Survival of Whitebark Pine Seedlings Grown from Direct Seeding: Implications for Regeneration and Restoration under Climate Change
Abstract
1. Introduction
2. Materials and Methods
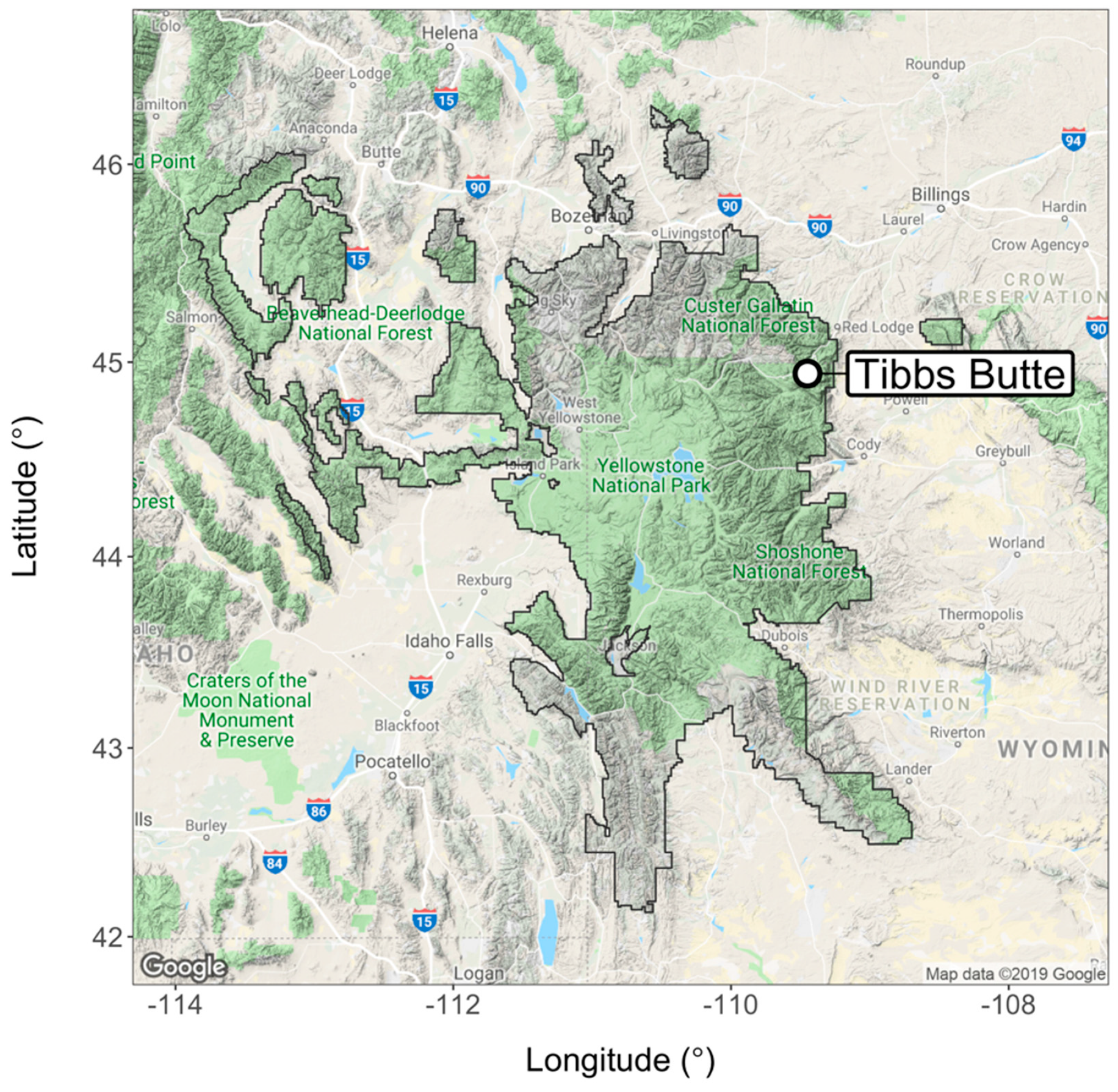
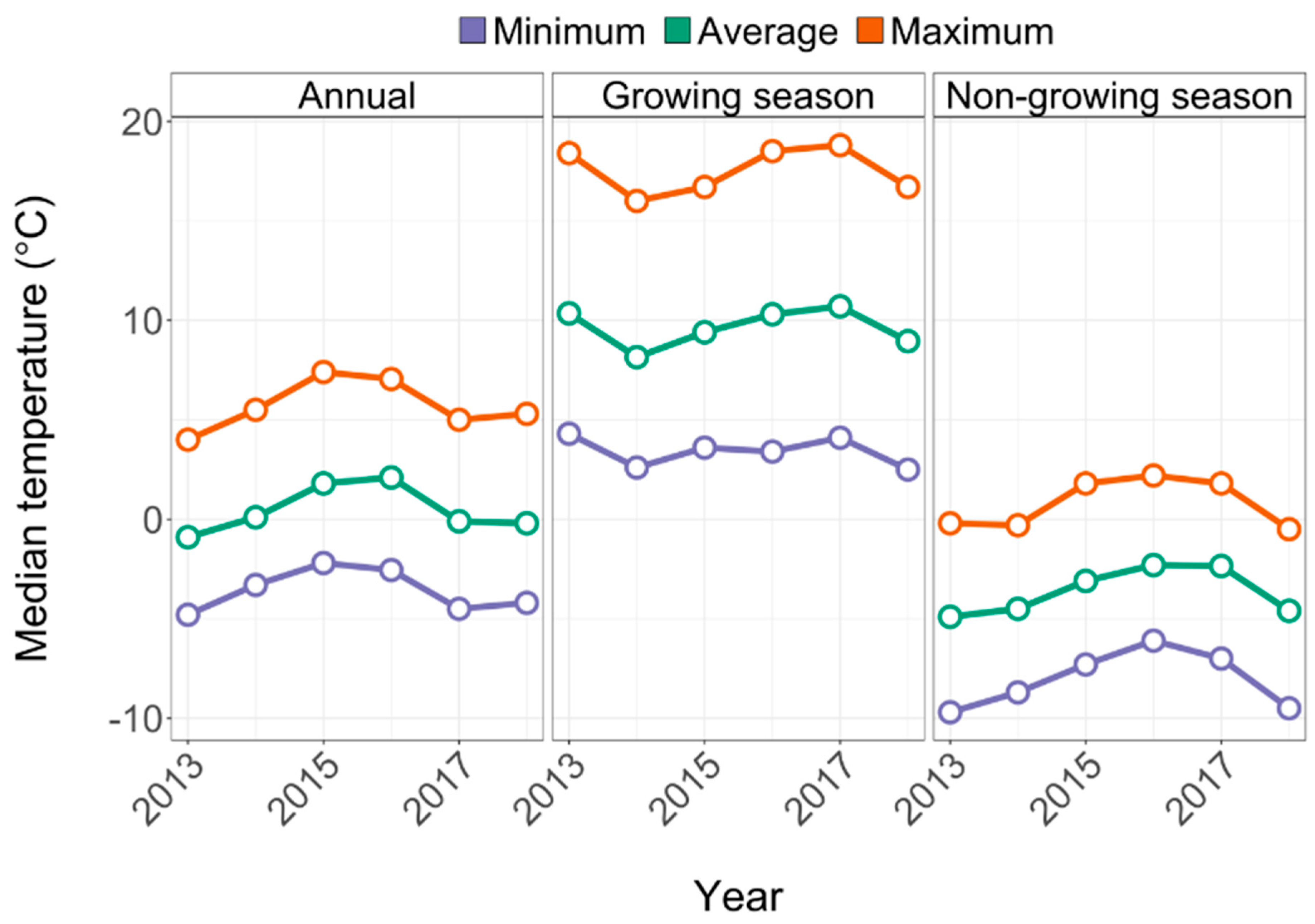
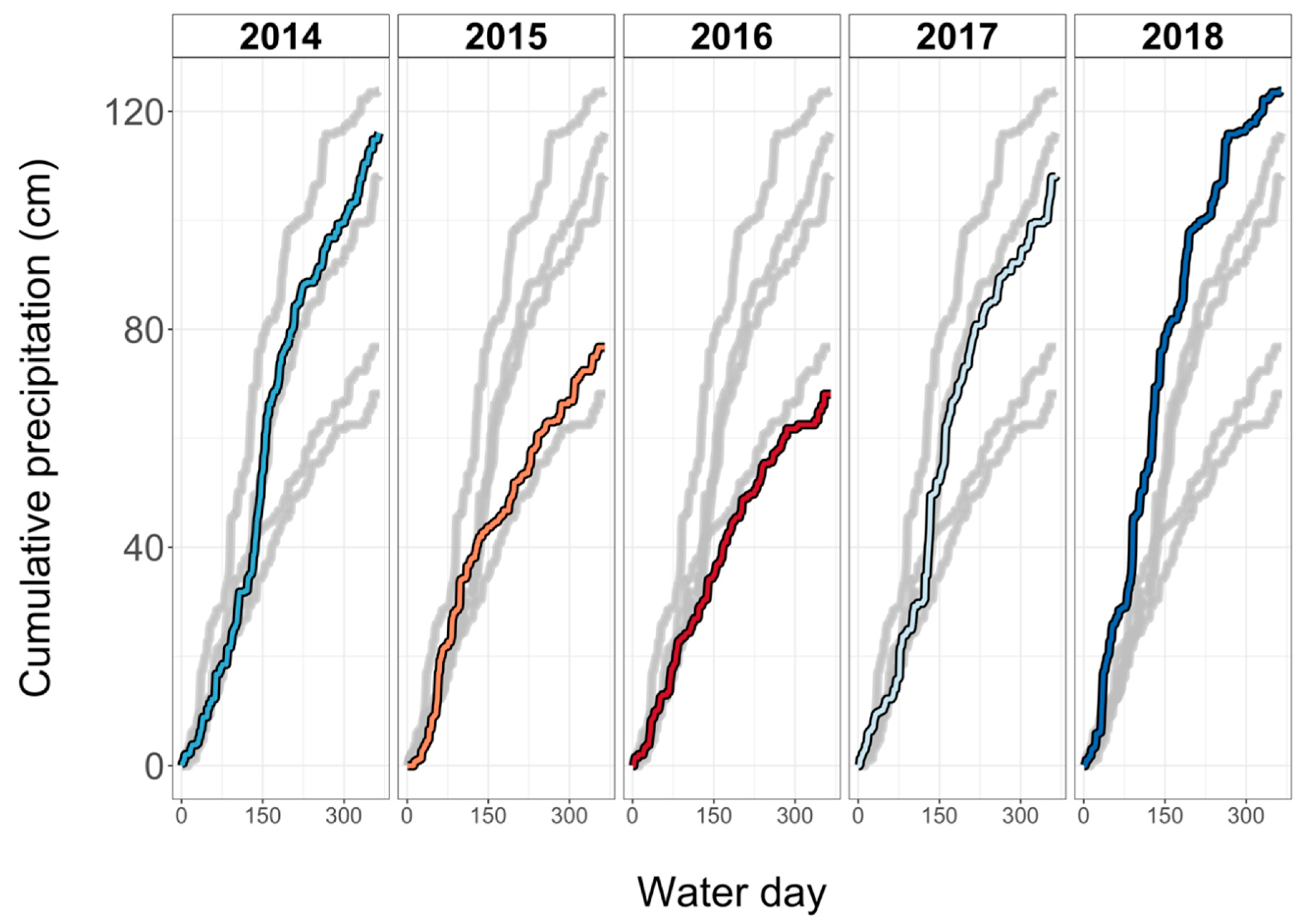
2.1. Study Area
2.2. Direct Sowing and Cache Surveys
2.3. Data Analysis
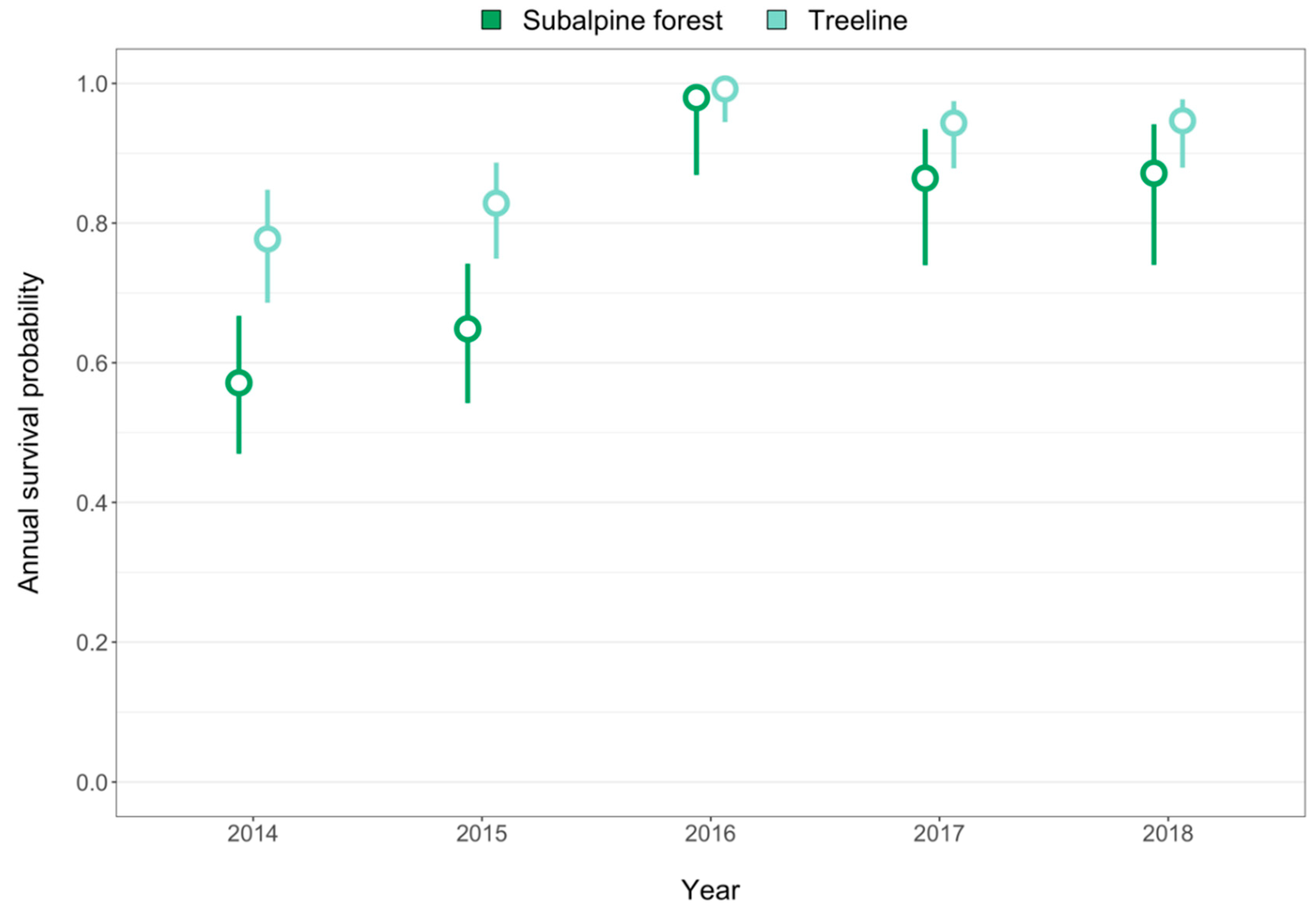
3. Results
4. Discussion
4.1. Implications for Treeline Dynamics under Climate Change
4.2. Treeline Restoration
4.3. Increased Moisture Stress Corresponded to Increased Seedling Survival
4.4. Nurse Objects
4.5. Direct Seeding Could Be a Viable Restoration Technique for Whitebark Pine
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Mantgem, P.J.; Stephenson, N.L.; Byrne, J.C.; Daniels, L.D.; Franklin, J.F.; Fule, P.Z.; Harmon, M.E.; Larson, A.J.; Smith, J.M.; Taylor, A.H.; et al. Widespread increase of tree mortality tates in the Western United States. Science 2009, 323, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Stevens-Rumann, C.S.; Kemp, K.B.; Higuera, P.E.; Harvey, B.J.; Rother, M.T.; Donato, D.C.; Morgan, P.; Veblen, T.T. Evidence for declining forest resilience to wildfires under climate change. Ecol. Lett. 2018, 21, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.M.; Daniels, L.D. Novel forest decline triggered by multiple interactions among climate, an introduced pathogen and bark beetles. Glob. Chang. Biol. 2017, 23, 1926–1941. [Google Scholar] [CrossRef] [PubMed]
- Westerling, A.L.; Hidalgo, H.G.; Cayan, D.R.; Swetnam, T.W. Warming and earlier spring increase Western U.S. forest wildfire activity. Science 2006, 313, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Pederson, G.T.; Gray, S.T.; Woodhouse, C.A.; Betancourt, J.L.; Fagre, D.B.; Littell, J.S.; Watson, E.; Luckman, B.H.; Graumlich, L.J. The unusual nature of recent snowpack declines in the North American Cordillera. Science 2011, 333, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Turner, M.G.; Romme, W.H. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: The dynamics of bark beetle eruptions. Bioscience 2008, 58, 501–517. [Google Scholar] [CrossRef]
- Weed, A.S.; Ayres, M.P.; Hicke, J.A. Consequences of climate change for biotic disturbances in North American forests. Ecol. Monogr. 2013, 83, 441–470. [Google Scholar] [CrossRef]
- Roy, B.A.; Alexander, H.M.; Davidson, J.; Campbell, F.T.; Burdon, J.J.; Sniezko, R.; Brasier, C. Increasing forest loss worldwide from invasive pests requires new trade regulations. Front. Ecol. Environ. 2014, 12, 457–465. [Google Scholar] [CrossRef]
- Lovett, G.M.; Weiss, M.; Liebhold, A.M.; Holmes, T.P.; Leung, B.; Lambert, K.F.; Orwig, D.A.; Campbell, F.T.; Rosenthal, J.; McCullough, D.G.; et al. Nonnative forest insects and pathogens in the United States: Impacts and policy options. Ecol. Appl. 2016, 26, 1437–1455. [Google Scholar] [CrossRef]
- Millar, C.I.; Stephenson, N.L. Temperate forest health in an era of emerging megadisturbance. Science 2015, 349, 823–826. [Google Scholar] [CrossRef]
- Tomback, D.F.; Achuff, P. Blister rust and western forest biodiversity: Ecology, values and outlook for white pines. For. Pathol. 2010, 40, 186–225. [Google Scholar] [CrossRef]
