Forest Decline Triggered by Phloem Parasitism-Related Biotic Factors in Aleppo Pine (Pinus halepensis)
Abstract
1. Introduction
2. Materials and Methods
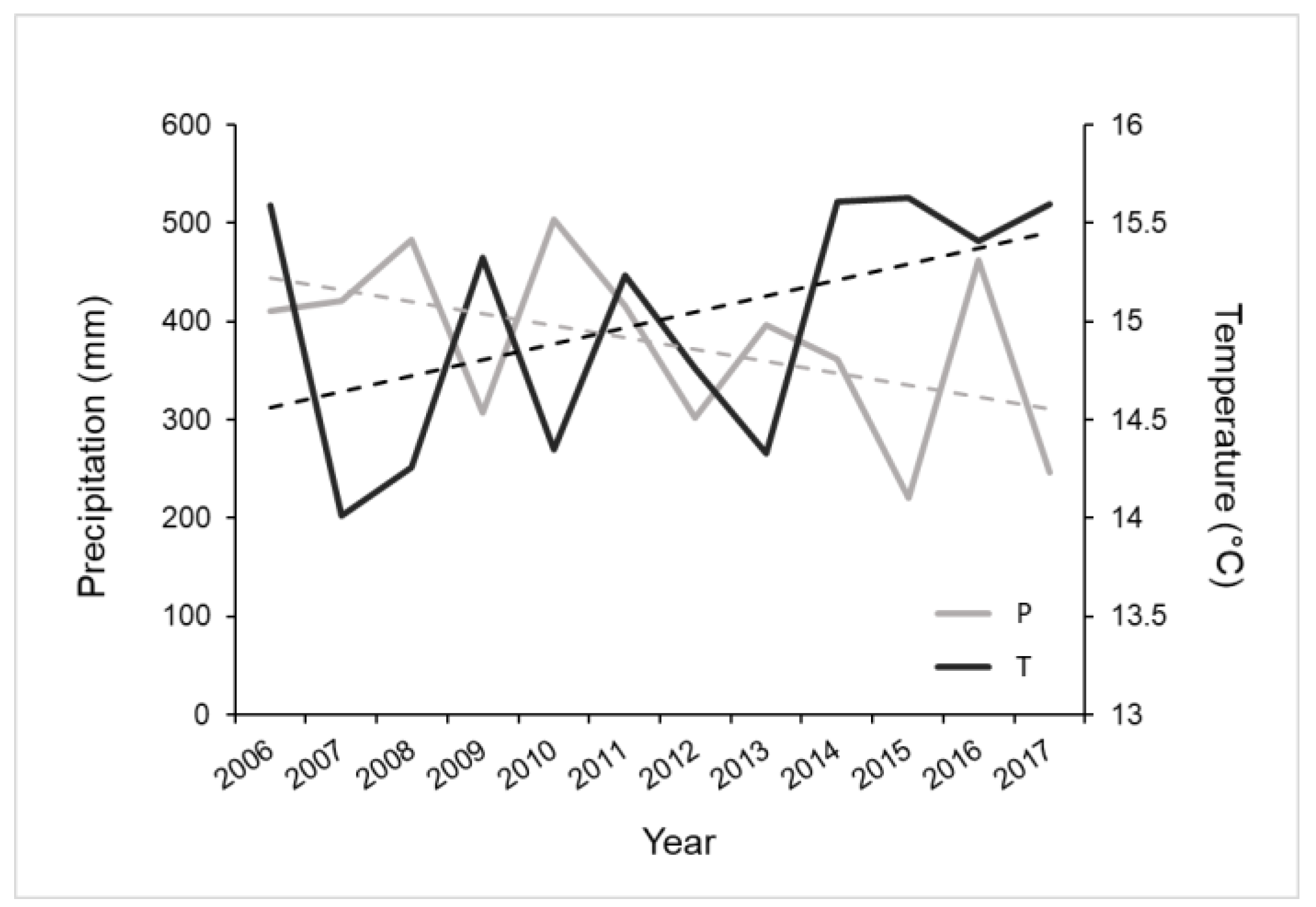
2.1. Experimental Set-Up
2.2. Measurements
2.2.1. The C. P. pini Infection PCR Test
2.2.2. Induced Defense Compounds
2.2.3. Mortality Rates
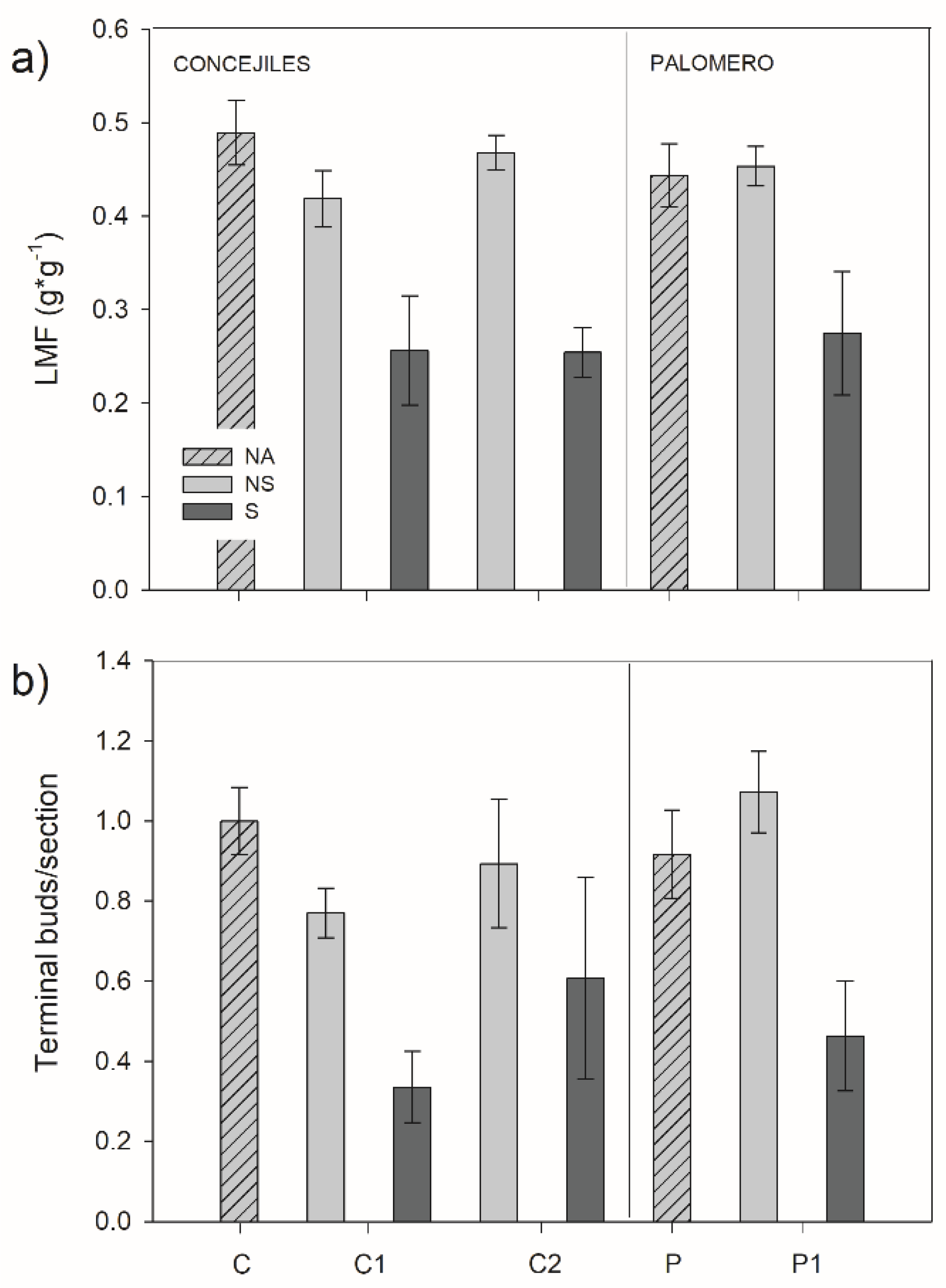
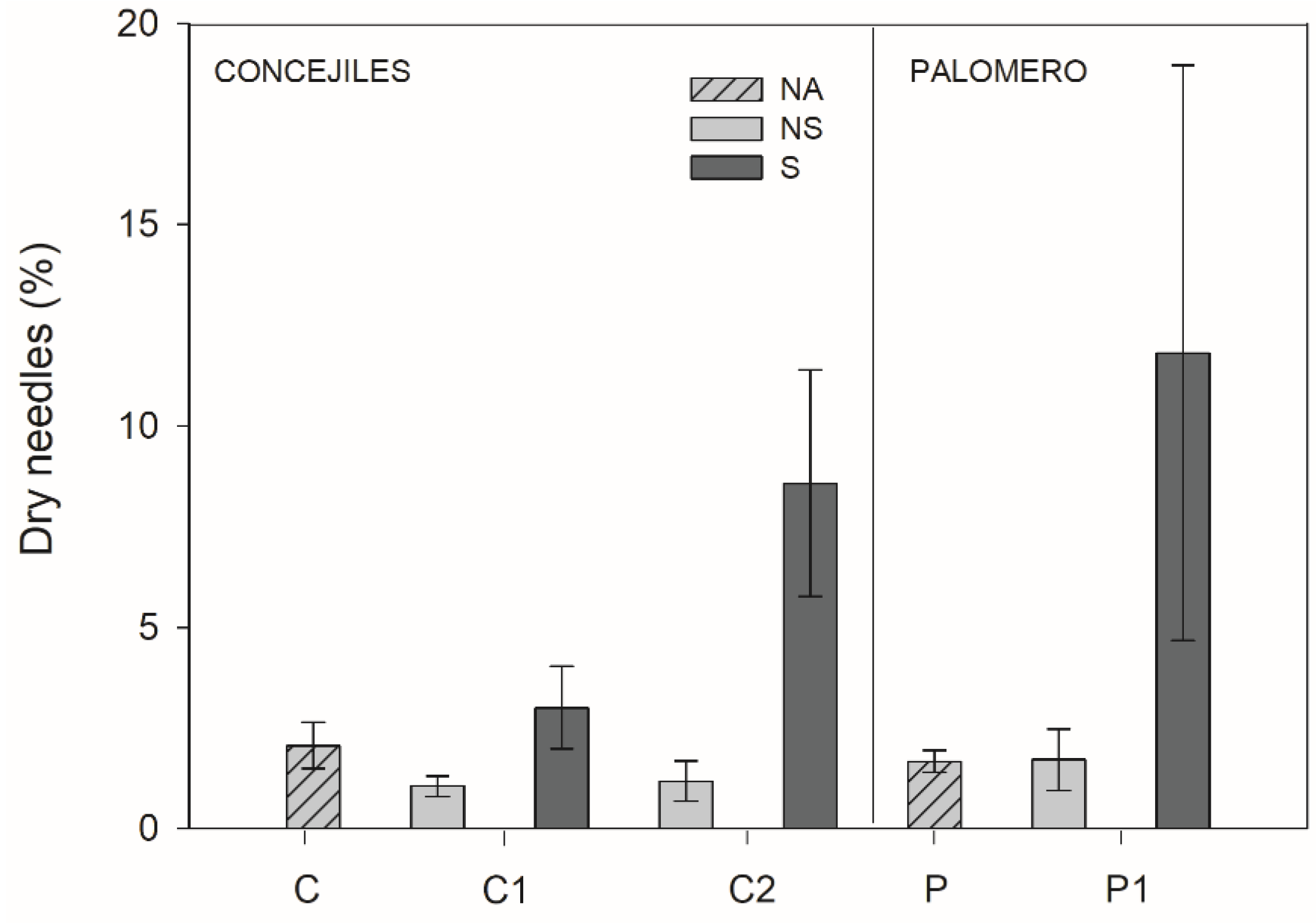
2.2.4. Morphological Traits
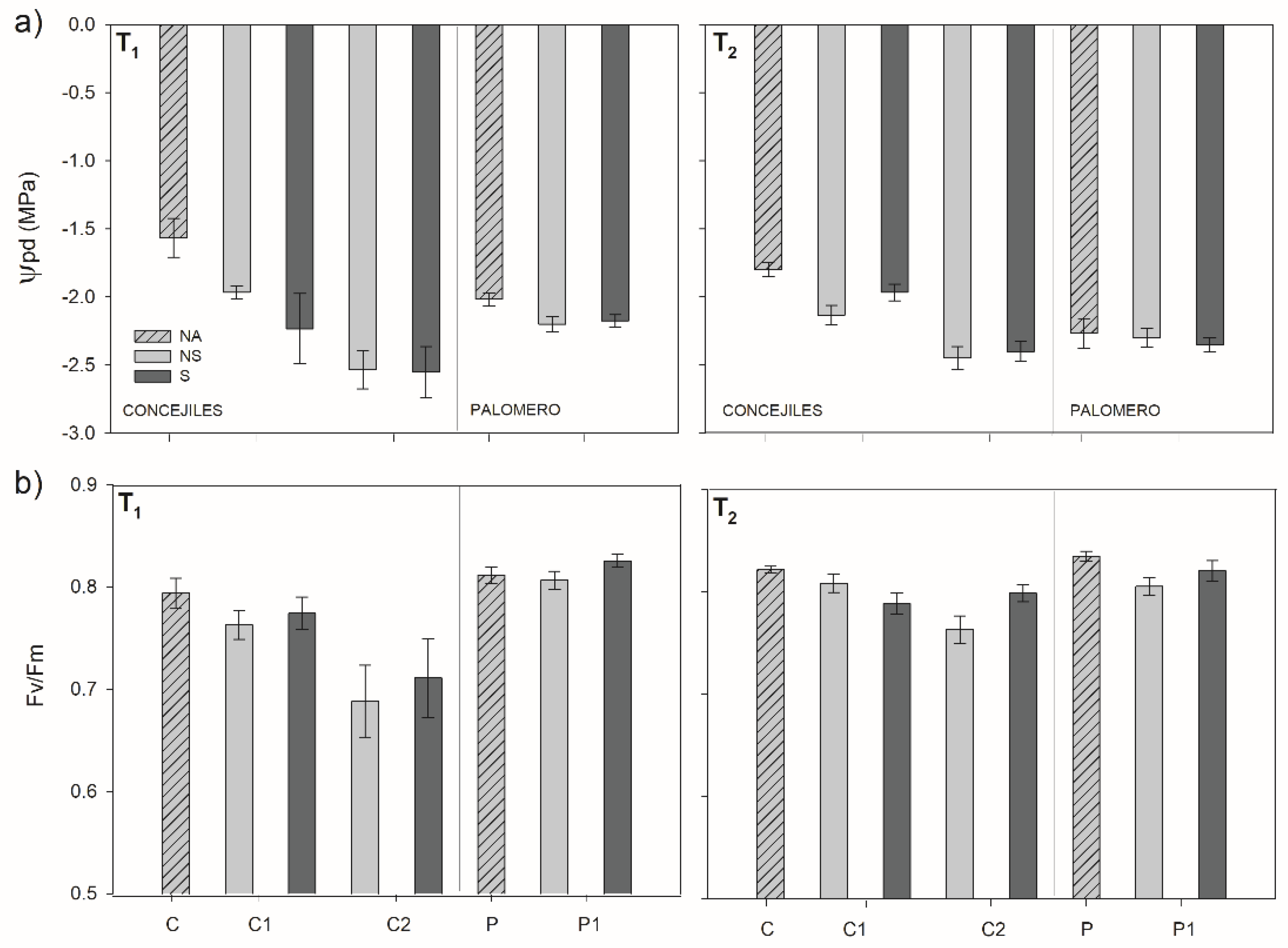
2.2.5. Ecophysiological Traits
2.2.6. Data Analysis
3. Results
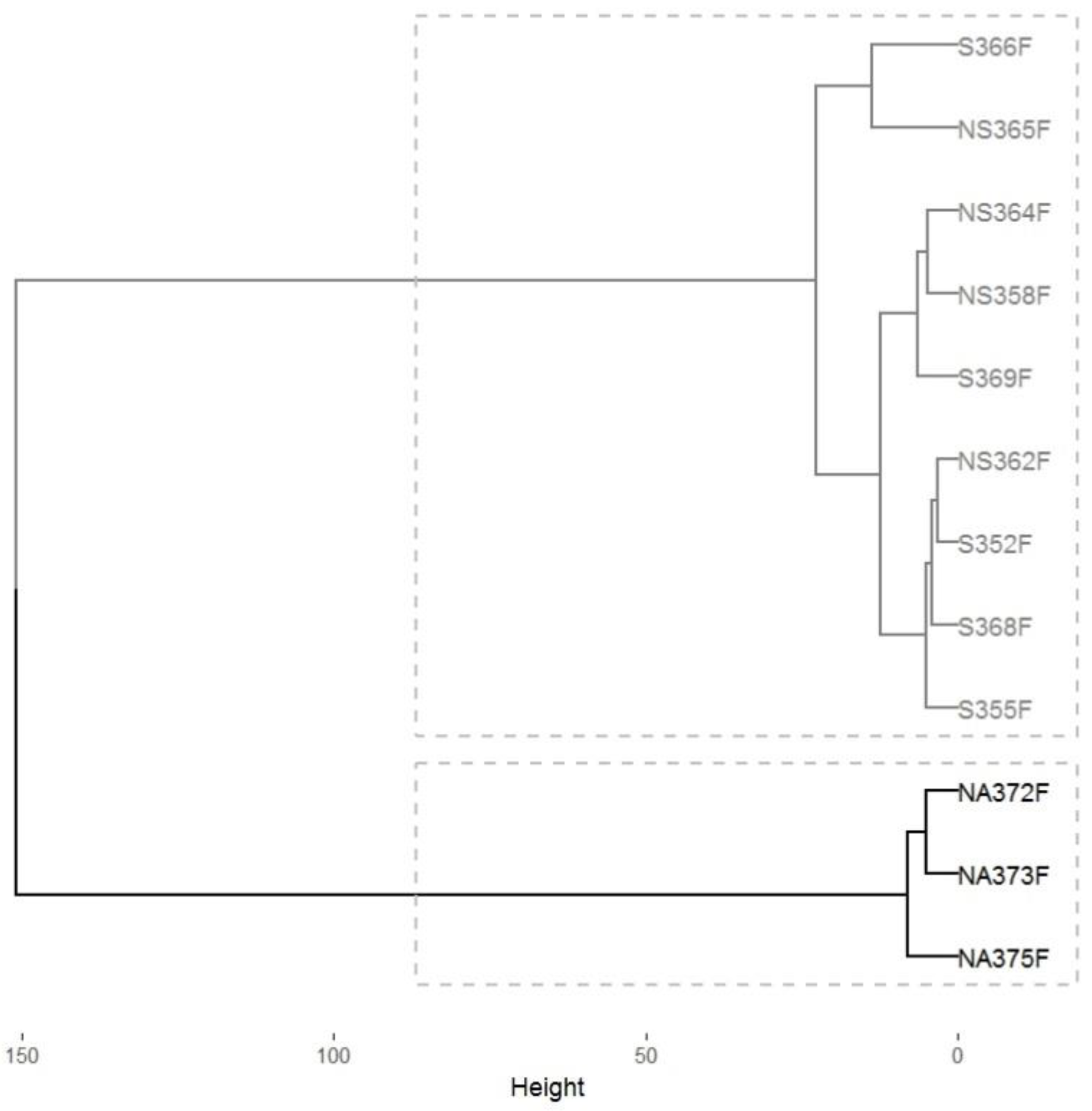
3.1. Analyzing C. P. pini Infection
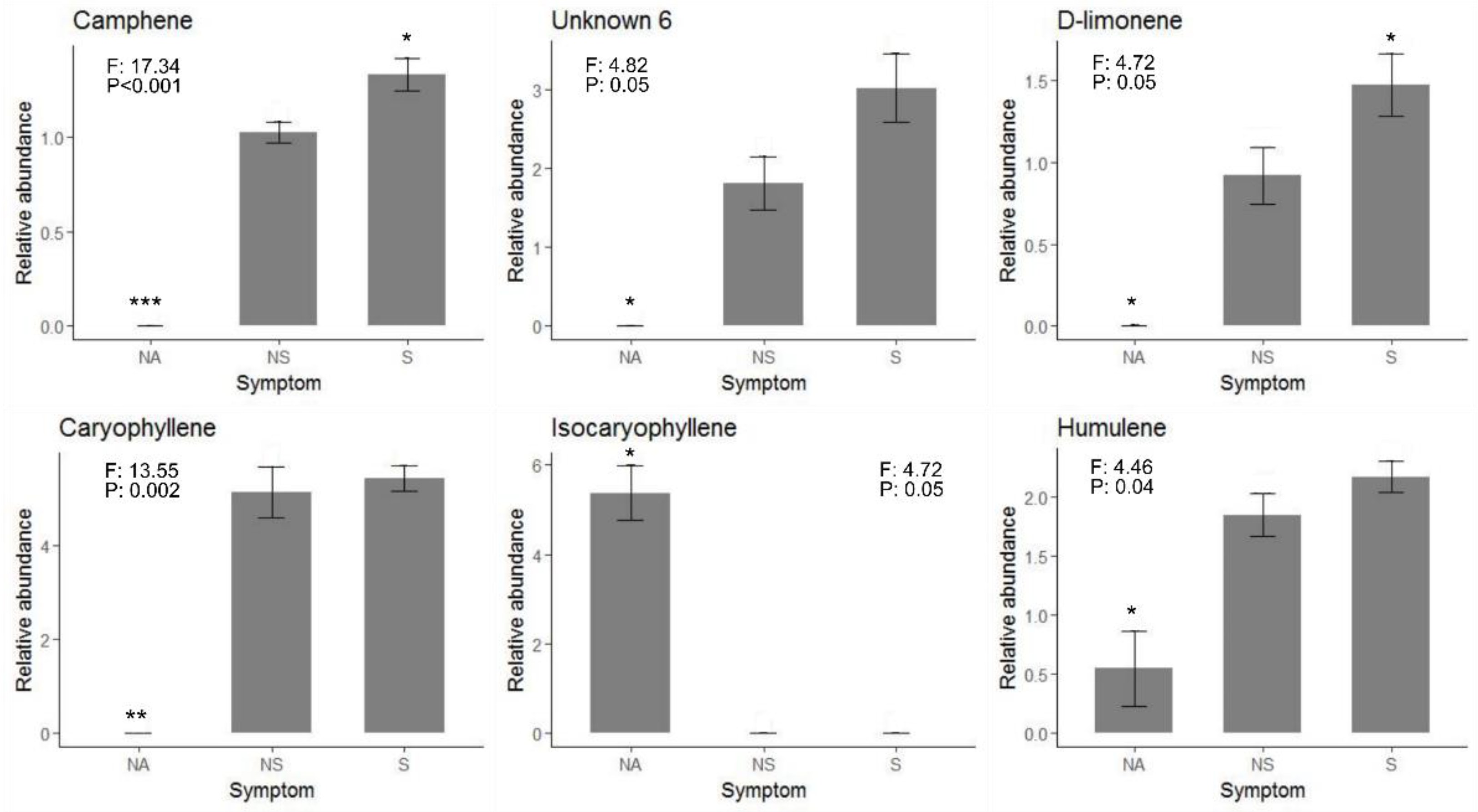
3.2. Induced Defense Compounds
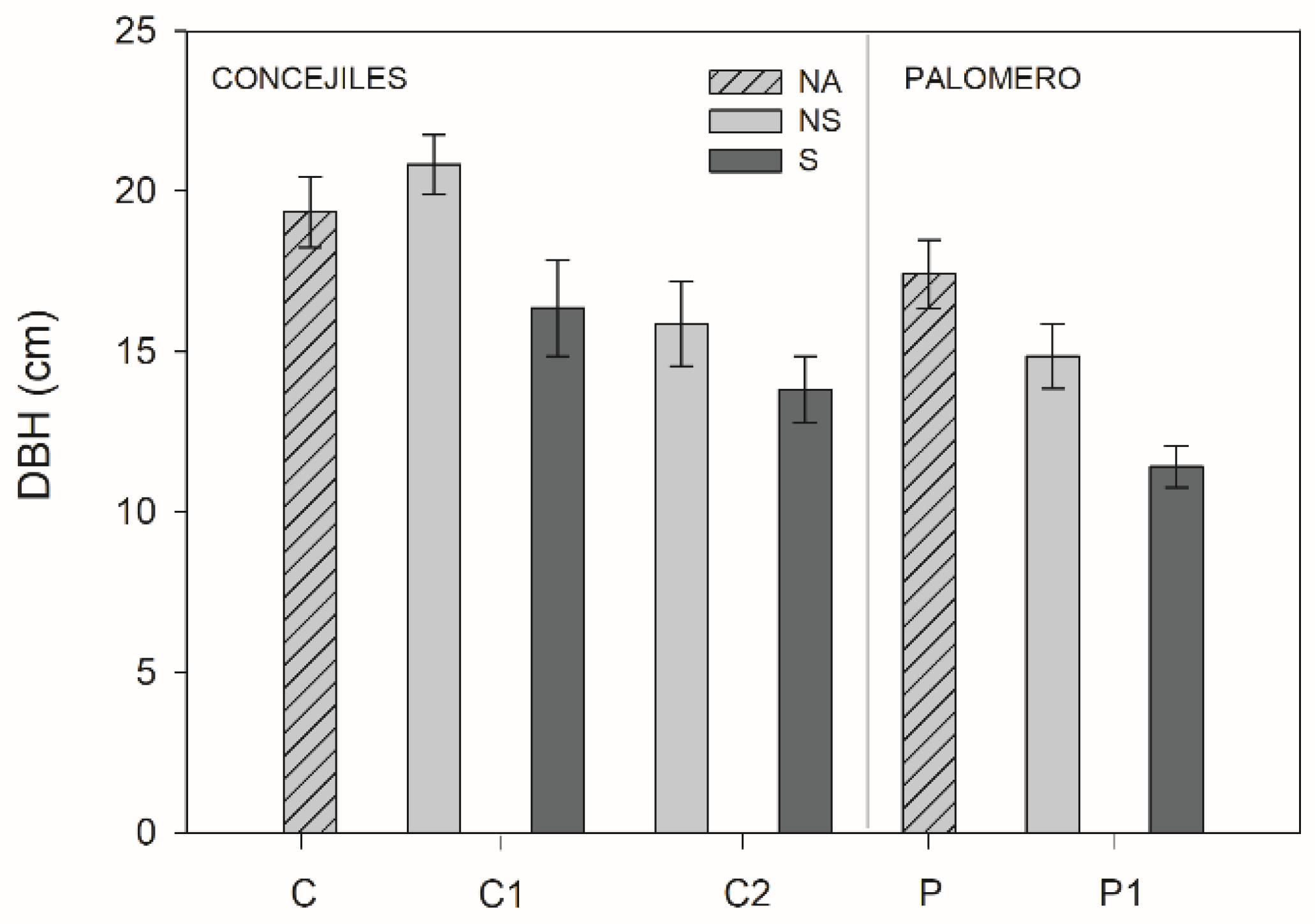
3.3. Morphological Traits
3.4. Plant Water Status and PSII Functionality
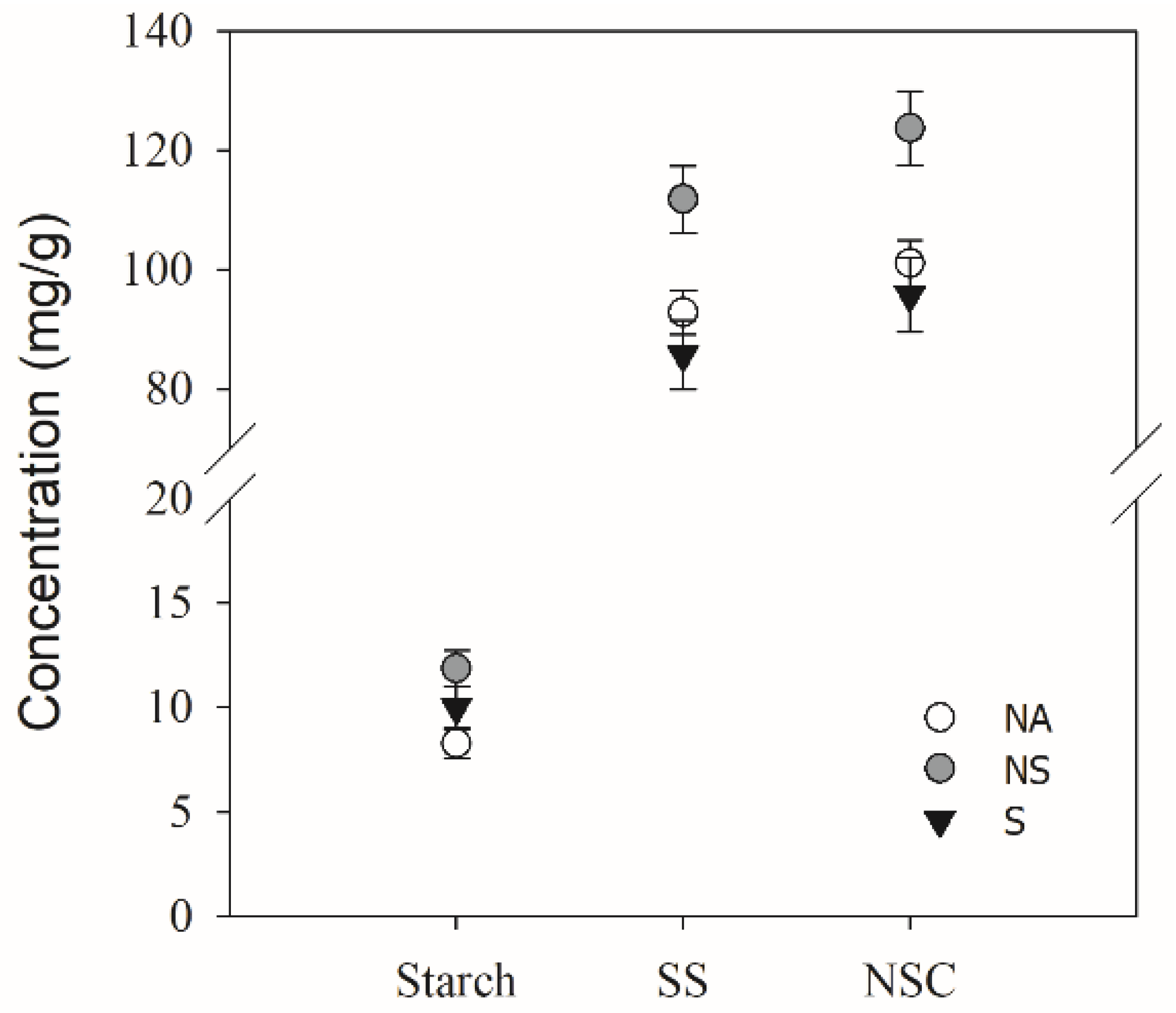
3.5. Carbohydrate Concentration
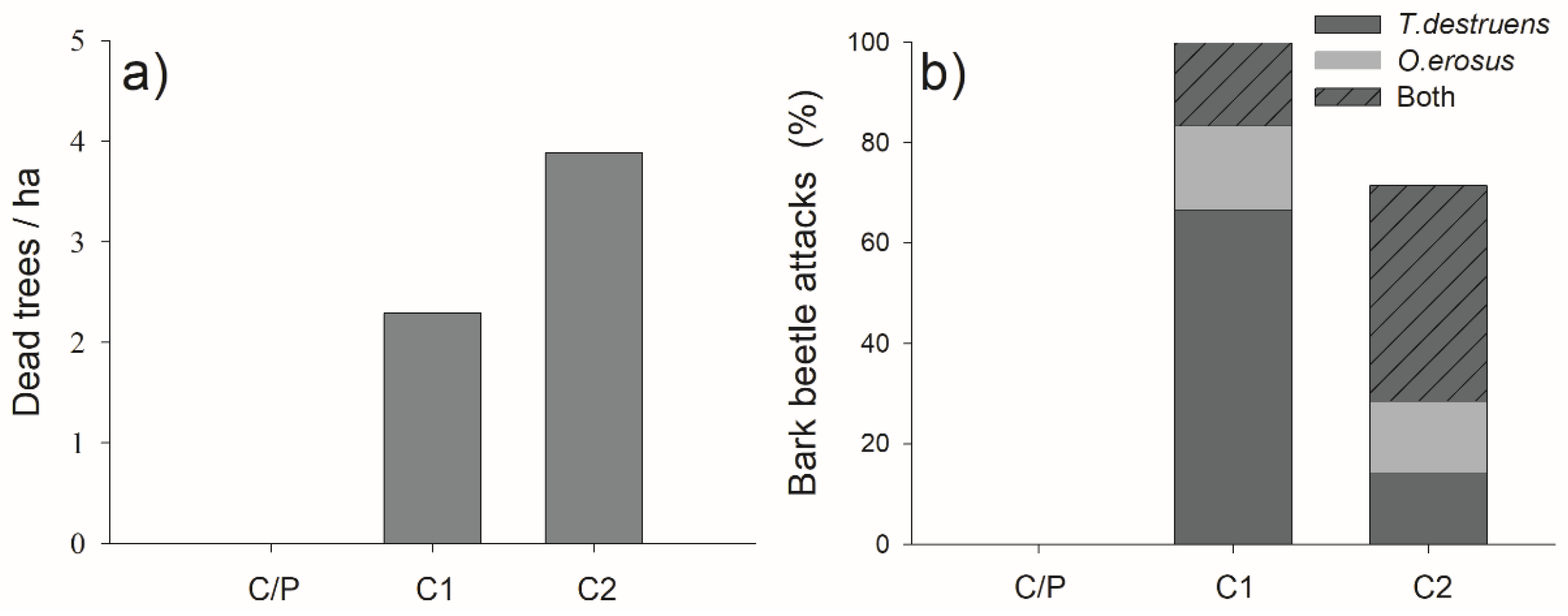
3.6. Pine Mortality and Bark Beetle Attacks
4. Discussion
4.1. Phytoplasma Infection and Induced Defense Mechanisms
4.2. Effects of C.P. pini Infection on the Morphological and Eco-Physiological Functional Traits
4.3. Bark Beetle Infection and Mortality Associated to C. P pini Disease
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change, (IPCC). Summary for Policymakers. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013; pp. 1–30. [Google Scholar]
- Peñuelas, J.; Filella, I.; Sabate, S.; Gracia, C. Natural systems: Terrestrial ecosystems. In Report on Climate Change in Catalonia; Llebot, J.E., Ed.; Institut d’Estudis Catalans: Barcelona, Spain, 2005; pp. 517–553. [Google Scholar]
- Giannakopoulos, C.; Le Sage, P.; Bindi, M.; Moriondo, M.; Kostopoulou, E.; Goodess, C.M. Climatic changes and associated impacts in the Mediterranean resulting from a 2 °C global warming. Glob. Planet. Chang 2009, 68, 209–224. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Gaylord, M.L.; Kolb, T.E.; Pockman, W.T.; Plaut, J.A.; Yepez, E.A.; Macalady, A.K.; McDowell, N.G. Drought predisposes piñon-juniper woodlands to insect attacks and mortality. New Phytol. 