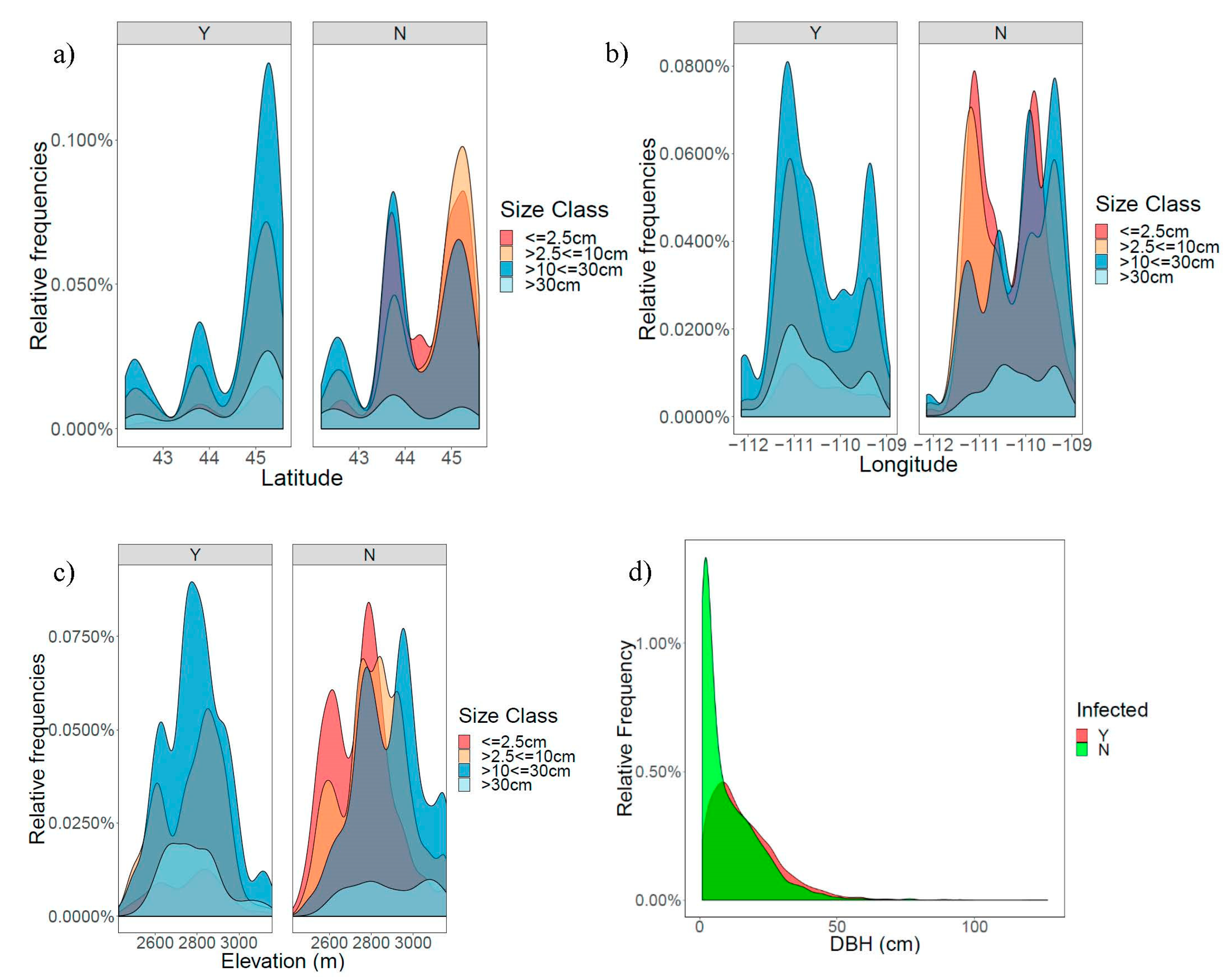
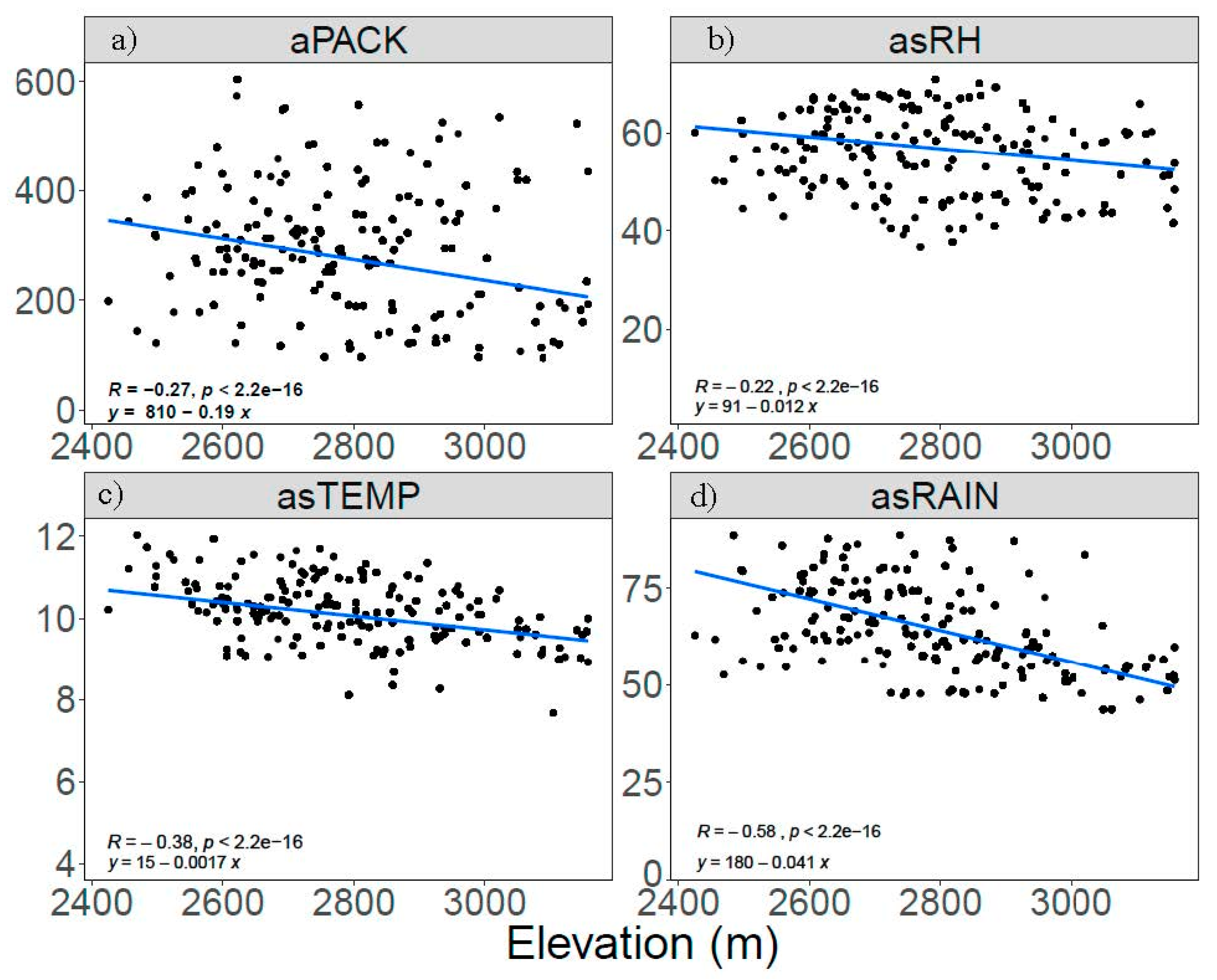
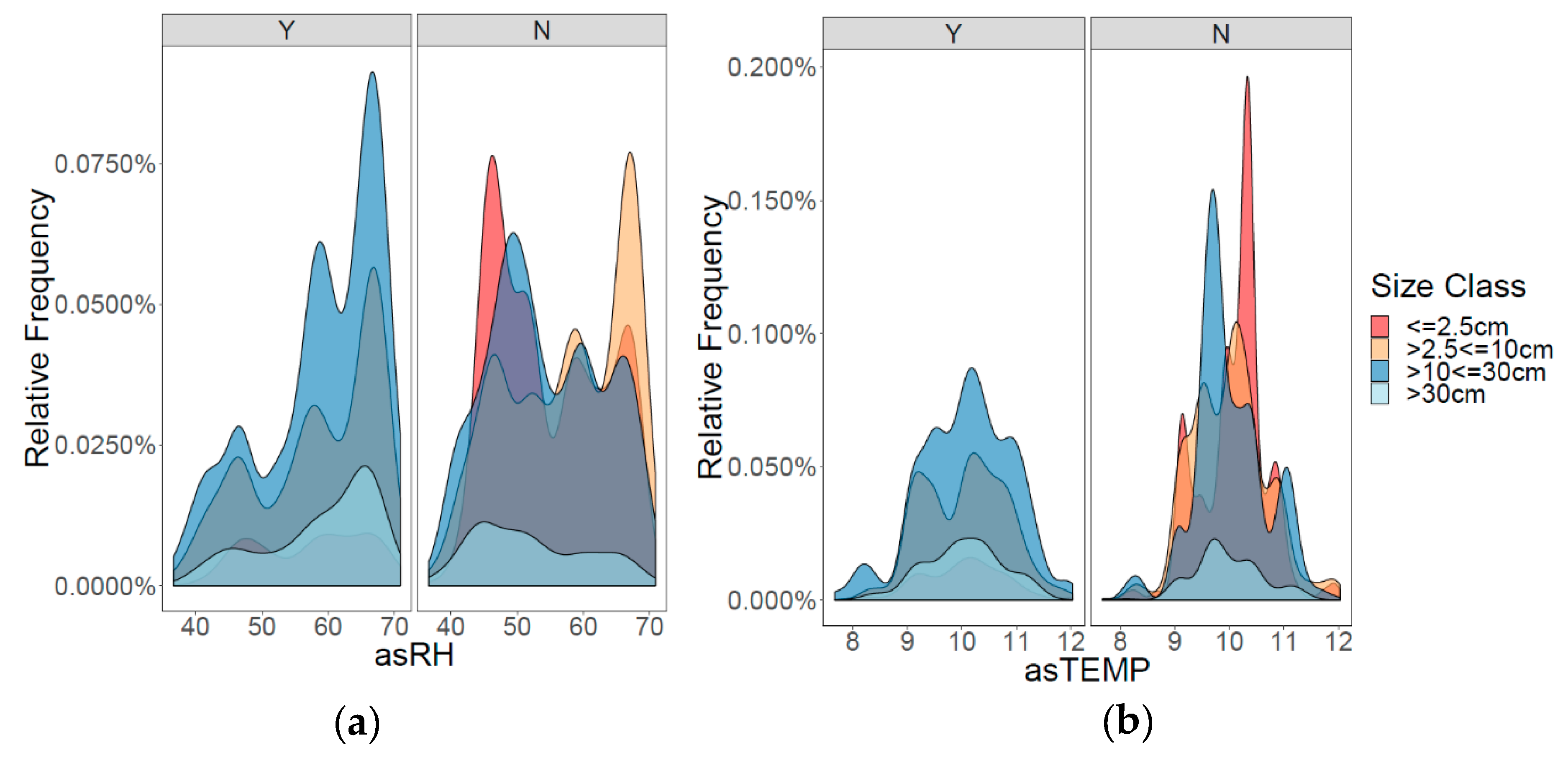
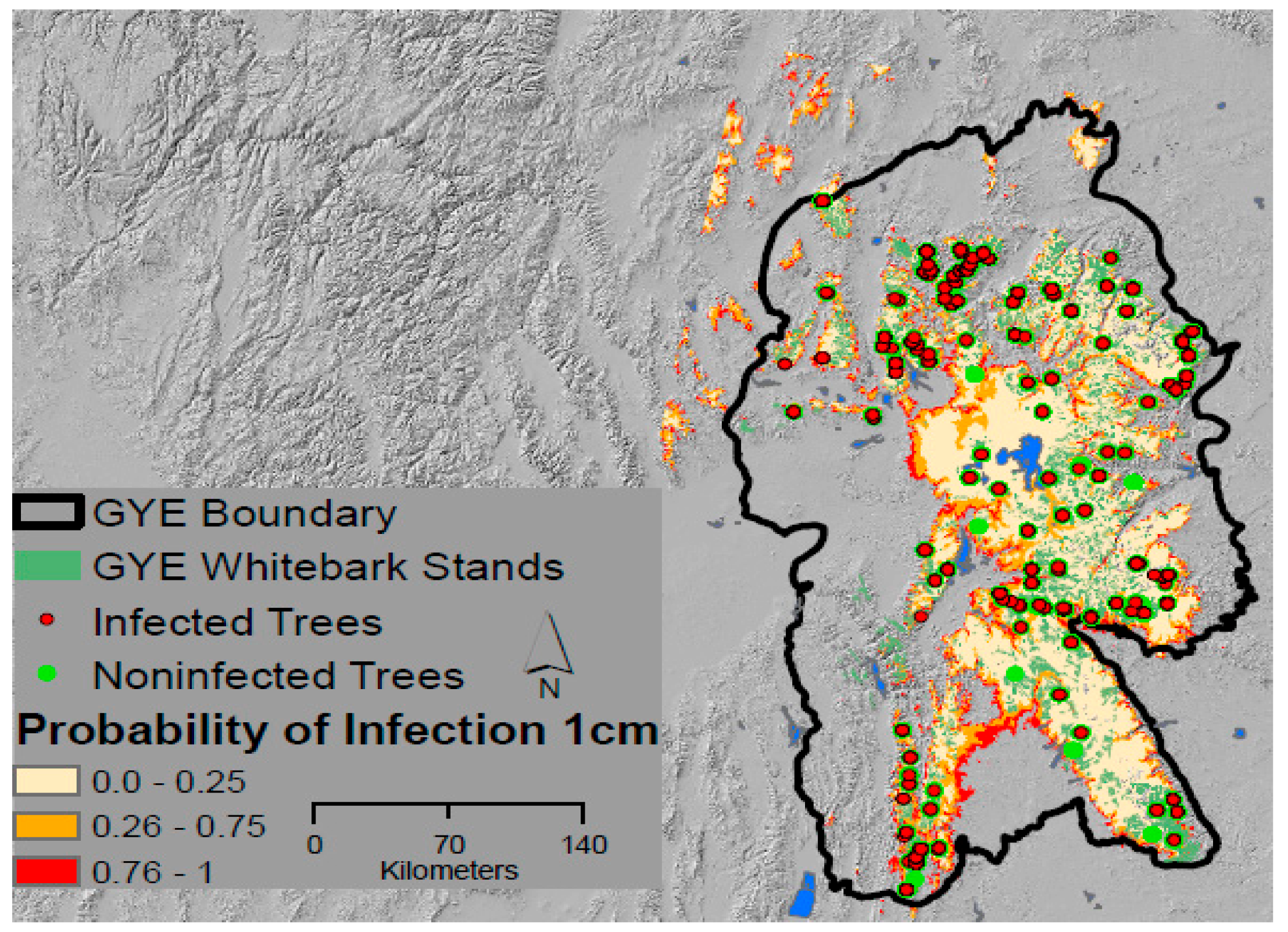
3.1. Patterns of Infection and Climate in the GYE
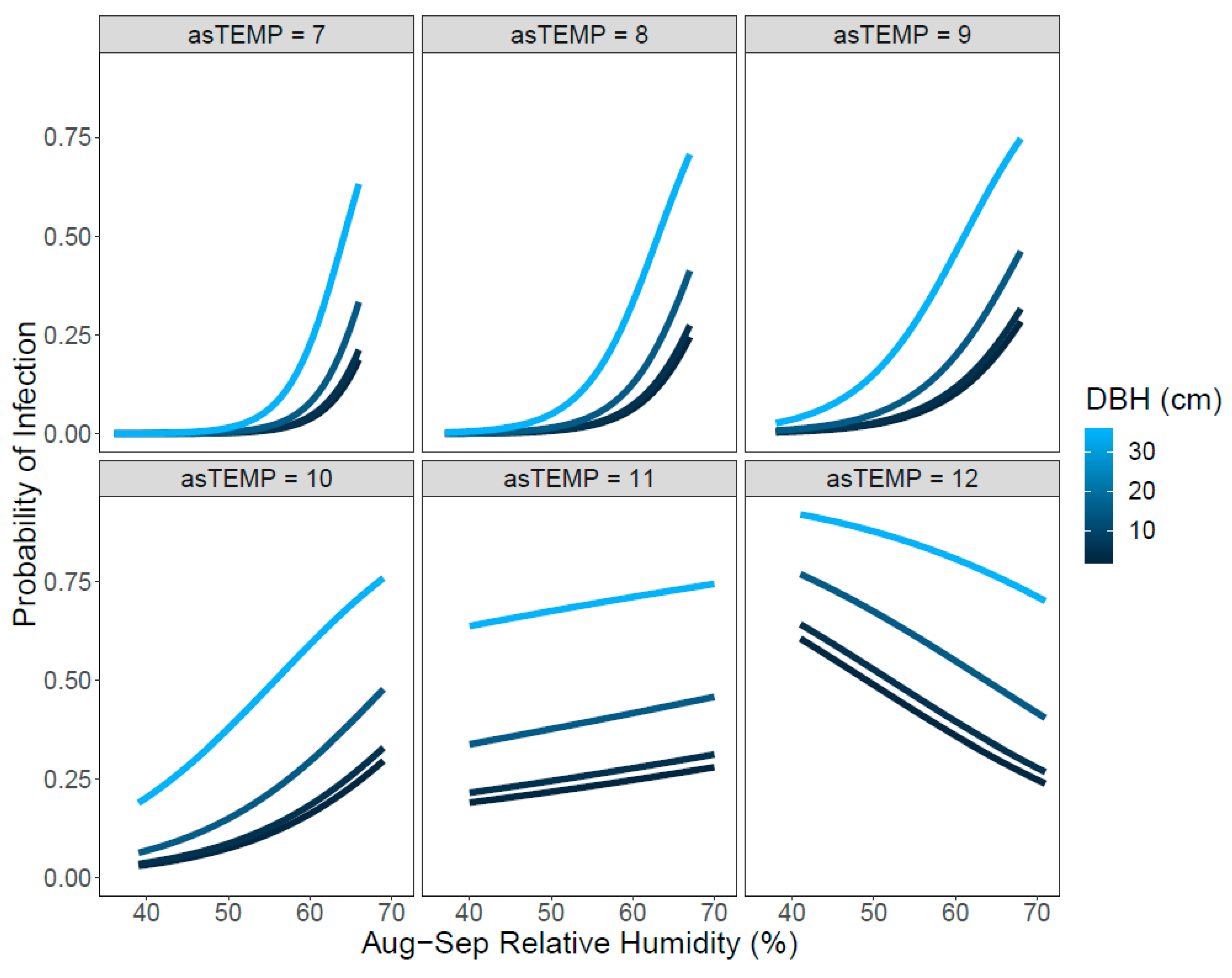
3.2. Climatic Correlates of White Pine Blister Rust Infection
3.3. Climate Limits on White Pine Blister Rust Infection
4. Discussion
4.1. Climate and Scale
4.2. Climate Influence
4.3. Regional Patterns
4.4. Limitations and Opportunities
4.5. Management Relevance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sturrock, R.N.; Frankel, S.J.; Brown, A.V.; Hennon, P.E.; Kliejunas, J.T.; Lewis, K.J.; Worrall, J.J.; Woods, A.J. Climate change and forest diseases. Plant Pathol. 2011, 60, 133–149. [Google Scholar] [CrossRef]
- Garbelotto, M.; Pautasso, M. Impacts of exotic forest pathogens on Mediterranean ecosystems: Four case studies. Eur. J. Plant Pathol. 2012, 133, 101–116. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 129. [Google Scholar] [CrossRef]