- Shanahan, E.; Irvine, K.M.; Thoma, D.; Wilmoth, S.; Ray, A.; Legg, K.; Shovic, H. Whitebark pine mortality related to white pine blister rust, mountain pine beetle outbreak, and water availability. Ecosphere 2016, 7, e01610. [Google Scholar] [CrossRef]
- Smith, C.M.; Langor, D.W.; Myrholm, C.; Weber, J.; Gillies, C.; Stuart-Smith, J. Changes in white pine blister rust infection and mortality in limber pine over time. Can. J. For. Res. 2013, 43, 90–96. [Google Scholar] [CrossRef]
- Buotte, P.C.; Hicke, J.A.; Preisler, H.K.; Abatzoglou, J.T.; Raffa, K.F.; Logan, J.A. Climate influences on whitebark pine mortality from mountain pine beetle in the Greater Yellowstone Ecosystem. Ecol. Appl. 2016, 26, 2507–2524. [Google Scholar] [CrossRef]
- Leirfallom, S.B.; Keane, R.E.; Tomback, D.F.; Dobrowski, S.Z. The effects of seed source health on whitebark pine (Pinus albicaulis) regeneration density after wildfire. Can. J. For. Res. 2015, 45, 1597–1606. [Google Scholar] [CrossRef]
- Tomback, D.F.; Arno, S.F.; Keane, R.E. The compelling case for management intervention. In Whitebark Pine Communities: Ecology and Restoration; Tomback, D.F., Arno, S.F., Keane, R.E., Eds.; Island Press: Washington, DC, USA, 2001; pp. 3–28. [Google Scholar]
- Shepherd, B.; Jones, B.; Sissons, R.; Cochrane, J.; Park, J.; Smith, C.M.; Stafl, N. Ten years of monitoring illustrates a cascade of effects of white pine blister rust and focuses whitebark pine restoration in the Canadian Rocky and Columbia Mountains. Forests 2018, 9, 138. [Google Scholar] [CrossRef]
- Nesmith, J.C.B.; Wright, M.; Jules, E.S.; McKinney, S.T. Whitebark and foxtail pine in Yosemite, Sequoia, and Kings Canyon National Parks: Initial assessment of stand structure and condition. Forests 2019, 10, 35. [Google Scholar] [CrossRef]
- Schrag, A.M.; Bunn, A.G.; Graumlich, L.J. Influence of bioclimatic variables on tree-line conifer distribution in the Greater Yellowstone Ecosystem: Implications for species of conservation concern. J. Biogeogr. 2008, 35, 698–710. [Google Scholar] [CrossRef]
- Chang, T.; Hansen, A.J.; Piekielek, N. Patterns and variability of projected bioclimatic habitat for Pinus albicaulis in the Greater Yellowstone Area. PLoS ONE 2014, 9, e111669. [Google Scholar] [CrossRef]
- Warwell, M.V.; Rehfeldt, G.E.; Crookston, N.L. Modeling contemporary climate profiles of whitebark pine (Pinus albicaulis) and predicting responses to global warming. In Proceedings of the Whitebark Pine: A Pacific Coast Perspective, Ashland, OR, USA, 27–31 August 2006; pp. 139–142. [Google Scholar]
- Keane, R.E.; Holsinger, L.M.; Mahalovich, M.F.; Tomback, D.F. Evaluating future success of whitebark pine ecosystem restoration under climate change using simulation modeling. Restor. Ecol. 2017, 25, 220–233. [Google Scholar] [CrossRef]
- Romme, W.H.; Turner, M.G. Implications of global climate change for biogeographic patterns in the Greater Yellowstone Ecosystem. Conserv. Biol. 1991, 5, 373–386. [Google Scholar] [CrossRef]
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.; Curtis-McLane, S. Adaptation, migration or extirpation: Climate change outcomes for tree populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef]
- Hutchins, H.E.; Lanner, R.M. The central role of Clark’s nutcracker in the dispersal and establishment of whitebark pine. Oecologia 1982, 55, 192–201. [Google Scholar] [CrossRef]