2013, 198, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Schuld, B.; Sanders, T.G.; Macinnis, C.; Boehmer, H.J.; Allen, C.D.; Bolte, A.; Crowther, T.W.; Hansen, M.C.; Medlyn, B.E.; et al. Monitoring global tree mortality patterns and trends. Report from the VW symposium Crossing scales and disciplines to identify global trends of tree mortality as indicators of forest health. New Phytol. 2018, 217, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 129. [Google Scholar] [CrossRef]
- Greenwood, S.; Ruiz-Benito, P.; Martínez-Vilalta, J. Tree mortality across biomes is promoted by drought intensity, lower wood density and higher specific leaf area. Ecol. Lett. 2017, 20, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Morillas, L.; Leuning, R.; Villagarcia, L.; Garcia, M.; Serrano-Ortiz, P.; Domingo, F. Improving evapotranspiration estimates in Mediterranean drylands: The role of soil evaporation. Water Resour. Res. 2013, 49, 6572–6586. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G.; Beerling, D.J.; Breshears, D.D.; Fisher, R.A.; Raffa, K.F.; Stitt, M. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol. Evol. 2011, 26, 523–532. [Google Scholar] [CrossRef]
- Vilagrosa, A.; Hernandez, E.I.; Luis, V.C.; Cochard, H.; Pausas, J.G. Physiological differences explain the co-existence of different regeneration strategies in Mediterranean ecosystems. New Phytol. 2014, 201, 1277–1288. [Google Scholar] [CrossRef]
- Grossiord, C.; Sevanto, S.; Borrego, I.; Chan, A.M.; Collins, A.D.; Dickman, L.T.; Hudson, P.J.; McBranch, N.; Michaletz, S.T.; Pockman, W.T.; et al. Tree water dynamics in a drying and warming world. Plant Cell Environ. 2017, 40, 1861–1873. [Google Scholar] [CrossRef] [PubMed]
- Vilagrosa, A.; Chirino, E.; Peguero-Pina, J.J.; Barigah, T.S.; Cochard, H.; Gil-Pelegrín, E. Xylem Cavitation and Embolism in Plants Living in Water-Limited Ecosystems. In Plants Response to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 63–109. [Google Scholar]
- Adams, H.D.; Zeppel, M.J.B.; Anderegg, W.R.L.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D.; et al. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Adams, H.D.; Anderegg, W.R.L.; Jansen, S.; Zeppel, M.J.B. Research frontiers in drought-induced tree mortality: Crossing scales and disciplines. New Phytol. 2015, 205, 965–969. [Google Scholar] [CrossRef] [PubMed]