- Holmes, T.P.; Aukema, J.E.; von Holle, B.; Liebhold, A.; Sills, E. Economic impacts of invasive species in forests: Past, present, and future. Ann. N. Y. Acad. Sci. 2009, 1162, 18–38. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Brockerhoff, E.G.; Garrett, L.J.; Parke, J.L.; Britton, K.O. Live plant imports: The major pathway for forest insect and pathogen invasions of the US. Front. Ecol. Environ. 2012, 10, 135–143. [Google Scholar] [CrossRef]
- Koteen, L. Climate change, whitebark pine, and grizzly bears in the Greater Yellowstone Ecosystem. In Wildlife Responses to Climate Change: North American Case Studies; Schneider, S.H., Root, T.L., Eds.; Island Press: Washington, DC, USA, 2002; pp. 343–387. [Google Scholar]
- Maloy, O.C. White Pine Blister Rust Control in North America: A Case History. Annu. Rev. Phytopathol. 1997, 35, 87–109. [Google Scholar] [CrossRef]
- Neuenschwander, L.F.; Byler, W.; Harvey, A.E.; McDonald, G.I.; Ortiz, D.S.; Osborne, H.L.; Snyder, G.C.; Zack, A. White Pine in the American West: A Vanishing Species—Can We Save It? General Technical Report RMRS-GTR-35; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 1999.