- Tomback, D.F. Foraging strategies of Clark’s nutcracker. Living Bird 1978, 16, 123–161. [Google Scholar]
- Tomback, D.F. Dispersal of whitebark pine seeds by Clark’s nutcracker: A mutualism hypothesis. J. Anim. Ecol. 1982, 51, 451–467. [Google Scholar] [CrossRef]
- McKinney, S.T.; Tomback, D.F. The influence of white pine blister rust on seed dispersal in whitebark pine. Can. J. For. Res. 2007, 37, 1044–1057. [Google Scholar] [CrossRef]
- Barringer, L.E.; Tomback, D.F.; Wunder, M.B.; McKinney, S.T. Whitebark pine stand condition, tree abundance, and cone production as predictors of visitation by Clark’s nutcracker. PLoS ONE 2012, 7, e37663. [Google Scholar] [CrossRef]
- Tomback, D.F.; Clary, J.K.; Koehler, J.; Hoff, R.J.; Arno, S.F. The effects of blister rust on post-fire regeneration of whitebark pine: The Sundance Burn of Northern Idaho (USA). Conserv. Biol. 1995, 9, 654–664. [Google Scholar] [CrossRef]
- Ellison, A.M.; Bank, M.S.; Clinton, B.D.; Colburn, E.A.; Elliott, K.; Ford, C.R.; Foster, D.R.; Kloeppel, B.D.; Knoepp, J.D.; Lovett, G.M.; et al. Loss of foundation species: Consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ. 2005, 3, 479–486. [Google Scholar] [CrossRef]
- Tomback, D.F.; Resler, L.M.; Keane, R.E.; Pansing, E.R.; Andrade, A.J.; Wagner, A.C. Community structure, biodiversity, and ecosystem services in treeline whitebark pine communities: Potential impacts from a non-native pathogen. Forests 2016, 7, 21. [Google Scholar] [CrossRef]
- Tomback, D.F.; Kendall, K.C. Biodiversity losses: The downward spiral. In Whitebark Pine Communities: Ecology and Restoration; Tomback, D.F., Arno, S.F., Keane, R.E., Eds.; Island Press: Washington, DC, USA, 2001; pp. 243–262. [Google Scholar]
- Schoettle, A.W.; Sniezko, R.A. Proactive intervention to sustain high-elevation pine ecosystems threatened by white pine blister rust. J. For. Res. 2007, 12, 327–336. [Google Scholar] [CrossRef]
- Keane, R.E.; Tomback, D.F.; Aubry, C.A.; Bower, A.D.; Campbell, E.M.; Cripps, C.L.; Jenkins, M.B.; Mahalovich, M.F.; McKinney, S.T.; Murray, M.P.; et al. A Range-Wide Restoration Strategy for Whitebark Pine (Pinus albicaulis); RMRS-GTR-279; USDA Forest Service Rocky Mountain Research Station: Fort Collins, CO, USA, 2012; p. 108. [Google Scholar]
- Sniezko, R.A.; Koch, J. Breeding trees resistant to insects and diseases: Putting theory into application. Biol. Invasions 2017, 19, 3377–3400. [Google Scholar] [CrossRef]
- Schwandt, J.W.; Lockman, I.B.; Kliejunas, J.T.; Muir, J.A. Current health issues and management strategies for white pines in the western United States and Canada. For. Pathol. 2010, 40, 226–250. [Google Scholar] [CrossRef]
- Sniezko, R.A. Resistance breeding against nonnative pathogens in forest trees—Current successes in North America. Can. J. Plant Pathol. 2010, 28, S270–S279. [Google Scholar] [CrossRef]
- Burns, K.S.; Schoettle, A.W.; Jacobi, W.R.; Mahalovich, M.F. Options for the Management of White Pine Blister Rust in the Rocky Mountain Region; RMRS-GTR-206; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2008; p. 26. [Google Scholar]
- Burr, K.E.; Eramian, A.; Eggleston, K. Growing whitebark pine seedlings for restoration. In Whitebark Pine Communities: Ecology and Restoration 1; Tomback, D.F., Arno, S.F., Keane, R.E., Eds.; Island Press: Washington, DC, USA, 2001; pp. 325–345. [Google Scholar]