- García de la Serrana, R.; Vilagrosa, A.; Alloza, J.A. Pine mortality in southeast Spain after an extreme dry and warm year: Interactions among drought stress, carbohydrates and bark beetle attack. Trees 2015, 29, 1791–1804. [Google Scholar] [CrossRef]
- Wong, C.M.; Daniels, L.D. Novel forest decline triggered by multiple interactions among climate, an introduced pathogen and bark beetles. Glob. Chang Biol. 2016, 23, 1926–1941. [Google Scholar] [CrossRef] [PubMed]
- Raffa, K.F.; Mason, C.J.; Bonello, P.; Cook, S.; Erbilgin, N.; Keefover-Ring, K.; Klutsch, J.G.; Villari, C.; Townsend, P.A. Defense sydromes in lodgepole-whitebark pine ecosystems related to degree of historical exposure to mountain pine beetles. Plant Cell Environ. 2017, 40, 1791–1806. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; Hussain, A.; Cale, J.A.; Erbilgin, N. Successful colonization of Lodgepole pine trees by mountain pine beetle increased monoterpene production and exhausted carbohydrate reserves. J. Chem. Ecol. 2018, 44, 209–214. [Google Scholar] [CrossRef]
- Savage, J.A.; Clearwater, M.J.; Haines, D.F.; Klein, T.; Mencuccini, M.; Sevanto, S.; Turgeon, R.; Zhang, C. Allocation, stress tolerance and carbon transport in plants: How does phloem physiology affect plant ecology? Plant Cell Environ. 2016, 39, 709–725. [Google Scholar] [CrossRef]
- Sevanto, S. Drought impacts on phloem transport. Curr. Opin. Plant Biol. 2018, 43, 76–81. [Google Scholar] [CrossRef]
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Turner, M.G.; Romme, W.H. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: The dynamics of bark beetle eruptions. BioScience 2008, 58, 501–517. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Hicke, J.A.; Fisher, R.A.; Allen, C.D.; Aukema, J.; Bentz, B.; Hood, S.; Lichstein, J.W.; Macalady, A.K.; McDowell, N.; et al. Tree mortality from drought, insects, and their interactions in a changing climate. New Phytol. 2015, 208, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Régnière, J.; Bentz, B. Modeling cold tolerance in the Mountain Pine Beetle, Dendroctonus ponderosae. J. Insect Physiol. 2007, 53, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Weed, A.S.; Ayres, M.P.; Hicke, J. Consequences of climate change for biotic disturbances in North American forests. Ecol. Monogr. 2013, 83, 441–470. [Google Scholar] [CrossRef]