- DTomback, F.; Anderies, A.J.; Carsey, K.S.; Powell, M.L.; Mellmann-Brown, S. Delayed seed germination in whitebark pine and regeneration patterns following the Yellowstone fires. Ecology 2001, 82, 2587–2600. [Google Scholar] [CrossRef]
- Kendall, J.M.; Asebrook, K.C. The War Against Blister Rust in Yellowstone National Park, 1945–1978. In Historical Perspectives on Science and Management in Yellowstone National Park; The George Wright Forum: Hancock, MI, USA, 1998; Volume 15, pp. 36–49. [Google Scholar]
- Kendall, K.; Keane, R.E. Whitebark pine decline: Infection, mortality, and population trends. In Whitebark Pine Communities: Ecology and Restoration; Tomback, D.F., Arno, S.F., Keane, R.E., Eds.; Island Press: Washington, DC, USA, 2001; pp. 221–242. [Google Scholar]
- Keane, R.E.; Arno, S.F. Rapid Decline of Whitebark Pine in Western Montana: Evidence from 20-Year Remeasurements. West. J. Appl. For. 1993, 8, 44–47. [Google Scholar]
- Zeglen, S. Whitebark pine and white pine blister rust in British Columbia, Canada. Can. J. For. Res. 2002, 32, 1265–1274. [Google Scholar] [CrossRef]