- Mahalovich, M.F.; Dickerson, G.A. Whitebark pine genetic restoration program for the Intermountain West (United States). In Proceedings of the IUFRO Working Party: Breeding and Genetic Resources of Five-Needle Pines: Growth, Adaptability and Pest Resistance, Medford, OR, USA, 23–27 July 2001; Sniezko, R.A., Samman, S., Schlarbaum, S.E., Kriebel, H.B., Eds.; RMRS-P-32. USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2004; pp. 181–187. [Google Scholar]
- Tomback, D.F.; Achuff, P.; Schoettle, A.W.; Schwandt, J.W.; Mastrogiuseppe, R.J. The magnificent high-elevation five-needle white pines: Ecological roles and future outlook. In The Future of High-Elevation Five-Needle Pines in Western North America, Proceedings of the High Five Symposium, Missoula, MT, USA, 28–30 June 2010; Keane, R.E., Tomback, D.F., Murray, M.P., Smith, C.M., Eds.; RMRS-P-63; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2011; pp. 2–28. ISBN 1570-0232. [Google Scholar]
- Keane, R.E. The importance of wilderness to whitebark pine research and management. In Proceedings of the Wilderness Science in a Time of Change Conference, Missoula, MT, USA, 23–27 May 1999; McCool, S.F., Cole, D.N., Borrie, W.T., O’Loughlin, J., Eds.; RMRS-P-15-VOL-3. USDA Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 2000; pp. 84–92. [Google Scholar]
- Shoal, R.; Ohlson, T.; Aubry, C. Land Managers Guide to Whitebark Pine Restoration in the Pacific Northwest Region 2009–2013; USDA Forest Service, Pacific Northwest Region: Portland, OR, USA, 2008; p. 46. [Google Scholar]
- Pansing, E.R.; Tomback, D.F.; Wunder, M.B.; French, J.P.; Wagner, A.C. Microsite and elevation zone effects on seed pilferage, germination, and seedling survival during early whitebark pine recruitment. Ecol. Evol. 2017, 7, 9027–9040. [Google Scholar] [CrossRef]
- DeMastus, C.R. Effective Methods of Regenerating Whitebark Pine (Pinus albicaulis) through Direct Seeding; Masters of Science, Montana State University: Bozeman, MT, USA, 2013. [Google Scholar]
- Schwandt, J.W.; Chadwick, K.; Kearns, H.; Jensen, C. Whitebark pine direct seeding trials in the Pacific Northwest. In The Future of High-Elevation Five-Needle Pines in Western North America, Proceedings of the High Five Symposium, Missoula, MT, USA, 28–30 June 2011; Keane, R.E., Tomback, D.F., Murray, M.P., Smith, C.M., Eds.; RMRS-P-63; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2011; pp. 357–361. [Google Scholar]
- Schwandt, J.W.; Tomback, D.F.; Keane, R.E.; McCaughey, W.W.; Kearns, H.S.J. First year results of a whitebark pine seed planting trial near Baker City, OR. In Proceedings of the Conference Whitebark Pine: A Pacific Coast Perspective, Ashland, OR, USA, 27–31 August 2006; Goheen, E.M., Sniezko, R.A., Eds.; Pacific Northwest Region, Forest Service, U. S. Department of Agriculture: Portland, OR, USA; pp. 132–138. [Google Scholar]
- Maher, E.L.; Germino, M.J. Microsite differentiation among conifer species during seedling establishment at alpine treeline. Ecoscience 2006, 13, 334–341. [Google Scholar] [CrossRef]
- McCaughey, W.W.; Tomback, D.F. The natural regenerration process. In Whitebark Pine Communities: Ecology and Restoration; Tomback, D.F., Arno, S.F., Keane, R.E., Eds.; Island Press: Washington, DC, USA, 2001; pp. 105–120. [Google Scholar]