- Netherer, S.; Matthews, B.; Katzensteiner, K.; Blackwell, E.; Henschke, P.; Hietz, P.; Schopf, A. Do water-limiting conditions predispose Norway spruce to bark beetle attack? New Phytol. 2015, 205, 1128–1141. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Ogaya, R.; Boada, M.; Jump, A.S. Migration, invasion and decline: Changes in recruitment and forest structure in a warming-linked shift of European beech forest in Catalonia (NE Spain). Ecography 2007, 30, 829–837. [Google Scholar] [CrossRef]
- Weintraub, P.G.; Beanland, L.A. Insect vectors of phytoplasmas. Annu. Rev. Entomol. 2006, 51, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Gallego, D.; Torres, E.; Campo, M.T. Candidatus Phytoplasma pini, posible agente causante de la fisiopatía de pino carrasco. In Proceedings of the 2nd Reunión Científica de Sanidad Forestal, Plasencia, Spain, 21–22 September 2011. [Google Scholar]
- Seemüller, E.; Garnier, M.; Schneider, B. Mycoplasmas of plants and insects. In Molecular Biology and Pathology of Mycoplasmas; Razin, S., Herrmann, R., Eds.; Kluwer Academic/Plenum Publishers: London, UK, 2002; pp. 91–116. [Google Scholar]
- Chang, C.J. Pathogenicity of Aster Yellows Phytoplasma and Spiroplasma citri on Periwinkle. Phytopathology 1988, 88, 1347–1350. [Google Scholar] [CrossRef]
- Lepka, P.; Stitt, M.; Moll, E.; Seemüller, E. Effect of phytoplasmal infection on concentration and translocation of carbohydrates and amino acids in periwinkle and tobacco. Physiol. Mol. Plant Pathol. 1999, 55, 59–68. [Google Scholar] [CrossRef]
- Bertamini, F.; Grando, M.S.; Muthuchelian, K.; Nedunchezhian, N. Effect of phytoplasmal infection on photosystem II efficiency and thylakoid membrane protein changes in field grown apple (Malus pumila) leaves. Physiol. Mol. Plant Pathol. 2002, 61, 349–356. [Google Scholar] [CrossRef]
- Kamińska, M.; Berniak, H.; Obdrzalek, J. New natural host plants of Candidatus Phytoplasma pini in Poland and the Czech Republic. Plant Pathol. 2011, 60, 1023–1029. [Google Scholar] [CrossRef]