- Tomback, D.F.; Achuff, P. Blister rust and western forest biodiversity: Ecology, values and outlook for white pines. For. Pathol. 2010, 40, 186–225. [Google Scholar] [CrossRef]
- Shanahan, E.; Irvine, K.M.; Thoma, D.; Wilmoth, S.; Ray, A.; Legg, K.; Shovic, H. Whitebark pine mortality related to white pine blister rust, mountain pine beetle outbreak, and water availability. Ecosphere 2016, 7, e01610. [Google Scholar] [CrossRef]
- Logan, J.A.; Macfarlane, W.W.; Willcox, L. Effective monitoring as a basis for adaptive management: A case history of mountain pine beetle in Greater Yellowstone Ecosystem whitebark pine. iForest Biogeosci. For. 2009, 2, 19–22. [Google Scholar] [CrossRef]
- MacFarlane, W.W.; Logan, J.A.; Kern, W.R. An innovative aerial assessment of Greater Yellowstone Ecosystem mountain pine beetle-caused whitebark pine mortality. Ecol. Appl. 2013, 23, 421–437. [Google Scholar] [CrossRef]
- Powell, J.A.; Logan, J.A. Insect seasonality: Circle map analysis of temperature-driven life cycles. Theor. Popul. Biol. 2005, 67, 161–179. [Google Scholar] [CrossRef]