- Mellmann-Brown, S. The Regeneration of Whitebark Pine in the Timberline Ecotone of the Beartooth Plateau, Montana and Wyoming. Ph.D. Dissertation, Westfälischen Wilhelms-Universität, Münster, Germany, 2002. [Google Scholar]
- Wagner, A.C.; Tomback, D.F.; Resler, L.M.; Pansing, E.R. Whitebark pine prevalence and ecological function in treeline communities of the Greater Yellowstone Ecosystem, USA: Potential disruption by white pine blister rust. Forests 2018, 9, 635. [Google Scholar] [CrossRef]
- National Park Service—Park Unit Boundaries. Available online: https://public-nps.opendata.arcgis.com/datasets/national-park-service-park-unit-boundaries?geometry=-135.857%2C38.031%2C-74.817%2C49.124 (accessed on 10 June 2019).
- USDA Forest Service. National Forest Administrative Units in the GYE. Available online: https://www.sciencebase.gov/catalog/item/58518df6e4b0f99207c4f136 (accessed on 10 June 2019).
- Kahle, D.; Wickham, H. ggmap: Spatial Visualization with ggplot2. R J. 2013, 5, 144–161. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Use R!), 2nd ed.; Springer: New York, NY, USA, 2016; ISBN 978-0-387-98140-6. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- National Resource Conservation Service SNOTEL. Available online: https://wcc.sc.egov.usda.gov/nwcc/site?sitenum=326 (accessed on 11 June 2019).
- Frich, P.; Alexander, L.V.; Della-Marta, P.; Gleason, B.; Haylock, M.; Tank Klein, A.M.G.; Peterson, T. Observed coherent changes in climatic extremes during the second half of the twentieth century. Clim. Res. 2002, 19, 193–212. [Google Scholar] [CrossRef]
- Mahalovich, M.F.; Hipkins, V.D. Molecular Genetic Variation in Whitebark Pine (Pinus albicaulis Engelm.) in the Inland West. In The Future of High-Elevation, Five-Needle White Pines Western North America, Proceedings of the High Five Symposium, Missoula, MT, USA, 28–30 June 2010; Keane, R.E., Tomback, D.F., Murray, M.P., Smith, C.M., Eds.; RMRS-P-63; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2011; pp. 118–132. ISBN 1570-0232. [Google Scholar]
- ESRI. ArcGIS Desktop: Release 10; Environmental Systems Research Institute: Redlands, CA, USA, 2011. [Google Scholar]
- Tomback, D.F.; Sund, S.K.; Hoffmann, L.A. Post-fire regeneration of Pinus albicaulis: Height-age relationships, age structure, and microsite characteristics. Can. J. For. Res. 1993, 23, 113–119. [Google Scholar] [CrossRef]
- Furnier, G.R.; Knowles, P.; Clyde, M.A.; Dancik, B.P. Effects of avian seed dispersal on the genetic structure of whitebark pine populations. Evolution 2006, 41, 607–612. [Google Scholar] [CrossRef]
- Tomback, D.F.; Linhart, Y.B. The evolution of bird-dispersed pines. Evol. Ecol. 1990, 4, 185–219. [Google Scholar] [CrossRef]
- Laake, J.L. RMark: An R Interface for Analysis of Capture-recapture Data with MARK; AFSC Processed Report; NOAA: Seattle, WA, USA, 2013. [Google Scholar]
- White, G.C.; Burnham, K.P. Program mark: Survival estimation from populations of marked animals. Bird Study 1999, 46, S120–S139. [Google Scholar] [CrossRef]