- Schneider, B.; Torres, E.; Martin, M.P.; Schröder, M.; Behnke, H.D.; Seemüller, E. Candidatus Phytoplasma pini, a novel taxon from Pinus silvestris and Pinus halepensis. Int. J. Syst. Evol. Microbiol. 2005, 55, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Śliwa, H.; Kaminska, M.; Korszun, S.; Adler, P. Detection of Candidatus Phytoplasma pini in Pinus sylvestris trees in Poland. J. Phytopathol. 2008, 156, 88–92. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Gallego, D.; Sánchez-García, F.J.; Pajares, J.A. Ethanol accumulation during severe drought may signal tree vulnerability to detection and attack by bark beetles. Can. J. For. Res. 2014, 44, 554–561. [Google Scholar] [CrossRef]
- Vilagrosa, A.; Morales, F.; Abadía, A.; Bellot, J.C.; Cochard, H.; Gil-Pelegrine, E. Are symplast tolerance to intense drought conditions and xylem vulnerability to cavitation coordinated? An integrated analysis of photosynthetic, hydraulic and leaf level processes in two Mediterranean drought-resistant species. Environ. Exp. Bot. 2010, 69, 233–242. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; ISBN 3-900051-07-0. Available online: http://www.R-project.org (accessed on 30 May 2018).
- Valiunas, D.; Jomantiene, R.; Ivanauskas, A.; Urbonaite, I.; Sneideris, D.; Davis, E.R. Molecular identification of phytoplasmas infecting diseased pine trees in the UNESCO-protected Curonian Spit of Lithuania. Forests 2015, 6, 2469–2483. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Nordlander, G. Limonene inhibits attraction to alpha-pinene in the pine weevils Hylobius abietis and H. pinastri. J. Chem. Ecol. 1990, 16, 1307–1320. [Google Scholar] [CrossRef]
- Suga, T.; Ohtaa, S.; Munesada, K.; Ide, N.; Kurokawa, M.; Shimizu, M.; Ohta, E. Endogenous pine wood nematicidal substances in pines, Pinus massoniana, P. strobus and P. palustris. Phytochemistry 1993, 33, 1395–1401. [Google Scholar] [CrossRef]
- Klepzig, K.D.; Smalley, E.B.; Raffa, K.F. Interactions of ecologically similar saprogenic fungi with healthy and abiotically stressed conifers. Ecol. Manag. 1996, 86, 163–169. [Google Scholar] [CrossRef]
- Mita, E.; Tsitsimpikou, C.; Tsiveleka, L.; Petrakis, P.V.; Ortiz, A.; Vagias, C.; Roussis, V. Seasonal variation of oleoresin terpenoids from Pinus halepensis and Pinus pinea and host selection of the scale insect Marchalina hellenica (Homoptera, Coccoidea, Margarodidae, Coelostonidiinae). Holzforschung 2002, 56, 572–578. [Google Scholar] [CrossRef]