- Gibson, K.E. Mountain Pine Beetle: Conditions and Issues in the Western United States, 2003. In Mountain Pine Beetle Symposium: Challenges and Solutions, October 30–31; Natural Resources Canada: Kelowna, BC, Canada, 2003; pp. 57–61. [Google Scholar]
- Bentz, B.J.; Regniere, J.; Fettig, C.J.; Hansen, E.M.; Hayes, J.L.; Hicke, J.A.; Kelsey, R.G.; Negron, J.F.; Seybold, S.J. Climate Change and Bark Beetles of the Western United States and Canada: Direct and Indirect Effects. Bioscience 2010, 60, 602–613. [Google Scholar] [CrossRef]
- Buotte, P.C.; Hicke, J.A.; Preisler, H.K.; Abatzoglou, J.T.; Raffa, K.F.; Logan, J.A. Climate influences on whitebark pine mortality from mountain pine beetle in the Greater Yellowstone Ecosystem. Ecol. Appl. 2016, 26, 2505–2522. [Google Scholar] [CrossRef]
- Schwandt, J.W.; Kearns, H.S.J.; Byler, J.W. Impacts of White Pine Blister Rust and Competition on Natural Whitebark Pine Regeneration in Northern Idaho 1995–2012; U.S. Forest Service: Washington, DC, USA, 2013.