- Pollock, K.H.; Winterstein, S.R.; Conroy, M.J. Estimation and analysis of survival distributions for radio-tagged animals. Biometrics 1989, 45, 99–109. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Chamberlain, T.C. The Method of Multiple Working Hypotheses and the Agony of Choice. Science 1890, 15, 92–96. [Google Scholar]
- Burnham, K.P.; Anderson, D.R.; Huyvaert, K.P. AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: Berlin, Germany, 2002; ISBN 0-387-95364-7. [Google Scholar]
- Dochtermann, N.A.; Jenkins, S.H. Developing multiple hypotheses in behavioral ecology. Behav. Ecol. Sociobiol. 2011, 65, 37–45. [Google Scholar] [CrossRef]
- Petrie, M.D.; Wildeman, A.M.; Bradford, J.B.; Hubbard, R.M.; Lauenroth, W.K. A review of precipitation and temperature control on seedling emergence and establishment for ponderosa and lodgepole pine forest regeneration. For. Ecol. Manag. 2016, 361, 328–338. [Google Scholar] [CrossRef]
- Tomback, D.F.; Blakeslee, S.C.; Wagner, A.C.; Wunder, M.B.; Resler, L.M.; Pyatt, J.C.; Diaz, S. Whitebark pine facilitation at treeline: Potential interactions for disruption by an invasive pathogen. Ecol. Evol. 2016, 6, 5144–5157. [Google Scholar] [CrossRef]
- Casper, A.M.A.; Jacobi, W.R.; Schoettle, A.W.; Burns, K.S. Restoration planting options for limber pine (Pinus flexilis James) in the Southern Rocky Mountains. J. Torrey Bot. Soc. 2016, 143, 21–37. [Google Scholar] [CrossRef]
- Resler, L.M.; Tomback, D.F. Blister rust prevalence in krummholz whitebark pine: Implications for treeline dynamics, Northern Rocky Mountains, Montana, USA. Arctic Antarct. Alp. Res. 2008, 40, 161–170. [Google Scholar] [CrossRef]
- Tomback, D.F.; Chipman, K.G.; Resler, L.M.; Smith-McKenna, E.K.; Smith, C.M. Relative abundance and functional role of whitebark pine at treeline in the Northern Rocky Mountains. Arctic Antarct. Alp. Res. 2014, 46, 407–418. [Google Scholar] [CrossRef]
- Tomback, D.F.; Anderies, A.J.; Carsey, K.S.; Powell, M.L.; Mellmann-Brown, S. Delayed seed germination in whitebark pine and regeneration patterns following the Yellowstone fires. Ecology 2001, 82, 2587–2600. [Google Scholar] [CrossRef]
- Grace, J.; Berninger, F.; Nagy, L. Impacts of climate change on the tree line. Ann. Bot. 2002, 90, 537–544. [Google Scholar] [CrossRef]
- Harsch, M.A.; Hulme, P.E.; McGlone, M.S.; Duncan, R.P. Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecol. Lett. 2009, 12, 1040–1049. [Google Scholar] [CrossRef]
- Holtmeier, F.-K.; Broll, G. Treeline advance—Driving processes and adverse factors. Landsc. Online 2007, 1, 1–33. [Google Scholar] [CrossRef]
- Malanson, G.P.; Butler, D.R.; Fagre, D.B.; Walsh, S.J.; Tomback, D.F.; Daniels, L.D.; Resler, L.M.; Smith, W.K.; Weiss, D.J.; Peterson, D.L.; et al. Alpine treeline of Western North America: Linking organism-to-landscape dynamics. Phys. Geogr. 2007, 28, 378–396. [Google Scholar] [CrossRef]
- Coops, N.C.; Waring, R.H. Estimating the vulnerability of fifteen tree species under changing climate in Northwest North America. Ecol. Modell. 2011, 222, 2119–2129. [Google Scholar] [CrossRef]
- Hansen, A.; Ireland, K.; Legg, K.; Keane, R.E.; Barge, E.; Jenkins, M.; Pillet, M. Complex challenges of maintaining whitebark pine in Greater Yellowstone under climate change: A call for innovative research, management, and policy approaches. Forests 2016, 7, 54. [Google Scholar] [CrossRef]