- Giunta, A.D.; Runyon, J.B.; Jenkins, M.J.; Teich, M. Volatile and within-needle terpene changes to Douglas-fir trees associated with Douglas-fir beetle (Coleoptera: Curculionidae) attack. Environ. Entomol. 2016, 45, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Achotegui-Castells, A.; Danti, R.; Llusià, J.; Della Rocca, G.; Barberini, S.; Peñuelas, J. Strong induction of minor terpenes in italian cypress, Cupressus sempervirens, in response to infection by the fungus Seiridium cardinale. J. Chem. Ecol. 2015, 41, 224–243. [Google Scholar] [CrossRef] [PubMed]
- Pastor, C. Deformaciones en Pino Relacionadas con Candidatus Phytoplasma Pini en la Comunidad Valenciana. Master’s Thesis, Universidad Politecnica de Valencia, Valencia, Spain, 2015. [Google Scholar]
- Baquedano, F.J.; Castillo, F.J. Comparative ecophysiological effects of drought on seedlings of the Mediterranean water-saver Pinus halepensis and water-spenders Quercus coccifera and Quercus ilex. Trees 2006, 20, 689. [Google Scholar] [CrossRef]
- Oliva, J.; Stenlid, J.; Martínez-Vilalta, J. The effect of fungal pathogens on the water and carbon economy of trees: Implications for drought-induced mortality. New Phytol. 2014, 203, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Galiano, L.; Martínez-Vilalta, J.; Lloret, F. Carbon reserves and canopy defoliation determine the recovery of Scots pine 4 year after a drought episode. New Phytol. 2011, 190, 750–759. [Google Scholar] [CrossRef]
- Aguadé, D.; Poyatos, R.; Gómez, M.; Oliva, J.; Martínez-Vilalta, J. The role of defoliation and root rot pathogen infection in driving the mode of drought-related physiological decline in Scots pine (Pinus sylvestris L.). Tree Physiol. 2015, 35, 229–242. [Google Scholar] [CrossRef]
- Hartmann, H.; Ziegler, W.; Trumbore, S. Lethal drought leads to reduction in nonstructural carbohydrates in Norway spruce tree roots but not in the canopy. Funct. Ecol. 2013, 27, 413–427. [Google Scholar] [CrossRef]
- Mitchell, P.J.; O’Grady, A.P.; Tissue, D.T.; White, D.A.; Ottenschlaeger, M.L.; Pinkard, E.A. Drought response strategies define the relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality. New Phytol. 2013, 197, 862–872. [Google Scholar] [CrossRef]