- Shepherd, B.; Jones, B.; Sissons, R.; Cochrane, J.; Park, J.; Smith, C.M.; Stafl, N. Ten years of monitoring illustrates a cascade of effects of white pine blister rust and focuses whitebark pine restoration in the canadian Rocky and Columbia Mountains. Forests 2018, 9, 138. [Google Scholar] [CrossRef]
- Kearns, H.S.J.; Jacobi, W.R.; Reich, R.M.; Flynn, R.L.; Burns, K.S.; Geils, B.W. Risk of white pine blister rust to limber pine in Colorado and Wyoming, USA. For. Pathol. 2014, 44, 21–38. [Google Scholar] [CrossRef]
- Smith, J.P.; Hoffman, J.T. Site and stand characteristics related to white pine blister rust in high-elevation forests of southern Idaho and western Wyoming. West. N. Am. Nat. 2001, 61, 409–416. [Google Scholar]
- Hansen, A.; Ireland, K.; Legg, K.; Keane, R.; Barge, E.; Jenkins, M.; Pillet, M. Complex challenges of maintaining Whitebark pine in Greater Yellowstone under climate change: A call for innovative research, management, and policy approaches. Forests 2016, 7, 54. [Google Scholar] [CrossRef]
- Kinloch, B.B. Forest Pathology for the Last Century: A Retrospective and Directions for the Future—White Pine Blister Rust in North America: Past and Prognosis. Phytopathology 2003, 93, 1044–1047. [Google Scholar] [CrossRef]
- Van Arsdel, E.P.; Geils, B.W.; Zambino, P.J. Epidemiology for hazard rating of white pine blister rust. In Proceedings of the 53rd Western International Forest Disease Work Conference, Jackson, WY, USA, 26–30 September 2005; pp. 49–69. [Google Scholar]
- Mielke, J.L. White Pine Blister Rust in Western North America; Bulletin No. 52; Yale School of Forestry: New Haven, CT, USA, 1943. [Google Scholar]
- Van Arsdel, E.P.; Riker, A.J.; Patton, R.F. Elevation effects on temperature and rainfall correlated with blister rust distribution in southwestern Wisconsin. Phytopathology 1956, 47, 307–318. [Google Scholar]
- Van Arsdel, E.P. Micrometeorology and Plant Disease Epidemiology. Phytopathology 1965, 55, 945–950. [Google Scholar]
- Zambino, P.J. Biology and pathology of Ribes and their implications for management of white pine blister rust. For. Pathol. 2010, 40, 264–291. [Google Scholar] [CrossRef]
- Mahalovich, M. Grizzly bears and whitebark pine in the Greater Yellowstone Ecosystem. In Future Status of Whitebark Pine: Blister Rust Resistance, Mountain Pine Beetle and Climate Change; Report Number: 2470 RRM-NR-WP-13-01; USDA Forest Service Northern Rocky Mountain, Southwestern, and Intermountain Regions: Washington, DC, USA, 2013. [Google Scholar]
- Hoff, R.J. How To Recognize Blister Rust Infection On Whitebark Pine. For. Sci. 1992, 406, 1–8. [Google Scholar]
- Campbell, E.M.; Antos, J.A. Distribution and severity of white pine blister rust and mountain pine beetle on whitebark pine in British Columbia. Can. J. For. Res. 2000, 30, 1051–1059. [Google Scholar] [CrossRef]
- Kearns, H.S.J.; Jacobi, W.R. The distribution and incidence of white pine blister rust in central and southeastern Wyoming and northern Colorado. Can. J. For. Res. 2007, 37, 462–472. [Google Scholar] [CrossRef]
- Newcomb, M. White Pine Blister Rust Whitebark Pine and Ribes Species in the Greater Yellowstone Area. Bachelor’s Theses, University of Montana, Missoula, MT, USA, 2003. [Google Scholar]
- Larson, E.R. Influences of the biophysical environment on blister rust and mountain pine beetle, and their interactions, in whitebark pine forests. J. Biogeogr. 2011, 453–470. [Google Scholar] [CrossRef]