- Ibáñez, I.; Clark, J.S.; LaDeau, S.; Hille Ris Lambers, J. Exploiting temporal variability to understand tree recruitment response to climate change. Ecol. Monogr. 2007, 77, 163–177. [Google Scholar] [CrossRef]
- Andersen, A.N. How important is seed predation to recruitment in stable populations of long-lived perennials? Oecologia 1989, 81, 310–315. [Google Scholar] [CrossRef]
- Eriksson, O.; Ehrlén, J. Seed and microsite limitation of recruitment in plant populations. Oecologia 1992, 91, 360–364. [Google Scholar] [CrossRef]
- Elliott, G.P. Extrinsic regime shifts drive abrupt changes in regeneration dynamics at upper treeline in the Rocky Mountains, USA. Ecology 2012, 93, 1614–1625. [Google Scholar] [CrossRef]
- Kullman, L.; Öberg, L. Post-Little Ice Age tree line rise and climate warming in the Swedish Scandes: A landscape ecological perspective. J. Ecol. 2009, 97, 415–429. [Google Scholar] [CrossRef]
- Kullman, L. Tree line population monitoring of Pinus sylvestris in the Swedish Scandes, 1973–2005: Implications for tree line theory and climate change ecology. J. Ecol. 2007, 95, 41–52. [Google Scholar] [CrossRef]
- Millar, C.I.; Westfall, R.D.; Delany, D.L. Response of subalpine conifers in the Sierra Nevada, California, U.S.A., to 20th-century warming and decadal climate variability. Arctic Antarct. Alp. Res. 2004, 36, 181–200. [Google Scholar] [CrossRef]
- Hamann, A.; Wang, T. Potential effects of climate change on ecosystem and tree species distribution in British Columbia. Ecology 2006, 87, 2773–2786. [Google Scholar] [CrossRef]
- Loarie, S.R.; Duffy, P.B.; Hamilton, H.; Asner, G.P.; Field, C.B.; Ackerly, D.D. The velocity of climate change. Nature 2009, 462, 1052–1055. [Google Scholar] [CrossRef]
- Liang, Y.; Duveneck, M.J.; Gustafson, E.J.; Serra-Diaz, J.M.; Thompson, J.R. How disturbance, competition, and dispersal interact to prevent tree range boundaries from keeping pace with climate change. Glob. Chang. Biol. 2018, 24, e335–e351. [Google Scholar] [CrossRef]
- McLane, S.C.; Aitken, S.N. Whitebark pine (Pinus albicaulis) assisted migration potential: Testing establishment north of the species range. Ecol. Appl. 2012, 22, 142–153. [Google Scholar] [CrossRef]
- Roberts, D.R.; Hamann, A. Climate refugia and migration requirements in complex landscapes. Ecography 2016, 39, 1238–1246. [Google Scholar] [CrossRef]
- Bansal, S.; Reinhardt, K.; Germino, M.J. Linking carbon balance to establishment patterns: Comparison of whitebark pine and Engelmann spruce seedlings along an herb cover exposure gradient at treeline. Plant Ecol. 2011, 212, 219–228. [Google Scholar] [CrossRef]
- Cripps, C.L.; Alger, G.; Sissons, R. Designer niches promote seedling survival in forest restoration: A 7-year study of whitebark pine (Pinus albicaulis) seedlings in Waterton Lakes National Park. Forests 2018, 9, 477. [Google Scholar] [CrossRef]
- Pyatt, J.C.; Tomback, D.F.; Blakeslee, S.C.; Wunder, M.B.; Resler, L.M.; Boggs, L.A.; Bevency, H.D. The importance of conifers for facilitation at treeline: Comparing biophysical characteristics of leeward microsites in whitebark pine communities. Arctic Antarct. Alp. Res. 2016, 48, 427–444. [Google Scholar] [CrossRef]
- Schupp, E.W. Seed-seedling conflicts, habitat choice, and patterns of plant recruitment. Am. J. Bot. 1995, 82, 399–409. [Google Scholar] [CrossRef]