- Klein, T.; Hoch, G.; Yakir, D.; Körner, C. Drought stress, growth and nonstructural carbohydrate dynamics of pine trees in a semi-arid forest. Tree Physiol. 2014, 34, 981–992. [Google Scholar] [CrossRef]
- Goodsman, D.W.; Lusebrink, I.; Landhäusser, S.M.; Erbilgin, N.; Lieffers, V.J. Variation in carbon availability, defense chemistry and susceptibility to fungal invasion along the stems of mature trees. New Phytol. 2013, 197, 586–594. [Google Scholar] [CrossRef]
- Piper, F.I.; Fajardo, A.; Hoch, G. Single-provenance mature conifers show higher non-structural carbohydrate storage and reduced growth in a drier location. Tree Physiol. 2017, 37, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Croisé, L.; Lieutier, F. Effects of drought on the induced defence reaction of Scots pine to bark beetle-associated fungi. Ann. Sci. 1993, 50, 91–97. [Google Scholar] [CrossRef]
- Bonello, P.; Gordon, T.R.; Herms, D.A.; Wood, D.L.; Erbilgin, N. Nature and ecological implications of pathogen-induced systemic resistance in conifers: A novel hypothesis. Physiol. Mol. Plant Pathol. 2006, 68, 95–104. [Google Scholar] [CrossRef]
- Viiri, H.; Annila, E.; Kitunen, V.; Niemela, P. Induced responses in stilbenes and terpenes in fertilized Norway spruce after inoculation with blue-stain fungus. Ceratocystis polonica. Trees 2001, 15, 112–122. [Google Scholar] [CrossRef]
- Croteau, R.; Loomis, W.D. Biosynthesis and metabolism of monoterpenes. Int. Flavours Food Addit. 1975, 6, 292–296. [Google Scholar]
- Waring, R.H.; Pitman, G.B. Physiological stress in Lodgepole pine as a precursor for Mountain pine beetle attack. Z. Angew. Entomol. 1983, 96, 265–270. [Google Scholar] [CrossRef]
- Mulock, P.; Christiansen, E. The threshold of successful attack by Ips typographus on Picea abies: A field experiment. Ecol. Manag. 1986, 14, 125–132. [Google Scholar] [CrossRef]
| Parameters | Mean ± Error |
|---|---|
| OM (%) | 0.81 ± 0.44 |
| N (%) | 0.19 ± 0.00 |
| P (ppm) | 13.2 ± 1.9 |
| K (ppm) | 22.4 ± 8.5 |
| Fe (%) | 0.63 ± 0.18 |
| Carbonates (%) | 42.6 ± 5.9 |
| Stoniness (%) | 30.2 ± 5.1 |
| Texture: | |