- Smith-Mckenna, E.K.; Resler, L.M.; Tomback, D.F.; Zhang, H.; Malanson, G.P. Topographic Influences on the Distribution of White Pine Blister Rust in Pinus albicaulis Treeline Communities. Ecoscience 2013, 20, 215–229. [Google Scholar] [CrossRef]
- Tercek, M.T.; Gray, S.T.; Nicholson, C.M. Climate zone delineation: Evaluating approaches for use in natural resource management. Environ. Manag. 2012, 49, 1076–1091. [Google Scholar] [CrossRef]
- Marston, R.A.; Anderson, J.E. Society for Conservation Biology Watersheds and Vegetation of the Greater Yellowstone Ecosystem. Conserv. Biol. 1991, 5, 338–346. [Google Scholar] [CrossRef]
- Thornton, P.E.; Thornton, M.M.; Mayer, B.W.; Wei, Y.; Devarakonda, R.; Vose, R.S.; Cook, R.B. Daymet: Daily Surface Weather Data on a 1-km Grid for North America, Version 3; ORNL DAAC: Oak Ridge, TN, USA, 2016. [Google Scholar]
- Lutz, J.A.; van Wagtendonk, J.W.; Franklin, J.F. Climatic water deficit, tree species ranges, and climate change in Yosemite National Park. J. Biogeogr. 2010, 37, 936–950. [Google Scholar] [CrossRef]
- Dilts, T.E.; Weisberg, P.J.; Dencker, C.M.; Chambers, J.C. Functionally relevant climate variables for arid lands: A climatic water deficit approach for modelling desert shrub distributions. J. Biogeogr. 2015, 42, 1986–1997. [Google Scholar] [CrossRef]
- Allen, M.; Pereira, R.G.; Raes, L.S.; Smith, D. FAO Irrigation and Drainage Paper. Crop Evapotranspiration (Guidelines for Computing Crop Water Requirements); Paper No. 56; Food and Agriculture Organization: Rome, Italy, 2006. [Google Scholar]
- The R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Zuur, G.M.; Leno, A.; Walker, E.N.; Saveliev, N.; Smith, A.A. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Lohr, S.L. Sampling: Design and Analysis, 2nd ed.; Brooks/Cole Cengage Learning: Boston, MA, USA, 2010. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- McDonald, G.I.; Hoff, R.J.J. Blister rust: An introduced plague. In Whitebark Pine Communities: Ecology and Restoration; Tomback, K.R.E., Arno, D.F., Eds.; Island Press: Washington, DC, USA, 2001; pp. 193–220. [Google Scholar]
- Helmbrecht, D.J.; Keane, R.E.; Gray, K.L. Modeling and Mapping White Pine Blister Rust Infection in Whitebark Pine; Poster; USDA Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory: Fort Collins, CO, USA, 2007. [Google Scholar]
- Minder, J.R.; Mote, P.W.; Lundquist, J.D. Surface temperature lapse rates over complex terrain: Lessons from the Cascade Mountains. J. Geophys. Res. 2010, 115, 1–13. [Google Scholar] [CrossRef]
- Burns, K.S.; Schoettle, A.W.; Jacobi, W.R.; Mahalovich, M.F. White Pine Blister Rust in the Rocky Mountain Region and Options for Management; Biological Evaluation R2-07-04; USDA Forest Service Rocky Mountain Region: Golden, CO, USA, 2007. [Google Scholar]
- Shanahan, E.; Legg, K.; Daley, R. Status of Whitebark Pine in the Greater Yellowstone Ecosystem A Step-Trend Analysis with Comparisons from 2004 to 2015; National Park Service: Fort Collins, CO, USA, 2017. [Google Scholar]
- Kearns, H.S.J.; Jacobi, W.R.; Geils, B.W. A method for estimating white pine blister rust canker age on limber pine in the central Rocky Mountains. For. Pathol. 2009, 39, 177–191. [Google Scholar] [CrossRef]