- Douterlungne, D.; Ferguson, B.G.; Siddique, I.; Soto-Pinto, L.; Jímenez-Ferrer, G.; Gavito, M.E. Microsite determinants of variability in seedling and cutting establishment in tropical forest restoration plantations. Restor. Ecol. 2015, 23, 861–871. [Google Scholar] [CrossRef]
- Scott, G.L.; McCaughey, W.W. Whitebark pine guidelines for planting prescriptions. In National Proceedings of the Forest and Conservation Nursery Associations; RMRS-P-43; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2006; pp. 84–90. [Google Scholar]
- McCaughey, W.W.; Scott, G.L.; Izlar, K.L. Whitebark pine planting guidelines. West. J. Appl. For. 2009, 24, 163–166. [Google Scholar]
- Greater Yellowstone Coordinating Committee. Whitebark Pine Subcommittee Whitebark Pine Strategy for the Greater Yellowstone Area; Greater Yellowstone Coordinating Committee: Bozeman, MT, USA, 2011. [Google Scholar]
- Jungck, E. (USDA Forest Service). Personal communication, 2019.
| Model |
|---|
| ASR ~ 1 |
| ASR ~ elevation zone + year |
| ASR ~ elevation zone × nurse object |
| ASR ~ nurse object + elevation zone + year |
| No. Caches with 1+ Seedlings | ||||||
|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
| Treeline | 66 | 75 | 47 | 47 | 45 | 42 |
| Subalpine | 73 | 63 | 34 | 32 | 28 | 26 |
| TOTAL | 139 | 138 | 81 | 79 | 73 | 68 |
| Model | Parameters | AICc | ΔAICc | Model Weight | Evidence Ratio |
|---|---|---|---|---|---|
| ~year + elevation zone | 6 | 428.88 | — | 0.658 | — |
| ~year + elevation zone + object | 8 | 430.19 | 1.31 | 0.342 | 1.93 |
| ~object × elevation zone | 6 | 474.14 | 45.26 | 9.77 × 10−11 | 6.73 × 109 |
| ~1 | 1 | 491.16 | 62.28 | 1.97 × 10−14 | 3.35 × 1013 |
| Model | Parameter | Estimate | 95% CI |
|---|---|---|---|
| Elevation zone + year | Intercept | 0.287 | −0.122, 0.696 |
| Treeline | 0.962 | 0.474, 1.45 * | |
| 2015 | 0.326 | −0.208, 0.860 | |
| 2016 | 3.59 | 1.579, 5.60 * | |
| 2017 | 1.57 | 0.702, 2.43 * | |
| 2018 | 1.63 | 0.709, 2.54 * | |
| Elevation zone + year + nurse object | Intercept | 0.0560 | −0.444, 0.552 |
| Rock | 0.352 | −0.199, 0.904 | |
| Tree | 0.480 | −0.142, 1.10 | |
| Treeline | 0.965 | 0.475, 1.45 * | |
| 2014/2015 | 0.317 | −0.220, 0.854 | |
| 2015/2016 | 3.57 | 1.56, 5.58 * | |
| 2016/2017 | 1.54 | 0.672, 2.40 * | |
| 2017/2018 | 1.61 | 0.689, 2.53 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pansing, E.R.; Tomback, D.F. Survival of Whitebark Pine Seedlings Grown from Direct Seeding: Implications for Regeneration and Restoration under Climate Change. Forests 2019, 10, 677. https://doi.org/10.3390/f10080677
Pansing ER, Tomback DF. Survival of Whitebark Pine Seedlings Grown from Direct Seeding: Implications for Regeneration and Restoration under Climate Change. Forests. 2019; 10(8):677. https://doi.org/10.3390/f10080677
Chicago/Turabian StylePansing, Elizabeth R., and Diana F. Tomback. 2019. "Survival of Whitebark Pine Seedlings Grown from Direct Seeding: Implications for Regeneration and Restoration under Climate Change" Forests 10, no. 8: 677. https://doi.org/10.3390/f10080677
APA StylePansing, E. R., & Tomback, D. F. (2019). Survival of Whitebark Pine Seedlings Grown from Direct Seeding: Implications for Regeneration and Restoration under Climate Change. Forests, 10(8), 677. https://doi.org/10.3390/f10080677