| Sand (%) | 32.4 ± 2.7 |
| Silt + Clay (%) | 68.7 ± 3.2 |
| Variables | Disease Degree F (P) |
|---|---|
| DBH | 0.93 (0.343) |
| LMF | 0.47 (0.500) |
| Terminal buds | 0.00 (0.948) |
| Dry needles | 0.93 (0.343) |
| WPpd | T1: 13.91 (0.001) |
| T2: 9.89 (0.004) | |
| Fv/Fm | T1: 3.37 (0.078) |
| T2: 11.01 (0.003) | |
| Starch | 7.35 (0.015) |
| Soluble sugars | 4.66 (0.046) |
| Total NSC | 5.66 (0.030) |
| Variables | Stand (S) F (P) | Disease Degree (D) F (P) | S × D F (P) | Post hoc Stand |
|---|---|---|---|---|
| DBH | 12.80 (<0.001) | 13.60 (0.001) | 0.61 (0.549) | P1 = C2 ≤ C1 |
| LMF | 0.25 (0.779) | 30.00 (<0.001) | 0.20 (0.821) | nd |
| Terminal buds | 1.31 (0.284) | 13.62 (0.001) | 0.60 (0.554) | nd |
| Dry needles | 1.12 (0.340) | 6.21 (0.018) | 0.85 (0.439) | nd |
| WPpd | T1: 5.09 (0.012) | 0.52 (0.477) | 0.58 (0.567) | C2 ≤ P1 ≤ C1 |
| T2: 10.71 (<0.001) | 0.05 (0.824) | 1.84 (0.190) | C2 ≤ P1 ≤C1 | |
| Fv/Fm | T1: 12.54 (<0.001) | 0.85 (0.362) | 0.03 (0.970) | C2 < C1, P1 |
| T2: 3.03 (0.076) | 0.39 (0.540) | 0.87 (0.438) | nd | |
| Starch | 3.04 (0.076) | 2.47 (0.136) | 0.64 (0.542) | nd |
| Soluble sugars | 4.88 (0.022) | 18.69 (0.001) | 2.02 (0.166) | nd |
| Total NSC | 5.73 (0.013) | 19.18 (<0.001) | 2.20 (0.143) | C1 ≤ P1≤ C2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morcillo, L.; Gallego, D.; González, E.; Vilagrosa, A. Forest Decline Triggered by Phloem Parasitism-Related Biotic Factors in Aleppo Pine (Pinus halepensis). Forests 2019, 10, 608. https://doi.org/10.3390/f10080608
Morcillo L, Gallego D, González E, Vilagrosa A. Forest Decline Triggered by Phloem Parasitism-Related Biotic Factors in Aleppo Pine (Pinus halepensis). Forests. 2019; 10(8):608. https://doi.org/10.3390/f10080608
Chicago/Turabian StyleMorcillo, Luna, Diego Gallego, Eudaldo González, and Alberto Vilagrosa. 2019. "Forest Decline Triggered by Phloem Parasitism-Related Biotic Factors in Aleppo Pine (Pinus halepensis)" Forests 10, no. 8: 608. https://doi.org/10.3390/f10080608
APA StyleMorcillo, L., Gallego, D., González, E., & Vilagrosa, A. (2019). Forest Decline Triggered by Phloem Parasitism-Related Biotic Factors in Aleppo Pine (Pinus halepensis). Forests, 10(8), 608. https://doi.org/10.3390/f10080608