- Van Arsdel, E.P. Environment in Relation to White Pine Blister Rust Infection. In Biology of Rust Resistance in Forest Trees: Proceedings of a NATO-IUFRO Advanced Study Institute; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1972; Volume 9, pp. 479–493. [Google Scholar]
- Chang, T.; Hansen, A.J.; Piekielek, N. Patterns and Variability of Projected Bioclimatic Habitat for Pinus albicaulis in the Greater Yellowstone Area. PLoS ONE 2014, 9, e111669. [Google Scholar] [CrossRef]
| Class | Model | (Intercept) | Recent DBH (cm) | Elev. (m) | Longitude | Latitude | Aspect | Slope | asRAIN | asRH | asTEMP | aPACK | asRH:asTEMP | df | logLik | AICc | ∆AIC | Weight | R2 Conditional | R2 Marginal |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| climate | m17 | −1.459 | 0.062 | 0.738 | 0.541 | −0.435 | 6 | −2888 | 5789 | 0.0 | 1.00 | 0.20 | 0.40 | |||||||
| climate | m15 | −1.275 | 0.062 | 0.641 | 0.448 | 5 | −2900 | 5810 | 21.0 | 0.00 | 0.16 | 0.41 | ||||||||
| climate | m10 | −1.286 | 0.062 | 0.656 | 0.452 | 0.071 | 6 | −2900 | 5811 | 22.5 | 0.00 | 0.16 | 0.41 | |||||||
| climate | m8 | −1.284 | 0.062 | −0.110 | 0.733 | 0.490 | 0.120 | 7 | −2899 | 5812 | 23.8 | 0.00 | 0.17 | 0.41 | ||||||
| climate | m9 | −1.284 | 0.062 | −0.110 | 0.733 | 0.490 | 0.120 | 7 | −2899 | 5812 | 23.8 | 0.00 | 0.17 | 0.41 | ||||||
| location | m3 | −1.325 | 0.063 | −0.335 | −0.303 | 5 | −2901 | 5813 | 24.3 | 0.00 | 0.15 | 0.40 | ||||||||
| location | m4 | −1.304 | 0.063 | −0.291 | −0.287 | 0.114 | 6 | −2901 | 5814 | 25.3 | 0.00 | 0.15 | 0.40 | |||||||
| location | m6 | −1.303 | 0.063 | −0.290 | −0.283 | 0.088 | 0.035 | 0.111 | 8 | −2900 | 5817 | 28.2 | 0.00 | 0.15 | 0.41 | |||||
| location | m2 | −1.329 | 0.063 | −0.498 | 4 | −2905 | 5817 | 28.4 | 0.00 | 0.13 | 0.40 | |||||||||
| location | m13 | −1.306 | 0.063 | −0.511 | 4 | −2906 | 5820 | 30.8 | 0.00 | 0.14 | 0.42 | |||||||||
| climate | m19 | −1.229 | 0.063 | 0.433 | 4 | −2910 | 5828 | 39.2 | 0.00 | 0.13 | 0.42 | |||||||||
| climate | m11 | −1.236 | 0.063 | 0.442 | 0.046 | 5 | −2910 | 5830 | 41.1 | 0.00 | 0.13 | 0.42 | ||||||||
| climate | m21 | −1.307 | 0.063 | 0.292 | 4 | −2914 | 5836 | 46.9 | 0.00 | 0.11 | 0.42 | |||||||||
| climate | m20 | −1.329 | 0.063 | 0.230 | 4 | −2915 | 5837 | 48.6 | 0.00 | 0.11 | 0.43 | |||||||||
| climate | m1 | −1.289 | 0.063 | 3 | −2917 | 5841 | 52.1 | 0.00 | 0.10 | 0.43 | ||||||||||
| climate | m12 | −1.286 | 0.063 | −0.020 | 4 | −2917 | 5843 | 54.1 | 0.00 | 0.10 | 0.43 | |||||||||
| location | m14 | −0.341 | −0.427 | 3 | −3084 | 6174 | 385.5 | 0.00 | 0.04 | 0.30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thoma, D.P.; Shanahan, E.K.; Irvine, K.M. Climatic Correlates of White Pine Blister Rust Infection in Whitebark Pine in the Greater Yellowstone Ecosystem. Forests 2019, 10, 666. https://doi.org/10.3390/f10080666
Thoma DP, Shanahan EK, Irvine KM. Climatic Correlates of White Pine Blister Rust Infection in Whitebark Pine in the Greater Yellowstone Ecosystem. Forests. 2019; 10(8):666. https://doi.org/10.3390/f10080666
Chicago/Turabian StyleThoma, David P., Erin K. Shanahan, and Kathryn M. Irvine. 2019. "Climatic Correlates of White Pine Blister Rust Infection in Whitebark Pine in the Greater Yellowstone Ecosystem" Forests 10, no. 8: 666. https://doi.org/10.3390/f10080666
APA StyleThoma, D. P., Shanahan, E. K., & Irvine, K. M. (2019). Climatic Correlates of White Pine Blister Rust Infection in Whitebark Pine in the Greater Yellowstone Ecosystem. Forests, 10(8), 666. https://doi.org/10.3390/f10080666